Abstract
Cyclic esters and amides (lactones and lactams) are important active ingredients and polymer building blocks. In recent years, numerous biocatalytic methods for their preparation have been developed including enzymatic and chemoenzymatic Baeyer–Villiger oxidations, oxidative lactonisation of diols, and reductive lactonisation and lactamisation of ketoesters. The current state of the art of these methods is reviewed.
Keywords: Baeyer–Villiger oxidation, biocatalysis, lactams, lactones, oxidative Lactonisation
1. Introduction
Lactones and lactams represent two very diverse product classes widespread in natural products, active pharmaceutical ingredients, cosmetics, flavours and fragrances. In addition, lactones and lactams are important building blocks in the synthesis of polyesters and polyamides.1 Hence, it is not very astonishing that today's organic chemistry toolbox is well filled with synthetic procedures for their synthesis.
In recent years, their synthesis also caught the attention of biotechnologists resulting in a rich portfolio of biocatalytic alternatives complementing the existing chemical ones. The promise of these methods lies with the (suspected) environmental benignity of biocatalysis and ‐more importantly‐ with the intrinsic selectivity of enzymes. Hence, biocatalysis in principle offers more selective routes yielding products of higher quality in fewer process steps.
The aim of this contribution is to critically summarise the current state of the art of this dynamically developing field.
1.1. The major biocatalytic synthesis strategies
At present, there are three major biocatalytic routes for the synthesis of lactones: (1) Baeyer–Villiger oxidations, (2) oxidative lactonisations of diols and (3) the reductive cyclisation of γ‐ and δ‐ketoesters. In the following these methods will be briefly outlined.
For the biocatalytic Baeyer–Villiger oxidation, two very different enzyme classes are at hand: the Baeyer–Villiger monooxygenases (BVMOs) and hydrolases following the so‐called perhydrolase pathway.
BVMOs activate molecular oxygen within the enzymes’ active sites as organic peroxide, which then attacks the carbonyl group forming the lactone product and water. For the reductive activation of O2 BVMOs utilise reduced nicotinamide cofactors (NADH or NADPH) (Scheme 1). Very similar to the chemical Baeyer–Villiger oxidation, the BVMO oxidation proceeds via the so‐called Criegee intermediate. In contrast to the chemical reaction, the migration tendency of the two substituents of the carbonyl group is not only determined by their chemical reactivity but can also be influenced by the amino acids in the enzyme active site. Hence, BVMO‐catalysed Baeyer–Villiger oxidations very frequently give access to complementary products as compared to their chemical counterparts. Furthermore, BVMO‐catalysed Baeyer–Villiger oxidations proceed highly enantiospecific and enantioselective, making them valuable tools for the synthesis of enantiomerically pure lactones.
Scheme 1.

Baeyer–Villiger monooxygenase (BVMO)‐catalysed oxidative lactonisation of cyclic ketones.
One consequence of the catalytic mechanism of BVMOs is that these enzymes depend on the constant, stoichiometric supply of reducing equivalents in form of the reduced, natural nicotinamide adenine dinucleotide cofactor (NADH or NADPH). The high cost of this cofactor prevents any commercial application in stoichiometric amounts. Fortunately, today, a broad range of in situ regeneration systems for the reduced nicotinamide cofactor are available allowing for its use in catalytic amounts (vide infra).
As mentioned above, the catalytic mechanism of the hydrolase‐mediated Baeyer–Villiger oxidation differs significantly from the mechanism of the above‐described BVMOs. Hydrolase‐mediated Baeyer–Villiger oxidations comprise a two‐step, chemoenzymatic reaction mechanism (Scheme 2). In the first step, carboxylic acid (esters) are transformed into their percarboxylic acid derivates. This reaction is facilitated by the so‐called perhydrolase activity of many hydrolases. The resulting percarboxylic acid then performs the classical Baeyer–Villiger oxidation.
Scheme 2.
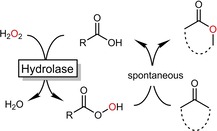
Chemoenzymatic Baeyer–Villiger oxidations exploiting the perhydrolase pathway of hydrolases.
The chemoenzymatic nature of this reaction also entails that none of the selectivity advantages of biocatalytic reactions can be expected for this approach. Possibly, using enantiomerically pure carboxylic acids may overcome this limitation.
Oxidative lactonisations of diols have been reported mostly using alcohol dehydrogenases (ADHs) as catalysts. ADHs catalyse the reversible, NAD(P)+‐dependent abstraction of a hydride equivalent from OH‐substituted C−H‐bonds. In case of 1,4‐ or 1,5‐diols, the primarily formed aldehyde can undergo spontaneous cyclisation leading to a hemiacetal (lactol), which itself can be oxidised once again by the ADH (Scheme 3).
Scheme 3.

Oxidative lactonisation of diols using alcohol dehydrogenases (ADHs).
Again, the issue of NAD(P)+ costs and their use in catalytic amounts arises. But again this challenge can, in principle, be considered to be solved through the use of established in situ regeneration systems for oxidized nicotinamide cofactors.
Finally, the reductive cyclisation of γ‐ and δ‐ketoesters is worth being discussed here. In this approach, reduction or reductive amination of the keto group in γ‐ and δ‐ketoesters leads to γ‐ and δ‐hydroxy‐ or amino esters, which spontaneously and irreversibly cyclise to the corresponding lactones or lactams, respectively (Scheme 4).
Scheme 4.

Reduction or reductive amination of ketoacids for the synthesis of lactones or lactams.
2. Biocatalytic Baeyer–Villiger oxidations using BVMOs
2.1. Mechanistic considerations
BVMOs are flavin containing enzymes. The catalytically active species is a deprotonated peroxoflavin, which is formed in a sequence of NAD(P)H‐dependent reduction of the resting state (oxidised) flavin group and reductive activation of molecular oxygen (Scheme 5). The latter then nucleophilically attacks the carbonyl group of the substrate forming the Criegee intermediate. In contrast to the chemical reaction, the migration tendency of the two substituents of the carbonyl group is not only determined by their chemical reactivity but can also be influenced by the active site architecture of the enzyme. Hence, BVMO‐catalysed Baeyer–Villiger (BV) oxidations very frequently give access to complementary products as compared to their chemical counterparts.
Scheme 5.

Simplified representation of the catalytic mechanism of BVMOs.
2.2. Sources for BVMOs and new, improved BVMOs
Despite the early realisation that a biological counterpart to BV‐oxidation occurs in microorganisms, the discovery and cloning of cyclohexanone monooxygenase (CHMO) and the subsequent studies into the substrate scope and selectivity and specificity of CHMO truly realised the synthetic potential of BVMOs. In recent years the number of available BVMOs has expanded significantly and now includes several eukaryotic BVMOs from fungi,2 moss and algae.3 In addition to the ever growing collection of BVMOs, considerable effort into the directed evolution of several BVMOs has further expanded their substrate scope and improved or altered their stereoselectivity/specificity.4
Despite the widespread use of CHMO, the practical application of this BVMO (and many others) remains limited due to its operational and thermal instability. Various researchers have therefore focused on improving the stability of CHMO through rational design and directed evolution.5 Until recently, phenylacetone monooxygenase (PAMO) was the only truly stable BVMO. Unfortunately PAMO displays a rather narrow substrate scope and is unreactive with most of the cyclic ketones accepted by CHMO, limiting its use for lactone synthesis. Again, evolution studies were able to expand the substrate scope of PAMO to include cyclic ketones such as cyclohexanone6 and 2‐phenylcyclohexanone.7 The discovery of new, more stable BVMOs such as PockeMO2a from Thermothelomyces thermophile and TmCHMO8 from Thermocrispum municipal will potentially greatly improve practical applications of BVMOs in lactone synthesis.
2.3. Practical issues for the application of BVMOs
Some issues frequently observed using BVMOs are (1) their dependency on the reduced nicotinamide cofactors and (2) inhibitory effects of the reagents (substrates and products).
The “cofactor challenge” particularly concerns Baeyer–Villiger oxidations using cell‐free BVMOs. This general limitation to oxidoreductases has been known for decades9 resulting in a broad range of in situ NAD(P)H regeneration systems. Obviously “the usual suspects” such as formate dehydrogenase (FDH) or glucose dehydrogenase (GluDH) can be applied as in situ NAD(P)H regeneration systems. More elegantly, however, sequential cascades employing alcohol dehydrogenases (ADHs) and BVMOs enable redox‐neutral transformation of lactones from the corresponding alcohols without using any auxiliary cosubstrates for example, glucose or formic acid (Scheme 6).
Scheme 6.
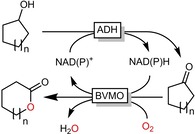
Sequential, redox‐neutral cascade combining ADHs and BVMOs to transform cycloalkanols into lactones.
Reported first as early as the 1990s,10 this approach is now receiving renewed attention by various groups envisioning the cost‐efficient synthesis of lactone building blocks for polyesters.11 Next to this sequential, redox‐neutral cascade some of us have recently developed a convergent analogue (vide infra).
In vivo reactions, that is, BVMO reactions using whole, metabolically active cells are also enjoying great popularity particularly because no additional cofactors have to be supplied since the microbial metabolism provides the reduced nicotinamide cofactors needed for catalysis.12
Inhibitory effects of substrates and products are frequently observed in BVMO‐catalysed reactions. While substrate inhibition can be addressed by a substrate feeding strategy,13 product inhibition needs more elaborate strategies to remove the products from the reaction mixtures (Scheme 7). Solid extraction resins for example have been demonstrated13a,13b as well as hydrophobic organic solvents.14
Scheme 7.
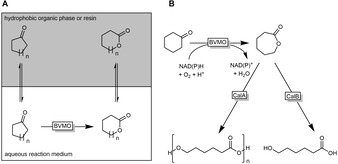
Common product removal strategies to alleviate inhibitory effects on BVMOs. A: in situ extraction of the product into a hydrophobic organic phase or to a resin; B: hydrolysis or oligomerisation of the lactone product.
To overcome the product inhibition observed with ϵ‐caprolactone, researchers have used lipases to remove the inhibiting lactone. For example, lipase A (CalA) from Candida antarctica oligomerises ϵ‐caprolactone15 whereas CalB can be employed for the hydrolysis of the lactone to 6‐hydroxyhexanoic acid.16
2.4. BVMO‐catalysed oxidations
The substrate scope of BVMOs has now grown to include simple cyclic ketones from cyclobutanone to cylopentadecanone, highly substituted cyclic ketones including various monoterpenones, fused and bridged bicyclic ketones to various steroids (Scheme 8).17
Scheme 8.
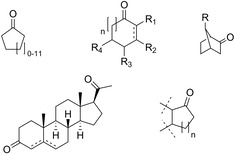
Representation of the wide substrate scope of BVMOs.
As mentioned above, one of the major advantages of BVMO‐catalysis for the oxidative lactonisation of (cyclo)ketones is the ability to form both the normal and the (chemically disfavoured) “abnormal” lactone, depending on which BVMO is used (Scheme 9).
Scheme 9.
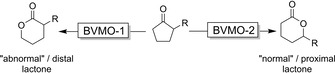
Use of complementary BVMOs (from natural or man‐made diversity) to produce either the ‘normal’ or the ‘abnormal’ lactone.
Nowadays, numerous BVMOs and evolved variants are available and regiocomplementary reactions from the same starting material are often possible.18
One of the most often used substrates to evaluate the activity of BVMOs is rac‐(cis)‐bicyclo[3.2.0]hept‐2‐en‐6‐one of which the products are important chiral synthons in prostaglandin synthesis. This seemingly universal substrate for BVMOs can simultaneously assess the ability of BVMOs for regiospecificity as well as enantioselectivity (Scheme 10).
Scheme 10.
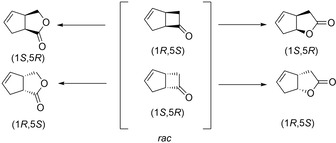
Asymmetric (enantioseletive and regiospecific) oxygenation of rac‐(cis)‐bicyclo[3.2.0]hept‐2‐en‐6‐one by BVMOs.
Cyclohexanone monooxygenase (CHMO), for example performs the regiodivergent conversion of rac‐(cis)‐bicyclo[3.2.0]hept‐2‐en‐6‐one with the (1R,5S) enantiomer being transformed into the normal lactone whereas the (1S,5R) enantiomer is transformed into the abnormal lactone.19 Other BVMOs such as MO14 from Rhodococcus jostii shows high enantioselectivity for the racemate resulting in kinetic resolutions (KRs) yielding the enantiomerically pure normal lactone of the (1R, 5S) enantiomer while leaving the (1S,5R)‐ketone in 96 % ee.20 Similar kinetic resolutions have been shown with other BVMOs as well as the ability to obtain the abnormal lactone (1S,5R) in high ee.2c
The popularity of rac‐(cis)‐bicyclo[3.2.0]hept‐2‐en‐6‐one as model substrate also explains it prevalence as starting material in preparative‐scale BVMO oxidations (Table 1).
Table 1.
Notable preparative scale whole cell biotransformations using BVMOs.
| Biocatalyst | Strategy | Volume, Time | Substrate loading/feeding | STY (g L−1 h−1) |
Conversion/ Yield [%] |
|---|---|---|---|---|---|
| CHMO21 | SF[b]
PR[c] |
50 L, 20 h | 900 g, | 1.02 | >99 %, 58 %[a] |
| CHMO22 | SF[b] | 55 L, 3.5 h | 247.5 g, 1 g L−1 h−1 |
1.09 | 76 %, 210 g |
| CHMO23 | SF[b] | 200 L, 7 h | 900 g, 0.6–1.1 g L−1 h−1 |
0.64 | 4.5 g L−1 |
[a] Isolated Yield. [b] Substrate feeding (SF). [c] Product removal (PR).
Further examples of KRs using BVMOs include p‐alkene cyclohexanone derivates24 or dihydrocarvone.4b The intrinsic disadvantage of KRs of maximal yields of 50 % in some cases can be overcome using prochiral starting materials such as p‐alkyl cyclohexanones.25 Another approach is to design dynamic kinetic resolutions (DKRs) where the racemic starting material, next to the enantioselective Baeyer–Villiger oxidation also undergoes a racemisation reaction. Obviously, in case of α‐substituted (cyclo)ketones this can be achieved easily by adjusting the pH of the reaction mixture to alkaline values or using anion exchange resins at more ambient pH (Scheme 11).26
Scheme 11.

DKR of α‐substituted ketones at alkaline pH benefitting from the high enantioselectvity of the BVMO for one enantiomer and the fast racemisation of the starting material.26a,26b
In recent years, embedding BVMO‐catalysed reactions into larger cascade reactions has attracted significant interest. Particularly, in situ generation of the cycloketone substrate has been investigated by various groups. For example, a cascade comprising a cytochrome P450 monooxygenase and an ADH yielded cyclohexanone from the corresponding cycloalkane (Scheme 12 A);27 in a similar redox‐neutral approach allylic alcohols can be transformed into cycloketones (Scheme 12 B).28 The latter approach represents an interesting method to transform limonene wastes (from orange peels) into carvolactone as a building block for functional polymers.
Scheme 12.
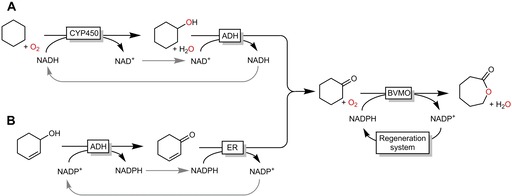
BVMO‐catalysed Baeyer–Villiger oxidations with in situ generated cycloketones. A: from cycloalkanes with cytochrome P450 monooxygenase (CYP450)‐catalysed hydroxylation followed by ADH‐catalysed oxidation or B: from allylic alcohols by ADH‐catalysed oxidation and ene reductase (ER)‐catalysed reduction. Both pre‐cascades as redox‐neutral.
3. Chemoenzymatic oxidations via the perhydrolase approach
Already in the 1990s, Björkling and co‐workers reported the so‐called perhydrolase reaction of esterases as promiscuous activity of many hydrolases.29 Especially serine hydrolases accept nucleophiles other than water or alcohols to hydrolyse the enzyme acyl‐intermediate. In case of H2O2 as alternative nucleophile, peracids are formed, which can undergo a range of oxidative transformations, including Baeyer–Villiger oxidations (Scheme 2).30
Compared to the BVMO‐catalysed Baeyer–Villiger oxidation, the chemoenzymatic variant bears a range of intrinsic advantages making it interesting for industrial application: First, this reaction does not require any cofactors and corresponding regeneration systems. Second, a broad range of immobilised hydrolases are commercially available (though the immobilised lipase from Candida antarctica, tradename Novozyme 435 is by far the most popular). Third, many of these preparations are active and stable under non‐aqueous conditions. Especially the latter is of utmost importance to attain high substrate loadings. Table 2 gives a representative, yet incomplete overview of reported chemoenzymatic Baeyer–Villiger oxidations.
Table 2.
Selection of products obtained from the chemoenzymatic Baeyer–Villiger oxidation of cycloketones.

| ||||
|---|---|---|---|---|
| Product | Hydrolase | Acyl donor/ H2O2 source | Yield/ Remarks | Ref. |

|
CalB (50 mg mmol−1) | Ethylacetate (0.66 equiv.)/ UHP (1.5 equiv.) | 960 mm | 31 |

|
CalB (25 mg mmol−1) | Ethylacetate (0.66 equiv.)/ UHP (2 equiv.) | high | 32 |

|
CalB on MWCNTs (75 mg mmol−1) or silica immobilised |
Octanoic acid (>10 equiv.)/ 30 % H2O2 (2 equiv.) | 33 | |

|
CalB in ILs | Octanoic acid (0.13 equiv.)/ 50 % H2O2 (1.7 equiv.) | 83 % | 34 |

|
Acyl transferase from Mycobacterium smegmatis | Ethylacetate/ 50 % H2O2 | 84–99 % | 35 |
MWCNTs: Multi‐walled carbon nanotubes.
Frequently, concentrated H2O2 solutions are used to minimise the water concentration. Similarly, also urea‐H2O2 (UHP) is a popular (anhydrous) H2O2 source. Nevertheless, even under anhydrous conditions and using UHP, water accumulation cannot be prevented since it constitutes the stoichiometric by‐product. This may lead to hydrolysis of the lactone product. To some extent, this can be overcome by reaction engineering and/or protein engineering.34, 36
Under carefully chosen reaction conditions, this generally undesired side reaction can, however, also be exploited for a combined Baeyer–Villiger oxidation and ring opening polymerisation sequence.37
Another issue necessitating further attention in the future is the poor affinity of hydrolases for H2O2 necessitating high H2O2 concentrations to achieve high reaction rates. Such high H2O2 concentrations (several hundred millimolar) also lead to oxidative inactivation of the enzymes.
Envisioning chiral lactone products, the chemoenzymatic approach appears less suited as generally racemic products are formed. A promising approach to solve this shortcoming may be to use chiral (per)acids for the chemical Baeyer–Villiger oxidation. Using methyl‐substituted carboxylic acids, Chrobok and co‐workers achieved optical purities of up to 96 % in the conversion of 4‐methyl cyclohexanone.38
Overall, the perhydrolase approach appears to be an interesting approach for the preparative scale Baeyer–Villiger oxidation if the optical purity of the products is of lesser importance.
4. Biocatalytic oxidative lactonisations
4.1. ADH‐catalysed reactions
The oxidative lactonisation of (mostly 1,4‐ and 1,5‐) diols is a double oxidation process. In the first step one alcohol functionality is oxidised to the corresponding aldehyde, which is in equilibrium with the intramolecular hemiacetal (lactol). The latter can be oxidised once more to the desired lactone product (Scheme 3).
The first example for an ADH‐catalysed version of this reaction sequence was reported as early as 1977 by Jones and co‐workers.39 Since the ADH‐oxidation steps are principally stereospecific, often enantiomerically pure lactone products are obtained either via kinetic resolution of racemic diol starting materials or using the meso‐trick. Later the same group also demonstrated the preparative applicability of this approach.40
Since these pioneering works, other groups have further contributed to the development and application of this reaction system.
Table 3 gives a representative overview of products reported from ADH‐catalysed oxidative lactonisations.
Table 3.
Representative examples for ADH‐catalysed oxidative lactonisations.

| ||||
|---|---|---|---|---|
| Product | Bioatalyst | Regeneration system | Remark | Ref. |

|
HLADH | Glucose dehydrogenase | 50 mmol scale, 84 %, 2LPS | 41 |

|
Enantio‐complementary ADHs | whole cells | several mgs | 42 |

|
HLADH | FMN | kinetic resolution | 39a |

|
HLADH | FMN | kinetic resolution 69 %, yield several mgs |
43 |

|
HLADH | LMS |
n=0: 99 % n=1: 80 % n=2: 26 % |
44 |
HLADH: Horse‐liver alcohol dehydrogenase. FMN: Flavin mononucleotide. LMS: Laccase mediator system. 2LPS: Two‐liquid‐phase‐system.
As cofactor‐dependent reactions, ADH‐catalysed oxidative lactonisations necessitate efficient in situ NAD(P)+ regeneration systems. Jones and others used a simple aerobic regeneration system with flavins as NAD(P)H oxidation catalysts.39, 40, 42, 45 The first oxidation step [that is, the hydride transfer from NAD(P)H to flavin mononucleotide (FMN)] is painfully slow necessitating high concentrations of the flavin “catalyst” and requiring long reaction times. This can be alleviated very significantly by photochemical activation of the flavin catalyst.46 Also other catalysts can be used to reoxidise NAD(P)H such as 2,2′‐azino‐bis(3‐ethylbenzothiazoline‐6‐sulfonic acid) (ABTS). The resulting reduced ABTS itself can be reoxidised electrochemically47 or using laccases.44, 48
Next to cofactor regeneration issues, substrate‐ and product inhibition as well as undesired hydrolysis of the lactone products in aqueous media are also frequently observed. Again, these challenges can be overcome by use of the two‐liquid‐phase‐system (2LPS) approach (Scheme 13).41, 44
Scheme 13.
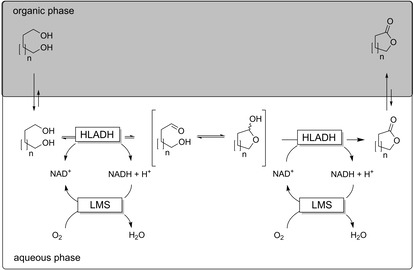
Application of the 2LPS approach in oxidative lactonisation reactions.
Recently, some of us combined the aforementioned BVMO‐catalysed Baeyer–Villiger oxidation of cycloketones with the ADH‐catalysed lactonisation of diols to produce lactones in a convergent, redox‐neutral cascade reaction (Scheme 14).49 The proof‐of‐concept was first shown for the synthesis of ϵ‐caprolactone up to mg scales.49b,49c
Scheme 14.
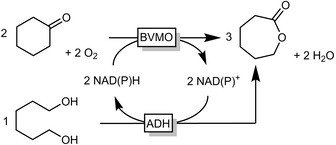
A convergent, redox‐neutral cascade combining an ADH and a BVMO for the synthesis of ϵ‐caprolactone.49b,49c
4.2. Chemoenzymatic reactions using the laccase‐TEMPO system
Gotor‐Fernández and co‐workers explored an interesting chemoenzymatic alternative to the above‐mentioned ADH‐catalysed oxidative lactonisation (Scheme 15).50 Here 2,2,6,6‐tetramethylpiperidinoxyl (TEMPO) serves as a chemical oxidant for the hydroxyl/lactol group and is itself regenerated aerobically by laccases.
Scheme 15.
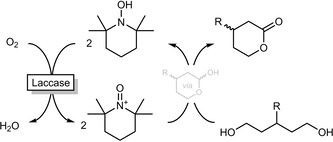
TEMPO‐catalysed oxidative lactonisation of 1,5‐diols with aerobic, laccase‐catalysed regeneration of TEMPO.50
This methodology may be of interest especially for the lactonisation of racemic diols to racemic lactones. Here, the usually valued high stereoselectivity of enzymes may become an issue due to lowered conversion because of the KR character of the ADH‐reaction.
5. Biocatalytic reductive lactonisations
While the aforementioned methodologies have been oxidative, also reductive lactonisation of γ‐keto acid(ester)s, especially the fructose‐derived levulinic acid, has received some attention.
Hilterhaus, Liese and co‐workers for example designed a reaction cascade transforming levulinic acid into (S)‐γ‐valerolactone (Scheme 16).51
Scheme 16.
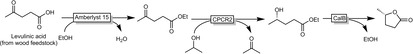
Chemoenzymatic cascade transforming levulinic acid into enantiomerically pure (S)‐γ‐valerolactone. CPCR2: Carbonyl reductase 2 from Candida parapsilosis.
Using stereocomplementary ADHs, Lavandera, Gotor and co‐workers broadened the scope of this reaction to both enantiomers of γ‐ and δ‐lactones.52
More recently, the same group also reported the synthesis of γ‐ and δ‐lactames by substituting the ADH‐catalysed ketoreduction by an ω‐transaminase‐catalysed reductive amination (Scheme 17) of γ‐ or δ‐ketoesters.53
Scheme 17.
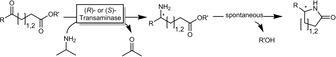
Reductive lactamisation of some γ‐ or δ‐ketoesters using stereocomplementary transaminases. Both enantiomers were obtained essentially enantiomerically pure.53
The following table (Table 4) shows some selected examples (but not all) representing the reductive biocatalytic oxidations and reductive aminations (Scheme 4).
Table 4.
Representative examples for reductive lactonisations catalysed by alcohol dehydrogenases (ADHs) and reductive aminations by transaminases (TAs).
| Product | Biocatalyst | Substrate loading, Time | Yield, Selectivity | Ref. |
|---|---|---|---|---|

|
CPCR2 CalB |
90 %, >99 % ee | 51 | |

|
LBADH | 50 mm, 24 h | 90 %, >99 % ee | 52 |

|
LBADH | 50 mm, 24 h | >80 %, >99 % ee | 52 |

|
ADH‐A | 50 mm, 24 h | 90 %, >99 % ee | 52 |

|
ADH‐A | 50 mm, 24 h | >80 %, >99 % ee | 52 |

|
RasADH | 50 mm, 24 h | 97 %, >99 % ee | 52 |

|
YqjM ADHT |
≈420 mm, (15 mL scale), <24 h | 90 %, >99 % ee, 0.66 g | 54 |

|
ArS‐TA | 25 mm, 24 h | >99 %, >99 % ee | 53 |

|
ATA‐024 | 15 mm, 24 h | 99 %, >99 % ee | 53 |

|
ATA‐237 | 15 mm, 24 h | 94 %, 95 % ee | 53 |

|
ATA‐117 | 50 g L−1 (50 mL scale), <15 h | >99 % conv., >90 % yield, >99 % ee | 55 |
CPCR2: Candida parapsilosis carbonyl reductase 2. CalB: Candida antarctica lipase B. LBADH: Lactobacillus brevis alcohol dehydrogenase. ADH‐A: Alcohol dehydrogenase from Rhodococcus ruber. RasADH: Ralstonia alcohol dehydrogenase. YqjM: Ene reductase from Bacillus subtilis. ADHT: ADH from Thermoanaerobacter sp. ATA: Amine transaminase.
6. Conclusions
Lactones and lactams represent an important class of substances with a wide range of applications in industry. Although discovered decades ago, enzymatic synthesis of these crucial building blocks has been receiving increased interest during the past few years. Not only do these biocatalytic routes offer an apparent green alternative to current chemical methods, alternative regiomer synthesis as well as the superior enantioselectvity displayed by these biocatalysts provide access to important chiral synthons not easily accessible through traditional chemical routes. The challenges related to substrate and product inhibition, poor enzyme stability and narrow substrate scope have been largely dealt with through modern molecular biology techniques and/or reaction engineering. Considering the redox‐reactions, the required nicotinamide cofactors can be recycled by diverse efficient in situ regeneration methods including the redox‐neutral cascades, which makes the use of enzymes also economically feasible.
We believe that the rediscovered (chemo)enzymatic synthesis of lactones and lactams will be one of the major focuses in biocatalysis with the aim of synthesis of these industrial intermediates with high volumetric productivities and economical as well as ecological efficiency.
Conflict of interest
The authors declare no conflict of interest.
Biographical Information
Frank Hollmann studied Chemistry at the University of Bonn (Germany). After his Ph.D. at the Swiss Federal Institute of Technology (ETH Zurich, Switzerland supervised by Prof. Andreas Schmid) and a postdoctoral stay with Prof. Manfred T. Reetz at the Max‐Planck Institute for Coal Research (Mülheim an der Ruhr, Germany) he joined Evonik as R&D manager. Since 2008, he has been a member of the Biocatalysis group at the Delft University of Technology. His research interests focus around the application of redox enzymes for organic synthesis.

Biographical Information
Selin Kara studied Chemical Engineering and Food Engineering at the Middle East Technical University (METU, Turkey). After her M.Sc. study in Biotechnology and Ph.D. at the Hamburg University of Technology (TUHH, Germany), she moved to the Netherlands for her postdoctoral research with Frank Hollmann at the Delft University of Technology (TU Delft, The Netherlands). In 2013, she started her Habilitation (venia legendi) at the Technical University of Dresden (TU Dresden, Germany) with Prof. Dr. M. Ansorge‐Schumacher. In May 2018, she finalised her Habilitation at TUHH with Prof. Dr. A. Liese and since July 2018 she has been an Associate Prof. at the Aarhus University (AU, Denmark).

Biographical Information
Diederik J. Opperman obtained his Ph.D. in Biochemistry from the University of the Free State (UFS, South Africa) in 2008. He then joined the research group of Professor Manfred T. Reetz at Max‐Planck Institute for Coal Research (Mülheim an der Ruhr, Germany) as a postdoctoral co‐worker in directed evolution. He is currently an Associate Professor at the UFS with a research focus on the structure–function relationship of biocatalysts.

Biographical Information
Yonghua Wang serves as professor and vice‐dean in the Department of Food Science and Engineering at the South China University of Technology (Guangzhou, China). Her research interests focus on enzyme and lipid engineering. Amongst others distinctions, she was awarded as “Distinguished Young Scholars” by the Chinese National Science Fund (2017), the “Ten Thousand Talent Program” (2016), as “Leading young Scientist” by the Chinese Ministry of Science and Technology (2014) and as “Leading Scientist and Innovator” by the Guangdong Province Special Support fund.

Acknowledgements
F.H. gratefully acknowledge funding by the European Research Commission (ERC consolidator grant, No. 648026), the European Union (H2020‐BBI‐PPP‐2015‐2‐1‐720297) and the Netherlands Organisation for Scientific Research (VICI grant No. 724.014.003). S.K. gratefully acknowledges financial support from German Research Foundation (Deutsche Forschungsgemeinschaft, grant No. KA4399/1‐1) and Fonds der Chemischen Industrie (FCI). D.J.O. acknowledges the National Research Foundation (NRF, South Africa) for funding (grant No. 112094).
F. Hollmann, S. Kara, D. J. Opperman, Y. Wang, Chem. Asian J. 2018, 13, 3601.
References
- 1. Liu X., He L. N., Top. Curr. Chem. 2017, 375, 32. [DOI] [PubMed] [Google Scholar]
- 2.
- 2a. Fürst M. J. L. J., Savino S., Dudek H. M., Castellanos J. R. G., de Souza C. G., Rovida S., Fraaije M. W., Mattevi A., J. Am. Chem. Soc. 2017, 139, 627–630; lit b> [DOI] [PubMed] [Google Scholar]; Mthethwa K. S., Kassier K., Engel J., Kara S., Smit M. S., Opperman D. J., Enzyme Microb. Technol. 2017, 106, 11–17; [DOI] [PubMed] [Google Scholar]
- 2c. Ferroni F. M., Smit M. S., Opperman D. J., J. Mol. Catal. B 2014, 107, 47–54. [Google Scholar]
- 3. Beneventi E., Ottolina G., Carrea G., Panzeri W., Fronza G., Lau P. C. K., J. Mol. Catal. B 2009, 58, 164–168. [Google Scholar]
- 4.
- 4a. Balke K., Beier A., Bornscheuer U. T., Biotechnol. Adv. 2018, 36, 247–263; [DOI] [PubMed] [Google Scholar]
- 4b. Balke K., Schmidt S., Genz M., Bornscheuer U. T., ACS Chem. Biol. 2016, 11, 38–43; [DOI] [PubMed] [Google Scholar]
- 4c. Li G. Y., Furst M., Mansouri H. R., Ressmann A. K., Ilie A., Rudroff F., Mihovilovic M. D., Fraaije M. W., Reetz M. T., Org. Biomol. Chem. 2017, 15, 9824–9829; [DOI] [PubMed] [Google Scholar]
- 4d. Zhang Z.-G., Parra L. P., Reetz M. T., Chem. Eur. J. 2012, 18, 10160–10172; [DOI] [PubMed] [Google Scholar]
- 4e. Dudek H. M., Fink M. J., Shivange A. V., Dennig A., Mihovilovic M. D., Schwaneberg U., Fraaije M. W., Appl. Microbiol. Biotechnol. 2014, 98, 4009–4020. [DOI] [PubMed] [Google Scholar]
- 5.
- 5a. Opperman D. J., Reetz M. T., ChemBioChem 2010, 11, 2589–2596; [DOI] [PubMed] [Google Scholar]
- 5b. van Beek H. L., Wijma H. J., Fromont L., Janssen D. B., Fraaije M. W., FEBS Open Bio 2014, 4, 168–174; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5c. Schmidt S., Genz M., Balke K., Bornscheuer U. T., J. Biotechnol. 2015, 214, 199–211. [DOI] [PubMed] [Google Scholar]
- 6. Parra L. P., Acevedo J. P., Reetz M. T., Biotechnol. Bioeng. 2015, 112, 1354–1364. [DOI] [PubMed] [Google Scholar]
- 7.
- 7a. van Beek H. L., Gonzalo G. D., Fraaije M. W., Chem. Commun. 2012, 48, 3288–3290; [DOI] [PubMed] [Google Scholar]
- 7b. Bocola M., Schulz F., Leca F., Vogel A., Fraaije M. W., Reetz M. T., Adv. Synth. Catal. 2005, 347, 979–986. [Google Scholar]
- 8. Romero E., Castellanos J. R. G., Mattevi A., Fraaije M. W., Angew. Chem. Int. Ed. 2016, 55, 15852–15855; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 16084–16087. [Google Scholar]
- 9. Whitesides G. M., Wong C. H., Angew. Chem. Int. Ed. Engl. 1985, 24, 617–638; [Google Scholar]; Angew. Chem. 1985, 97, 617–638. [Google Scholar]
- 10.
- 10a. Grogan G., Roberts S., Willetts A., Biotechnol. Lett. 1992, 14, 1125–1130; [Google Scholar]
- 10b. Willetts A. J., Knowles C. J., Levitt M. S., Roberts S. M., Sandey H., Shipston N. F., J. Chem. Soc. Perkin Trans. 1 1991, 1608–1610. [Google Scholar]
- 11.
- 11a. Kohl A., Srinivasamurthy V., Bottcher D., Kabisch J., Bornscheuer U. T., Enzyme Microb. Technol. 2018, 108, 53–58; [DOI] [PubMed] [Google Scholar]
- 11b. Staudt S., Bornscheuer U. T., Menyes U., Hummel W., Gröger H., Enzyme Microb. Technol. 2013, 53, 288–292; [DOI] [PubMed] [Google Scholar]
- 11c. Mallin H., Wulf H., Bornscheuer U. T., Enzyme Microb. Technol. 2013, 53, 283–287; [DOI] [PubMed] [Google Scholar]
- 11d. Aalbers F. S., Fraaije M. W., Appl. Microbiol. Biotechnol. 2017, 101, 7557–7565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.
- 12a. Stewart J. D., Curr. Org. Chem. 1998, 2, 195–216; [Google Scholar]
- 12b. Clouthier C. M., Kayser M. M., J. Mol. Catal. B 2007, 46, 32–36. [Google Scholar]
- 13.
- 13a. Hilker I., Gutierrez M. C., Furstoss R., Ward J., Wohlgemuth R., Alphand V., Nat. Protoc. 2008, 3, 546–554; [DOI] [PubMed] [Google Scholar]
- 13b. Rudroff F., Alphand V., Furstoss R., Mihovilovic M. D., Org. Process Res. Dev. Org. Proc. Res. Dev. 2006, 10, 599–604; [Google Scholar]
- 13c. Lee W. H., Park J. B., Park K., Kim M. D., Seo J. H., Appl. Microbiol. Biotechnol. 2007, 76, 329–338. [DOI] [PubMed] [Google Scholar]
- 14.
- 14a. Schulz F., Leca F., Hollmann F., Reetz M. T., Beilstein J. Org. Chem. 2005, 1, DOI: 10.1186/1860-5397-1181-1110; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14b. Reimer A., Wedde S., Staudt S., Schmidt S., Höffer D., Hummel W., Kragl U., Bornscheuer U. T., Gröger H., J. Heterocycl. Chem. 2017, 54, 391–396. [Google Scholar]
- 15. Schmidt S., Scherkus C., Muschiol J., Menyes U., Winkler T., Hummel W., Gröger H., Liese A., Herz H. G., Bornscheuer U. T., Angew. Chem. Int. Ed. 2015, 54, 2784–2787; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 2825–2828. [Google Scholar]
- 16.
- 16a. Scherkus C., Schmidt S., Bornscheuer U. T., Gröger H., Kara S., Liese A., Biotechnol. Bioeng. 2017, 114, 1215–1221; [DOI] [PubMed] [Google Scholar]
- 16b. Scherkus C., Schmidt S., Bornscheuer U. T., Gröger H., Kara S., Liese A., ChemCatChem 2016, 8, 3446–3452. [Google Scholar]
- 17.
- 17a. Bucko M., Gemeiner P., Schenkmayerova A., Krajcovic T., Rudroff F., Mihovilovic M. D., Appl. Microbiol. Biotechnol. 2016, 100, 6585–6599; [DOI] [PubMed] [Google Scholar]
- 17b. Balke K., Kadow M., Mallin H., Sass S., Bornscheuer U. T., Org. Biomol. Chem. 2012, 10, 6249–6265; [DOI] [PubMed] [Google Scholar]
- 17c. Leisch H., Morley K., Lau P. C. K., Chem. Rev. 2011, 111, 4165–4222; [DOI] [PubMed] [Google Scholar]
- 17d. Kayser M. M., Tetrahedron 2009, 65, 947–974; [Google Scholar]
- 17e. Mihovilovic M. D., Curr. Org. Chem. 2006, 10, 1265–1287. [Google Scholar]
- 18. Mihovilovic M. D., Kapitan P., Kapitanova P., ChemSusChem 2008, 1, 143–148. [DOI] [PubMed] [Google Scholar]
- 19. Zambianchi F., Pasta P., Carrea G., Colonna S., Gaggero N., Woodley J. M., Biotechnol. Bioeng. 2002, 78, 489–496. [DOI] [PubMed] [Google Scholar]
- 20. Summers B. D., Omar M., Ronson T. O., Cartwright J., Lloyd M., Grogan G., Org. Biomol. Chem. 2015, 13, 1897–1903. [DOI] [PubMed] [Google Scholar]
- 21. Hilker I., Wohlgemuth R., Alphand V., Furstoss R., Biotechnol. Bioeng. 2005, 92, 702–710. [DOI] [PubMed] [Google Scholar]
- 22. Doig S. D., Avenell P. J., Bird P. A., Gallati P., Lander K. S., Lye G. J., Wohlgemuth R., Woodley J. M., Biotechnol. Prog. 2002, 18, 1039–1046. [DOI] [PubMed] [Google Scholar]
- 23. Baldwin C. V. F., Wohlgemuth R., Woodley J. M., Org. Process Res. Dev. 2008, 12, 660–665. [Google Scholar]
- 24. Zhang Z.-G., Roiban G.-D., Acevedo J. P., Polyak I., Reetz M. T., Adv. Synth. Catal. 2013, 355, 99–106. [Google Scholar]
- 25. Reetz M. T., Brunner B., Schneider T., Schulz F., Clouthier C. M., Kayser M. M., Angew. Chem. Int. Ed. 2004, 43, 4078–4081; [Google Scholar]; Angew. Chem. 2004, 116, 4170–4173. [Google Scholar]
- 26.
- 26a. Berezina N., Kozma E., Furstoss R., Alphand V., Adv. Synth. Catal. 2007, 349, 2049–2053; [Google Scholar]
- 26b. Rioz-Martínez A., de Gonzalo G., Pazmino D. E. T., Fraaije M. W., Gotor V., J. Org. Chem. 2010, 75, 2073–2076; [DOI] [PubMed] [Google Scholar]
- 26c. Gutiérrez M.-C., Furstoss R., Alphand V., Adv. Synth. Catal. 2005, 347, 1051–1059. [Google Scholar]
- 27.
- 27a. Pennec A., Hollmann F., Smit M. S., Opperman D. J., ChemCatChem 2015, 7, 236–239; [Google Scholar]
- 27b. Karande R., Salamanca D., Schmid A., Buehler K., Biotechnol. Bioeng. 2018, 115, 312–320. [DOI] [PubMed] [Google Scholar]
- 28.
- 28a. Oberleitner N., Ressmann A. K., Bica K., Gartner P., Fraaije M. W., Bornscheuer U. T., Rudroff F., Mihovilovic M. D., Green Chem. 2017, 19, 367–371; [Google Scholar]
- 28b. Oberleitner N., Peters C., Rudroff F., Bornscheuer U. T., Mihovilovic M. D., J. Biotechnol. 2014, 192, 393–399; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28c. Oberleitner N., Peters C., Muschiol J., Kadow M., Sass S., Bayer T., Schaaf P., Iqbal N., Rudroff F., Mihovilovic M. D., Bornscheuer U. T., ChemCatChem 2013, 5, 3524–3528. [Google Scholar]
- 29.
- 29a. Björkling F., Frykman H., Godtfredsen S. E., Kirk O., Tetrahedron 1992, 48, 4587–4592; [Google Scholar]
- 29b. Björkling F., Godtfredsen S. E., Kirk O., J. Chem. Soc. Chem. Commun. 1990, 1301–1303. [Google Scholar]
- 30. Lemoult S. C., Richardson P. F., Roberts S. M., J. Chem. Soc. Perkin Trans. 1 1995, 89–91. [Google Scholar]
- 31. González-Martinez D., Rodríguez-Mata M., Mendez-Sánchez D., Gotor V., Gotor-Fernández V., J. Mol. Catal. B 2015, 114, 31–36. [Google Scholar]
- 32.
- 32a. Rios M. Y., Salazar E., Olivo H. F., J. Mol. Catal. B 2008, 54, 61–66; [Google Scholar]
- 32b. Ríos M. Y., Salazar E., Olivo H. F., Green Chem. 2007, 9, 459–462. [Google Scholar]
- 33.
- 33a. Markiton M., Boncel S., Janas D., Chrobok A., ACS Sustainable Chem. Eng. 2017, 5, 1685–1691; [Google Scholar]
- 33b. Drozdz A., Chrobok A., Baj S., Szymanska K., Mrowiec-Białon J., Jarzebski A. B., Appl. Catal. A 2013, 467, 163–170. [Google Scholar]
- 34. Kotlewska A. J., van Rantwijk F., Sheldon R. A., Arends I., Green Chem. 2011, 13, 2154–2160. [Google Scholar]
- 35. Drozdz A., Hanefeld U., Szymanska K., Jarzebski A., Chrobok A., Catal. Commun. 2016, 81, 37–40. [Google Scholar]
- 36.
- 36a. Wang X.-P., Zhou P.-F., Li Z.-G., Yang B., Hollmann F., Wang Y.-H., Sci. Rep. 2017, 7, 44599; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36b. Drozdz A., Erfurt K., Bielas R., Chrobok A., New J. Chem. 2015, 39, 1315–1321. [Google Scholar]
- 37. Zhong J. R., Xu F., Wang J. F., Li Y. Y., Lin X. F., Wu Q., RSC Adv. 2014, 4, 8533–8540. [Google Scholar]
- 38. Drozdz A., Chrobok A., Chem. Commun. 2016, 52, 1230–1233. [DOI] [PubMed] [Google Scholar]
- 39.
- 39a. Irwin A. J., Jones J. B., J. Am. Chem. Soc. 1977, 99, 1625–1630; [DOI] [PubMed] [Google Scholar]
- 39b. Irwin A. J., Jones J. B., J. Am. Chem. Soc. 1977, 99, 556–561. [DOI] [PubMed] [Google Scholar]
- 40. Lok K. P., Jakovac I. J., Jones J. B., J. Am. Chem. Soc. 1985, 107, 2521–2526. [Google Scholar]
- 41. Matos J. R., Wong C. H., J. Org. Chem. 1986, 51, 2388–2389. [Google Scholar]
- 42. Boratyński F., Dancewicz K., Paprocka M., Gabryś B., Wawrzeńcz C., PLoS One 2016, 11, e0146160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Boratyński F., Janik-Polanowicz A., Szczepańska E., Olejniczak T., Sci. Rep. 2018, 8, 468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kara S., Spickermann D., Schrittwieser J. H., Weckbecker A., Leggewie C., Arends I. W. C. E., Hollmann F., ACS Catal. 2013, 3, 2436–2439. [Google Scholar]
- 45. Boratynski F., Kiełbowicz G., Wawrzenczyk C., J. Mol. Catal. B 2010, 65, 30–36. [Google Scholar]
- 46.
- 46a. Rauch M., Schmidt S., Arends I. W. C. E., Oppelt K., Kara S., Hollmann F., Green Chem. 2017, 19, 376–379; [Google Scholar]
- 46b. Gargiulo S., Arends I. W. C. E., Hollmann F., ChemCatChem 2011, 3, 338–342. [Google Scholar]
- 47. Schröder I., Steckhan E., Liese A., J. Electroanal. Chem. 2003, 541, 109–115. [Google Scholar]
- 48.
- 48a. Kochius S., Ni Y., Kara S., Gargiulo S., Schrader J., Holtmann D., Hollmann F., ChemPlusChem 2014, 79, 1554–1557; [Google Scholar]
- 48b. Könst P., Kara S., Kochius S., Holtmann D., Arends I. W. C. E., Ludwig R., Hollmann F., ChemCatChem 2013, 5, 3027–3032; [Google Scholar]
- 48c. Aksu S., Arends I. W. C. E., Hollmann F., Adv. Synth. Catal. 2009, 351, 1211–1216. [Google Scholar]
- 49.
- 49a. Huang L., Romero E., Ressmann A. K., Rudroff F., Hollmann F., Fraaije M. W., Kara S., Adv. Synth. Catal. 2017, 359, 2142–2148; [Google Scholar]
- 49b. Bornadel A., Hatti-Kaul R., Hollmann F., Kara S., Tetrahedron 2016, 72, 7222–7228; [Google Scholar]
- 49c. Bornadel A., Hatti-Kaul R., Hollmann F., Kara S., ChemCatChem 2015, 7, 2442–2445. [Google Scholar]
- 50. Díaz-Rodríguez A., Martinez-Montero L., Lavandera I., Gotor V., Gotor-Fernández V., Adv. Synth. Catal. 2014, 356, 2321–2329. [Google Scholar]
- 51. Götz K., Liese A., Ansorge-Schumacher M., Hilterhaus L., Appl. Microbiol. Biotechnol. 2013, 97, 3865–3873. [DOI] [PubMed] [Google Scholar]
- 52. Díaz-Rodríguez A., Borzęcka W., Lavandera I., Gotor V., ACS Catal. 2014, 4, 386–393. [Google Scholar]
- 53. Mourelle-Insua Á., Zampieri L. A., Lavandera I., Gotor-Fernández V., Adv. Synth. Catal. 2018, 360, 686–695. [Google Scholar]
- 54. Classen T., Korpak M., Schölzel M., Pietruszka J., ACS Catal. 2014, 4, 1321–1331. [Google Scholar]
- 55. Truppo M. D., Rozzell J. D., Turner N. J., Org. Process Res. Dev. 2010, 14, 234–237. [Google Scholar]


