Abstract
Introduction
Elevated lipid concentrations were observed in adults with headaches. However, studies in children are scarce. Recent data suggest new potential risk factors for atherosclerosis, which may be associated with headaches. The aim of the study was to analyse the blood levels of lipids and new markers of atherosclerosis in children with idiopathic headaches.
Material and methods
The study population comprised 65 children (39 with idiopathic headaches and 26 healthy children). Total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL) cholesterol and triacylglycerol (TG) levels were measured in every patient. Brain-derived neurotrophic factor (BDNF), soluble CD40 ligand (sCD40L), endothelial plasminogen activator inhibitor (serpin E1/PAI I) and vascular endothelial growth factor (VEGF) blood level measurements were performed in 34 children.
Results
Children with headaches had higher BMI z-scores (0.2 vs. –1.14; p = 0.006). TC level was lower in patients with headaches (121.04 mg/dl vs. 146.87 mg/dl, p = 0.019). No differences in concentrations of TG, HDL or LDL were found. BDNF was significantly higher in the studied group (171.57 pg/ml vs. 64.04 pg/ml, p = 0.012). The VEGF was higher in boys with headaches than in girls (368.27 pg/ml vs. 142.86 pg/ml, p = 0.011). There were no differences in levels of VEGF, sCD40L or PAI-1 between groups.
Conclusions
Children with headaches have lower total cholesterol and higher BDNF levels than controls. No significant difference in levels of triacylglycerols, HDL cholesterol, LDL cholesterol, VEGF, sCD40L or PAI-1 was found between children with headaches and controls.
Keywords: children, atherosclerosis, dyslipidemia, headache
Introduction
Headaches are a common disorder that affects 40% to 70% of children [1]. It is a significant health concern with complex and multi-faceted diagnostics and treatment. Vasospasm and accompanying ischemia play an important role in the primary headache pathophysiology [2]. Migraine is suggested to be one of the first signs of atherosclerosis and disorders in lipid metabolism. Disturbances in lipid levels are known risk markers for cardiovascular and cerebrovascular diseases. Elevated lipid concentrations were observed in adult patients with headaches. Some authors report a correlation between migraine and increased risk of ischaemic stroke in adult patients [3]. However, there are only a few studies conducted in paediatric patients. Some small clinical studies showed a relation between dyslipidaemia and migraine in children [4, 5]. Recent data suggest that new potential risk factors for atherosclerosis and platelet aggregation, such as brain-derived neurotrophic factor (BDNF), sCD40L (soluble CD40 ligand), serpin E1/PAI I (endothelial plasminogen activator inhibitor) and vascular endothelial growth factor (VEGF), may also play a role in the pathogenesis of atherosclerosis and platelet aggregation [6–17]. Exploring new ideas about headache pathophysiology may provide additional treatment opportunities, especially in medical prognosis and prevention of vascular risk factors.
The aim of the present study was to analyse levels of total cholesterol, triacylglycerols, high-density lipoprotein (HDL) cholesterol and low-density lipoprotein (LDL) cholesterol in children with idiopathic headaches. We have also tried to estimate the occurrence of selected risk factors of atherosclerosis, inflammation and procoagulant state (BDNF, sCD40L, TIMP1, serpin E1/PAI I, VEGF) in some of the patients.
Material and methods
Participants
The study population comprised 65 children, divided into two groups: 39 children with idiopathic headaches (aged 7–18 years, 26 girls and 13 boys) and 26 healthy children (aged 7–18, 13 girls and 13 boys). Patients with idiopathic headaches were recruited from the Department of Paediatric Neurology. The control group consisted of healthy children admitted to the Department of Paediatric Endocrinology for the diagnostics of short stature. Short stature was defined as a height that is 2 standard deviations (SD) or more below the mean height for individuals of the same sex and chronological age in a given population. All of the patients from the control group presented familial short stature or constitutional delay of growth and development. Other causes of short stature, such as chronic diseases or malnutrition, were excluded. The design of the study has been approved by the Local Ethical Committee. The study was conducted according to the Declaration of Helsinki and informed consent was obtained from parents or guardians of all children. Signed personal permission was also required for any participant aged 16 years or over. The research was funded within the project no. KNW-2-050/D/5/N.
Biochemical analyses
Antecubital venous blood was collected after 12 h of fasting and samples were centrifuged within 2 h after being drawn. Lipid parameters (total cholesterol (TC), HDL, LDL and triglycerides (TG)) were measured using enzymatic methods (commercial Pointe Scientific kits) immediately in fasting plasma for every patient. The rest of the plasma was stored at –80°C for further assays. BDNF, sCD40L, serpin E1/PAI-1 and VEGF blood levels were analysed using ELISA (R&D Systems) according to the manufacturer’s recommendations in 34 children: 25 patients with headaches (17 girls and 8 boys) and 9 healthy children (5 girls and 4 boys).
Statistical analysis
Data were analysed using Statistica 12.0 software. The normality of distribution of quantitative data was evaluated by the Shapiro-Wilk W test and for the homogeneity of variance the Levene test was used. To compare the mean values of age between analysed subgroups two tests were used: Student’s t-test when the distributions of the data were normal and the Mann-Whitney U test when these distributions differed from the normal distribution or the assumption of homogeneity was violated.
Results
Table I shows general characteristics of analysed groups of children as well as mean values of biochemical parameters. Children with idiopathic headaches had a significantly higher body mass index (BMI) z-score than healthy patients (0.2 vs. –1.14; p = 0.006). We observed that the total cholesterol level was lower in the patients with idiopathic headaches compared to controls (121.04 mg/dl vs. 146.87 mg/dl) and the difference was statistically significant (p = 0.019). We did not observe any differences in concentrations of triacylglycerols, HDL cholesterol or LDL cholesterol between children with headaches and the controls. When we analysed girls and boys separately, we found a significant difference in total cholesterol level between girls with headaches and healthy girls (115.44 mg/dl vs. 144.58 mg/dl, p = 0.014). In boy subgroups, no differences in lipid levels were observed. TG/HDL-C ratio was higher in children with headaches (2.36 vs. 0.71, p = 0.02) than in controls. There were no differences in LDL-to-HDL or total cholesterol-to-HDL ratio.
Table I.
Characteristics of paediatric patients with idiopathic headache and controls
| Parameter | Paediatric patients with idiopathic headache (n = 39) | Controls (n = 26) | P-value |
|---|---|---|---|
| Sex, n (%): | |||
| Girls | 26 (66.67) | 13 (50.00) | 0.205 |
| Boys | 13 (33.33) | 13 (50.00) | |
| Age, mean ± SD [years] | 12.08 ±4.34 | 10.98 ±2.90 | 0.255 |
| BMI z-score, mean ± SD | 0.2 ±1.11 | –1.14 ±1.45 | 0.006 |
| Total cholesterol, mean ± SD [mg/dl] | 121.04 ±41.62 | 146.87 ±48.85 | 0.026 |
| HDL-cholesterol, mean ± SD [mg/dl] | 71.32 ±38.08 | 75.90 ±34.19 | 0.653 |
| LDL-cholesterol, mean ± SD [mg/dl] | 95.27 ±35.15 | 110.87 ±42.48 | 0.124 |
| Triacylglycerols, mean ± SD [mg/dl] | 68.97 ±38.39 | 69.66 ±30.85 | 0.939 |
| TG/HDL-C, mean ± SD | 2.36 ±3.91 | 0.71 ±0.38 | 0.02 |
| LDL-C/HDL-C, mean ± SD | 2.68 ±3.33 | 1.52 ±0.35 | 0.60 |
| TC/HDL-C, mean ± SD | 3.53 ±4.06 | 2.00 ±1.07 | 0.51 |
| Parameter | N = 25 | N = 9 | P-value |
| Sex, n (%): | |||
| Girls | 17 (68.0) | 5 (55.55) | 0.526 |
| Boys | 8 (32.0) | 4 (44.45) | |
| Age, range [years] | 7–18 | 7–18 | |
| BMI z-score, mean ± SD | 0.046 ±1.04 | –1.58 ±2.04 | 0.014 |
| BDNF, mean, median, IQR [pg/ml] | 171.57, 103.33, 315.47 | 64.04, 2.67, 606.56 | 0.012 |
| VEGF, mean, median, IQR [pg/ml] | 214.99, 132.60, 265.81 | 181.99, 173.25, 193.53 | 0.906 |
| sCD40L, mean, median, IQR [pg/ml] | 2168.91, 826.96, 4231.71 | 2230.73, 1492.87, 478.83 | 0.148 |
| PAI-1, mean, median, IQR [ng/ml] | 82.50, 19.36, 58.55 | 18.52, 10.58, 21.98 | 0.148 |
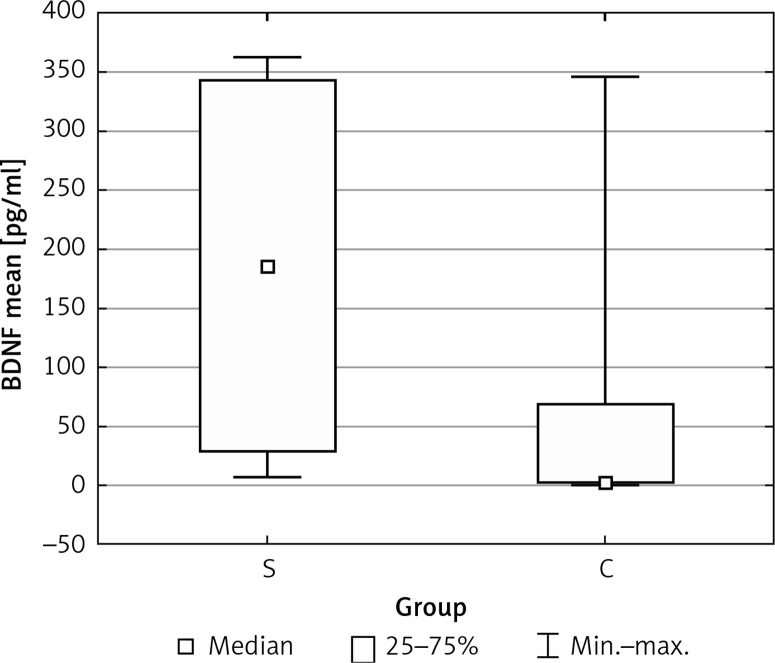
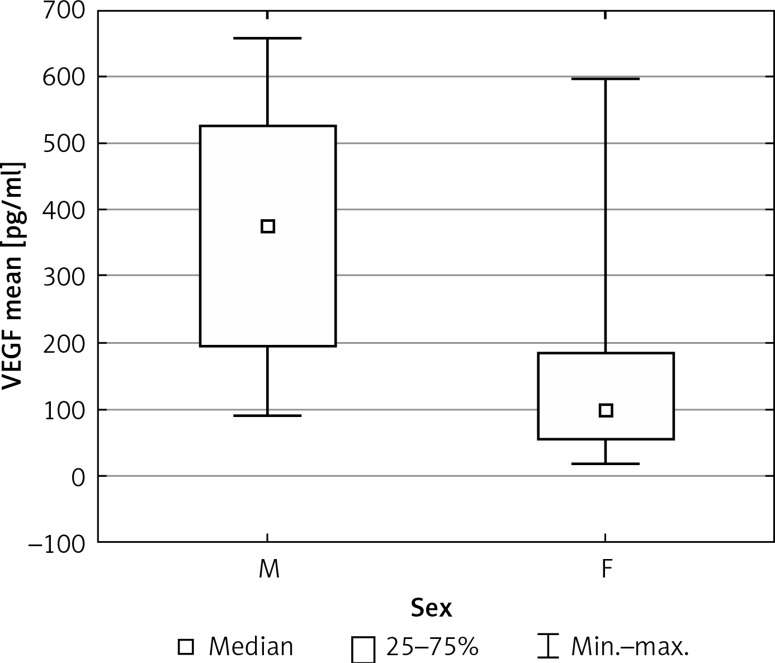
Mean BDNF blood levels were significantly higher in the studied group than in the healthy subjects (171.57 vs. 64.04 pg/ml, p = 0.012) (Figure 1). In the sex-matched subgroups we found mean VEGF blood levels to be significantly higher among boys with headaches than in the group of girls (368.27 vs. 142.86 pg/ml, p = 0.011) (Figure 2). There were no significant differences in levels of VEGF, sCD40L or PAI-1 between groups.
Figure 1.
Mean values of blood BDNF levels in the study (S) and control (C) groups
Figure 2.
Mean values of blood VEGF levels (pg/ml) in the study group (M – males, F – females)
Discussion
To the best of our knowledge, this is the first study concerning the correlation between mentioned factors and headaches in the literature. According to the International Classification of Headache Disorders, 3rd edition (beta version) created by the International Headache Society, the group of idiopathic headaches consists of migraine, tension-type headaches, trigeminal autonomic cephalalgias and other idiopathic headaches disorders. The risk factors of stroke occur more frequently in adult patients with migraine than in the healthy population [18]. Studies in children did not confirm such a correlation. However, it was suggested that headaches may be the first manifestation of vascular disturbances in paediatric patients. Some recent studies reported that idiopathic headaches could be the first symptom of early stages of atherosclerosis. Moreover, higher prevalence of migraine among young individuals with a history of stroke, silent infarct-like brain lesions and cerebrovascular disorders during headaches attacks was reported [19–21].
Dyslipidaemia is one of the recognized risk factor for cardiovascular and cerebrovascular diseases. A previous study of Kopyta et al. showed that serum triacylglycerol levels are risk factors for ischaemic stroke in Polish paediatric patients [22]. Some authors suggest that primary headaches could be associated with lipid disturbances. Previously elevated lipid concentrations were observed in adult patients with headaches. Some authors found a correlation between migraine and increased risk of ischaemic stroke in groups of adult patients [3]. Available data for children are insufficient and controversial. A large US study revealed no correlation between lipid levels and the occurrence of primary headaches in children [23]. In contrast, Glueck and Bates reported a relation between severe migraine headaches and LDL cholesterol and HDL cholesterol levels in boys with primary and familial dyslipoproteinaemias [3]. In our study total blood cholesterol level was lower in the patients with idiopathic headaches. In the study of Ozdemir et al. [24] concerning lipid levels in children with epilepsy, a slightly lower level of total cholesterol was also observed in the study group than in controls. In adults suffering from migraine lower total cholesterol and HDL cholesterol levels were demonstrated when compared to non-migrainous headaches sufferers [25]. In contrast to the widely known risk related to a high cholesterol level, some recent studies reported that low total cholesterol levels may be associated with higher risk of mortality due to haemorrhagic stroke and heart failure in adults [26]. This mechanism is not well understood and may involve, inter alia, decreased ability to remove endotoxins and increased metabolic stress [27]. We have also observed that patients with idiopathic headaches had a higher TG/HDL-C ratio compared to controls. Low HDL cholesterol and high triacylglycerol levels are known to be associated with cardiovascular diseases and diabetes mellitus type 2 [28, 29]. Moreover, some authors reported that TG/HDL-C ratio could help to identify patients at high risk for metabolic disturbances and structural changes in blood vessels [30]. However, more clinical studies are needed to better understand these effects, especially in the paediatric population.
In our study we noted a difference in BMI between groups. Obesity in children is a significant health concern worldwide. According to Ogden et al. 16.3% of American children and adolescents had a BMI value fulfilling criteria for overweight [31]. Obesity is known to be a risk factor for numerous medical conditions, including hypertension and cerebrovascular and cardiovascular disease in adulthood [32–34]. In the adult population migraine occurs more frequently in obese patients, and obese migraineurs have greater risk of transformation from episodic to chronic migraine headaches [35–37]. Data regarding effects of obesity and headaches in children are more limited. However, an increasing number of reports suggest that obesity could also be a risk factor for migraine in the paediatric population [38–40]. Our study confirmed these findings.
The BDNF is widely known for its role in the central nervous system. Elevated BDNF levels were observed in ischaemic changes in the brain [41], and they may play an important role in coronary atherosclerosis development [42]. Vascular endothelial growth factor (VEGF) is regarded as an important factor in angiogenesis, vasculogenesis and tissues remodelling [43]. Nevertheless, some recent studies suggest its role in energy haemostasis as well as its influence on adipose tissue function. The increased expression of VEGF is thought to protect against insulin resistance and diet-induced obesity [44]. In our study the BDNF levels were significantly higher in the group of children with headaches. Moreover, VEGF, sCD40L and PAI-1 showed a tendency to be elevated in the study group, but with no significant difference. Cerebrovascular and cardiovascular diseases may remain asymptomatic until middle or even old age. The first manifestations of these disturbances very often engender the risk of serious damage to physical and mental health, such as stroke and heart attack. Therefore, prevention and searching for new potential markers of vascular diseases play an important role in medical practice.
In conclusion, children with idiopathic headaches have lower total cholesterol and higher BDNF levels than healthy children. There are no significant differences in levels of triacylglycerols, HDL cholesterol and LDL cholesterol, VEGF, sCD40L and PAI-1 between children with idiopathic headaches and controls.
The present study has some limitations. First, there was a small number of examined patients. The low number of participants did not allow us to make reliable conclusions, especially about sex-dependent differences. However, the preliminary data showed promising potential for further study and may enrich the knowledge of headache molecular characteristics. It is crucial to increase the number of patients to make more convincing conclusions. Secondly, morphological bias may influence the results. We are aware that the blood levels of some factors may vary depend on anthropometric parameters. However, we recruited our control group from children with short stature from the Endocrinology Department, because wide differential diagnosis of these patients allowed us to exclude many diseases and pathological states that certainly interfere with the results.
It would also be valuable to assess age-related ranges for BDNF, VEGF, sCD40L and PAI-1 blood levels, as well as to measure the ultrasonic intima-media complex thickness to give a more complete picture of vascular disturbances in children with idiopathic headaches. This area is planned to be a subject of research of our subsequent study.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Brna PM, Dooley JM. Headaches in the pediatric population. Semin Pediatr Neurol. 2006;13:222–30. doi: 10.1016/j.spen.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Villalon CM, Centurion D, Valdivia LF, de Vries P, Saxena PR. Migraine: pathophysiology, pharmacology, treatment and future trends. Curr Vasc Pharmacol. 2003;1:71–84. doi: 10.2174/1570161033386826. [DOI] [PubMed] [Google Scholar]
- 3.Scher AI, Gudmundsson LS, Sigurdsson S, et al. Migraine headache in middle age and late life brain infarcts. JAMA. 2009;24:2563–70. doi: 10.1001/jama.2009.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glueck MD, Bates SR. Migraine in children: association with primary and familial dyslipoproteinemias. Pediatrics. 1986;77:316–21. [PubMed] [Google Scholar]
- 5.Nelson KB, Richardson AK, He J, Lateef TM, Khoromi S, Merikangas KR. Headache and biomarkers predictive of vascular disease in a representative sample of US children. Arch Pediatr Adolesc Med. 2010;164:358–62. doi: 10.1001/archpediatrics.2010.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaldakov GN, Fiore M, Hristova MG, Aloe L. Metabotrophic potential of neurotrophins: implication in obesity and related diseases? Med Sci Monit. 2003;9:19–21. [PubMed] [Google Scholar]
- 7.Mannia L, Nikolova V, Vyagova D, Chaldakov GN, Aloe L. Reduced plasma levels of NGF and BDNF in patients with acute coronary syndromes. Int J Cardiol. 2005;102:169–71. doi: 10.1016/j.ijcard.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 8.Li ST, Pan J, Hua XM, et al. Endothelial nitric oxide synthase protects neurons against ischemic injury through regulation of brain-derived neurotrophic factor expression. CNS Neurosci Therap. 2014;20:154–64. doi: 10.1111/cns.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu CP, Sheu WH, Lee IT, et al. Effects of weight loss on epicardial adipose tissue thickness and its relationship between serum soluble CD40 ligand levels in obese men. Clin Chim Acta. 2013;421:98–103. doi: 10.1016/j.cca.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Baena-Fustegueras JA, Pardina E, Balada E, et al. Soluble CD40 ligand in morbidly obese patients effect of body mass index on recovery to normal levels after gastric bypass surgery. JAMA Surg. 2013;148:151–6. doi: 10.1001/jamasurgery.2013.419. [DOI] [PubMed] [Google Scholar]
- 11.Unek IT, Bayraktar F, Solmaz D, et al. The levels of soluble CD40 ligand and C-reactive protein in normal weight, overweight and obese people. Clin Med Res. 2010;8:89–95. doi: 10.3121/cmr.2010.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang JH, Zhang YW, Zhang P, et al. CD40 ligand as a potential biomarker for atherosclerotic instability. Neurol Res. 2013;35:693–700. doi: 10.1179/1743132813Y.0000000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reuter B, Rodemer C, Grudzenski S, et al. Temporal profile of matrix metalloproteinases and their inhibitors in a human endothelial cell culture model of cerebral ischemia. Cerebrovasc Dis. 2013;35:514–20. doi: 10.1159/000350731. [DOI] [PubMed] [Google Scholar]
- 14.Karasek D, Vaverkova H, Halenka M, et al. Prothrombotic markers in asymptomatic dyslipidemic subjects. J Thromb Thrombolysis. 2011;31:27–36. doi: 10.1007/s11239-010-0474-4. [DOI] [PubMed] [Google Scholar]
- 15.Soinne L, Saimanen E, Malmberg-Ceder K, et al. Association of the fibrinolytic system and hemorheology with symptoms in patients with carotid occlusive disease. Cerebrovasc Dis. 2005;20:172–9. doi: 10.1159/000087201. [DOI] [PubMed] [Google Scholar]
- 16.Gürger M, Atescelik M, Yılmaz M, et al. Can we define migraine patients with blood high-sensitivity C-reactive protein and galectin-3 levels in the emergency department? Arch Med Sci. 2018;14:307–12. doi: 10.5114/aoms.2016.60984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hogas S, Bilha S, Branisteanu D, et al. Potential novel biomarkers of cardiovascular dysfunction and disease: cardiotrophin-1, adipokines and galectin-3. Arch Med Sci. 2016;13:897–913. doi: 10.5114/aoms.2016.58664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsai CL, Chou CH, Lee PJ, et al. The potential impact of primary headache disorders on stroke risk. J Headache Pain. 2016;17:108. doi: 10.1186/s10194-016-0701-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larrosa-Campo D, Ramon-Carbajo C, Para-Prieto M, Calleja-Puerta S, Cernuda-Morollon E, Pascual J. Migraine as a vascular risk factor. Rev Neurol. 2012;55:349–58. [PubMed] [Google Scholar]
- 20.Guillan M, Alonso-Canovas A, Gonzalez-Valcarcel J, et al. Stroke mimics treated with thrombolysis: further evidence on safety and distinctive clinical features. Cerebrovasc Dis. 2012;34:115–20. doi: 10.1159/000339676. [DOI] [PubMed] [Google Scholar]
- 21.Partap S. Stroke and cerebrovascular complications in childhood cancer survivors. Semin Pediatr Neurol. 2012;19:18–24. doi: 10.1016/j.spen.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Kopyta I, Sarecka-Hujar B, Emich-Widera E, Marszał E, Zak I. Association between lipids and fibrinogen levels and ischemic stroke in the population of the Polish children with arteriopathy and cardiac disorders. Wiad Lek. 2010;63:17–23. [PubMed] [Google Scholar]
- 23.Nelson KB, Richardson AK, He J, et al. Headache and biomarkers predictive of vascular disease in a representative sample of US children. Arch Pediatr Adolesc Med. 2010;164:358–62. doi: 10.1001/archpediatrics.2010.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozdemir O, Yakut A, Dinleyici EC, Aydogdu SD, Yarar C, Colak O. Serum asymmetric dimethylarginine (ADMA), homocysteine, vitamin B(12), folate levels, and lipid profiles in epileptic children treated with valproic acid. Eur J Pediatr. 2011;170:873–7. doi: 10.1007/s00431-010-1366-5. [DOI] [PubMed] [Google Scholar]
- 25.Winsvold BS, Hagen K, Aamodt AH, Stovner LJ, Holmen J, Zwart JA. Headache, migraine and cardiovascular risk factors: the HUNT study. Eur J Neurol. 2011;18:504–11. doi: 10.1111/j.1468-1331.2010.03199.x. [DOI] [PubMed] [Google Scholar]
- 26.Nago N, Ishikawa S, Goto T, et al. Cholesterol is associated with mortality from stroke, heart disease, and cancer: the Jichi Medical School Cohort Study. J Epidemiol. 2011;21:67–74. doi: 10.2188/jea.JE20100065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pikula A, Beiser AS, Chen TC, et al. Serum BDNF and VEGF levels are associated with Risk of Stroke and Vascular Brain Injury: Framingham Study. Stroke. 2013;44:2768–75. doi: 10.1161/STROKEAHA.113.001447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonora E, Kiechl S, Willeit J, et al. Prevalence of insulin resistance in metabolic disorders: the Bruneck Study. Diabetes. 1998;47:1643–9. doi: 10.2337/diabetes.47.10.1643. [DOI] [PubMed] [Google Scholar]
- 29.Sun GZ, Li Z, Guo L, Sun YX. High prevalence of dyslipidemia and associated risk factors among rural Chinese adults. Lipids Health Dis. 2014;13:189. doi: 10.1186/1476-511X-13-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pacifico L, Bonci E, Andreoli G, et al. Association of serum triglyceride-to-HDL cholesterol ratio with carotid artery intima-media thickness, insulin resistance and nonalcoholic fatty liver disease in children and adolescents. Nutr Metab Cardiovasc Dis. 2014;24:737–43. doi: 10.1016/j.numecd.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003–2006. JAMA. 2008;299:2442–3. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- 32.Li C, Ford ES, Zhao G, Croft JB, Balluz LS, Mokdad AH. Prevalence of self-reported clinically diagnosed sleep apnea according to obesity status in men and women: National Health and Nutrition Examination Survey, 2005–2006. Prev Med. 2010;51:18–23. doi: 10.1016/j.ypmed.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 33.Jiraskova N, Rozsival P. Idiopathic intracranial hypertension in pediatric patients. Clin Ophthalmol. 2008;2:723–6. doi: 10.2147/opth.s1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spear BA, Barlow SE, Ervin C, et al. Recommendations for treatment of child and adolescent overweight and obesity. Pediatrics. 2007;120:254–88. doi: 10.1542/peds.2007-2329F. [DOI] [PubMed] [Google Scholar]
- 35.Bigal ME, Lipton RB. Obesity is a risk factor for transformed migraine but not chronic tension-type headache. Neurology. 2006;67:252–7. doi: 10.1212/01.wnl.0000225052.35019.f9. [DOI] [PubMed] [Google Scholar]
- 36.Bigal ME, Lipton RB, Holland PR, Goadsby PJ. Obesity, migraine, and chronic migraine: possible mechanisms of interaction. Neurology. 2007;68:1851–61. doi: 10.1212/01.wnl.0000262045.11646.b1. [DOI] [PubMed] [Google Scholar]
- 37.Peres MF, Lerario DD, Garrido AB, Zukerman E. Primary headaches in obese patients. Arq Neuropsiquiatr. 2005;63:931–3. doi: 10.1590/s0004-282x2005000600005. [DOI] [PubMed] [Google Scholar]
- 38.Hershey AD, Powers SW, Nelson TD, et al. Obesity in the pediatric headache population: a multicenter study. Headache. 2009;49:170–7. doi: 10.1111/j.1526-4610.2008.01232.x. [DOI] [PubMed] [Google Scholar]
- 39.Kinik ST, Alehan F, Erol I, Kanra AR. Obesity and paediatric migraine. Cephalalgia. 2010;30:105–9. doi: 10.1111/j.1468-2982.2009.01907.x. [DOI] [PubMed] [Google Scholar]
- 40.Pinhas-Hamiel O, Frumin K, Gabis L, et al. Headaches in overweight children and adolescents referred to a tertiary-care center in Israel. Obesity. 2008;16:659–63. doi: 10.1038/oby.2007.88. [DOI] [PubMed] [Google Scholar]
- 41.Lasek-Bal A, Jedrzejowska-Szypulka H, Rozycka J, et al. Low concentration of BDNF in the acute phase of ischemic stroke as a factor in poor prognosisin terms of functional status of patients. Med Sci Monit. 2015;21:3900–5. doi: 10.12659/MSM.895358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chaldakov GN, Fiore M, Stankulov IS, et al. NGF, BDNF, leptin, and mast cells in human coronary atherosclerosis and metabolic syndrome. Arch Physiol Biochem. 2001;109:357–60. doi: 10.1076/apab.109.4.357.4249. [DOI] [PubMed] [Google Scholar]
- 43.Roy H, Bhardwaj S, Yla-Herttuala S. Biology of vascular endothelial growth factors. FEBS Lett. 2006;580:2879–87. doi: 10.1016/j.febslet.2006.03.087. [DOI] [PubMed] [Google Scholar]
- 44.Elias I, Franckhauser S, Bosch F. New insights into adipose tissue VEGF-A actions in the control of obesity and insulin resistance. Adipocyte. 2013;2:109–12. doi: 10.4161/adip.22880. [DOI] [PMC free article] [PubMed] [Google Scholar]