Abstract
MET amplification is a frequently observed genomic aberration in solid tumors. We conducted a phase I trial to evaluate dose-limiting toxicity (DLT) and recommended phase II dose (RP2D) for the combination therapy. The following dose levels were tested in this single-arm phase I study: docetaxel as an intravenous infusion over 1 hour at 60 mg/m2 once every 3 weeks of a 21-day schedule plus savolitinib (level 1, 200 mg qd; level 2, 400 mg qd; level 3, 600 mg qd; level 4800 mg qd). In total, there were 17 patients enrolled on to this study [7 gastric cancer (GC) patients, 5 melanoma patients, 3 sarcoma patients, and 2 rectal cancer patients]. Most of the patients (14 of 17) were heavily pretreated (≥third line or greater lines of treatment). For the first 3 cohorts (200 mg savolitinib + docetaxel 60 mg/m2, 400 mg savolitinib + docetaxel 60 mg/m2, 600 mg savolitinib + docetaxel 60 mg/m2), there were no DLTs. In the fourth dose cohort (800 mg savolitinib + docetaxel 60 mg/m2), one DLT occurred with generalized edema grade 3 that required intensive management. One GC patient with both MET overexpression (3+) and MET amplification (MET/CEP7 ratio, 7.3) achieved a durable partial response for 297 days, and another MET-amplified GC patient (MET/CEP7 ratio, 7.6) achieved stable disease for 86 days. Due to the higher incidence of G4 neutropenia in cohort 4 (800 mg), we recommend savolitinib 600 mg qd in combination with docetaxel 60 mg/m2 as the RP2D for phase II trial. The combination therapy demonstrated a very promising antitumor activity with durable responses in MET amplified GC patients.
Introduction
Mesenchymal epithelial transition factor (c-MET) is a tyrosine kinase receptor that, along with hepatocyte growth factor (HGF) as its ligand, is involved in multiple cellular processes including carcinogenesis and tumor progression in various tumors including gastric cancer (GC) [1], [2], [3]. Studies demonstrate that many types of human cancers, including gastric, colorectal, renal, breast, pancreatic, lung, thyroid, and hepatocellular carcinoma, have inappropriate activation of the MET pathway due to elevated HGF expression or due to overexpression, amplification, or activating mutations of the MET gene [4], [5]. Given that MET plays a critical role in cancer progression, inhibition of MET could have a considerable impact on the treatment of solid cancer patients with aberrant MET pathway. For instance, approximately 5% of GC patients have increased copy numbers of the MET gene [2], [3], [6], [7], [8], [9], [10].
Savolitinib (AZD6094, HMPL-504, volitinib) is a potent and selective small molecule MET kinase inhibitor which inhibits MET kinase at the enzyme and cell levels with IC50s of 4 nM for both enzyme and MET phosphorylation in the cell. Consistent with its potent enzyme and cell activity, savolitinib was found to inhibit cell growth in vitro against tumors with MET gene amplification in the absence of HGF stimulation, with IC50s generally below 10 nM. Savolitinib is currently being investigated as a targeted therapy for patients with non–small-cell lung cancer in combination with osimertinib and as a monotherapy for patients with advanced or metastatic papillary renal cell carcinoma (PRCC) [11]. Of 44 MET-driven PRCC patients, there were 8 confirmed partial responders in a recent phase II trial [11]. Based on this trial, the phase III SAVOIR study which compares sunitinib versus savolitinib is currently ongoing in MET-amplified PRCC (ClinicalTrial.gov.Identifier: NCT# 03091192).
The most commonly used salvage chemotherapy regimens in metastatic GC were docetaxel, weekly paclitaxel, or irinotecan at the time of study design [12]. Combined volitinib and docetaxel therapy also showed efficacy benefit in cMET dysregulated xenograft models [13]. Hence, we designed a dose-finding phase I study to evaluate the maximal tolerated dose (MTD) of savolitinib in combination with a fixed dose of docetaxel in refractory cancer patients.
Patients and Methods
Patients
Patients enrolled in this study had measurable, histologically confirmed refractory metastatic solid cancer. The trial was conducted in accordance with the Declaration of Helsinki and the Guidelines for Good Clinical Practice (ClinicalTrial.gov.Identifier: NCT# 02447406). The trial protocol was approved by the institutional review board of Samsung Medical Center (Seoul, Korea), and all patients provided written informed consent before enrolment. This trial was part of the VIKTORY trial—targeted agent eValuation in gastrIc cancer basKeT kORean studY (ClinicalTrial.gov.Identifier: NCT# 02299648). The phase I component of the trial was in unselected patients who had histologically or cytologically confirmed diagnosis of relapsed or refractory locally advanced or metastatic solid tumors for whom no alternative effective standard therapy was available or for whom standard therapy is considered unsuitable or intolerable, as the primary aim was to assess safety and establish the RP2D. To be eligible to participate in this study, patients were required to be ≥20 years old, have at least one measurable lesion according to Response Evaluation Criteria in Solid Tumors 1.1, and have an Eastern Cooperative Oncology Group performance status of 0 or 1 (7). Adequate hematologic function, hepatic function, and renal function were required.
Study Design and Treatment
This prospective open-label trial was designed as a single-arm phase I study at an academic cancer center. Treatment was administered as follows: docetaxel as an intravenous infusion over 1 hour at 60 mg/m2 once every 3 weeks of a 21-day schedule plus savolitinib at the following dose levels: level 1, 200 mg qd; level 2, 400 mg qd; level 3, 600 mg qd; level 4800 mg qd Up to 15 subjects were planned to be enrolled in the phase Ib component of the study, with the final sample size being dependent on the number of subjects who experienced dose-limiting toxicities (DLTs), the safety data at each dose level based on DLTs, and other safety data. Savolitinib was administered according to a modified Fibonacci design, following the conventional 3 + 3 design. DLT was evaluated during the first cycle of treatment for each patient.
Definition of DLT
Hematologic toxicity was defined as grade 4 neutropenia lasting >5 days, febrile neutropenia of any grade or duration as defined by National Cancer Institute Common Terminology Criteria for Adverse Events 4.0, platelets <25 × 103/μl or platelets <50 × 103/μl with bleeding requiring medical intervention, or grade 4 anemia. Nonhematologic toxicity was defined as any grade 4 nonhematologic adverse events, grade ≥ 3 headache lasting ≥7 days despite optimal supportive care, grade ≥ 3 fatigue, grade ≥ 3 edema lasting ≥7 days despite prophylactic and/or symptomatic treatment, and grade ≥ 3 abnormal liver function tests.
Tumor and Toxicity Assessment
At baseline, the medical history, physical examination, blood tests, urinalysis, electrocardiography, echocardiogram, chest X-ray, and abdomen and pelvis computed tomography scan results of the patients were reviewed. Physical examinations, chest X-rays, and blood tests were repeated before beginning each cycle of chemotherapy. Tumor responses were evaluated every two cycles according to the Response Evaluation Criteria in Solid Tumors 1.1 criteria. Toxicities were graded based on the National Cancer Institute Common Terminology Criteria for Adverse Events 4.0.
MET Immunohistochemistry (IHC)
MET IHC was performed using the rabbit monoclonal primary antibody, CONFIRM anti-total MET (SP44) (Ventana Medical Systems, Tucson, AZ), and the Ventana BenchMark XT automated slide processing system (Ventana Medical Systems) according to the manufacturer's protocol. The results were evaluated by an expert pathologist (K.M.K.) without prior knowledge of the clinicopathological or molecular data. For MET, we applied a scoring system for GC that we developed as previously described [2]. MET overexpression was defined as 2+ or 3+ according to our previously published criteria [2].
MET Fluorescent In Situ Hybridization (FISH)
FISH was performed using dual-color DNA-specific MET/CEP7 probes (Abnova, Walnut, CA) as described previously [2], [3]. A pathologist counted the numbers of MET and chromosome 7 centromere probe (CEP7) signals (1 for individual signals, 6 for small clusters, and 12 for big clusters) in 20 interphase tumor cell nuclei, and the mean number of MET and CEP7 copies per nucleus were determined, along with the ratio. Normal MET/CEP7 signals (one to two copies per cell) in the various non-neoplastic cells served as the internal positive control. We defined MET gene amplification as a MET/CEP7 ratio >2.0 in 20 tumor nuclei, and polysomy-7 was regarded as negative for gene amplification.
Results
Patient Characteristics and Dose Escalation
Patient characteristics are provided in Table 1. In total, there were 17 patients enrolled on to this study. Of the 17 patients, there were 7 GC patients, 5 melanoma patients, 3 sarcoma patients, and 2 rectal cancer patients. Of 17 patients, 14 (82.4%) patients received the study regimen as third line or greater lines of treatment, suggesting that most of the patients were heavily pretreated. One patient withdrew consent before study drug administration (cohort 4), and one patient was not evaluable for DLT since <75% of the planned dose was administered (cohort 3). For the first 3 cohorts (200 mg savolitinib + docetaxel 60 mg/m2, 400 mg savolitinib + docetaxel 60 mg/m2, 600 mg savolitinib + docetaxel 60 mg/m2), there were no DLTs (Table 2). In the fourth dose cohort (800 mg savolitinib + docetaxel 60 mg/m2), one DLT occurred with generalized edema grade 3 that required intensive management, but the patient fully recovered 2 weeks after cessation of the investigational drugs. After recruiting three more patients in cohort 4, no DLTs were observed.
Table 1.
Baseline Characteristics and Treatment Outcome
| No | Subject # | Gender | Age | Disease | Pathology | Lines of Treatment |
|---|---|---|---|---|---|---|
| 1 | B6_001 | M | 46 | Gastric cancer | Moderate differentiated adenocarcinoma | 4th |
| 2 | B6_002 | M | 58 | Gastric cancer | Moderate differentiated adenocarcinoma | 4th |
| 3 | B6_003 | M | 59 | Gastric cancer | Moderate differentiated adenocarcinoma | 3rd |
| 4 | B6_004 | F | 42 | Gastric cancer | Poorly differentiated tubular adenocarcinoma | 2nd |
| 5 | B6_005 | F | 48 | Rectal cancer | Moderate differentiated adenocarcinoma | 6th |
| 6 | B6_006 | M | 22 | Sarcoma | Fibrous histiocytoma | 6th |
| 7 | B6_007 | M | 45 | Rectal cancer | Moderate differentiated adenocarcinoma | 6th |
| 8 | B6_008 | M | 34 | Gastric cancer | Poorly differentiated adenocarcinoma | 2nd |
| 9 | B6_009 | F | 33 | Sarcoma | Leiomyosarcoma | 7th |
| 10 | B6_010 | M | 48 | Melanoma | Melanoma | 3rd |
| 11 | B6_011 | M | 68 | Melanoma | Melanoma | 3rd |
| 12 | B6_012 | F | 48 | Melanoma | Melanoma | 3rd |
| 13 | B6_013 | M | 44 | Sarcoma | Angiosarcoma | 3rd |
| 14 | B6_015 | M | 48 | Gastric cancer | Signet ring cell carcinoma | 3rd |
| 15 | B6_016 | M | 52 | Melanoma | Melanoma | 4th |
| 16 | B6_017 | M | 52 | Melanoma | Melanoma | 3rd |
| 17 | B6_018 | M | 68 | Gastric cancer | Poorly differentiated adenocarcinoma | 2nd |
Table 2.
Treatment Outcome
| Cohort | Subject # | MET IHC | MTE FISH (MET:CEP7) | DLT | Best Response | Duration of Treatment (Days) |
|---|---|---|---|---|---|---|
| 1 (200 mg) |
B6_001 | 1 + | - | None | NE | 34 |
| B6_002 | 2 + | - | None | SD | 93 | |
| B6_003 | 3 + | 7.6 | None | SD | 86 | |
| B6_004 | 3 + | 7.3 | None | PR | 297 | |
| 2 (400 mg) |
B6_005 | 2 + | - | None | PD | 42 |
| B6_006 | NA | - | None | SD | 126 | |
| B6_007 | 1 + | - | None | PD | 82 | |
| 3 (600 mg) |
B6_008 | 3 + | 1.6 | None | PD | 48 |
| B6_009 | 0 | - | None | SD | 217 | |
| B6_010 | 1 + | - | None | NE | 30 | |
| B6_011 | 0 | - | None | PD | 21 | |
| 4 (800 mg) |
B6_012 | 0 | - | Yes | PD | 14 |
| B6_013 | NA | - | None | PD | 34 | |
| B6_015 | 0 | - | None | SD | 231 | |
| B6_016 | 1 + | - | None | PD | 45 | |
| B6_017 | NA | - | None | PD | 28 | |
| B6_018 | 3 + | 1.0 | None | PD | 41 |
NA, not available; NE, not evaluable.
For toxicity, the most commonly detected toxicity was neutropenia (Table 3). The protocol allowed G-CSF support once neutropenia was detected, and the patient was followed up every 1 to 3 days until recovery from neutropenia was observed. Although the incidence of grade 3 or grade 4 neutropenia was high, there was no episode of neutropenic fever in this study. In addition, in the 800-mg cohort, the incidence of toxicity was increased especially pyrexia, generalized edema, and grade 4 neutropenia (nonfebrile). All incidences of pyrexia were fully reversed when savolitinib was discontinued. After dose reduction of savolitinib in two patients, pyrexia was no longer observed in the patient who experienced drug fever. The fever pattern in two patients was clinically similar: no infectious sign, no chilling sensation, and no alterations in other vital signs except for body temperature. Based on these dose escalation results, we concluded the dose level to be savolitinib 600 mg + docetaxel 60 mg/m2 as the recommended phase II dose and schedule.
Table 3.
Toxicity Profile
| Dose Level | Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|---|
| 200 mg | Oral mucositis | 1 | 1 | 0 | 0 |
| Fatigue | 1 | 2 | 0 | 0 | |
| Rash | 0 | 1 | 0 | 0 | |
| Neutropenia | 0 | 1 | 2 | 3* | |
| Vomit | 1 | 0 | 0 | 0 | |
| 400 mg | Vomit | 1 | 0 | 0 | 0 |
| Neutropenia | 1 | 0 | 2 | 1 | |
| Peripheral edema | 0 | 1 | 0 | 0 | |
| Myalgia | 3 | 0 | 0 | 0 | |
| Skin rash | 1 | 0 | 0 | 0 | |
| 600 mg | Pruritis | 1 | 0 | 0 | 0 |
| Neutropenia | 0 | 0 | 4 | 2 | |
| General weakness | 1 | 0 | 0 | 0 | |
| Peripheral edema | 0 | 1 | 0 | 0 | |
| 800 mg | Pyrexia | 1 | 1 | 0 | 0 |
| Rash | 1 | 0 | 0 | 0 | |
| Generalized edema | 0 | 0 | 1 | 0 | |
| Anorexia | 1 | 0 | 0 | 0 | |
| Headache | 2 | 0 | 0 | 0 | |
| Diarrhea | 1 | 0 | 0 | 0 | |
| Nausea/vomit | 3 | 0 | 0 | 0 | |
| Neutropenia | 0 | 0 | 1 | 4* |
G4 neutropenia not lasting >5 days.
Drug Efficacy
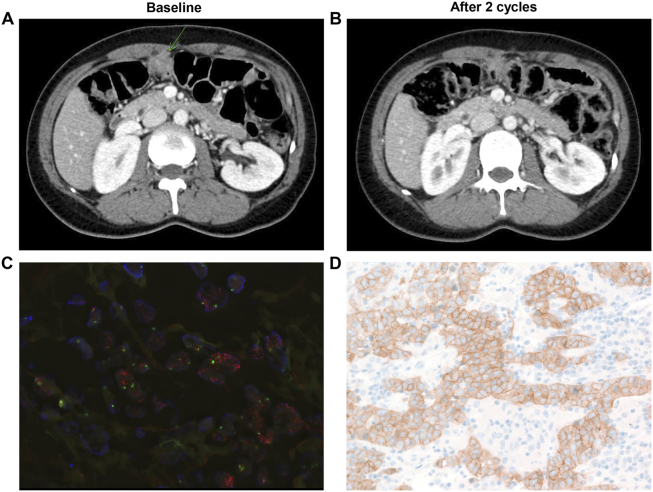
Since this trial was a dose-finding phase I trial, we did not enroll patients based on MET amplification. As an exploratory analysis, we tested MET protein overexpression by IHC in all cases where tissues were available (Table 2). Of the 14 patients with available tissue specimens, there were 4 patients with MET 3+ (all GC), 2 MET 2+ (1 GC, 1 rectal cancer), 4 MET 1+ (1 GC, 1 rectal, 2 melanoma), and 4 MET 0 patients (2 melanoma, 1 GC, 1 sarcoma). Of the four patients with MET 3+, two patients had confirmed MET amplification by FISH (Table 1). Of note, one GC patient (B6-004) with both MET overexpression (3+) and amplification (MET/CEP7 ratio, 7.3) achieved a durable PR for 297 days, and another MET-amplified GC patient (MET/CEP7 ratio, 7.6, B6-003) achieved stable disease for 86 days. Computed tomographic findings and tumor characteristics are shown in Figure 1.
Figure 1.
A) Baseline CT of MET-amplified GC patient (arrow indicates peritoneal seeding); B) After 2 cycles of docetaxel + savolitinib, the patient achieved PR; 3) MET FISH; 4) MET IHC.
Discussion
In this Phase I trial, we demonstrated that docetaxel in combination with the MET inhibitor savolitinib is a feasible combination regimen in heavily pretreated solid tumor patients. DLT, by definition, was observed in one patient who developed severe (grade 3) generalized edema (at 800 mg) after study drug administration. However, generalized edema was completely reversed when the study drug was stopped. Two other patients who experienced peripheral edema were grade 2, which was medically manageable. Due to the higher incidence of G4 neutropenia in cohort 4 (800 mg), we recommend savolitinib at 600 mg qd in combination with docetaxel 60 mg/m2 as the RP2D for the phase II trial.
Of note, although this trial was not a biomarker preselected study, we found one GC patient who had MET amplification by next-generation sequencing and FISH (Figure 1) who achieved a durable response. The RILOMET-1 trial compared the combination of epirubicin/cisplatin/capecitabine (ECX) and rilotumumab versus ECX alone as first-line chemotherapy in patients with MET+ GC but demonstrated no survival advantage [14]. Further development of rilotumumab in MET-amplified GC patients is not being pursued based on this study. Phase I trial of AMG337, a MET kinase inhibitor, demonstrated a response rate of 62% in 13 MET-amplified GC patients [15]. As part of the GC specific umbrella trial, the VIKTORY (targeted agent eValuation in gastric cancer basket KORea), we are currently enrolling MET amplified patients into two separate trials: savolitinib monotherapy, NCT#02449551, and phase II docetaxel/savolitinib trial, NCT# 02447406. We observed promising antitumor activity with durable response which lasted for 297 days in MET-amplified GC patient. The antitumor efficacy of savolitinib with or without docetaxel in a subset of MET-amplified GC population is anticipated to be analyzed in Q4 of 2018.
Acknowledgements
This work was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI14C2750, HI14C3418).
References
- 1.Lee J, Jain A, Kim P, Lee T, Kuller A, Princen F, In G, Kim SH, Park JO, Park YS. Activated cMET and IGF1R-driven PI3K signaling predicts poor survival in colorectal cancers independent of KRAS mutational status. PLoS One. 2014;9 doi: 10.1371/journal.pone.0103551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ha SY, Lee J, Kang SY, Do IG, Ahn S, Park JO, Kang WK, Choi MG, Sohn TS, Bae JM. MET overexpression assessed by new interpretation method predicts gene amplification and poor survival in advanced gastric carcinomas. Mod Pathol. 2013;26:1632–1641. doi: 10.1038/modpathol.2013.108. [DOI] [PubMed] [Google Scholar]
- 3.Lee J, Seo JW, Jun HJ, Ki CS, Park SH, Park YS, Lim HY, Choi MG, Bae JM, Sohn TS. Impact of MET amplification on gastric cancer: possible roles as a novel prognostic marker and a potential therapeutic target. Oncol Rep. 2011;25:1517–1524. doi: 10.3892/or.2011.1219. [DOI] [PubMed] [Google Scholar]
- 4.Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nat Rev Cancer. 2012;12:89–103. doi: 10.1038/nrc3205. [DOI] [PubMed] [Google Scholar]
- 5.Comoglio PM, Giordano S, Trusolino L. Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat Rev Drug Discov. 2008;7:504–516. doi: 10.1038/nrd2530. [DOI] [PubMed] [Google Scholar]
- 6.Lee J, Ou SH, Lee JM, Kim HC, Hong M, Kim SY, Jang J, Ahn S, Kang SY, Lee S. Gastrointestinal malignancies harbor actionable MET exon 14 deletions. Oncotarget. 2015;6:28211–28222. doi: 10.18632/oncotarget.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ha SY, Lee J, Jang J, Hong JY, Do IG, Park SH, Park JO, Choi MG, Sohn TS, Bae JM. HER2-positive gastric cancer with concomitant MET and/or EGFR overexpression: a distinct subset of patients for dual inhibition therapy. Int J Cancer. 2015;136:1629–1635. doi: 10.1002/ijc.29159. [DOI] [PubMed] [Google Scholar]
- 8.Lee J, Kim KM, Kang WK, Ou SH. Innovative personalized medicine in gastric cancer: time to move forward. Clin Genet. 2014;86:37–43. doi: 10.1111/cge.12408. [DOI] [PubMed] [Google Scholar]
- 9.Cancer Genome Atlas Research Network Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee J, Kim KM. Biomarkers for gastric cancer: molecular classification revisited. Precis Future Med. 2017;1:59–68. [Google Scholar]
- 11.Choueiri TK, Plimack E, Arkenau HT, Jonasch E, Heng DYC, Powles T, Frigault MM, Clark EA, Handzel AA, Gardner H. Biomarker-based phase II trial of savolitinib in patients with advanced papillary renal cell cancer. J Clin Oncol. 2017;35:2993–3001. doi: 10.1200/JCO.2017.72.2967. [DOI] [PubMed] [Google Scholar]
- 12.Kang JH, Lee SI, Lim DH, Park KW, Oh SY, Kwon HC, Hwang IG, Lee SC, Nam E, Shin DB. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol. 2012;30:1513–1518. doi: 10.1200/JCO.2011.39.4585. [DOI] [PubMed] [Google Scholar]
- 13.Gavine PR, Ren Y, Han L, Lv J, Fan S, Zhang W, Xu W, Liu YJ, Zhang T, Fu H. Volitinib, a potent and highly selective c-Met inhibitor, effectively blocks c-Met signaling and growth in c-MET amplified gastric cancer patient-derived tumor xenograft models. Mol Oncol. 2015;9:323–333. doi: 10.1016/j.molonc.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cunningham D, Tebbutt NC, Davidenko I, Murad AM, Al-Batran SE, Ilson DH, Tjulandin S, Gotovkin E, Karaszewska B, Bondarenko I. Phase III, randomized, double-blind, multicenter, placebo (P)-controlled trial of rilotumumab (R) plus epirubicin, cisplatin and capecitabine (ECX) as first-line therapy in patients (pts) with advanced MET-positive (pos) gastric or gastroesophageal junction (G/GEJ) cancer: RILOMET-1 study. J Clin Oncol. 2015;33 [abstract 4000] [Google Scholar]
- 15.Kwak EL, LoRusso P, Hamid O, Janku F, Kittaneh M, Catenacci DVT, Chan E, Bekaii-Saab TS, Amore B, Hwang YC. Clinical activity of AMG 337, an oral MET kinase inhibitor, in adult patients (pts) with MET-amplified gastroesophageal junction (GEJ), gastric (G), or esophageal (E) cancer. J Clin Oncol. 2015;33 [abstrr 1] [Google Scholar]