Abstract
Watson–Crick like G‐U mismatches with tautomeric Genol or Uenol bases can evade fidelity checkpoints and thereby contribute to translational errors. The 5‐oxyacetic acid uridine (cmo5U) modification is a base modification at the wobble position on tRNAs and is presumed to expand the decoding capability of tRNA at this position by forming Watson–Crick like cmo5Uenol‐G mismatches. A detailed investigation on the influence of the cmo5U modification on structural and dynamic features of RNA was carried out by using solution NMR spectroscopy and UV melting curve analysis. The introduction of a stable isotope labeled variant of the cmo5U modifier allowed the application of relaxation dispersion NMR to probe the potentially formed Watson–Crick like cmo5Uenol‐G base pair. Surprisingly, we find that at neutral pH, the modification promotes transient formation of anionic Watson–Crick like cmo5U−‐G, and not enolic base pairs. Our results suggest that recoding is mediated by an anionic Watson–Crick like species, as well as bring an interesting aspect of naturally occurring RNA modifications into focus—the fine tuning of nucleobase properties leading to modulation of the RNA structural landscape by adoption of alternative base pairing patterns.
Keywords: NMR spectroscopy, relaxation dispersion, RNA, RNA modifications
The fidelity of translation relies on the ability of the rigid ribosomal decoding center to recognize the shape of Watson–Crick base pairs.1 Mismatches can evade these fidelity mechanisms by spatial mimicry of the Watson–Crick like shape through tautomerization and ionization.2 The propensity to adopt such Watson–Crick like base pairs can be tuned by modifications of the canonical bases at the wobble position to rewire3 and change the efficiency of translational decoding.4 Uridine 5‐oxyacetic acid (cmo5U) is one such modification that based on X‐ray crystallographic and biochemical data has been proposed to increase the efficiency of translation of G‐ending codons by promoting formation of Watson–Crick like cmo5Uenol‐G base pairs.5 However, because hydrogen atoms could not be visualized by crystallography, the precise nature of the Watson–Crick like species could not be unambiguously determined.
Herein, we have synthesized a 15N isotopically labeled version of the cmo5U modification that allows the application of relaxation dispersion NMR to probe the formation of Watson–Crick like base pairs. Surprisingly, we find that the modification promotes the transient formation of anionic cmo5U−‐G base pairs via decreasing the pK a of the nucleoside. Our results provide key mechanistic insights into how cmo5U influences base‐pairing properties and modulates decoding, and lay out a framework for studying the impacts of other RNA modifications.1
Our first efforts focused on the synthesis of a 15N2‐cmo5U phosphoramidite. The synthetic access is loosely based on published syntheses but represents the first comprehensive description of the chemical synthesis of the cmo5U RNA building block. The synthesis started from 15N2‐uridine, which was prepared according to published procedures by using 15N2‐urea.6 In the next steps, the 5‐hydroxyl group was installed,7 followed by protection of the sugar hydroxyls and installation of the 5‐oxyacetic acid side‐chain as an ester functionality.8 Removal of the 2′,3′‐ketal protecting group was followed by the regioselective incorporation of the 2′‐tert‐butyl‐dimethylsilyl group by using a transient 3′,5′‐di‐tert‐butylsilyl protection. Then, after removal of the methyl ester functionality, the 4‐nitrophenylethylester was obtained via a Steglich esterification. The remaining steps were the introduction of the 5′‐(4,4′‐dimethoxy)‐trityl group and phosphitylation of the 3′‐hydroxyl group yielding the desired stable isotope modified cmo5U RNA building block. A detailed synthetic scheme and further experimental details on the synthetic steps can be found in the Supporting Information.
Then, the cmo5U modification was introduced into 20 nt RNA model system to probe changes in the overall thermodynamic stability. The cmo5U‐modified RNAs were characterized by anion‐exchange chromatography and mass spectrometry (Figures S1 and S2 in the Supporting Information). Four RNA constructs differing at the central U5‐A16 base pair were synthesized and subjected to UV melting analysis to give the melting temperatures and thermodynamic parameters of the 20 nt hairpins—the U‐A hp, the U⋅G hp, the cmo5U‐A hp and the cmo5U⋅G hp (Figure 1 a–c and Figure S3 in the Supporting Information). As was expected, the U‐A hairpin comprising only canonical base pairs showed the highest melting transition at 85 °C and the most negative free energy of −12.2 kcal mol−1, closely followed by the U⋅G hp (T m=83.3 °C, ΔG 298 K=−11.3 kcal mol−1). The cmo5U‐modified hairpins showed slightly decreased stabilities in‐line with previously conducted free‐energy calculations.9 The cmo5U‐A hp, although forming a canonical base pair exhibits an even lower thermodynamic stability than the U⋅G hp (ΔT m U⋅G/cmo5U‐A=1.3 °C, ΔG 298 K,U⋅G/cmo5U‐A=0.5 kcal mol−1). The UV melting experiment gives information on the global thermodynamic stabilities of the stem‐loop folds, but no structural features and local residue‐specific dynamics can be delineated from the UV data.
Figure 1.
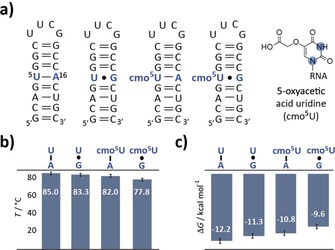
Thermodynamic stability of the unmodified and cmo5U‐modified RNAs. a) Secondary structures of the four RNAs under investigation. The inset shows the structure of the cmo5U modification. The nitrogen atoms highlighted in blue are 15N labeled. b) Bar graph of the melting temperatures of the hairpin RNAs. c) Bar graph of the free energies at 298 K of the hairpin RNAs.
However, solution NMR spectroscopy is perfectly suited to study structure and dynamics in biomolecules at atomic resolution.6, 10 Based on NMR data, the U‐A hp forms a standard A‐form RNA stem capped with an extra‐stable UUCG loop. The U⋅G hp was extensively investigated earlier and in the major populated ground state a U⋅G wobble base pair was observed.11 Basically, the same base pairing properties were observed for the cmo5U‐modified stem‐loop RNAs (Figure 2). The cmo5U‐A hp showed slightly shifted imino proton resonances compared to the U‐A wildtype sequence, which could be assigned using a 1H‐homonuclear NOESY experiment (Figure 2 a, upper trace). Noteworthy, the N3H of resonance is shifted from 13.6 ppm (U‐A base pair) to 14.1 ppm (cmo5U‐A base pair) pointing toward a more de‐shielded magnetic environment. We further demonstrated the Watson–Crick like base pairing via an HNN‐COSY with residue‐specific 15N3 and 15N1 labeling of cmo5U5 and A16, respectively (Figure 2 a). The cmo5U⋅G wobble base pair was confirmed by 1H‐15N‐correlation NMR spectroscopy making use of 15N3 and 15N1 labeling of cmo5U5 and G16 (Figure 2 b). Again, a 0.5 ppm de‐shielding effect for the cmo5U imino proton resonance (U⋅G hp U5 H3 11.6 ppm; cmo5U⋅G hp U5 H3 12.1 ppm) was induced by the 5‐oxyacetic acid modification.
Figure 2.
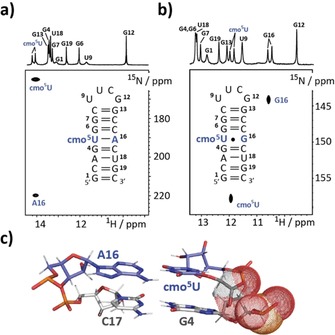
Structural features of cmo5U‐modified hairpins. a) Canonical cmo5U‐A base pair is observed in an HNN‐COSY experiment at 25 °C. b) 1H‐15N HSQC data showing chemical shift signatures for the formation of a cmo5U‐G wobble base pair (at 5 °C). c) Structural model of the G4‐C17 and cmo5U5‐A16 base pairs in the cmo5U‐A hp. Interfering negative charges of the 5‐oxyacetate and the phosphate backbone are shown in dot representation.
We then addressed individual base pair exchange kinetics in all four hairpins by the application of CLEANEX‐PM experiments (Table S1 and Figure S4 in the Supporting Information).12 The slow to very slow imino proton exchange rates between 0.8 to 10 s−1 for the U‐A hp are in‐line with the high thermodynamic stability of the stem loop structure and reveal no dynamic hotspot. In the U⋅G hp the wobble base pair represents a dynamic base pair opening hot spot with the U5‐N3H displaying the highest exchange rate with bulk water protons (22.15 s−1) followed by its interaction partner G16‐N3H (10.14 s−1). The destabilization effect introduced by the U⋅G wobble appears to be only localized as other imino proton exchange rates are only marginally affected (e.g., U18).
The water‐proton‐exchange NMR experiment of cmo5U‐A hp gave slightly to strongly elevated imino proton water‐exchange rates compared to its unmodified U‐A counterpart. The cmo5U modification strongly influences the stability of the 5′‐preceding base pair—in this case, the G4‐C17 base pair. We observed an almost tenfold increase in the water‐exchange rate of the G4‐N1H proton. A structural model of the cmo5U‐A hairpin gives a potential explanation for the destabilization of the 5′‐preceding base pair. The model reveals that the negatively charged 5‐oxyacetate side chain gets in proximity to the likewise negatively charged phosphate group of G4 (Figure 2 c). The repulsion effect induced by this unfavorable electrostatic interaction gives a rational for the tenfold weakening of the G4‐C17 base pair and the concomitant global fold destabilization, as has been seen in the UV melting curve analysis. In the thermodynamically most unstable hairpin, the highly dynamic hotspot at the cmo5U⋅G mismatch site was directly reflected in the imino proton water‐exchange rates. We found strongly elevated exchange rates at the mismatch site. Most prominently, the cmo5U5‐N3H resonance was strongly broadened at 25 °C preventing the determination of the exchange rate with water protons. The G16 N1H water exchange is sixfold enhanced compared to the U⋅G wobble base pair (59.88 vs. 10.14 s−1). Again, the destabilization effect introduced by the cmo5U⋅G wobble is highly localized, because other imino proton water‐exchange rates are only marginally affected.
Finally, we addressed the postulated stabilization of the rare enolic uridine state by the cmo5U modification via 15N‐R1ρ relaxation dispersion NMR experiments. We previously showed in the hpU⋅G sequence context (Figure 1 a) that the U⋅G wobble mismatch exists in dynamic equilibrium with two equally populated and rapidly exchanging U‐Genol and Uenol‐G base pairs, with the anionic species falling below detection limits at neutral pH (p B<0.05 %) and only becoming detectable (p B≈0.3 %) at high pH>7.9.11
We examined how replacement of the U⋅G mismatch with a cmo5U⋅G base pair affects these dynamics. Strikingly, we found that at neutral pH, the modification significantly increased 15N RD at both cmo5U5‐N3 and G16‐N1 indicating that it promotes the exchange process to WC‐like mismatches (by ca. fivefold, Figure 3 a and Table S2 in the Supporting Information). As a negative control, no RD was observed for cmo5U‐A hp (Figure S5 in the Supporting Information). Unlike the data for unmodified U⋅G hp, which could be interpreted in terms of two‐state exchange between the wobble and two rapidly exchanging U‐Genol and Uenol‐G base pairs, the RD data for the cmo5U⋅G hp called for a three‐state fit giving two ESs (Table S2 in the Supporting Information). ES1 (p B=0.16±0.03 % and k ex GS‐B=5949±709 s−1) has Δω (cmo5U5‐N3)=16.0±4.8 ppm and Δω (G16‐N1)=40.4±4.1 ppm consistent with a mixture of rapidly exchanging cmo5U‐Genol and cmo5Uenol‐G and species in a 70:30 ratio. ES2 (p c=0.24±0.03 % and k ex GS‐C=976±683 s−1) has Δω (cmo5U5‐N3)=55.4±2.9 and Δω (G16‐N1)=−0.02±3.5 consistent with the formation of a cmo5U−‐G anionic base pair. This was confirmed through measurement of RD profiles at higher pH 8.0 (Table S2 and Figure S6 in the Supporting Information). Therefore, the modification increases the population of the anionic species by >twofold such that it becomes the dominant species in the ES at neutral pH (ca. 60 %), although minimally impacting the population of the tautomeric species (ca. twofold) or tautomeric equilibrium (from 50:50 to 70:30 cmo5U‐Genol/cmo5Uenol‐G). These findings are in‐line with a recent computational study, in which no evidence for the stabilization of the enolic uridine state by the oxyacetic acid modification was found.13 They also mirror the effects of 5‐bromo‐2′‐deoxyuridine (5BrdU), which is a mutagenic agent in DNA that causes increased mis‐incorporation via an anionic species.14 We got further support for the preference of the anionic Watson–Crick like base pairing from the pK a values determined for the cmo5U nucleoside (Figures S7 and S8 in the Supporting Information). By using pH‐dependent 13C NMR data, the pK a of the 5‐oxyacetic acid side‐chain was found to be 2.8. Thus, at physiological pH the 5‐oxyacetic acid is negatively charged in‐line with the observed destabilization effect found for the cmo5U‐A RNA. The pK a of the imino proton H3 in cmo5U is 8.7, that is, the proton is more acidic than in the unmodified uridine nucleoside (pK a 9.2–9.6) by almost a factor of ten. This higher acidity favors the anionic Watson–Crick like base pair state, as has been seen in the relaxation dispersion data. Further, the higher acidity of the N3‐bound proton rationalizes the observed de‐shielding effect by the change in its electronic environment.
Figure 3.
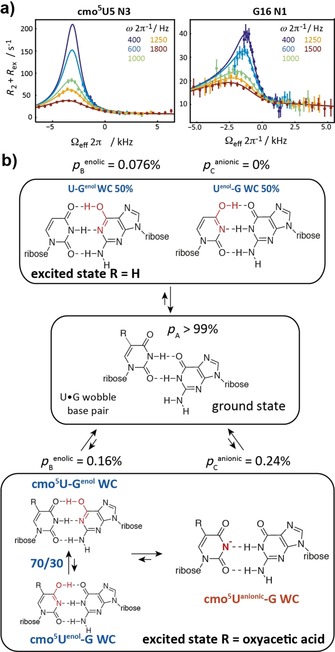
Probing the excited states in cmo5U‐modified RNA. a) 15N‐R1ρ relaxation dispersion experiments for N3 of cmo5U5 and N1 of G16 with a three‐state global fit at pH 6.9 and 10° C. b) Summary on ground/excited state equilibria for the unmodified U‐G and the cmo5U‐G wobble base pairs.
To conclude, we successfully synthesized a 15N stable isotope labeled variant of a cmo5U RNA building block that allowed to obtain unprecedented insights into the structure and dynamics of RNA carrying this naturally occurring modification via solution NMR spectroscopy. We found a generally destabilizing effect for double helical RNA stem structures by the cmo5U modification very likely due to the additional negative charge introduced by the oxyacetic acid sidechain. The destabilization is reflected in a lowered melting temperature and higher free energy values, and also slightly enhanced imino water proton exchange rates. We further probed the presumed stabilizing effect of the 5‐oxyacetic acid modification on the U‐enol tautomeric form by 15N‐R1ρ relaxation dispersion NMR spectroscopy. We found that an anionic Watson–Crick like cmo5U−‐G base pair represents the major species in the excited state. Thus, we have evidence that in a previous work the cmo5Uenol‐G WC‐like base pair was misinterpreted and is in fact an anionic cmo5U‐G base pair.5a This anionic Watson–Crick base pair between the mRNA and the tRNA anticodon loop might be further stabilized by the controlled conditions regarding pH or metal ion positioning within the ribosome, thus giving an explanation for the expansion of the decoding capacity via the cmo5U modification by a lowered N3H pK a value.10 The electron density map in the previous work of Weixlbaumer et al. is also compatible with the anionic base pair formed by N3 and O2 of cmo5U and N1H and N2H of G (Figure 3 b).5a
Further, the effects of cmo5U in RNA are reminiscent to that of 5‐BrdU in DNA, which also promotes formation of an anionic base pair by lowering the pK a.14 Thus, cmo5U can be regarded as nature's version of 5‐BrdU. Although 5‐BrdU is a known chemotherapeutic agent that acts by promoting G point mutations, the function of cmo5U is the enhanced reading capability of G at the third codon position by the formation of an anionic base pair. To summarize, the results highlight an important aspect of the function of naturally occurring RNA modifications—the alteration of the folding landscape of RNA by fine‐tuning nucleobases properties leading to alternative base pairing patterns. We are further confident that solution NMR spectroscopy will give important insights into the function of naturally occurring RNA modifications, which is especially important in the light of the recent finding that RNA epigenetics plays an important role in many cellular processes.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
This work was supported by the Austrian Science Fund (FWF, project P28725 and P30370 to CK) and the Austrian Research Promotion Agency FFG (West Austrian BioNMR, 858017). H.M.A. acknowledges funding from NIH (RO1 GM089846).
E. Strebitzer, A. Rangadurai, R. Plangger, J. Kremser, M. A. Juen, M. Tollinger, H. M. Al-Hashimi, C. Kreutz, Chem. Eur. J. 2018, 24, 18903.
Contributor Information
Dr. Hashim M. Al‐Hashimi, Email: hashim.al.hashimi@duke.edu
Dr. Christoph Kreutz, Email: christoph.kreutz@uibk.ac.at.
References
- 1. Rozov A., Demeshkina N., Westhof E., Yusupov M., Yusupova G., Trends Biochem. Sci. 2016, 41, 798–814. [DOI] [PubMed] [Google Scholar]
- 2.
- 2a. Rozov A., Westhof E., Yusupov M., Yusupova G., Nucleic Acids Res. 2016, 44, 6434–6441; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2b. Rozov A., Wolff P., Grosjean H., Yusupov M., Yusupova G., Westhof E., Nucleic Acids Res. 2018, 46, 7425–7435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.
- 3a. Cantara W. A., Murphy F. V., Demirci H., Agris P. F., Proc. Natl. Acad. Sci. USA 2013, 110, 10964; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3b. Muramatsu T., Nishikawa K., Nemoto F., Kuchino Y., Nishimura S., Miyazawa T., Yokoyama S., Nature 1988, 336, 179; [DOI] [PubMed] [Google Scholar]
- 3c. Ikeuchi Y., Kimura S., Numata T., Nakamura D., Yokogawa T., Ogata T., Wada T., Suzuki T., Suzuki T., Nat. Chem. Biol. 2010, 6, 277. [DOI] [PubMed] [Google Scholar]
- 4.
- 4a. Kurata S., Weixlbaumer A., Ohtsuki T., Shimazaki T., Wada T., Kirino Y., Takai K., Watanabe K., Ramakrishnan V., Suzuki T., J. Biol. Chem. 2008, 283, 18801–18811; [DOI] [PubMed] [Google Scholar]
- 4b. Vendeix F. A. P., Murphy F. V., Cantara W. A., Leszczyńska G., Gustilo E. M., Sproat B., Malkiewicz A., Agris P. F., J. Mol. Biol. 2012, 416, 467–485; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4c. Nedialkova D. D., Leidel S. A., Cell 2015, 161, 1606–1618; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4d. Rozov A., Demeshkina N., Khusainov I., Westhof E., Yusupov M., Yusupova G., Nat. Commun. 2016, 7, 10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.
- 5a. Weixlbaumer A., F. V. Murphy IV , Dziergowska A., Malkiewicz A., Vendeix F. A. P., Agris P. F., Ramakrishnan V., Nat. Struct. Mol. Biol. 2007, 14, 498; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5b. Näsvall S. J., Chen P., Björk G. R., RNA 2007, 13, 2151–2164; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5c. Näsvall S. J., Chen P., Björk G. R., RNA 2004, 10, 1662–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wunderlich C. H., Spitzer R., Santner T., Fauster K., Tollinger M., Kreutz C., J. Am. Chem. Soc. 2012, 134, 7558–7569. [DOI] [PubMed] [Google Scholar]
- 7. Ueda T., Chem. Pharm. Bull. 1960, 8, 455–458. [Google Scholar]
- 8.
- 8a. Andrzej J. M., Nawrot B., Sochacka E., Z. Naturforsch. B 1987, 42, 360; [Google Scholar]
- 8b. Sierzputowska-Gracz H., Sochacka E., Malkiewicz A., Kuo K., Gehrke C. W., Agris P. F., J. Am. Chem. Soc. 1987, 109, 7171–7177. [Google Scholar]
- 9. Vendeix F. A. P., Munoz A. M., Agris P. F., RNA 2009, 15, 2278–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.
- 10a. Szymanski E. S., Kimsey I. J., Al-Hashimi H. M., J. Am. Chem. Soc. 2017, 139, 4326–4329; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10b. Juen M. A., Wunderlich C. H., Nußbaumer F., Tollinger M., Kontaxis G., Konrat R., Hansen D. F., Kreutz C., Angew. Chem. Int. Ed. 2016, 55, 12008–12012; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 12187–12191. [Google Scholar]
- 11. Kimsey I. J., Petzold K., Sathyamoorthy B., Stein Z. W., Al-Hashimi H. M., Nature 2015, 519, 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hwang T.-L., van Zijl P. C. M., Mori S., J. Biomol. NMR 1998, 11, 221–226. [DOI] [PubMed] [Google Scholar]
- 13. Hartono Y. D., Ito M., Villa A., Nilsson L., J. Phys. Chem. B 2018, 122, 1152–1160. [DOI] [PubMed] [Google Scholar]
- 14. Kimsey I. J., Szymanski E. S., Zahurancik W. J., Shakya A., Xue Y., Chu C.-C., Sathyamoorthy B., Suo Z., Al-Hashimi H. M., Nature 2018, 554, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


