Abstract
The agglutination titers of Brachyspira pilosicoli (B. pilosicoli) and Brachyspira aalborgi (B. aalborgi) were examined in colitis patients with human intestinal spirochetes. Among three cases of colitis patients, the titer of B. pilosicoli was extremely high in two cases while the titer of B. aalborgi was extremely high in one case. These three cases had symptoms of colitis, such as watery diarrhea, and we diagnosed the case as Brachyspira- related colitis. These findings suggest that the agglutination titers of Brachyspira may be useful in cases of Brachyspira- related colitis. Severe symptoms, such as abdominal pain and diarrhea, were observed in cases with high antibody titer of B. aalborgi, as well as B. pilosicoli, indicating that B. aalborgi could also cause symptomatic colitis.
Keywords: agglutination, antibody, titer, Brachyspira
Introduction
Human intestinal spirochaetosis (HIS) was first reported by Harland and Lee in 1967.(1) HIS is a disease of the colorectum caused by the two kinds of gram-negative bacteria Brachyspira aalborgi (B. aalborgi) and Brachyspira pilosicoli (B. pilosicoli).(2) B. pilosicoli causes disease in humans and in various animals, however B. aalborgi induces colonic spirochaetosis only in humans.(3,4)
In clinical practice, HIS is often diagnosed upon pathological examination with a finding of so-called fringe formation.(5) Definitive diagnosis requires the use of polymerase chain reaction, electron microscopy,(6) imprint cytology,(7) and detection of serum antibody titers to B. aalborgi and to B. pilosicoli with agglutination titers.(8) We also isolated B. aalborgi and observed the genetic and immunological characteristics.(9) All isolates also reacted with strain ATCC51139 and NCTC11492 strongly, but the reaction profiles attributed to our isolates were slightly different from those of ATCC51139 and NCTC11492. In 2017, we reported that a Brachyspira-like organism in an Akoya (pearl) oyster did not react with anti-Treponema pallidum (T. pallidum) serum but reacted with anti-B. aalborgi and anti-B. pilosicoli sera. This finding indicates that the antigenic features of Brachyspira sp. are different from those to T. pallidum.(10)
On the other hand, when we investigated the antibody to B. hyodysenteriae by dark field microscopy.(11) the reaction was serotype-specific.(12) We have been using this test in cases of human intestinal spirochetes, and the results indicate that the test is very useful and the reaction is serotype- or species-specific. We attempted to investigate the antibodies to the human brachyspiras and showed high titers in colitis patients with human intestinal spirochetes.
Materials and Methods
B. pilosicoli ATCC51139, Kyoto-C, and B. aalborgi NCTC11492, Yokosuka 24-d, B. ibaraki (one lineage of B. aalborgi) were used for the agglutination test. The organisms were grown anaerobically on 4% sheep blood agar containing 400 µg/ml spectinomycin using a GasPak Anaerobic System (Mitsubishi Gas Chemical Co., Inc., Tokyo, Japan) as described previously.(13) Dark field agglutination tests were carried out as described previously.(11) B. pilosicoli was grown anaerobically for 7 days and B. aalborgi was grown anaerobically for 30 days. The cells grown on blood agar were harvested with physiological saline and after centrifugation, the precipitate was suspended in physiological saline. The cells were adjusted to MacFarland No.1. The sera collected from patients with diarrhea were diluted two-fold limiting dilution from 100 to 12,800. Into each well of the ceramic agglutination plate, an aliquot (0.05 µl) if the diluted serum pipetted and then 0.05 µl of the brachyspiral cell suspensions were dispensed into each well. After incubation for 60 min, the ceramic plates were left for 2 h. and then observed under the dark field microscope. Furthermore, the agglutination was observed again after 24 h and 48 h.
Informed consent was obtained from all subjects, and the experimental protocol was approved by the Ethics Committee of Tokyo Medical University Ibaraki Medical Center.
Results
Comparison of agglutinability between B. pilosicoli and B. aalborgi
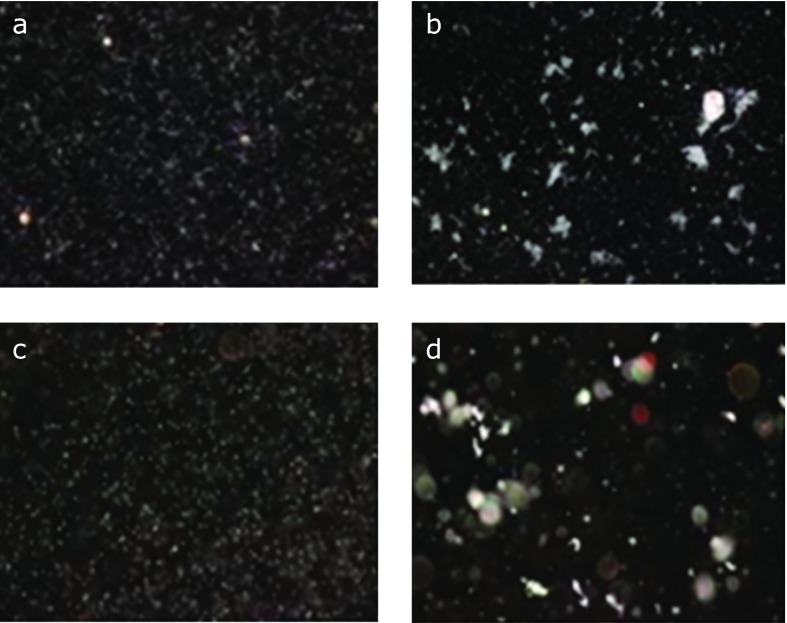
Serum obtained from a patient with colitis was diluted at 1:100 and the agglutinability was compared between B. pilosicoli and B. aalborgi antigens adjusted to MacFarland No. 1. The results are shown in Fig. 1. Plates a and c were negative controls using physiological saline instead of human serum. Plates b and d showed positive agglutination using human serum from the colitis patient. In the agglutination reaction with B. pilosicoli, free cells were observed and the agglutination was a soft aggregate of the cells, while in agglutination with B. aalborgi, no free cells were observed and the agglutination formed a rigid cell aggregate (Fig. 2).
Fig. 1.
Comparison of agglutinability to between B. pilosicoli and B. aalborgi. (A, B) B. pilosicoli; (C, D) B. aalborgi. (A) and (C) were negative control (physiological saline was used instead of human serum). (B) and (D) were positive agglutination (human serum from a patient with colitis was used).
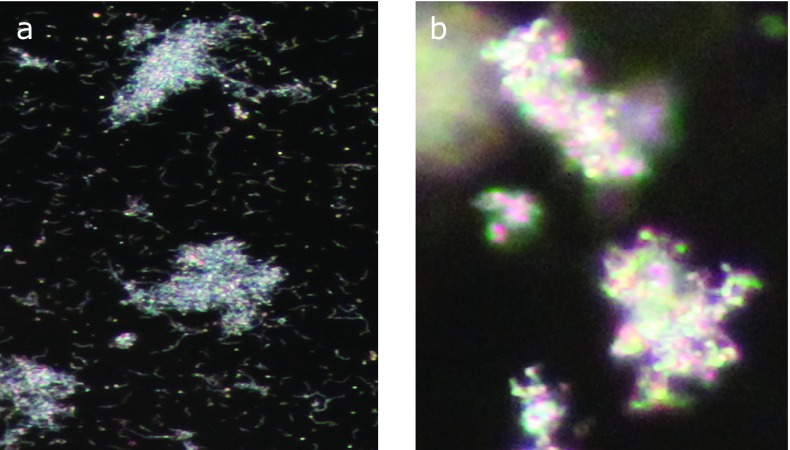
Fig. 2.
Comparison of intensity of the agglutinability between to B. aalborgi and to B. pilosicoli. (A) in agglutination with B. pilosicoli, free cells were observed and the agglutination was soft agglegate of the cells, while in agglutination with B. aalborgi, no free cells were observed and the agglutination rigid aggregate. As the results, the antibody titer to B. aalborgi was higher than that to B. pilosicoli.
Antibody titers on the agglutination reaction
The antibody titer to B. pilosicoli was higher than that to B. aalborgi in cases 1 and 2. By contrast, the antibody titer to B. aalborgi was higher than that to B. pilosicoli in case 3 (Table 1).
Table 1.
Diagnosis of patients with colitis by dark field agglutination test
|
Agglutination titers to |
|||
|---|---|---|---|
| Patients | Specimens | B. p (Kyoto-C) | B. a (B. ibaraki) |
| Case 1 | 3,200 (after 24 h) | 200> (after 24 h) | |
| Case 2 | 6,400 (after 24 h) | 200> (after 24 h) | |
| Specimens | B. p (ATCC51139) | B. a (B. ibaraki) | |
| Case 3 | 80 (after 24 h) | 640 (after 24 h) | |
Agglutination titers were investigated under dark field microscopy. B. p, Brachyspira pilosicoli; B. a, Brachyspira aalborgi.
Clinical feature of symptomatic patients with colitis who showed the high antibody titer of B. pilosicoli, or B. aalborgi
The endoscopic and pathological findings of cases are shown in Fig. 3, 4, 5. The profiles of symptomatic patients with colitis with the high antibody titer for B. pilosicoli, or B. aalborgi are shown in Table 2. All three cases exhibited symptoms of colitis, including intense diarrhea, and colonoscopy revealed edematous mucosa with multiple erythematous spots in the colonic mucosa. These colonoscopic findings were mainly distributed in the ascending and transverse colon.
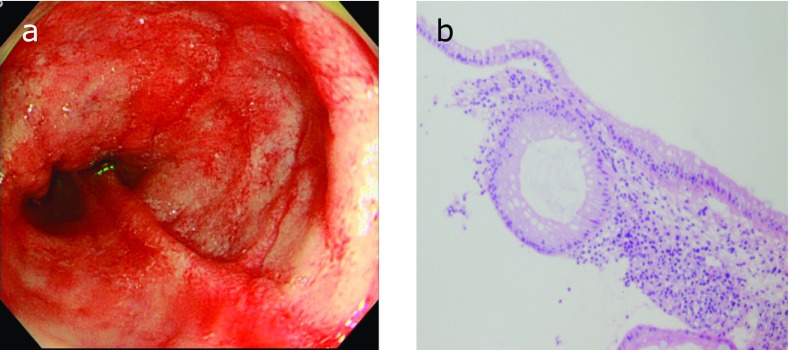
Fig. 3.
Case 1. Colonoscopy shows edematous mucosa with multiple erythematous spots in ascending colon (A) and histology of colonic biopsy specimens did not show fringe formation on the luminal side of colonic surface epithelium (hematoxylin-eosin stain) (B).
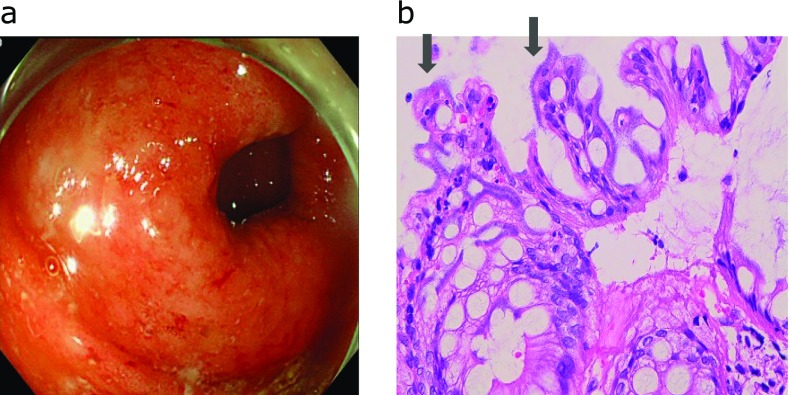
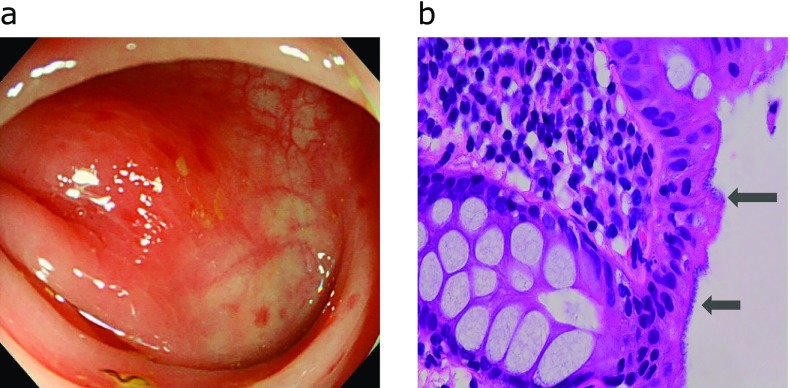
Fig. 4.
Case 2. Colonoscopy shows edematous mucosa with multiple erythematous spots in transverse colon (A) and histology of colonic biopsy specimens showed fringe formation on the luminal side of colonic surface epithelium (hematoxylin-eosin stain). Arrow showed human intestinal spirochetes (B).
Fig. 5.
Colonoscopy shows edematous mucosa with multiple erythematous spots in transverse colon (A) and histology of colonic biopsy specimens showed fringe formation on the luminal side of colonic surface epithelium (hematoxylin-eosin stain). Arrow showed human intestinal spirochetes (B).
Table 2.
Clinical and endoscopical findings of three cases
| Case | Age/Gender | Underlying disease | Clinical symptom | Endoscopic findings | Colonic distribution of HIS | Severity of histopathological inflammation (microscopic findings) | Dignosis of HIS |
|---|---|---|---|---|---|---|---|
| 1 | 31/male | Ulcerative colitis | Watery diarrhea Abdominal pain | Edematous mucosa with multiple erythematous spots | A/C, T/C | Moderate | B. p |
| 2 | 53/male | None | Watery diarrhea Abdominal pain | Edematous mucosa with multiple erythematous spots | T/C | Moderate | B. p |
| 3 | 58/male | None | Watery diarrhea Abdominal pain | Edematous mucosa with multiple erythematous spots | A/C, T/C, S/C | Mild | B. a |
B. p, Brachyspira pilosicoli; B. a, Brachyspira aalborgi.
Discussion
Previous studies have investigated the incidence of HIS in the colon; indicating its possible correlation with various diseases. HIS has been associated with carcinoma of the large intestine, adenomatous polyps, hemorrhoids with a metaplastic polyp, and ulcerative colitis.(14) Another study has demonstrated that HIS was observed in cases of carcinoma or polyp of the colon and ulcerative colitis.(15) We also have reported cases of HIS infection in ulcerative colitis patients undergoing long-term steroid therapy.(16)
We previously investigated the agglutination titers of five species of Brachyspira and showed high titers of B. aalborgi in the serum of patients.(8) In our present study, we demonstrated high antibody titers of B. pilosicoli, or B. aalborgi in patients with human intestinal spirochaetosis-related colitis. In clinical practice, human intestinal spirochaetosis is often detected in cases of colonic polyps in the absence of symptoms, such as diarrhea. In our present cases, the patients exhibited the symptoms of colitis, including severe diarrhea, and HIS was detected in histopathological examination in two cases and all three cases exhibited high antibody titers for B. pilosicoli, or B. aalborgi. These results indicate that these patients could be diagnosed as colitis induced by human intestinal spirochetes.
The endoscopic findings of HIS-related colitis have been reported in several HIS case reports; edematous mucosa with multiple erythematous spots are observed in the ascending and in the proximal transverse colon and are not apparent in the distal colon or the rectum.(5,17) Another previous report investigated the relationship between HIS infection and colonoscopic findings and the colonic distribution and showed that the colonoscopic findings in HIS patients exhibited either non-specific ulceration of the ileocecal valve or extensive areas of ischemic ulcers.(18) The colonic distribution of 8 HIS-related cases of colitis has also been reported and 5 cases of the eight were throughout the entire colon, one case was right colon predominant, one case was rectal sparing and one case was right colon sparing.(18) It has been also reported that colonoscopy revealed the edematous mucosa with multiple erythematous spots in the cecum and transverse colon and several ulcers were found in the transverse colon in HIS infectious colitis in an immunocompromised host.(19) In the present cases, the colonoscopic findings exhibited edematous mucosa with multiple erythematous spots, which were similar to previous reports.
It has been suggested that B. pilosicoli infection causes clinical symptoms, such as abdominal pain and watery diarrhea.(20) In the previous case report of B. pilosicoli infection, the patient had severe symptoms, such as acute onset abdominal pain and watery diarrhea.(17) In another report from Japan, PCR analysis of B. pilosicoli and B. aalborgi were examined in asymptomatic HIS cases, and showed that more of the asymptomatic HIS patients were infected with B. aalborgi.(21) These studies have suggested that patients with B. pilosicoli infection exhibit more severe symptoms, such as abdominal pain and watery diarrhea, than patients with B. aalborgi infection. In the present cases, severe symptoms, such as abdominal pain and diarrhea, were observed in cases with high antibody titers for both B. pilosicoli and B. aalborgi, indicating that B. aalborgi could also cause symptomatic colitis.
The recent investigation has demonstrated the probable association between proton pump inhibitor (PPI) use and alternation of gut microbiota, indicating that PPIs may predispose the enteric infection.(22) From these valuable reported results, the effect of PPI use on B. pilosicoli and B. aalborgi infection should be investigated in the next study.
The limitation of this study is that the agglutination titers of B. pilosicoli and B. aalborgi were examined in only three cases of colitis patients with human intestinal spirochetes. Therefore, accumulation of human intestinal spirochetes cases examined with agglutination titers of B. pilosicoli and B. aalborgi is desirable.
In conclusion, in cases of HIS infection, the differential diagnosis of B. pilosicoli and B. aalborgi is clinically meaningful because it has been suggested that the clinical feature of these two kinds of infection are different. Severe symptoms, such as abdominal pain and diarrhea, were observed in cases with high antibody titer of B. pilosicoli and B. aalborgi, indicating that B. aalborgi could also cause symptomatic colitis.
References
- 1.Harland WA, Lee FD. Intestinal spirochaetosis. Br Med J. 1967;3:718–719. doi: 10.1136/bmj.3.5567.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korner M, Gebbers JO. Clinical significance of human intestinal spirochetosis--a morphologic approach. Infection. 2003;31:341–349. doi: 10.1007/s15010-003-3145-y. [DOI] [PubMed] [Google Scholar]
- 3.Smith JL. Colonic spirochetosis in animals and humans. J Food Prot. 2005;68:1525–1534. doi: 10.4315/0362-028x-68.7.1525. [DOI] [PubMed] [Google Scholar]
- 4.Kraaz W, Pettersson B, Thunberg U, Engstrand L, Fellstrom C. Brachyspira aalborgi infection diagnosed by culture and 16S ribosomal DNA sequencing using human colonic biopsy specimens. J Clin Microbiol. 2000;38:3555–3560. doi: 10.1128/jcm.38.10.3555-3560.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakamura S, Kuroda T, Sugai T, et al. The first reported case of intestinal spirochaetosis in Japan. Pathol Int. 1998;48:58–62. doi: 10.1111/j.1440-1827.1998.tb03829.x. [DOI] [PubMed] [Google Scholar]
- 6.Weisheit B, Bethke B, Stolte M. Human intestinal spirochetosis: analysis of the symptoms of 209 patients. Scand J Gastroenterol. 2007;42:1422–1427. doi: 10.1080/00365520701245629. [DOI] [PubMed] [Google Scholar]
- 7.Ogata S, Higashiyama M, Adachi Y, et al. Imprint cytology detects floating Brachyspira in human intestinal spirochetosis. Hum Pathol. 2010;41:249–254. doi: 10.1016/j.humpath.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 8.Abe Y, Hirane A, Yoshizawa A, Nakajima H, Adachi Y. The specific antibody to Brachyspira aalborgi in serum obtained from a patient with intestinal spirochetosis. J Vet Med Sci. 2006;68:1089–1091. doi: 10.1292/jvms.68.1089. [DOI] [PubMed] [Google Scholar]
- 9.Adachi Y, Tachibana H, Ogawa Y, Nakamura S. Isolation of the human intestinal spirochetes in patients with diarrhea resembling Brachyspira aalborgi. Proc Congr Int Pig Vet Soc. 2000;16:59. [Google Scholar]
- 10.Matsuyama T, Yasuike M, Fujiwara A, et al. A Spirochaete is suggested as the causative agent of Akoya oyster disease by metagenomic analysis. PLoS One. 2017;12:e0182280. doi: 10.1371/journal.pone.0182280. doi: 10.1371/journal.pone.0182280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kashiwazaki M, Adachi Y, Kume T. Microscopic agglutination test for antibody against Treponema hyodysenteriae. Natl Inst Anim Health Q (Tokyo). 1980;20:114–115. [PubMed] [Google Scholar]
- 12.Adachi Y, Kume T, Yamamoto S, Watase H. Comparison of antigenic properties among severe strains of weak beta-hemolytic treponeme isolated from swine and a dog. Zbl Bakt Hyg Abt Orig. 1980;A248:541–547. [PubMed] [Google Scholar]
- 13.Songar JG, Kinyon JM, Harris DL. Selective medium for isolation of Treponema hyodysenteriae. J Clin Microbiol. 1976;4:57–60. doi: 10.1128/jcm.4.1.57-60.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delladetsima K, Markaki S, Papadimitriou K, Antonakopoulos GN. Intestinal spirochaetosis. Light and electron microscopic study. Pathol Res Pract. 1987;182:780–782. doi: 10.1016/S0344-0338(87)80042-0. [DOI] [PubMed] [Google Scholar]
- 15.Jensen TK, Boye M, Ahrens P, et al. Diagnostic examination of human intestinal spirochetosis by fluorescent in situ hybridization for Brachyspira aalborgi, Brachyspira pilosicoli, and other species of the genus Brachyspira (Serpulina). J Clin Microbiol. 2001;39:4111–4118. doi: 10.1128/JCM.39.11.4111-4118.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwamoto J, Ogata S, Honda A, et al. Human intestinal spirochaetosis in two ulcerative colitis patients. Intern Med. 2014;53:2067–2071. doi: 10.2169/internalmedicine.53.2386. [DOI] [PubMed] [Google Scholar]
- 17.Umeno J, Matsumoto T, Nakamura S, et al. Intestinal spirochetosis due to Brachyspira pilosicoli: endoscopic and radiographic features. J Gastroenterol. 2007;42:253–256. doi: 10.1007/s00535-006-1988-6. [DOI] [PubMed] [Google Scholar]
- 18.Esteve M, Salas A, Fernandez-Bañares F, et al. Intestinal spirochetosis and chronic watery diarrhea: clinical and histological response to treatment and long-term follow up. J Gastroenterol Hepatol. 2006;21:1326–1333. doi: 10.1111/j.1440-1746.2006.04150.x. [DOI] [PubMed] [Google Scholar]
- 19.Takezawa T, Hayashi S, Adachi Y, et al. Human intestinal spirochetosis in an immunocompromised host: evaluation of eradication therapy by endoscopy, histopathology and bacteriology. Clin J Gastroenterol. 2012;5:69–73. doi: 10.1007/s12328-011-0265-2. [DOI] [PubMed] [Google Scholar]
- 20.Trivett-Moore NL, Gilbert GL, Law CL, Trott DJ, Hampson DJ. Isolation of Serpulina pilosicoli from rectal biopsy specimens showing evidence of intestinal spirochetosis. J Clin Microbiol. 1998;36:261–265. doi: 10.1128/jcm.36.1.261-265.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato H, Nakamura S, Habano W, Wakabayashi G, Adachi Y. Human intestinal spirochaetosis in northern Japan. J Med Microbiol. 2010;59:791–796. doi: 10.1099/jmm.0.017376-0. [DOI] [PubMed] [Google Scholar]
- 22.Takagi T, Naito Y, Inoue R, et al. The influence of long-term use of proton pump inhibitors on the gut microbiota: an age-sex-matched case-control study. J Clin Biochem Nutr. 2018;62:100–105. doi: 10.3164/jcbn.17-78. [DOI] [PMC free article] [PubMed] [Google Scholar]