Abstract
Spontaneous pneumothorax is rarely associated with cancer. We describe a 73 year old man who presented with recurrent tumor in the right neck, mediastinal lymphadenopathy and bilateral pulmonary nodules after thyroidectomy. He was treated with lenvatinib and presented with bilateral pneumothoraces. Anaplastic thyroid cancer is an aggressive subtype of thyroid cancer that has limited response to cytotoxic chemotherapy and poor prognosis. Recent reports show that targeted therapy with a multiple receptor tyrosine kinase inhibitor, lenvatinib, may have improvement in progression-free survival, but rarely pneumothorax has been reported in those with lung metastases. Various mechanisms have been postulated, but necrosis of pulmonary lesions and/or subpleural micrometastases leading to bilateral pleural defects likely resulted in the development of pneumothoraces for our patient.
Keywords: Lenvatinib, Pneumothorax, Anaplastic thyroid cancer, Targeted therapy
1. Introduction
Anaplastic thyroid cancer is an aggressive subtype of thyroid cancer that has limited response to cytotoxic chemotherapy and poor prognosis (with the exception of those harboring the BRAF V600E mutation). Recent reports show that targeted therapy with a multiple receptor tyrosine kinase (RTK) inhibitor, lenvatinib, may have improvement in progression-free survival [1,2]. Adverse events typically include fatigue, hypertension, weight loss, diarrhea and anorexia [3]. Unilateral spontaneous pneumothorax associated with lenvatinib therapy is exceedingly rare, and this potentially life-threatening toxicity has been cited in isolated case reports among patients with pulmonary metastases [4]. Bilateral pneumothorax associated with this agent is even more unusual. A causal association is implied based on the temporal relationship between drug initiation and the development of spontaneous pneumothorax, coupled with the development of tumor cavitation and/or fistula formation, the subpleural location of the metastatic tumors and the exclusion of competing causes of pneumothorax. Herein we describe a rare case of anaplastic thyroid cancer treated with lenvatinib complicated with bilateral pneumothoraces.
2. Case report
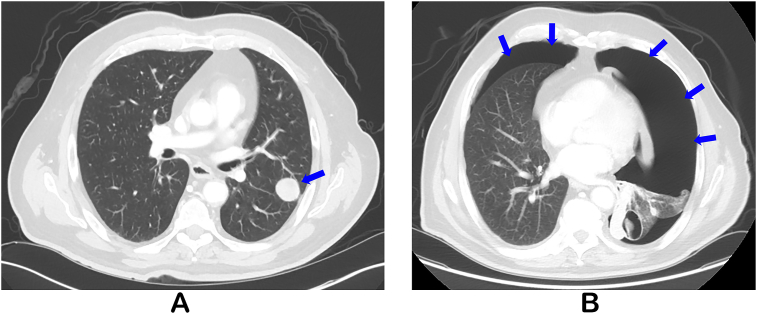
A 73 year old man presented with recurrent tumor in the right neck, mediastinal lymphadenopathy, and bilateral pulmonary nodules (Fig. 1A) four weeks following total thyroidectomy for anaplastic thyroid carcinoma. BRAF mutation was not found in his tumor and therefore lenvatinib was promptly initiated. At routine imaging two months later, bilateral pneumothoraces (Fig. 1B), and increased pulmonary metastases with cavitation were found. He was relatively asymptomatic and hemodynamically stable with saturations of 94% on room air. Upon further questioning, he reported developing mild dyspnea four days prior to the imaging studies without associated cough, wheezing, chest pain or recent trauma.
Fig. 1.
Computed tomography (CT) of the chest at the following time points: presentation (A) with multiple new bilateral pulmonary parenchymal nodules (blue arrow) with the largest measuring 2.6 cm on the left; after therapy with lenvatinib (B) with large left and moderate right pneumothorax (blue arrows).
Due to the safety concerns with bilateral pneumothoraces, a left-sided chest tube was urgently placed, and lenvatinib was held. A right-sided chest tube was also placed the next day and subsequently radiation therapy to the tumor bed in the neck was performed due to progression in the neck during the drug hold. Lenvatinib was resumed at a lower dose after chemical pleurodesis performed via the left-sided chest tube. He was then discharged to hospice as per his wishes.
3. Discussion
In general aside from iatrogenic causes, spontaneous pneumothorax with malignancy is rare. Without antecedent trauma, pneumothorax in those with normal lungs is considered primary, and in those with underlying lung disease would be considered secondary [5]. Spontaneous pneumothorax has been described most frequently with lung cancers, and etiologies were thought to be multifactorial including the tumor itself, changes of the tumor with therapies such a radiation, and pre-existing parenchymal abnormalities such as obstructive lung disease [6]. A few reports have described spontaneous pneumothorax as a sequelae of treatment in those with pulmonary metastases and chemosensitive malignancies, and rarely as a presentation of the cancer itself [[7], [8], [9]].
Certain therapies have been implicated in the development of spontaneous pneumothorax as well. The incidence of pneumothorax associated with lenvatinib have been rare, and one series described pneumothorax in two patients with lung metastases [3]. Another case report described a right pneumothorax approximately 34 days after initiation of lenvatinib in a patient with anaplastic thyroid cancer and lung metastases [4]. Lenvatinib impedes the kinase activities of various receptors including vascular endothelial growth factor, fibroblast growth factor, platelet-derived growth factor receptor α, RET and KIT, to inhibit pathogenic angiogenesis, tumor growth, and cancer progression. Various mechanisms have been postulated for the development of spontaneous pneumothorax in cancer patients which may include: development of bronchopleural fistula within the necrotic tumor; tumor-induced rupture of a subpleural bleb; direct pleural invasion by the tumor [6]. Another angiogenesis inhibitor and RTK agent, pazopanib, has been described in those with sarcoma and pulmonary metastases [10]. However, there are conflicting reports regarding the association of the pneumothorax to pazopanib, and some have advocated no causality with the drug, rather the presence of cavitary lung nodules/masses and pleural-based disease were the risk factors [11].
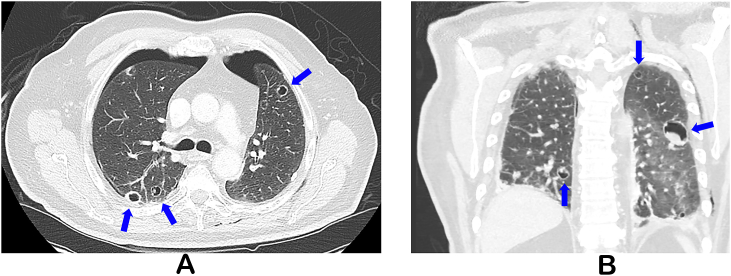
With the advent of new targeted therapies, patients may develop a myriad of respiratory symptoms, so it is important to have a high clinical suspicion for various pulmonary manifestations. For example, certain therapies such as bevacizumab either independently or in combination with radiation may result in fistulous formations [12,13]. The development of spontaneous pneumothorax in our patient was particularly unique. He did have bilateral lung metastases and was receiving a medication associated with pneumothorax. Furthermore, anaplastic thyroid cancer is notorious for aggressive pathology. Thus although levantinib may be the culprit for his pneumothorax, the location of his lung metastates and underlying malignancy likely contributed as well. In our patient, we suspect necrosis of pulmonary lesions and/or subpleural micrometastases leading to bilateral pleural defects likely resulted in the development of pneumothoraces (Fig. 2A and B) [4]. Thus, a high clinical suspicion for the development of pneumothorax in those with lung lesions and lenvatinib therapy should be maintained.
Fig. 2.
Computed tomography (CT) of the chest after placement of bilateral chest tubes with bilateral cavitating pulmonary masses (A, coronal view B).
In the event of pneumothorax, definitive management with pleural intervention and pleurodesis would be recommended. One case report described recurrent pneumothorax that improved after pleurodesis, and the medication was continued [4]. In our patient after placement of chest tube and pleurodesis, the medication was resumed, but he was discharged to hospice. In general lenvatinib may be resumed with dose reduction after pneumothorax has been addressed; however, monitoring for recurrent pneumothorax would be imperative.
In conclusion, previous reports have described unilateral pneumothorax described with lenvatinib, but simultaneous bilateral spontaneous pneumothorax is extremely rare in general and has not been described with this therapy [14]. Although we suspect lenvatinib contributed significantly, the presence of underlying lung metastases and an aggressive malignancy further promoted this adverse event. Thus, close monitoring of anaplastic thyroid cancer with lung metastases on lenvatinib for pulmonary symptoms and pneumothorax would be advised.
Conflicts of interest
MEC has received research funding from Eisai.
Author contributions
FIK, MEC, LB, VRS, SAF: conception and design, acquisition of radiological and pathological data, drafting the article, critical revision of intellectual content and final approval of the version to be published.
Funding
This research is supported in part by the National Institutes of Health through MD Anderson's Cancer Center Support Grant (CA016672).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmcr.2019.01.006.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Schlumberger M., Tahara M., Wirth L.J., Robinson B., Brose M.S., Elisei R., Habra M.A., Newbold K., Shah M.H., Hoff A.O., Gianoukakis A.G., Kiyota N., Taylor M.H., Kim S.B., Krzyzanowska M.K., Dutcus C.E., de las Heras B., Zhu J., Sherman S.I. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N. Engl. J. Med. 2015;372:621–630. doi: 10.1056/NEJMoa1406470. [DOI] [PubMed] [Google Scholar]
- 2.Iniguez-Ariza N.M., Ryder M.M., Hilger C.R., Bible K.C. Salvage lenvatinib therapy in metastatic anaplastic thyroid cancer. Thyroid. 2017;27:923–927. doi: 10.1089/thy.2016.0627. [DOI] [PubMed] [Google Scholar]
- 3.Berdelou A., Borget I., Godbert Y., Nguyen T., Garcia M.E., Chougnet C.N., Ferru A., Buffet C., Chabre O., Huillard O., Leboulleux S., Schlumberger M. Lenvatinib for the treatment of radioiodine-refractory thyroid cancer in real-life practice. Thyroid. 2018;28(1):72–78. doi: 10.1089/thy.2017.0205. [DOI] [PubMed] [Google Scholar]
- 4.Yamazaki H., Iwasaki H., Yamashita T., Yoshida T., Suganuma N., Yamanaka T., Masudo K., Nakayama H., Kohagura K., Rino Y., Masuda M. A case of pneumothorax after treatment with lenvatinib for anaplastic thyroid cancer with lung metastasis. Case Rep. Endocrinol. 2018;2018:7875929. doi: 10.1155/2018/7875929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henry M., Arnold T., Harvey J., Pleural Diseases Group SoCCBTS BTS guidelines for the management of spontaneous pneumothorax. Thorax. 2003;58(Suppl 2):ii39–52. doi: 10.1136/thorax.58.suppl_2.ii39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan S.N., Okuno S.H., Jatoi A. Causes and outcomes of spontaneous pneumothoraces in solid tumor cancer patients: an update for the medical oncologist. J. Thorac. Oncol. 2006;1:335–338. [PubMed] [Google Scholar]
- 7.Laurencet F.M., Zulian G.B., Dietrich P.Y. Pneumothorax following induction chemotherapy for a germ cell tumour. Eur. J. Cancer. 1997;33:169–170. doi: 10.1016/s0959-8049(96)00296-1. [DOI] [PubMed] [Google Scholar]
- 8.Stein M.E., Haim N., Drumea K., Ben-Itzhak O., Kuten A. Spontaneous pneumothorax complicating chemotherapy for metastatic seminoma. A case report and a review of the literature. Cancer. 1995;75:2710–2713. doi: 10.1002/1097-0142(19950601)75:11<2710::aid-cncr2820751112>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 9.Arias de la Vega F., Illarramendi J.J., Martinez E., Vila E., Martinez-Penuela J.M., Dominguez M.A. Spontaneous pneumothorax as the first manifestation of Ewing's sarcoma. Pediatr. Pulmonol. 1995;19:182–184. doi: 10.1002/ppul.1950190307. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura T., Matsumine A., Kawai A., Araki N., Goto T., Yonemoto T., Sugiura H., Nishida Y., Hiraga H., Honoki K., Yasuda T., Boku S., Sudo A., Ueda T. The clinical outcome of pazopanib treatment in Japanese patients with relapsed soft tissue sarcoma: a Japanese Musculoskeletal Oncology Group (JMOG) study. Cancer. 2016;122:1408–1416. doi: 10.1002/cncr.29961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabath B., Muhammad H.A., Balagani A., Ost D.E., Vakil E., Ahmed T., Vial M.R., Grosu H.B. Secondary spontaneous pneumothorax in patients with sarcoma treated with Pazopanib, a case control study. BMC Cancer. 2018;18:937. doi: 10.1186/s12885-018-4858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spigel D.R., Hainsworth J.D., Yardley D.A., Raefsky E., Patton J., Peacock N., Farley C., Burris H.A., 3rd, Greco F.A. Tracheoesophageal fistula formation in patients with lung cancer treated with chemoradiation and bevacizumab. J. Clin. Oncol. 2010;28:43–48. doi: 10.1200/JCO.2009.24.7353. [DOI] [PubMed] [Google Scholar]
- 13.Wong J., Gutierrez C., Shannon V.R., Eapen G.A., Faiz S.A. Bronchomediastinal fistula after endobronchial ultrasound-guided transbronchial needle aspiration. Am. J. Respir. Crit. Care Med. 2016;194:114–115. doi: 10.1164/rccm.201601-0144IM. [DOI] [PubMed] [Google Scholar]
- 14.Graf-Deuel E., Knoblauch A. Simultaneous bilateral spontaneous pneumothorax. Chest. 1994;105:1142–1146. doi: 10.1378/chest.105.4.1142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.