Abstract
Introduction:
Psoriatic arthritis (PsA) is a chronic inflammatory disease that can result in significant disability. With the emergence of tumor necrosis factor inhibitors (TNFi), therapeutic outcomes in PsA have improved substantially. The clinical efficacy and the inhibition of radiographic progression demonstrated by TNFi have transformed the management of PsA. However, there is still an unmet need for a subset of patients who do not respond adequately to TNFi.
Areas covered:
This review provides an overview of the pharmacokinetics of TNFi, the efficacy of TNFi in PsA, and the role of immunogenicity of TNFi in the treatment of PsA. In addition, we address the use of TNFi in the setting of other medications utilized in the treatment of PsA and the potential future role of biosimilars.
Expert commentary:
Monoclonal antibodies exhibit complex and widely variable pharmacokinetics. The study of factors that can affect the pharmacokinetics, such as immunogenicity, is valuable to further define and understand the use of TNFi in PsA, especially in the subset of patients who do not respond adequately to these agents or lose effectiveness over time.
Keywords: Adalimumab, certolizumab, etanercept, golimumab, immunogenicity, infliximab, methotrexate, monoclonal antibodies, psoriatic arthritis, tumor necrosis factor inhibitors
1. Introduction
Psoriatic arthritis (PsA) is a chronic inflammatory disease that affects the joints, periarticular structures, skin, and nails. The disease can result in permanent joint damage and disability. The prevalence of PsA ranges from 0.06% to 0.25% in developed countries such as the US, the UK, and Western Europe. It is common among patients with psoriasis with a prevalence ranging from 6% to 41% [1]. Treatment of PsA has evolved substantially since the 1990s with introduction of the tumor necrosis factor inhibitors (TNFi). This review will focus on the pharmacology and clinical efficacy of the TNFi in PsA.
2. Psoriatic arthritis
2.1. Clinical manifestations
PsA is a heterogeneous condition encompassing a wide range of clinical manifestations that include the key domains of peripheral and axial arthritis, inflammation at tendon/ligament insertion sites (enthesitis), diffuse swelling of an entire finger or toe (dactylitis), nail disease, and psoriasis [2]. The incidence and prevalence of cardiovascular disease and diabetes is increased in PsA [3]. Inflammatory bowel disease and ophthalmic disease, particularly uveitis, are considered extra-articular manifestations of the disease [3,4].
2.2. Risk factors
Although PsA can occur prior to developing psoriasis, psoriasis usually precedes PsA in the vast majority of patients by approximately 10 years [5,6]. Obesity has been associated with an increased risk of developing PsA not only in patients with psoriasis, but also among patients in the general population [7,8]. Nail disease has been suggested as a potential risk factor for PsA but may also be just an early feature of the disease [1]. Intergluteal/perianal psoriasis and scalp lesions in psoriasis patients may be associated with a greater likelihood of developing PsA [9]. Other potential associations include a family history of PsA and severe psoriatic dermatoses [10,11].
2.3. Importance of early diagnosis
Early diagnosis of PsA is crucial for prevention of disease progression [12] and may also influence development of comorbidities. Early PsA has been defined as within 1–2 years of the onset of symptoms [13]. Erosions and worse long-term physical outcomes have been demonstrated with even a 6-month delay in diagnosis [14–17]. Unlike conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), TNFi have been demonstrated to prevent or slow radiographic progression of PsA. Early institution of therapy within 6 months of disease initiation results in improved response to therapy and improved long-term outcomes [12,18].
2.4. Treatment
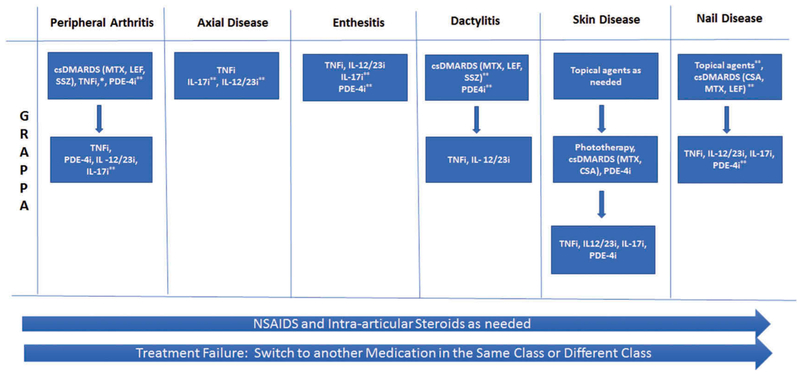
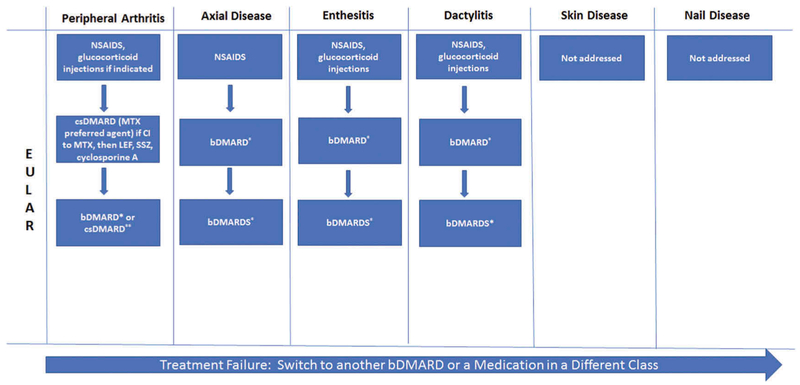
The most widely used consensus treatment recommendations are The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) and the European League Against Rheumatism (EULAR) recommendations (Figures 1 and 2). Overarching principles of therapy are similar and include shared decision-making with the patient, controlling symptoms and preventing damage, improving quality of life, minimizing or avoiding complications, and assessing comorbidities. A central feature in the treatment of PsA is considering all the domains involved when deciding on a treatment regimen. The GRAPPA and EULAR recommendations favor a ‘step-up’ approach for the treatment of PsA [4,19]. Nonsteroidal anti-inflammatory drugs (NSAIDs) and intra-articular corticosteroids may be effective in reducing pain from inflammation. Traditional oral therapies such as methotrexate (MTX), leflunomide (LEF), and sulfasalazine (SSZ) can decrease inflammation and improve symptoms. Both NSAIDs and csDMARDs are commonly used in PsA, but neither treat all domains of the disease. Other than the TICOPA study, a treat to target study in PsA [20], there is little data confirming the efficacy of MTX in PsA [21–23]. However, csDMARDs, particularly MTX, continue to be a mainstay of treatment even though they have not been shown to clearly inhibit radiographic progression and there is a paucity of efficacy data. MTX remains the most commonly used therapy for PsA and has good retention rates (e.g. 2-year retention rates of 65%, Lie et al. in 2010 [24]). The GRAPPA recommendations do not specifically delineate MTX as the csDMARD of choice or TNFi as the first biologic DMARD (bDMARD) of choice [4]. TNFi are the first-line bDMARD of choice in the EULAR recommendations on the basis of clinical data and evidence of efficacy and long-term safety data that is available compared to other biologic agents, such as the interleukin (IL)-17A inhibitor, secukinumab, and the IL-12/23 inhibitor, ustekinumab [19]. Furthermore, the TNFi have been shown to inhibit progressive joint destruction and are an effective treatment for all domains of the disease [25]. As there is a paucity of data on axial disease in PsA, recommendations are derived from data for axial spondyloarthritis (SpA) [26–30]. Both organizations recommend bDMARDs after NSAID failure for axial disease and enthesitis as csDMARDs are not efficacious in these two disease domains [4,19]. A distinction between the two sets of recommendations is that GRAPPA recommendations allow for an ‘expedited therapeutic route’ in which csDMARDs are bypassed and a bDMARD may be initiated early. This recommendation is based on (a) the efficacy of bDMARDs and relatively little data for traditional oral agents for long-term prevention of progression and (b) the relative lack of efficacy of oral agents for enthesitis, both particularly in the patient with poor prognostic factors (e.g. elevated C-reactive protein or high joint counts) [4].
Figure 1.
Simplified GRAPPA treatment recommendations [4].
*combination csDMARDS and TNFi common in clinical practice. **Conditional recommendations: At the time these recommendations were published, these drugs were not approved or recommendations were based on data from abstracts. csDMARD, conventional synthetic DMARD; CSA, cyclosporin A; GRAPPA, Group for Research and Assessment of Psoriasis and Psoriatic Arthritis; IL-17i, interleukin-17 inhibitor; IL-12/23i, interleukin-12/23 inhibitor; LEF, leflunomide; MTX, methotrexate; NSAIDS, nonsteroidal anti-inflammatory drugs; PDE-4i, phosphodiesterase 4 inhibitor; SSZ, sulfasalazine; tsDMARD, targeted synthetic DMARD; TNFi, tumor necrosis factor inhibitor
Figure 2.
Simplified EULAR treatment recommendations [19].
*bDMARD includes TNFi, IL-12/23i, IL-17i. The preference initially is a TNFi, but if contraindicated, can consider one of the others or a PDE-4i. **no adverse prognostic factors: can try a second csDMARD or combination therapy. bDMARD, biologic DMARD; csDMARD, conventional synthetic DMARD; CI, contraindicated; EULAR, European League Against Rheumatism; IL-17i, interleukin-17 inhibitor; IL-12/23i, interleukin-12/23 inhibitor; LEF, leflunomide; MTX, methotrexate; NSAIDS, nonsteroidal anti-inflammatory drugs; PDE-4i, phosphodiesterase 4 inhibitor; SSZ, sulfasalazine; tsDMARD, targeted synthetic DMARD; TNFi, tumor necrosis factor inhibitor
2.4.1. Defining treatment response
The primary outcome used in PsA randomized controlled trials (RCTs) is the American College of Rheumatology (ACR)-20% improvement criteria. Patients must achieve at least a 20% improvement in the tender and swollen joint counts and at least three of the five remaining outcome measures: Health Assessment Questionnaire-Disability Index, patient pain assessment, patient global assessment, physician global assessment, and C-reactive protein. Psoriasis severity is measured in RCTs using the Psoriasis Area and Severity Index (PASI) score. The PASI75 is an improvement of at least 75% in the PASI score [31]. PASI75 (or even PASI90) is generally a secondary outcome in RCTs examining therapies for PsA. While ACR20 and PASI are the most commonly used outcome measures in trials, these measures are not often used in clinical practice. Instead a variety of outcome measures are used including joint counts, enthesitis measures, dactylitis assessment, psoriasis severity, and patient-reported outcomes [6,32].
3. Tumor necrosis factor
In 1975, TNF was recognized as an endotoxin-induced glycoprotein that caused hemorrhagic necrosis of transplanted sarcomas in mice [33]. Since then, it has been associated with a wide range of biologic conditions and has been identified as an important pro-inflammatory cytokine [34]. Overexpression of TNF has been implicated in the pathogenesis of a wide variety of diseases, such as rheumatoid arthritis (RA), inflammatory bowel disease, psoriasis, and PsA [35,36]. TNF is a pleotropic cytokine that is produced by cells such as activated macrophages, T lymphocytes, monocytes, neutrophils, mast cells, endothelial cells, fibroblasts, and osteoclasts. It is a key driver of many inflammatory activities in the body and also contributes to cell proliferation, apoptosis, and angiogenesis [35,37]. Transmembrane TNF (tmTNF), a 26 kDa protein, is cleaved by a metalloproteinase, TNF-alpha-converting enzyme, and is ultimately released as a soluble cytokine, sTNF (17 kDa) [34,38–40]. sTNF and tmTNF can then bind to TNF receptor 1 (TNFR1, p55) or TNFR2, (p75) and exert biological effects on various cell types [34,38]. TNFR1 and TNFR2 use different signaling mechanisms; they have differing affinities to ligands and distinct cellular expression profiles [38]. These differences may contribute to varied biological responses [34,41].
4. Role of TNF in the pathogenesis of PsA
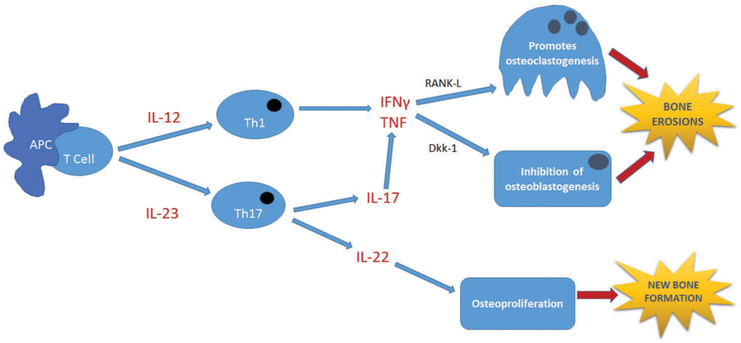
While genetic and environmental factors may play a role in the development of PsA, the immune response to such triggers is what sustains the disease. Inflammation in PsA is thought to be driven both by the Th1 and Th17 pathways. In both pathways, TNF superfamily proteins are important for sustaining inflammation [44]. When the inciting antigen is presented to the initial T-cell, unregulated IL-12 causes differentiation and propagation of Th1 cells and contributes to the release of pro-inflammatory cytokines, including TNF [45]. Conversely, IL-23 is thought to play a key role in the pathogenesis of PsA by triggering Th17 cell differentiation [46]. This leads to production of IL-22 and IL-17. IL-17 leads to upregulation of TNF [47]. TNF, along with several other cytokines, induces expression of receptor activator of nuclear factor-κB ligand, a member of the TNF superfamily, promoting osteoclastogenesis and eventually erosion formation [42]. TNF also induces expression of Dickkopf-related protein 1 (Dkk-1) by synovial fibroblasts, inhibiting osteoblastogenesis, further promoting erosions [42] (Figure 3).
Figure 3.
Pathogenesis of psoriatic arthritis.
The Th1 and Th17 pathways are important pathways involved in the pathogenesis of PsA. TNF, a pro-inflammatory cytokine, is a key player in osteoclastogenesis via RANK-L and in inhibition of osteoblastogenesis via Dkk-1. Both processes eventually lead to bone erosions [42]. In addition, IL-22 is involved in the pathologic formation of new bone (osteoproliferation) [43]. APC, antigen presenting cell; Dkk-1, dickkopf-related protein 1; IFNγ, interferon gamma; IL-12, interleukin-12; IL-17, interleukin-17; IL-22, interleukin-22; IL-23, interleukin-23; RANK-L, receptor activator of nuclear factor-κB ligand; T cell, T lymphocyte; Th1, type 1 T helper cell; Th17, T helper 17 cell; TNF, tumor necrosis factor
5. TNF inhibitors
Etanercept, infliximab, adalimumab, certolizumab pegol, and golimumab are the TNFi that have been approved for PsA in the US and UK. Etanercept was the first US FDA-approved TNFi for the treatment of PsA in January 2002.
5.1. Structure and mechanism of action of TNFi
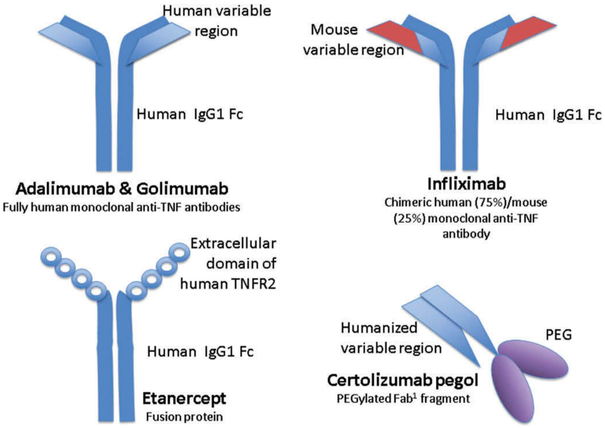
TNFi are monoclonal antibody (mAB) therapeutics directed at TNF. IgG mABs are large proteins that possess hydrophilic properties [48]. They consist of two unique heavy chains and two unique light chains each of which has constant and variable domains [48]. The heavy and light chains are linked by disulfide bonds and connected by disulfide bonds at the hinge region to a fragment crystallizable (Fc) region [49]. The fragment antigen-binding region (Fab) is the antigen-binding portion, and the Fc region is the portion which takes part in Fc-mediated actions, such as complement-dependent cytotoxicity and antibody-dependent cell-mediated cytotoxicity [50]. The Fc portion also binds to the neonatal Fc receptor (FcRn), which is integral in protecting the antibody from intracellular catabolism [49,51]. The hypervariable region at the top of the variable domain is where binding to the target antigen occurs [48,49].
With the exception of certolizumab and etanercept, the remaining three TNFi are full-length bivalent mABs [38] (Figure 4). Certolizumab is a humanized (exogenic hypervariable regions) IgG1 mAB with a Fab1 fragment [38,48]. The hinge region is modified and is linked to polyethylene glycol allowing for better solubility, half-life, bioavailability, and decreased immunogenicity. It has an affinity for both sTNF and tmTNF [52]. Unlike the other TNFi, certolizumab does not have an Fc portion and therefore does not take part in Fc-mediated actions [50]. In contrast to the other TNFi, etanercept is a genetically engineered soluble fusion protein that is composed of two extracellular portions of the p75 TNFR linked to the Fc portion of human IgG1 [38]. It binds to both sTNF and tmTNF at the receptor binding site, preventing the binding of TNF with the p75 receptor [38,53]. The short halflife of entanercept may in part be due to a difference in the conformation of the Fc region [38]. In addition, etanercept is the only TNFi of the five that also binds members of the lymphotoxin (LT) family (also members of the TNF superfamily), specifically LTα3 and LTαβ1 [38]. Infliximab is unique in that it is a chimeric IgG1k mouse and human mAB that consists of human constant regions of IgG1k and murine variable regions [38,54]. It binds to both sTNF and tmTNF with high affinity via the E–F loop, blocking the ability of TNF to bind to its receptors [55]. Adalimumab is a recombinant human IgG1 antibody. It occupies the TNFR-binding site of both sTNF and tmTNF with high affinity, preventing the binding of TNF to its receptors [54,55]. Like adalimumab, golimumab is a human immunoglobulin IgG1 mAB that binds to both sTNF and tmTNF [38,56] (Table 1).
Figure 4.
Simplified structures of TNFi.
Fab, fragment antigen-binding; Fc, fragment crystallizable region; IgG1, immunoglobulin G1; PEG, polyethylene glycol; TNF, tumor necrosis factor; TNFR2, tumor necrosis factor receptor 2
Table 1.
Basic characteristics of TNFi.
| TNFi | Structure | Protein type | Affinity | Fc portion? |
|---|---|---|---|---|
| Infliximab | Full-length bivalent mAB | Chimeric and human | sTNF and tmTNF | Yes |
| Etanercept | Genetically engineered Fc-fusion protein | Recombinant human | sTNF, tmTNF, LTα3, LTα2β1 | Yes |
| Adalimumab | Full-length bivalent mAB | Fully human | sTNF and tmTNF | Yes |
| Golimumab | Full-length bivalent mAB | Fully human | sTNF and tmTNF | Yes |
| Certolizumab pegol | Monovalent Fab1 antibody fragment | Humanized | sTNF and tmTNF | No |
Fab: fragment antigen-binding; Fc: fragment crystallizable region; LTα3: lymphotoxin alpha 3; LTα2β1: lymphotoxin alpha 2 beta 1; mAB: monoclonal antibody; sTNF: soluble tumor necrosis factor; tmTNF: transmembrane tumor necrosis factor; TNFi: tumor necrosis factor inhibitor.
5.2. General pharmacokinetic (PK) properties of TNFi
5.2.1. Absorption
As large protein molecules with poor membrane permeability, TNFi are administered parenterally. The oral bioavailability is very low as they are denatured in the acidic environment of the stomach or they undergo a rapid proteolytic cleavage in the gastrointestinal tract [49,57]. It is hypothesized that mABs are absorbed via the lymphatic system by convection and diffusion across blood vessels [49,51]. Absorption can take anywhere from about 1 to 8 days [58]. Most of the TNFi are administered subcutaneously (infliximab is intravenous (IV) only and golimumab can be given intravenously or subcutaneously), which can cause variability among patients in regard to the amount of drug absorbed [59].
5.2.2. Distribution
Given their large weight and hydrophilic nature, mABs usually have a small volume of distribution [49]. The molecules usually reside in the vascular and interstitial spaces and are distributed via paracellular movement by convection and via transcellular movement by endocytosis (phagocytosis, receptor-mediated endocytosis, or fluid-phase pinocytosis) [49,51]. Convective transport is driven by the blood-tissue hydrostatic pressure gradient. Osmotic pressure gradients and the characteristics of the paracellular pores also affect convective transport [58].
5.2.3. Metabolism and excretion
Since mABs are large molecules, they are not predominantly renally excreted. The PEG portion of certolizumab decreases its renal excretion secondary to increasing the size of the molecule [52]. Very little is also excreted in bile [49,51]. These molecules undergo catabolic metabolism via Fc-receptor-mediated elimination and target mediated elimination (clearance following binding to target) [49]. The Fc portion is also thought to contribute to the long half-life of most of these mABs since it interacts with the FcRn, which has a mechanism that protects these molecules from systemic elimination [60] (Table 2).
Table 2.
General pharmacokinetic properties of monoclonal antibodies.
| Absorption | Lymphatic system via convection and diffusion |
| Distribution | Small volume of distribution, paracellular and transcellular movement |
| Metabolism and excretion | Catabolic metabolism |
5.3. Pharmacokinetics of TNFi
TNFi differ in their PK properties. Underlying disease type or severity, body weight, immunogenicity, and the concomitant use of other medications such as MTX can impact PK parameters. Elimination of TNFi for the treatment of PsA generally follows linear kinetics and volume of distribution is that of the central compartment (~6 L) [48,61–66]. Table 3 outlines the pharmacokinetics of monoclonal antibodies utilized in rheumatic diseases with an emphasis, when available, on population PK parameters in PsA.
Table 3.
Pharmacokinetics of TNFi in rheumatologic diseases.
| Infliximab | Etanerceptc | Adalimumab | Golimumab | Certolizumab | |
|---|---|---|---|---|---|
| Administration | IV | SC | SC | SC | SC |
| Loading dose | 3–5 mg/kg at 0, 2, and 6 wks | – | – | – | 400 mg at 0, 2, and 4 wks |
| Maintenance dosages | 3–10 mg/kg every 4–8 wks | 50 mg weekly | 40 mg eow | 50 mg once a month | 200 mg eow or 400 mg once a month |
| Half-life (t1/2) | 8–10 daysa | 3–5 days | 14 days | 14 daysd | 14 days |
| Clearance (L/d) | 0.26b | 1.67 | 0.269 | 0.40d | 0.408 |
| Bioavailability | – | 58% | 64% | 53% | 80% |
| Cmax (μg/ml) | 192 ± 51b | 2.4 ± 1.5 | 4.7 ± 1.6 | 2.5 | 43–49 (after loading dose) |
| References | [38,64,67–70] | [53,65,70–72] | [65,70,73] | [61,74] | [65,66] |
Population PK in PsA for infliximab: the t1/2 life was 15.7 days [75].
Based on 5 mg/kg IV in RA patients.
PK of etanercept 50 mg once weekly is comparable to 25 mg twice a week SC [76].
Population pharmacokinetics in PsA were characterized using a one-compartment model. Clearance: 0.68 L/d, t1/2 life of golimumab was 12.5 days [77]; eow: every other week; SC: subcutaneous; IV: intravenous; TNFi: tumor necrosis factor inhibitor; wk: week.
5.3.1. Obesity
Obesity impacts the pharmacokinetics of TNFi. Higher disease activity is seen in obese PsA patients, and disease registries suggest that obesity is associated with a decreased response to TNFi [78]. PsA patients on TNFi that lost >5% from their baseline weight were found to be significantly more likely to achieve minimal disease activity than patients who did not lose weight [79]. Obesity may affect the pharmacokinetics of TNFi secondary to insufficient dosing, changes in volume of distribution, and increased drug elimination [48,78].
5.3.2. Immunogenicity
Immunogenicity, the ability of a substance to cause an immune response [49], can play a role in the varying pharmacokinetics of mABs. The underlying disease, duration of treatment, route of administration, concomitant medications, dose frequency, genetic predisposition, assay methodology, and the type of antibody can all affect the immunogenicity of TNFi [80–82]. Humanization of mABs may help to decrease immunogenicity [48,51]. Thus, the chimeric structure of infliximab can account for its high immunogenicity potential. A metaanalysis of TNFi immunogenicity in RA, inflammatory bowel disease, and SpA (PsA and ankylosing spondylitis) among patients using one of the five TNFi demonstrated that infliximab was the most and etanercept the least immunogenic [83]. There is sparse data regarding the extent of immunogenicity of golimumab and certolizumab in PsA.
The elimination rate of TNFi is impacted by immunogenicity. Antidrug antibodies will increase the elimination rate of TNFi [48,67,73,80]. They may form immune complexes with the drug accelerating its clearance [84]. Small studies have demonstrated a correlation between anti-adalimumab antibodies and decreased serum concentration and thus decreased clinical response [85,86]. Another small study demonstrated elevated levels of antidrug antibodies to adalimumab and infliximab, but not etanercept in PsA patients, which correlated with low therapeutic drug levels and thus decreased drug efficacy [87].
5.3.3. Concomitant use of MTX and immunogenicity
There is an association between MTX, a widely used therapy in PsA, and the development of antidrug antibodies. A metaanalysis by Thomas et al. showed that MTX can attenuate the formation of antibodies by 74% overall and antibodies decreased clinical response by 18% overall in SpA (based on four studies looking at infliximab, adalimumab, and etanercept) [83]. In RCTs of infliximab and golimumab, a greater proportion of patients on TNFi monotherapy were positive for antibodies compared to those taking concomitant MTX [88,89]. However, efficacy of TNFi is not generally impacted by MTX use [88–94]. Interestingly, a post-hoc analysis determined that patients who were taking combination MTX and golimumab had a 10% greater improvement in nail, dactylitis, and enthesitis scores compared to those not taking MTX [89]. In an observational cohort study of 375 patients with RA or PsA treated with adalimumab, trough concentrations were higher in patients concomitantly taking MTX and lower in patients on adalimumab monotherapy [95].
6. Key clinical trials of TNFi in PsA
TNFi in PsA were found to be efficacious with tolerable safety profiles in pivotal phase III trials (Table 4). The most common adverse events include injection site reactions, infusion reactions in infliximab, and infections [6]. All five TNFi demonstrated an inhibition in radiographic progression. In the GO-REVEAL 5-year study, concomitant MTX appeared to reduce radiographic progression [91]. Only the certolizumab trials included patients who were exposed to TNFi previously (19.8% of patients). Interestingly, improvements in ACR20 response rates at 12, 24, and 96 weeks were observed for both doses regardless of prior TNFi exposure [93,96].
Table 4.
Pivotal phase III trials of TNFi in psoriatic arthritis.
| Reference | Study size (n) |
Doses (vs. placebo) | % Achieving ACR20 response (tx/ placebo) (primary endpt wk) |
% Achieving PASI75 response (tx/placebo) (primary endpt wk) |
Inhibition of radiographic progression |
|
|---|---|---|---|---|---|---|
| Infliximab | IMPACT [97,98] | 104 | 5 mg/kg IV | 65.4/9.6 (16) | 68/0 (16) | 50 wks |
| IMPACT 2 [99,100] | 200 | 5 mg/kg IV | 58/11 (14) | 64/2 (14) | 6 months and 1 yr | |
| Etanercept | 12-wk study [101] | 60 | 25 mg SC 2x wk | 73/13 (12) | 26/0 (12) | Not studied |
| 24-wk study [94,102] | 205 | 25 mg SC 2x wk | 59/15 (12) | – | 12 months and 2 yrs | |
| Adalimumab | ADEPT [92] | 313 | 40 mg SC eow | 58/14 (12) | – | 24 wks |
| Golimumaba | GO-REVEAL [90,91] | 405 | 50 mg/100 mg | 51/45/9 (14) | 40/58/3 (14) | 24 wks and 256 wks |
| Certolizumabb | RAPID-PsA [93,96] | 409 | 200 mg/400 mg | 58/51.9/24.3 (12) | 46.7/47.4/14 (12) | 96 wks |
ACR20: American College of Rheumatology 20% improvement criteria; endpt: end point; eow: every other week; IV: intravenous; PASI75: ≥75% improvement in Psoriasis Area and Severity Index; SC: subcutaneous; tx: treatment; TNFi: tumor necrosis factor inhibitor; wk: week; yrs: years.
SC dosing every 4 weeks.
200 mg SC every 2 weeks; 400 mg SC every 4 weeks.
7. Other treatment options for PsA
A number of patients do not respond to TNFi and many more have a loss of response over time. Thus, recognition of the IL-23/IL-17 pathway in the pathogenesis of PsA and molecules that are targeting other cytokines in the pathway has been integral to the development of further medications to treat PsA.
7.1. Apremilast
Apremilast is an oral phosphodiesterase 4 inhibitor (PDE4i) that is approved for the treatment of PsA. PDE4 mediates the breakdown of cyclic adenosine monophosphate, which regulates inflammatory responses. Thus, PDE4 inhibitors demonstrate anti-inflammatory effects [103]. The clinical efficacy of apremilast in PsA patients who have already been treated with csDMARDs and/or bDMARDs or are on csDMARDs was studied extensively with several pivotal randomized placebo-controlled trials (Table 5). In the Psoriatic Arthritis Long-term Assessment of Clinical Efficacy (PALACE) phase III trials, in PALACE 1, 2, and 3, the primary end point, an ACR20 response at week 16, was achieved by significantly more patients taking apremilast 20 or 30 mg bid as compared to placebo regardless of prior treatment. bDMARD-naive patients had higher ACR20 response rates [104–106]. Sustained improvements were seen through week 52 in PALACE 1, 2, and 3. In PALACE 4, patients who were DMARD-naive were studied over a 52-week period. The primary end point was met for both doses at weeks 16 and 52 [107]. Studies suggested a lack of efficacy of apremilast in axial disease [108,109]. The most common adverse events were diarrhea and nausea [104–107].
Table 5.
Pivotal phase III trials for other treatment options in psoriatic arthritis.
| Reference | Study size(n) |
Doses (vs. placebo) | % Achieving ACR20 response (tx/placebo) (wk) |
% Achieving PASI75 response (tx/placebo) (wk) |
Less radiographic progression |
|
|---|---|---|---|---|---|---|
| Apremilast | PALACE 1 [104] | 504 | 30 mg bid/20 mg bid | 38.1/30.4/19 (16) | 21/17.6/4.6 (24) | Not assessed |
| PALACE 2 [105] | 484 | 30 mg bid/20 mg bid | 32.1/37.4/18.9 (16) | 22.1/18.8/2.7 (16) | Not assessed | |
| PALACE 3 [106] | 505 | 30 mg bid/20 mg bid | 41/28/18 (16) | 21/20/8 (16) | Not assessed | |
| PALACE 4 [107] | 527 | 30 mg bid/20 mg bid | 32.3/29.2/16.9 (16) | – | Not assessed | |
| Secukinumab | FUTURE 1 [110] | 606 | 75 mga/150 mga | 50.5/50/17.3 (24) | 64.8/61.1/8.3 (24) | Wk 24 and wk 52 |
| FUTURE 2 [111] | 397 | 300 mgb/150 mgb/75 mgb | 54/51/29/15 (24) | 63/48/28 (not significant)/16 (24) | Not assessed | |
| Ustekinumabc | PSUMMIT 1 [112,113] | 615 | 90mg/45mg | 49.5/42.4/22.8 (24) | 62.4/57.2/11 (24) | Wk 24 and 2 yrs |
| PSUMMIT 2 [114,115] | 312 | 90mg/45mg | 43.8/43.7/20.2 (24) | 55.6/51.3/5 (24) | Wk 24 and 1 yr |
Placebo or IV loading doses 10 mg/kg at baseline, week 2, and week 4 and then SC every 4 weeks.
SC loading doses 300, 150, 75 mg, or placebo once a week from baseline to week 4 and then SC every 4 weeks.
Placebo, 45 or 90 mg SC at baseline and 4 weeks and then every 12 weeks.
ACR20: American College of Rheumatology 20% improvement criteria; bid: twice a day; IV: intravenous; PASI75: ≥75% improvement in Psoriasis Area and Severity Index; SC: subcutaneous; tx: treatment; wk: week; yrs: years.
7.2. Secukinumab
Secukinumab is a human IgG1 mAB that binds to and neutralizes IL-17A. FUTURE 1 and 2 are key phase III, randomized, double-blind, placebo-controlled trials that have demonstrated the efficacy of secukinumab in the key domains of PsA (Table 5) [110,111]. MEASURE 1 and 2 are key phase III, randomized, double-blind, placebo-controlled trials that have demonstrated the efficacy of secukinumab in ankylosing spondylitis and thus should be effective for axial disease in PsA [116]. Additionally, secukinumab has been shown to decrease radiographic progression [110]. Efficacy was noted regardless of concomitant MTX use and among patients with prior TNF exposure, though the response was lower. Generally, numerically higher ACR responses were noted in the anti-TNF-naive populations. Efficacy was sustained through week 52. Candida infections were more common in secukinumab versus placebo, which may be because IL-17 plays a role in host defense against fungal infections [110,111].
7.3. Ustekinumab
Ustekinumab inhibits IL-12 and IL-23 by binding to the p40 subunit of IL-12 and IL-23. PSUMMIT 1 and PSUMMIT 2 are pivotal phase III, double-blind, placebo-controlled trials that studied ustekinumab in PsA patients and found that there was a significant improvement in joint and skin disease and less radiographic progression compared to placebo (Table 5). In PSUMMIT 1, ACR20 response rates were maintained at week 52 and efficacy was noted regardless of MTX use [112]. In contrast to PSUMMIT 1, in PSUMMIT 2, 58% of patients had been on TNFi previously. Clinical improvement was noted regardless of prior TNF exposure but was again lower (as has been seen in other studies of TNF inadequate responders). Anti-TNF-naive patients appeared to have a higher clinical response than anti-TNF-experienced patients [114]. Phase III, randomized, double-blind, placebo-controlled trials are underway to evaluate the efficacy and safety of ustekinumab in ankylosing spondylitis [117,118]. Ustekinumab has a tolerable safety profile with a low incidence of serious infections and sustained clinical improvement through week 100 [113].
8. Expert commentary
PsA is a heterogeneous, often debilitating disease that is associated with several comorbidities. Early intervention is vital to prevent disease progression. Although csDMARDs show variable efficacy in PsA [19,22], they have remained key medications in treatment largely in part due to cost considerations. With the emergence of TNFi, treatment options have vastly expanded for PsA patients. TNFi inhibit radiographic progression and are effective in treating all the domains of PsA [25]. However, some patients do not respond to TNFi or response may wane over time. Thus, the emergence of IL-17A inhibitors, IL-12/23 inhibitors, and small molecule treatments such as apremilast have provided a wider range of therapeutic options for PsA. Although apremilast has the advantage of being an oral medication with a relatively benign side effect profile, its effect on radiographic progression has not been examined. The TNFi agents etanercept, infliximab, adalimumab, and golimumab appear to have ACR20 advantage over newer non-TNFi biologics such as apremilast and ustekinumab, when compared using indirect methods [119]. There are no direct comparative efficacy trials between non-TNFi biologics; however, indirect comparisons suggest similar efficacy and safety among available agents [120]. There are also several new medications that are currently being evaluated for the treatment of PsA (Table 6).
Table 6.
Medications in development for PsA.
| Drug | Mechanism of action | Current phase in clinical trials |
|---|---|---|
| Ixekizumab | IL-17A inhibitor | SPIRIT-P2 Phase III (NCT02349295) |
| Abatacept | CTLA4-Ig | Phase III (NCT01860976) |
| Risankizumab | IL-23 inhibitor | Phase II (NCT02986373) |
| Guselkumab | IL-23 inhibitor | Phase II (NCT02319759) |
| Tofacitinib | Oral JAK inhibitor | Phase III (NCT01976364) |
| Tildrakizumab | IL-23 inhibitor | Unknown currently |
CTLA4-Ig: cytotoxic T lymphocyte-associated antigen-4 immunoglobulin fusion protein.
Even though there is an emergence of many new therapies in PsA, there is a subset of patients that do not adequately respond to available treatments. Thus, it would be of benefit to further study established therapies in PsA such as TNFi by assessing parameters that affect drug concentrations in this patient population. Given that mABs exhibit complex and widely variable pharmacokinetics, further population PK studies in PsA would be helpful in identifying covariates, such as age, immunogenicity, weight, comorbidities, and concomitant medications, which can influence dose–concentration–effect relationships [80]. Outside of weight-based dosing adjustment, individualization of dosing is currently not the standard for mABs in autoimmune disease. A model-based approach that links monoclonal exposure with disease state may eventually allow for more individualized dosing based upon disease phenotype, endotype (biomarker driven), and potentially, though less likely, genotype. In addition to drug concentrations, anti-monoclonal drug antibody levels can play a role on the effect of treatment discontinuation and adverse events such as infusion reactions, which have occurred at a higher incidence in antibody-positive patients [81,88]. It can provide insight into whether or not switching to another TNFi or a medication with a different mechanism of action in patients with poor clinical outcomes would be of greater benefit. Thus, having a better understanding of the factors associated with interindividual variability and the extent of that variability may eventually contribute to potential dosing strategies that can improve clinical outcomes, especially in patients with TNFi failure.
While combination treatment may be common in clinical practice, there is little data regarding its clinical efficacy [4,19]. However, decreased immunogenicity of TNFi with concomitant MTX may play a role in improving drug survival rates of TNFi [121,122]. In addition, one study noted higher drug levels in a small group of 26 patients on adalimumab combination therapy (with csDMARDs such as LEF, SSZ, or hydroxychloroquine) compared to patients using adalimumab monotherapy [95]. Prospective, randomized clinical trials of TNFi with various csDMARDs to assess trough antibody drug concentrations, antidrug antibody levels, the measurement of a clinical response (ACR20 response), and to further assess the potential long-term side effects of combination therapy in PsA would be of value.
9. Five-year view
With further understanding of the pathogenesis of PsA, novel treatment options are emerging. Over the last several years, many effective therapeutic options have been introduced and more are yet to come. Biosimilars, which are products similar to already approved drugs in regard to quality, safety, and efficacy [123], may help to alleviate the economic burden associated with TNFi. Few studies have evaluated infliximab, etanercept, and adalimumab biosimilars for PsA. These agents have been approved in the United States for PsA based on similar efficacy to the reference product in psoriasis and/or RA [123,124]. Immunogenicity has been the same, and in some cases less than reference products [125]. Switching established patients in ankylosing spondylitis and RA from infliximab to the biosimilar product CP-P13 is not associated with a loss of control [126,127]. Extrapolation from other disease states is complicated if alternate dosing regimens are used. RCTs or pragmatic trials specific to PsA may provide beneficial information regarding the efficacy and safety of biosimilars, but with current evidence the use of biosimilars in established or de novo patients appears to reasonable. Similar to their reference products, trials evaluating how the combination of a biosimilar with a csDMARD affects immunogenicity would be of interest. Long-term pharmacoepidemiology studies assessing predictors of response to biosimilars and the effectiveness of switching from the reference product to a biosimilar and vice versa will provide valuable information.
Key issues.
Psoriatic Arthritis is a chronic, debilitating disease associated with several comorbidities.
TNFi are a mainstay of treatment in PsA and inhibit radiographic progression.
Several factors affect the pharmacokinetic properties of TNFi, including underlying disease type or severity, body weight, immunogenicity, and the concomitant use of other medications such as MTX.
Identifying drug concentrations and anti-monoclonal drug antibody levels may help more quickly identify patients with TNFi failure and may provide insight regarding medication changes.
Assessing the effect of combination csDMARDS and TNFi on immunogenicity may contribute to future treatment recommendations.
While not tested specifically in PsA, biosimilars are expected to have similar efficacy and safety to reference products.
Acknowledgments
Funding
S Mantravadi was supported by National Institutes of Health Postdoctoral training grant no. T32GM008562.
Footnotes
Declaration of interest
A Ogdie discloses sources of support with Takeda, Novartis and Pfizer. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Papers of special note have been highlighted as either of interest (·) or of considerable interest (··) to readers.
- 1.Ogdie A, Weiss P. The epidemiology of psoriatic arthritis. Rheum Dis Clin North Am. 2015;41(4):545–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eder L, Gladman DD. Psoriatic arthritis: phenotypic variance and nosology. Curr Rheumatol Rep. 2013;15:316. [DOI] [PubMed] [Google Scholar]
- 3.Ogdie A, Schwartzman S, Husni ME. Recognizing and managing comorbidities in psoriatic arthritis. Curr Opin Rheumatol. 2015. March;27(2):118–126. [DOI] [PubMed] [Google Scholar]
- 4.Coates LC, Kavanaugh A, Mease PJ, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68(5):1060–1071. [DOI] [PubMed] [Google Scholar]; ·· Treatment recommendations in psoriatic arthritis.
- 5.Gladman DD, Antoni C, Mease P, et al. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005. March;64(Suppl 2):ii14–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med. 2017. March 9;376(10):957–970. [DOI] [PubMed] [Google Scholar]
- 7.Li W, Han J, Qureshi AA. Obesity and risk of incident psoriatic arthritis in US women. Ann Rheum Dis. 2012. May 05;71(8):1267–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jon Love T, Zhu Y, Zhang Y, et al. Obesity and the risk of psoriatic arthritis: a population-based study. Ann Rheum Dis. 2012;71(8):1273–1277. BMJ Publishing Group Ltd and European League Against Rheumatism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson FC, Icen M, Crowson CS, et al. Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: a population-based study. Arthritis Rheum. 2009. February 15;61(2):233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciurtin C, Roussou E. Cross-sectional study assessing family members of psoriatic arthritis patients affected by the same disease: differences between Caucasian, south Asian and Afro-Caribbean populations living in the same geographic region. Int J Rheum Dis. 2013. August;16(4):418–424. [DOI] [PubMed] [Google Scholar]
- 11.Tey HL, Ee HL, Tan AS, et al. Risk factors associated with having psoriatic arthritis in patients with cutaneous psoriasis. J Dermatol. 2010;37(5):426–430. [DOI] [PubMed] [Google Scholar]
- 12.Gladman DD, Thavaneswaran A, Chandran V, et al. Do patients with psoriatic arthritis who present early fare better than those presenting later in the disease? Ann Rheum Dis. 2011. December;70(12): 2152–2154. [DOI] [PubMed] [Google Scholar]
- 13.Gladman DD. Early psoriatic arthritis. Rheum Dis Clin North Am. 2012;38(2):373–386. [DOI] [PubMed] [Google Scholar]
- 14.Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis. 2015. June;74(6):1045–1050. [DOI] [PubMed] [Google Scholar]
- 15.Kane D, Stafford L, Bresnihan B, et al. A prospective, clinical and radiological study of early psoriatic arthritis: an early synovitis clinic experience. Rheumatology (Oxford). 2003. December;42(12):1460–1468. [DOI] [PubMed] [Google Scholar]
- 16.Kane D, Pathare S. Early psoriatic arthritis. Rheum Dis Clin North Am. 2005. November;31(4):641–657. [DOI] [PubMed] [Google Scholar]
- 17.Geijer M, Lindqvist U, Husmark T, et al. The Swedish early psoriatic arthritis registry 5-year followup: substantial radiographic progression mainly in men with high disease activity and development of dactylitis. J Rheumatol. 2015. November;42(11):2110–2117. [DOI] [PubMed] [Google Scholar]
- 18.Kirkham B, De Vlam K, Li W, et al. Early treatment of psoriatic arthritis is associated with improved patient-reported outcomes: findings from the etanercept PRESTA trial. Clin Exp Rheumatol. 2015. Jan-Feb;33(1):11–19. [PubMed] [Google Scholar]
- 19.Gossec L, Smolen JS, Ramiro S, et al. European league against rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016. March 01;75(3):499–510. [DOI] [PubMed] [Google Scholar]; ·· Treatment recommendations in psoriatic arthritis.
- 20.Coates LC, Helliwell PS. Methotrexate efficacy in the tight control in psoriatic arthritis study. J Rheumatol. 2016. February;43(2):356–361. [DOI] [PMC free article] [PubMed] [Google Scholar]; · A treat to target study that evaluated MTX efficacy.
- 21.Ash Z, Gaujoux-Viala C, Gossec L, et al. A systematic literature review of drug therapies for the treatment of psoriatic arthritis: current evidence and meta-analysis informing the EULAR recommendations for the management of psoriatic arthritis. Ann Rheum Dis. 2012;71(3):319–326. BMJ Publishing Group Ltd and European League Against Rheumatism. [DOI] [PubMed] [Google Scholar]
- 22.Kingsley GH, Kowalczyk A, Taylor H, et al. A randomized placebo-controlled trial of methotrexate in psoriatic arthritis. Rheumatology (Oxford). 2012. August;51(8):1368–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pincus T, Bergman M, Yazici Y. Limitations of clinical trials in chronic diseases: is the efficacy of methotrexate (MTX) underestimated in polyarticular psoriatic arthritis on the basis of limitations of clinical trials more than on limitations of MTX, as was seen in rheumatoid arthritis? Clin Exp Rheumatol. 2015;33:S82–93. [PubMed] [Google Scholar]
- 24.Lie E, Van Der Heijde D, Uhlig T, et al. Effectiveness and retention rates of methotrexate in psoriatic arthritis in comparison with methotrexate-treated patients with rheumatoid arthritis. Ann Rheum Dis. 2010. April;69(4):671–676. [DOI] [PubMed] [Google Scholar]
- 25.Mease PJ. Biologic therapy for psoriatic arthritis. Rheum Dis Clin America. 2015. November 41(4):723–738. [DOI] [PubMed] [Google Scholar]
- 26.Van Der Heijde D, Dijkmans B, Geusens P, et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT). Arthritis Rheum. 2005;52(2):582–591. [DOI] [PubMed] [Google Scholar]
- 27.Inman RD, Davis JC, Heijde DVD, et al. Efficacy and safety of golimumab in patients with ankylosing spondylitis: results of a randomized, double-blind, placebo-controlled, phase III trial. Arthritis Rheumatol. 2008;58(11):3402–3412. [DOI] [PubMed] [Google Scholar]
- 28.Landewe R, Braun J, Deodhar A, et al. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled phase 3 study. Ann Rheum Dis. 2014. January;73(1):39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Der Heijde D, Kivitz A, Schiff MH, et al. Efficacy and safety of adalimumab in patients with ankylosing spondylitis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2006;54(7):2136–2146. [DOI] [PubMed] [Google Scholar]
- 30.Braun J, Van Der Horst-Bruinsma, Irene E, et al. Clinical efficacy and safety of etanercept versus sulfasalazine in patients with ankylosing spondylitis: a randomized, double-blind trial. Arthritis Rheum. 2011. ;63(6):1543–1551. [DOI] [PubMed] [Google Scholar]
- 31.Abrouk M, Nakamura M, Zhu TH, et al. The impact of PASI 75 and PASI 90 on quality of life in moderate to severe psoriasis patients. J Dermatolog Treat. 2017;18:1–7. [DOI] [PubMed] [Google Scholar]
- 32.Orbai AM, Ogdie A. Patient-reported outcomes in psoriatic arthritis. Rheum Dis Clin North Am. 2016. May;42(2):265–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carswell EA, Old LJ, Kassel RL, et al. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975. September;72(9):3666–3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bradley J. TNF-mediated inflammatory disease. J Pathol. 2008;214 (2):149–160. [DOI] [PubMed] [Google Scholar]
- 35.Bazzoni F, Beutler B. The tumor necrosis factor ligand and receptor families. N Engl J Med. 1996;334:1717. [DOI] [PubMed] [Google Scholar]
- 36.Van Kuijk AW, Reinders-Blankert P, Smeets TJ, et al. Detailed analysis of the cell infiltrate and the expression of mediators of synovial inflammation and joint destruction in the synovium of patients with psoriatic arthritis: implications for treatment. Ann Rheum Dis. 2006. December;65(12):1551–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aggarwal BB, Gupta SC, Kim JH, Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood. 2012;119(3):651–665. American Society of Hematology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tracey D, Klareskog L, Sasso EH, et al. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008;117(2):244–279. [DOI] [PubMed] [Google Scholar]; ·· Reviews mechanism of action and pharmacology of TNFi.
- 39.Moss ML, Jin SL, Milla ME, et al. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature. 1997. February 20;385(6618):733–736. [DOI] [PubMed] [Google Scholar]
- 40.Black RA, Rauch CT, Kozlosky CJ, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997. February 20;385(6618):729–733. [DOI] [PubMed] [Google Scholar]
- 41.Chan FK, Chun HJ, Zheng L, et al. A domain in TNF receptors that mediates ligand-independent receptor assembly and signaling. Science. 2000;288(5475):2351–2354. American Association for the Advancement of Science. [DOI] [PubMed] [Google Scholar]
- 42.Schett G, Gravallese E. Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat Rev Rheumatol. 2012;8 (11):656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lories RJ, Schett G. Pathophysiology of new bone formation and ankylosis in spondyloarthritis. Rheum Dis Clin North Am. 2012. August;38(3):555–567. [DOI] [PubMed] [Google Scholar]
- 44.Croft M, Siegel RM, Beyond TNF. TNF superfamily cytokines as targets for the treatment of rheumatic diseases. Nat Rev Rheumatol. 2017;13(4):217–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coates LC, FitzGerald O, Helliwell PS, et al. Psoriasis, psoriatic arthritis, and rheumatoid arthritis: is all inflammation the same? Semin Arthritis Rheum. 2016. December;46(3):291–304. [DOI] [PubMed] [Google Scholar]
- 46.De Vlam K, Gottlieb AB, Mease PJ. Current concepts in psoriatic arthritis: pathogenesis and management. Acta Derm Venereol. 2014;94(6):627–637. [DOI] [PubMed] [Google Scholar]
- 47.Lories RJ, McInnes IB. Primed for inflammation: enthesis-resident T cells. Nat Med. 2012;18(7):1018. [DOI] [PubMed] [Google Scholar]
- 48.Ternant D, Bejan-Angoulvant T, Passot C, et al. Clinical pharmacokinetics and pharmacodynamics of monoclonal antibodies approved to treat rheumatoid arthritis. Clin Pharmacokinet. 2015;54(11):1107–1123. [DOI] [PubMed] [Google Scholar]
- 49.Dirks NL, Meibohm B. Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49(10):633–659. [DOI] [PubMed] [Google Scholar]
- 50.Nesbitt A, Fossati G, Bergin M, et al. Mechanism of action of certolizumab pegol (CDP870): in vitro comparison with other antitumor necrosis factor alpha agents. Inflamm Bowel Dis. 2007. November;13(11):1323–1332. [DOI] [PubMed] [Google Scholar]
- 51.Wang W, Wang E, Balthasar J. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84 (5):548–558. [DOI] [PubMed] [Google Scholar]
- 52.Pasut G Pegylation of biological molecules and potential benefits: pharmacological properties of certolizumab pegol. Biodrugs. 2014;28(1):15–23. [DOI] [PubMed] [Google Scholar]
- 53.Zhou HFCP. Clinical pharmacokinetics of etanercept: a fully humanized soluble recombinant tumor necrosis factor receptor fusion protein. J Clin Pharmacol. 2005. May;45(5):490–497. [DOI] [PubMed] [Google Scholar]
- 54.Wong M, Ziring D, Korin Y, et al. TNFα blockade in human diseases: mechanisms and future directions. Clin Immunol. 2008. February;126 (2):121–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu S, Liang S, Guo H, et al. Comparison of the inhibition mechanisms of adalimumab and infliximab in treating tumor necrosis factor alpha-associated diseases from a molecular view. J Biol Chem. 2013. September 20;288(38):27059–27067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ash Z, Emery P. Golimumab – a new tool in the armoury against inflammatory arthritis. Ann Med. 2011. March;43(2):133–141. [DOI] [PubMed] [Google Scholar]
- 57.Ferri N, Bellosta S, Baldessin L, et al. Pharmacokinetics interactions of monoclonal antibodies. Pharmacological Res. 2016;9(111): 592–599. [DOI] [PubMed] [Google Scholar]
- 58.Lobo ED, Hansen RJ, Balthasar JP. Antibody pharmacokinetics and pharmacodynamics. J Pharm Sci. 2004. November;93(11):2645–2668. [DOI] [PubMed] [Google Scholar]
- 59.Levy RA, Guzman R, Castaneda-Hernandez G, et al. Biology of anti-TNF agents in immune-mediated inflammatory diseases: therapeutic implications. Immunotherapy. 2016. December;8(12):1427–1436. [DOI] [PubMed] [Google Scholar]
- 60.Brambell FW, Hemmings WA, Morris IG. A theoretical model of gamma-globulin catabolism. Nature. 1964. September 26;203:1352–1354. [DOI] [PubMed] [Google Scholar]
- 61.Zhou H, Jang H, Fleischmann RM, et al. Pharmacokinetics and safety of golimumab, a fully human anti-TNF-α monoclonal antibody, in subjects with rheumatoid arthritis. J Clin Pharmacol. 2007;47(3):383–396. [DOI] [PubMed] [Google Scholar]
- 62.Zhuang Y, Lyn S, Lv Y, et al. Pharmacokinetics and safety of golimumab in healthy Chinese subjects following a single subcutaneous administration in a randomized phase I trial. Clin Drug Investig. 2013;33(11):795–800. [DOI] [PubMed] [Google Scholar]
- 63.Mould DR, Green B. Pharmacokinetics and pharmacodynamics of monoclonal antibodies. Biodrugs. 2010;24(1):23–39. [DOI] [PubMed] [Google Scholar]
- 64.Klotz U, Teml A, Schwab M. Clinical pharmacokinetics and use of infliximab. Clin Pharmacokinet. 2007;46(8):645–660. [DOI] [PubMed] [Google Scholar]
- 65.Dostalek M, Gardner I, Gurbaxani BM, et al. Pharmacokinetics, pharmacodynamics and physiologically-based pharmacokinetic modelling of monoclonal antibodies. Clin Pharmacokinet. 2013. February;52(2):83–124. [DOI] [PubMed] [Google Scholar]
- 66.Cimzia (certolizumab) [package insert]. Smyrna, GA: UCB Inc. 2016. [cited 2017 April]. Available from: https://www.cimzia.com/assets/pdf/Prescribing_Information.pdf [Google Scholar]
- 67.Remicade (infliximab) [package insert]. Horsham, PA: Janssen Biotech, Inc. 2013. [cited 2017 April]. Available from: https://www.remicade.com/shared/product/remicade/prescribing-information.pdf [Google Scholar]
- 68.Taylor PC. Pharmacology of TNF blockade in rheumatoid arthritis and other chronic inflammatory diseases. Curr Opin Pharmacol. 2010;10(3):308–315. [DOI] [PubMed] [Google Scholar]
- 69.Mewar D, Wilson AG. Treatment of rheumatoid arthritis with tumour necrosis factor inhibitors. Br J Pharmacol. 2011. ;162(4):785–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nestorov I. Clinical pharmacokinetics of TNF antagonists: how do they differ? Semin Arthritis Rheum. 2005. April 34(5, Supplement 1):12–18. [DOI] [PubMed] [Google Scholar]
- 71.Korth-Bradley JM, Rubin AS, Hanna RK, et al. The pharmacokinetics of etanercept in healthy volunteers. Ann Pharmacother. 2000. February;34(2):161–164. [DOI] [PubMed] [Google Scholar]
- 72.Enbrel (etanercept) [package insert]. Thousand Oaks, CA: Immunex Corporation; 2016. [cited 2017 April]. Available from: http://pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/enbrel/enbrel_pi.ashx [Google Scholar]
- 73.Humira (adalimumab) [package insert]. North Chicago, IL: AbbVie Inc. 2016. [cited 2017 April]. Available from: http://www.rxabbvie.com/pdf/humira.pdf [Google Scholar]
- 74.Simponi (golimumab) [package insert]. Horsham, PA: Janssen Biotech; 2013. [cited 2017 April]. Available from https://www.simpo nihcp.com/shared/product/simponi/prescribing-information.pdf [Google Scholar]
- 75.Passot C, Mulleman D, Bejan-Angoulvant T, et al. The underlying inflammatory chronic disease influences infliximab pharmacokinetics. Mabs. 2016. October;8(7):1407–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Keystone EC, Schiff MH, Kremer JM, et al. Once-weekly administration of 50 mg etanercept in patients with active rheumatoid arthritis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2004;50(2):353–363. [DOI] [PubMed] [Google Scholar]
- 77.Xu Z, Vu T, Lee H, et al. Population pharmacokinetics of golimumab, an anti-tumor necrosis factor-α human monoclonal antibody, in patients with psoriatic arthritis. J Clin Pharmacol. 2009;49 (9):1056–1070. [DOI] [PubMed] [Google Scholar]
- 78.Hojgaard P, Glintborg B, Kristensen LE, et al. The influence of obesity on response to tumour necrosis factor-alpha inhibitors in psoriatic arthritis: results from the DANBIO and ICEBIO registries. Rheumatology (Oxford). 2016. December;55(12):2191–2199. [DOI] [PubMed] [Google Scholar]
- 79.Di Minno MN, Peluso R, Iervolino S, et al. Weight loss and achievement of minimal disease activity in patients with psoriatic arthritis starting treatment with tumour necrosis factor alpha blockers. Ann Rheum Dis. 2014. June;73(6):1157–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gill KL, Machavaram KK, Rose RH, et al. Potential sources of intersubject variability in monoclonal antibody pharmacokinetics. Clin Pharmacokinet. 2016;55(7):789–805. [DOI] [PubMed] [Google Scholar]
- 81.Plasencia C, Pascual-Salcedo D, Nuño L, et al. Influence of immunogenicity on the efficacy of longterm treatment of spondyloarthritis with infliximab. Ann Rheum Dis. 2012;71(12):1955–1960. BMJ Publishing Group Ltd and European League Against Rheumatism.. [DOI] [PubMed] [Google Scholar]
- 82.Schellekens H. Immunogenicity of therapeutic proteins: clinical implications and future prospects. Clin Ther. 2002;24(11):1720–1740. [DOI] [PubMed] [Google Scholar]
- 83.Thomas SS, Borazan N, Barroso N, et al. Comparative immunogenicity of TNF inhibitors: impact on clinical efficacy and tolerability in the management of autoimmune diseases. A systematic review and meta-analysis. Biodrugs. 2015. August;29(4):241–258. [DOI] [PubMed] [Google Scholar]
- 84.Garces S, Demengeot J, Benito-Garcia E. The immunogenicity of anti-TNF therapy in immune-mediated inflammatory diseases: a systematic review of the literature with a meta-analysis. Ann Rheum Dis. 2013. December;72(12):1947–1955. [DOI] [PubMed] [Google Scholar]
- 85.Van Kuijk AW, De Groot M, Stapel SO, et al. Relationship between the clinical response to adalimumab treatment and serum levels of adalimumab and anti-adalimumab antibodies in patients with psoriatic arthritis. Ann Rheum Dis. 2010;69(3):624–625. [DOI] [PubMed] [Google Scholar]
- 86.Vogelzang EH, Kneepkens EL, Nurmohamed MT, et al. Anti-adalimumab antibodies and adalimumab concentrations in psoriatic arthritis; an association with disease activity at 28 and 52 weeks of follow-up. Ann Rheum Dis. 2014;73:2178–2182. BMJ Publishing Group Ltd and European League Against Rheumatism. [DOI] [PubMed] [Google Scholar]
- 87.Zisapel M, Zisman D, Madar-Balakirski N, et al. Prevalence of TNF-alpha blocker immunogenicity in psoriatic arthritis. J Rheumatol. 2015. January;42(1):73–78. [DOI] [PubMed] [Google Scholar]
- 88.Kavanaugh A, Krueger GG, Beutler A, et al. Infliximab maintains a high degree of clinical response in patients with active psoriatic arthritis through 1 year of treatment: results from the IMPACT 2 trial. Ann Rheum Dis. 2007;66(4):498–505. BMJ Publishing Group Ltd and European League Against Rheumatism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kavanaugh A, Van Der Heijde D, McInnes IB, et al. Golimumab in psoriatic arthritis: one-year clinical efficacy, radiographic, and safety results from a phase III, randomized, placebo-controlled trial. Arthritis Rheum. 2012;64(8):2504–2517. [DOI] [PubMed] [Google Scholar]
- 90.Kavanaugh A, McInnes I, Mease P, et al. Golimumab, a new human tumor necrosis factor alpha antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: twentyfour-week efficacy and safety results of a randomized, placebo-controlled study. Arthritis Rheum. 2009. April;60(4):976–986. [DOI] [PubMed] [Google Scholar]; ·· Phase III trial evaluating the efficacy of golimumab.
- 91.Kavanaugh A, McInnes IB, Mease P, et al. Clinical efficacy, radiographic and safety findings through 5 years of subcutaneous golimumab treatment in patients with active psoriatic arthritis: results from a long-term extension of a randomised, placebo-controlled trial (the GO-REVEAL study). Ann Rheum Dis. 2014. BMJ Publishing Group Ltd and European League Against Rheumatism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mease PJ, Gladman DD, Ritchlin CT, et al. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2005;52(10):3279–3289. [DOI] [PubMed] [Google Scholar]; ·· Phase III trial evaluating the efficacy of adalimumab.
- 93.Mease PJ, Fleischmann R, Deodhar AA, et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a phase 3 double-blind randomised placebo-controlled study (RAPID-PsA). Ann Rheum Dis. 2013. BMJ Publishing Group Ltd and European League Against Rheumatism.. [DOI] [PMC free article] [PubMed] [Google Scholar]; ·· Phase III trial evaluating the efficacy of certolizumab pegol
- 94.Mease PJ, Kivitz AJ, Burch FX, et al. Etanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progression. Arthritis Rheum. 2004;50(7):2264–2272. [DOI] [PubMed] [Google Scholar]
- 95.Vogelzang EH, Pouw MF, Nurmohamed M, et al. Adalimumab trough concentrations in patients with rheumatoid arthritis and psoriatic arthritis treated with concomitant disease-modifying antirheumatic drugs. Ann Rheum Dis. 2015;74(2):474–475. BMJ Publishing Group Ltd and European League Against Rheumatism. [DOI] [PubMed] [Google Scholar]
- 96.Mease P, Deodhar A, Fleischmann R, et al. Effect of certolizumab pegol over 96 weeks in patients with psoriatic arthritis with and without prior antitumour necrosis factor exposure. RMD Open. 2015;1(1). EULAR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Antoni CE, Kavanaugh A, Kirkham B. Sustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: results from the infliximab multinational psoriatic arthritis controlled trial (IMPACT). Arthritis Rheum. 2005;52:1227. [DOI] [PubMed] [Google Scholar]; ·· Phase III trial evaluating the efficacy of infliximab.
- 98.Kavanaugh A, Antoni CE, Gladman D. The infliximab multinational psoriatic arthritis controlled trial (IMPACT): results of radiographic analyses after 1 year. Ann Rheum Dis. 2006;65:1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Antoni C, Krueger GG, De Vlam K. Infliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT 2 trial. Ann Rheum Dis. 2005;64:1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Van Der Heijde D, Kavanaugh A, Gladman DD, et al. Infliximab inhibits progression of radiographic damage in patients with active psoriatic arthritis through one year of treatment: results from the induction and maintenance psoriatic arthritis clinical trial 2. Arthritis Rheum. 2007;56(8):2698–2707. [DOI] [PubMed] [Google Scholar]
- 101.Mease PJ, Goffe BS, Metz J, et al. Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. The Lancet. 2000;356(9227):385–390. [DOI] [PubMed] [Google Scholar]; ·· Phase III trial evaluating the efficacy of etanercept.
- 102.Mease PJ, Kivitz AJ, Burch FX, et al. Continued inhibition of radiographic progression in patients with psoriatic arthritis following 2 years of treatment with etanercept. J Rheumatol. 2006. April; 33(4):712–721. [PubMed] [Google Scholar]
- 103.Schafer P, Parton A, Gandhi A, et al. Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br J Pharmacol. 2010;159(4):842–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014. June;73(6):1020–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cutolo M, Myerson GE, Fleischmann RM, et al. A phase III, randomized, controlled trial of apremilast in patients with psoriatic arthritis: results of the PALACE 2 trial. J Rheumatol. 2016. September; 43(9):1724–1734. [DOI] [PubMed] [Google Scholar]
- 106.Edwards CJ, Blanco FJ, Crowley J, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: a phase III, randomised, controlled trial (PALACE 3). Ann Rheum Dis. 2016. June;75(6):1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wells AF, Edwards CJ, Adebajo AO, et al. Apremilast in the treatment of DMARD-naive psoriatic arthritis patients: results of a phase 3 randomized, controlled trial (PALACE 4). 2013. ACR/ARHP Annual Meeting; Abstract L4. [Google Scholar]
- 108.Pathan E, Abraham S, Van Rossen E, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in ankylosing spondylitis. Ann Rheum Dis. 2013;72(9):1475–1480. BMJ Publishing Group Ltd and European League Against Rheumatism. [DOI] [PubMed] [Google Scholar]
- 109.ClinicalTrials.gov. Study of apremilast to treat subjects with active ankylosing spondylitis (POSTURE); 2017. [cited 2017 May 3]. Available from https://www.clinicaltrials.gov/ct2/show/results/NCT01583374?term=apremilast&cond=ankylosing+spondylitis&rank=2§=X37016#limit
- 110.Mease PJ, McInnes IB, Kirkham B, et al. Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. N Engl J Med. 2015. October;373(14):1329–1339. [DOI] [PubMed] [Google Scholar]
- 111.McInnes IB, Mease PJ, Kirkham B, et al. Secukinumab, a human antiinterleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet. 2015. September 19–25;386(9999):1137–1146. [DOI] [PubMed] [Google Scholar]
- 112.McInnes IB, Kavanaugh A, Gottlieb AB, et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. The Lancet. 2013;382(9894):780–789. [DOI] [PubMed] [Google Scholar]
- 113.Kavanaugh A, Puig L, Gottlieb AB, et al. Maintenance of clinical efficacy and radiographic benefit through two years of ustekinumab therapy in patients with active psoriatic arthritis: results from a randomized, placebo-controlled phase III trial. Arthritis Care Res. 2015;67(12):1739–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ritchlin C, Rahman P, Kavanaugh A, et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis. 2014;73 (6):990–999. BMJ Publishing Group Ltd and European League Against Rheumatism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kavanaugh A, Ritchlin C, Rahman P, et al. Ustekinumab, an anti-IL-12/23 p40 monoclonal antibody, inhibits radiographic progression in patients with active psoriatic arthritis: results of an integrated analysis of radiographic data from the phase 3, multicentre, randomised, double-blind, placebo-controlled PSUMMIT-1 and PSUMMIT-2 trials. Ann Rheum Dis. 2014;73(6):1000–1006. BMJ Publishing Group Ltd and European League Against Rheumatism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Baeten D, Sieper J, Braun J, et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med. 2015. December 24;373 (26):2534–2548. [DOI] [PubMed] [Google Scholar]
- 117.ClinicalTrials.gov. A study to evaluate the efficacy and safety of ustekinumab in the treatment of anti-TNF (alpha) refractory participants with active radiographic axial spondyloarthritis.; 2017. [cited 2017 May 3]. Available from https://www.clinicaltrials.gov/ct2/show/record/NCT02438787?term=ustekinumab&rank=31
- 118.ClinicalTrials.gov. A study to evaluate the efficacy and safety of ustekinumab in the treatment of anti-TNFα naïve participants with active radiographic axial spondyloarthritis; 2017. [cited 2017 May 3]. Available from https://www.clinicaltrials.gov/ct2/show/record/NCT02437162?term=ustekinumab&rank=32
- 119.Ungprasert P, Thongprayoon C, Davis III JM. Indirect comparisons of the efficacy of biological agents in patients with psoriatic arthritis with an inadequate response to traditional disease-modifying anti-rheumatic drugs or to non-steroidal anti-inflammatory drugs: a meta-analysis. Semin Arthritis Rheum. 2016;45(4):428–438. [DOI] [PubMed] [Google Scholar]
- 120.Ungprasert P, Thongprayoon C, Davis JM III. Indirect comparisons of the efficacy of subsequent biological agents in patients with psoriatic arthritis with an inadequate response to tumor necrosis factor inhibitors: a meta-analysis. Clin Rheumatol. 2016;35(7):1795–1803. [DOI] [PubMed] [Google Scholar]
- 121.Fagerli KM, Lie E, Van Der Heijde D, et al. The role of methotrexate co-medication in TNF-inhibitor treatment in patients with psoriatic arthritis: results from 440 patients included in the NOR-DMARD study. Ann Rheum Dis. 2014. January;73(1):132–137. [DOI] [PubMed] [Google Scholar]
- 122.Behrens F, Canete JD, Olivieri I, et al. Tumour necrosis factor inhibitor monotherapy vs combination with MTX in the treatment of PsA: a systematic review of the literature (provisional abstract). SO: Database of Abstracts of Reviews of Effects; 2014. [DOI] [PubMed] [Google Scholar]
- 123.Chingcuanco F, Segal JB, Kim SC, et al. Bioequivalence of biosimilar tumor necrosis factor-alpha inhibitors compared with their reference biologics: a systematic review. Ann Intern Med. 2016. October 18;165(8):565–574. [DOI] [PubMed] [Google Scholar]
- 124.Cohen S, Kay J. Biosimilars: implications for rheumatoid arthritis therapy. Curr Opin Rheumatol. 2017;29(3):260–268. [DOI] [PubMed] [Google Scholar]
- 125.Emery P, Vencovsky J, Sylwestrzak A, et al. A phase III randomised, double-blind, parallel-group study comparing SB4 with etanercept reference product in patients with active rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis. 2017. January;76(1):51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Park W, Yoo DH, Miranda P, et al. Efficacy and safety of switching from reference infliximab to CT-P13 compared with maintenance of CT-P13 in ankylosing spondylitis: 102-week data from the PLANETAS extension study. Ann Rheum Dis. 2017. February;76(2):346–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yoo DH, Prodanovic N, Jaworski J, et al. Efficacy and safety of CT-P13 (biosimilar infliximab) in patients with rheumatoid arthritis: comparison between switching from reference infliximab to CT-P13 and continuing CT-P13 in the PLANETRA extension study. Ann Rheum Dis. 2017. February;76(2):355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]