Abstract
In Western society, couples increasingly delay parenthood until later in life. Overall, studies have focused on the reproductive performance of older parents or the impact of advanced maternal age on pregnancy outcomes, but few studies have examined how advanced paternal age (APA) affects offspring health. The aim of this study was to investigate the impact of increasing paternal age on offspring reproductive performance and long-term metabolic health in a mouse model. Here, the same adult B6D2F1/J male mice were mated at 4, 12, and 18 months of age with 6- to 10-week-old naturally cycling CF1 females to generate 3 offspring cohorts conceived at increasing paternal ages PA4, PA12, and PA18. The offspring resulting from mating the same fathers at different ages (n = 20 per age; 10 males and 10 females) were maintained up to 20 weeks of age and morphometric parameters, growth curve, and glucose tolerance were measured. We found that increasing paternal age was associated with a trend toward longer time to conception. Litter sizes were not significantly different. Reassuringly, metabolic parameters and growth curve were not different in the 3 cohorts of offspring. Most importantly, increased paternal age (PA4 vs PA18) was associated with a statistically significant decrease in sperm concentration, sperm motility, and anogenital distance in offspring. These changes raise concerns about the potential impact of APA on the reproductive fitness in males of the next generation.
Keywords: advanced paternal age, sperm, DOHaD
Introduction
As Western society has evolved in favor of career advancement and the pursuit of educational aims, couples increasingly delay parenthood until later in life. It is therefore of interest to ascertain the potential impact of advanced parental age on offspring health, metabolic parameters, and reproductive capabilities.
Overall, studies on the topic are limited and few human studies have explored the effects of older age on conception and offspring health. The majority of published analysis have focused on the reproductive performance of older parents or the impact of advanced maternal age on pregnancy outcomes (reviewed in the study by Jacobsson et al and Luke et al).1,2
Human studies indicate that older males display alterations in several reproductive parameters compared to younger males, including decreased androgen production3 and benign hypertrophy of the prostate gland.4 Testicular morphology and function decline with age, as does the quality of semen,5 while the incidence of sexual dysfunction increases with age.6 There is also evidence linking increased paternal age with diminished performance in assisted reproductive technology (ART) outcomes, but these results are more controversial.
The effect of advanced paternal age (APA) on offspring health has been studied to a lesser degree.7 Several studies have found an association between APA and psychiatric and academic morbidity8 or autism9,10 in offspring, as well as several other diseases such as cancer and a small group of mutation disorders collectively termed “paternal age effect disorders.”11–13
On the contrary, very few studies have studied the effects of APA on metabolic health or growth pattern of offspring. Some authors have described an association between APA and low birth weight in newborns7 and increased stature and altered serum lipid profiles.14
Animal studies highlight a relationship between APA and negative pregnancy outcomes. Natural conception using older male mice produces significantly smaller fetuses and placentae, with resulting offspring having decreased reproductive fitness and longevity, as well as altered behavioral traits.15,16 In addition, there is a trend toward lower implantation rates and increased pregnancy loss in rat offspring generated by older fathers17 or following embryo transfer of blastocysts generated in vitro using sperm from older males in mice.18
The finding of lower birth weight in offspring of older fathers has important implications since there is a well-established association between decreased birth weight and long-term metabolic complications, as set forth by the developmental origin of health and disease hypothesis.19
We therefore designed the present study to assess the metabolic health of offspring generated by fathers of increasing age. In particular, we analyzed whether APA, using the same male mice of increasing age, affects offspring growth and glucose tolerance, as well as sperm parameters. Reassuringly, increased paternal age did not predispose to consistent differences in growth kinetics or glucose tolerance. However and importantly, male offspring generated by older fathers had decreased sperm concentration, sperm motility, and reduced anogenital distance—factors known to affect fertility.
Materials and Methods
Animals
For mating, single male B6D2F1/J mice (F0) were housed with 6- to 10-week-old spontaneously ovulating CF1 female mice. Females were monitored daily and removed to a separate cage after detection of a copulatory plug, with the number of days cohoused recorded as the time to conception. The same 4 males (n = 4) were used for mating at 4, 12, and 18 months of age with young mothers (6-10 weeks of age), thus generating 3 cohorts of offspring generated by each father at different ages (each sire generated 1 litter per time point, PA4, PA12, PA18 groups).
To reduce variation and increase the sensitivity of statistical analyses of treatment,20 pups (F1) were randomly culled to a litter size of 7 to 9 at birth with no attempt to selectively cull sick or underweight pups. Additional culling was performed if necessary at weaning to reduce the total number of mice to 20 per cohort (10 per sex). Body weight, body length, and anogenital distance were measured at birth, and body weight was subsequently measured weekly until the end of the experiment. Organ weights were measured immediately following euthanization of the animals at 20 weeks of age.
All experiments were approved by the Institutional Animal Care and Use Committee of the University of California San Francisco. Animals were provided nesting material and housed in cages maintained under constant 12 hours light/dark cycle at 21°C to 23°C, with ad libitum access to water and standard chow (Pico-lab diet #5058: 23% protein; 22% fat, and 55% carbohydrate).
Intraperitoneal Glucose Tolerance Test and Insulin Measurement
Glucose homeostasis was investigated via intraperitoneal glucose tolerance testing (IPGTT) at 19 weeks of age as previously described.21,22 Animals were fasted 6 hours before receiving an intraperitoneal glucose bolus of 2 mg/g body weight, and both glucose and insulin levels were measured at 0 (fasting, before glucose injection), 15, 30, 60, and 120 minutes. To determine insulin levels, 5 to 10 μL of serum were assayed using an ultrasensitive insulin ELISA kit (Alpco, Salem, New Hampshire), as previously described.21,22
Sperm Analysis
Sperm concentration and motility were measured in cauda epididymal sperm collected from euthanized male offspring aged 20 weeks as described.23,24 Briefly, the caudal region of the epididymis was dissected out of the scrotum, washed in human tubal fluid medium (Specialty Media, Phillipsburg, New Jersey), and transferred to a culture dish containing 500 μL of 20 mM Tris–HCl, 130 mM NaCl, and 1 mM EDTA (pH 7.5). Several incisions were made in the cauda epididymis to allow sperm dispersal. Motility was assessed after a 30-minute incubation at 37°C in humidified air and 5% CO2. Sperm concentration (million/milliliter) was evaluated using a hemocytometer on spermatozoa that were washed, centrifuged, and resuspended in the same medium.23
Androgen Assay
Blood was collected via heart puncture in euthanized 20-week-old animals. Serum was immediately separated, snap frozen, and stored at −20°C until use. Testosterone levels were measured in 25 µL serum specimens using a mouse testosterone ELISA kit (Calbiotech Inc, Spring Valley, California), according to the manufacturer’s instructions.
Quantitative Real-Time Polymerase Chain Reaction
Expression of candidate genes associated with impaired spermatogenesis or male factor infertility18 was analyzed in semen from all male offspring per cohort at the time of sacrifice. Semen were passed through a 20-G hypodermic needle in 1 mL TRIzol reagent several times to facilitate RNA extraction. RNA was isolated via phase separation with the addition of 0.2 mL chloroform. Total RNA was then processed and purified using an RNeasy Mini Kit (Qiagen) and measured by NanoDrop spectrophotometry (Waltham, MA). Conversion of RNA to complementary DNA (cDNA) was performed using a commercially available first strand cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA) Expression of the genes protamine 1 and 2 (Prm1, Prm2), angiotensin-converting enzyme (AceV1), and sperm mitochondria-associated cysteine-rich protein (Smcp) was evaluated using previously reported primers.18 Glucose transporter 3 (Glut3) expression was measured with primers designed using PerlPrimer software: forward, CTCTTCAGGTCACCCAACTACGT; reverse, CCGCGTCCTTGAAGATTCC.25 Beta-actin was used as a reference gene: forward, AAGGCCAACCGTGAAAAGAT; reverse: GTGGTACGACCAGAGGCATAC.26 Gene expression was quantified using SYBR green PCR mix and analyzed via the log linear phase of the amplification curve using comparative threshold values.
Statistical Analysis
All data are presented as the mean (standard deviation [SD]) unless otherwise specified. To detect any differences among groups, a one-way analysis of variance (ANOVA) was used. If a 1-way ANOVA was significant, Tukey post hoc correction was applied to test for differences between groups. Linear regression analysis was performed to detect significant correlations with paternal age. All statistics were calculated using Prism software package (Graphpad, San Diego, California). A P value of <.05 was considered significant. Unless specifically reported, each mouse cohort was composed of 20 animals (10 per sex).
Results
Decreased Fecundity With APA
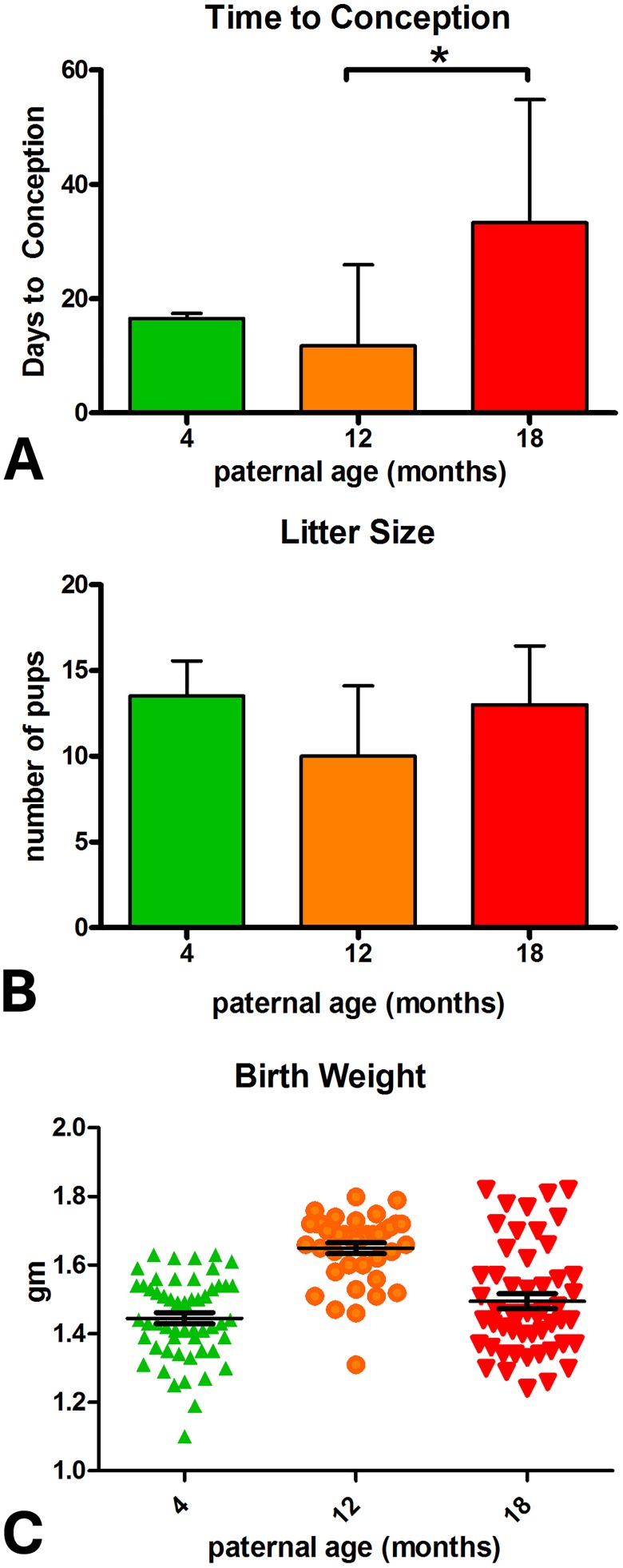
Increased paternal age was associated with greater time to conception, with 18-month-old males requiring significantly longer to produce litters than when they were 12 months of age (Figure 1A).
Figure 1.
Effect of paternal age on pregnancy and litter characteristics. A, Time to conception. B, Litter size. C, Birth weight (PA4: n = 54 [4], PA12: n = 40 [4], PA18: n = 52 [4]).
Effect of Paternal Age on Offspring Growth, Glucose Metabolism, and Organ Weight
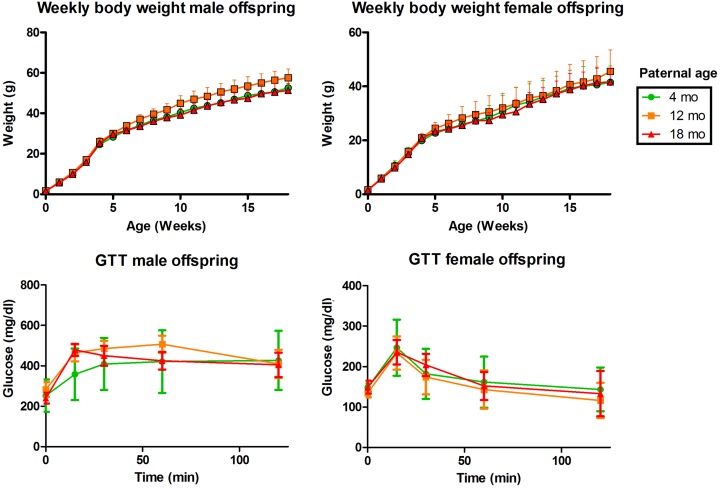
There was no effect of paternal age on litter size (Figure 1B). Birth weight was significantly higher in the PA12 group compared to PA4 or PA18 (Figure 1C). There was no difference between male and female weights, crown–rump length, or anogenital distance at birth for any PA group. Body weight did not differ between offspring of the various paternal age groups over the 20 weeks, with the exception of PA4 F1 females at 10 and 15 weeks (Figure 2A and B).
Figure 2.
Offspring growth and glucose metabolism. A, Body weight, males. B, Body weight, females. C, Glucose levels during the glucose tolerance tests, males. D, Glucose levels during the glucose tolerance test, females. *PA12 F1 were larger than those in the PA4 or PA18 groups.
Glucose levels at each time point (Figure 2C and D) and the area under the curve (not shown) quantified during the IPGTT were not different in either male or female offspring generated by fathers of different ages. Insulin levels were also not affected by paternal age, although PA12 females had slightly higher insulin levels compared to offspring from other groups (Supplemental Figure S1).
There were few differences in organ weights. Notably, adrenal and pancreatic weights were similar among all the offspring examined. The ovaries of the PA18 daughters were significantly larger than those in either the PA4 or PA12 groups (P < .05) but were similar in gross morphology to the other groups.
In males, PA18 gonadal fat weight was significantly less than either PA4 or PA12 (P < .05), the trend for smaller testis in PA12 and PA18 did not reach significance, and the liver weighed less in PA18 males compared to PA12 (but not PA4; Supplemental Table S1).
Sperm Parameters and Testosterone Levels of Adult Male Offspring
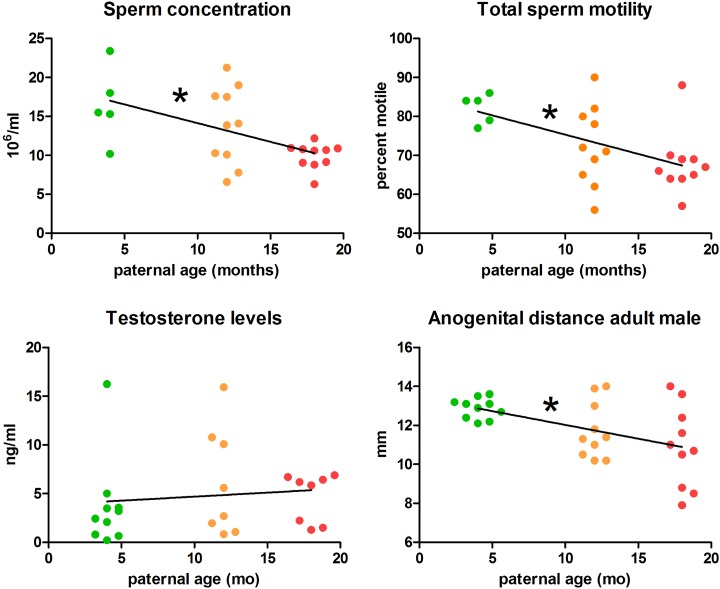
Increasing paternal age was associated with decreased sperm concentration and total motility in the offspring. Progressive motility was not so clearly affected by age, with sperm from the PA18 F1 cohort having lower motility than the PA12, but not PA4 group (Figure 3A and B). Male offspring produced by older sires additionally had a shorter anogenital distance in adulthood (Figure 3D). However, testis weight and androgen levels were similar among all F1 males, regardless of the age of their fathers (Figure 3D, Table 1).
Figure 3.
Reproductive physiology of adult male offspring. A, Sperm concentration. B, Total sperm motility. C, Testosterone levels (ng/mL). D, Anogenital distance measured at sacrifice. Slopes designated “*” are significantly different.
Table 1.
Testosterone Levels in F1 Males at 20 Weeks of Age.a
| Paternal Age (months) | 4 | 12 | 18 |
|---|---|---|---|
| Serum testosterone (ng/mL) | 3.76 (4.64) | 7.36 ± (7.42) | 3.94 ± (2.89) |
| Testis weight (mg) | 131.6 ± (12.2) | 114.7 ± (18.3) | 121.0 ± (14.8) |
an = 10 per group.
No Change in the Expression of Selected Genes in Offspring Spermatozoa
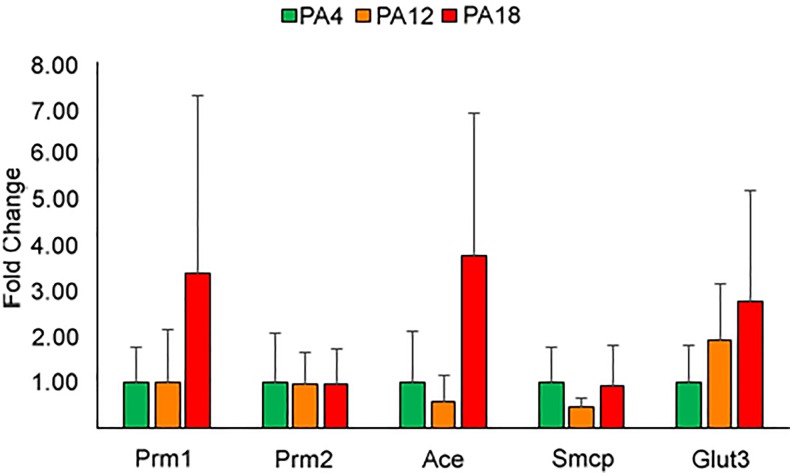
Based on the observed changes in sperm parameters, the expression of 5 genes involved in glucose transport and sperm physiology was evaluated in offspring sperm. No differences in gene expression were noted in the genes examined (Figure 4).
Figure 4.
Relative expression levels of sperm transcripts. The expression of genes Prm1, Prm2, Ace, Smcp, and Glut 3 was tested in offspring sperm and found not to be different in any of the cohorts examined. Bars represent standard deviation.
Discussion
This study was designed to examine the impact of APA on offspring growth, metabolism, and sperm parameters. To our knowledge, this is the first report aimed at assessing growth curve and glucose tolerance in offspring generated by fathers of increased age.
The first important finding is that somatic growth and glucose tolerance were similar between offspring generated by the same fathers at increasing age. This is particularly noteworthy in light of the current tendency to postpone pregnancy in our society.27 Furthermore, because developmental studies often reveal sexually dimorphic phenotypes,28 it is encouraging that both male and female F1 mice displayed normal growth parameters. Our results are particularly reassuring, given that other authors have found that male offspring generated by fathers older than 18 months generate significantly smaller fetuses.18 Lower birth weight is a well-documented marker of impaired metabolic health in adulthood, as established by the developmental origins of health and disease hypothesis.19
The 3 cohorts of mice examined in this study differed in very few parameters of somatic growth: PA12 offspring were larger at birth only, gonadal fat was significantly reduced in PA18 males compared to that of either PA4 or PA12 males, and liver weight in PA18 males was smaller than PA12 (but not PA4). In the present study, the ovaries from PA18 daughters were significantly larger but exhibited gross morphology similar to those of the PA4 and PA12 groups. It is unclear why ovarian weight increased with APA: These ovaries were not obviously polycystic. One possibility is that the increased ovarian mass may occur secondary to stroma hypertrophy, which has been associated with hyperandrogenism.29 Given that no other growth or metabolic parameters were affected between the groups, we cannot ascribe any clear biological significance to these findings.
A second major novel finding of this study is that sperm concentration and motility were diminished in the offspring generated by older fathers. The decline in total motility was linear over the different paternal ages investigated, but progressive motility appears to have been less affected. Although a decline in semen quality has been well-documented in older fathers/sires themselves,7 this has not previously been reported in the resulting offspring.
One related observation and possible explanation is that the anogenital distance measured at the time of sacrifice was significantly shorter in male offspring generated by older fathers (Figure 3D). There was no difference among female offspring. Of note, anogenital distance in adult men has been shown to be the strongest predictor of sperm count.30 Adult anogenital distance is generally believed to reflect the degree of androgen exposure in utero,31 with higher androgen levels associated with longer anogenital distance. However, evidence is emerging that the perineum is hormonally sensitive both perinatally and during puberty.32,33 Since there were no differences in anogenital distance at birth in the F1 males with older fathers, it is likely these animals experienced different androgenic exposures after puberty. This might suggest that offspring of older fathers would have had smaller testes and lower testosterone levels; however, testicular weight and testosterone levels measured at 20 weeks of age did not differ among the groups. There are several possible explanations for this result. Changes in testosterone may have been transient, occurring only during puberty. It is also possible that changes in the levels of other androgens or of the ratio between androgen(s) and estrogen were responsible for the altered anogenital distance. Finally, there could have been changes in 5α-reductase or in androgen receptors levels in the target tissues.
Analysis of the data in our aging cohort of fathers suggests that the pattern of decline of anogenital distance, sperm concentration, and sperm motility in offspring display a continuous downward slope rather than an age-related threshold effect. If this is confirmed, these data would indicate that there is not a specific time when a male mouse becomes an “old” sire, but instead that there is a growing negative effect of age. Indeed, older men show a continuous decrease in semen parameters with advancing age. Interestingly, reproductive outcome of older men using ART suggests that APA is associated with decreased fertilization, blastocyst formation, and pregnancy rates, as well as a greater risk of pregnancy loss and a lower live birth rate.7,34–41
However, we are not aware of human data monitoring semen parameters in the offspring of older fathers.42 A particularly relevant animal study showed that paternal age (up to 120 weeks) did not affect resulting male offspring (F1) reproductive performance or subsequent litter sizes.15 Female offspring from 30-month-old fathers displayed reduced reproductive potential with interbirth intervals longer than females of younger groups.
An important question to be addressed in future studies is to assess whether the decline in sperm parameters is associated with a decline in fertility in the offspring. Although we did not assess fertility in offspring (sperm data were obtained at sacrifice), we indirectly assessed sperm function by analyzing the expression of selected genes known to affect sperm physiology and competence. Prm1 and Prm2 are involved in DNA packaging,43 Ace is involved in binding of the spermatozoa to the zona,44 while Smcp affects motility.45 We also tested the expression of the facilitated glucose transporter Glut3, as glucose plays an important role in semen transport. In fact, decrease in sperm motility in offspring of diabetic sires is correlated with a decrease in Glut3.46 However, sperm from all F1 males, regardless of paternal age, did not show any alterations in messenger RNA expression of the testicular genes tested.
In order to better understand the mechanism of decline of semen quality in offspring of older fathers, it will be necessary to obtain a more detailed picture of endocrine profiles and testicular histology in these mice, especially at puberty. It might be also valuable to test spermatozoa for additional genes involved in sperm production and function or to analyze global gene expression.11
It is interesting to speculate on the potential mechanism(s) by which increasing paternal age might affect sperm count in offspring. It is known that sperm from older males harbor a greater number of point mutations in their DNA.47 Data from the 1000 genome project demonstrate that a significant percentage (∼75%) of the novo mutations are paternally derived and play an important role in autism spectrum disorders.48 Moreover, offspring of older fathers show an increase in the rate of de novo mutations in sperm.12
According to the “selfish spermatogonial selection” hypothesis, older males (with aging spermatogonia) have an increased number of spermatogonial clones with mutations that confer a mitotic advantage.13 The result is that the testis is populated by a relative abundance of mutated spermatogonia, which could explain the greater incidence of autism and neuropsychiatric disorders in the children of older fathers.10,12 Furthermore, sperm originating from older fathers might have a reduced ability to recover from oxidative damage: Defects in oxidative repair can impair sperm motility, DNA compaction, and maturation, leading to poor reproductive outcomes as shown in the peroxiredoxin 6 null mouse.49 Alternatively, the effects of APA could be secondary to epigenetic alterations being more common in sperm of older fathers.50 Indeed, altered DNA methylation48 or altered microRNA,51 which could be passed on to subsequent generations and affect offspring phenotype, have been observed in males exposed to different stress.52
This study has several strengths. First, the same fathers were used to generate all offspring cohorts, thereby controlling for interindividual paternal variation. We also performed extensive phenotyping in the offspring.
Among the limitations to this study, we documented paternal age effects up to 18 months (mice leave up to 24-36 months), and it is possible that additional effects exist when fathers age further. Similarly, offspring were monitored only up to 20 weeks of age, and it is possible that metabolic or growth alterations might occur at an older age. We also focused on male reproductive parameters and have not done extensive analysis of female ovulatory function and fertility. Future experiments should be designed with these caveats in mind.
In conclusion, this study is reassuring from a metabolic perspective, as the offspring of older fathers did not demonstrate any obvious abnormalities related to growth or glucose tolerance. However, the decline in anogenital distance and the decreased sperm concentration and motility observed with advancing paternal age are important findings and will be interesting areas of future investigation.
Supplemental Material
Supplemental Material, Supplementary_Table_S1A,B_Fig_S1 for Advanced Paternal Age Affects Sperm Count and Anogenital Distance in Mouse Offspring by Pedro Caballero-Campo, Wingka Lin, Rhodel Simbulan, Xiaowei Liu, Sky Feuer, Annemarie Donjacour, and Paolo F. Rinaudo in Reproductive Sciences
Footnotes
Authors’ Note: P. Caballero-Campo and W. Lin contributed equally.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Supported in part by RO1: HD 062803 - 01A1 to PFR, and Premio-Cátedra Dr Salvador Zubirán, México and Fundacion Tambre, Madrid, Spain to PCC.
Supplemental Material: Supplementary material is available for this article online.
References
- 1. Jacobsson B, Ladfors L, Milsom I. Advanced maternal age and adverse perinatal outcome. Obstet Gynecol. 2004;104(4):727–733. [DOI] [PubMed] [Google Scholar]
- 2. Luke B, Brown MB, Grainger DA, Stern JE, Klein N, Cedars MI; SART Writing Group. The effect of early fetal losses on singleton assisted-conception pregnancy outcomes. Fertil Steril. 2009;91(6):2578–2585. [DOI] [PubMed] [Google Scholar]
- 3. Mahmoud AM, Goemaere S, El-Garem Y, Van Pottelbergh I, Comhaire FH, Kaufman JM. Testicular volume in relation to hormonal indices of gonadal function in community-dwelling elderly men. J Clin Endocrinol Metab. 2003;88(1):179–184. [DOI] [PubMed] [Google Scholar]
- 4. Zenzmaier C, Untergasser G, Berger P. Aging of the prostate epithelial stem/progenitor cell. Exp Gerontol, 2008;43(11):981–985. [DOI] [PubMed] [Google Scholar]
- 5. Johnson SL, Dunleavy J, Gemmell NJ, Nakagawa S. Consistent age-dependent declines in human semen quality: a systematic review and meta-analysis. Ageing Res Rev. 2015;19:22–33. [DOI] [PubMed] [Google Scholar]
- 6. Johannes CB, Araujo AB, Feldman HA, Derby CA, Kleinman KP, McKinlay JB. Incidence of erectile dysfunction in men 40 to 69 years old: longitudinal results from the Massachusetts male aging study. J Urol. 2000;163(2):460–463. [PubMed] [Google Scholar]
- 7. Kovac JR, Addai J, Smith RP, Coward RM, Lamb DJ, Lipshultz L. The effects of advanced paternal age on fertility. Asian J Androl. 2013;15(6):723–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. D’Onofrio BM, Rickert ME, Frans E, et al. Paternal age at childbearing and offspring psychiatric and academic morbidity. JAMA Psychiatry, 2014;71(4):432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tsuchiya KJ, Matsumoto K, Miyachi T, et al. Paternal age at birth and high-functioning autistic-spectrum disorder in offspring. Br J Psychiatry. 2008;193(4):316–321. [DOI] [PubMed] [Google Scholar]
- 10. Sandin S, Schendel D, Magnusson P, et al. Autism risk associated with parental age and with increasing difference in age between the parents. Mol Psychiatry. 2016;21(5):693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garrido N, Garcia-Herrero S, Meseguer M. Assessment of sperm using mRNA microarray technology. Fertil Steril. 2013;99(4):1008–1022. [DOI] [PubMed] [Google Scholar]
- 12. Kong A, Frigge ML, Masson G, et al. Rate of de novo mutations and the importance of father’s age to disease risk. Nature. 2012;488(7412):471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goriely A, Wilkie AO. Paternal age effect mutations and selfish spermatogonial selection: causes and consequences for human disease. Am J Hum Genet. 2012;90(2):175–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Savage T, Derraik JG, Miles HL, Mouat F, Hofman PL, Cutfield WS. Increasing paternal age at childbirth is associated with taller stature and less favourable lipid profiles in their children. Clin Endocrinol (Oxf). 2014;80(2):253–260. [DOI] [PubMed] [Google Scholar]
- 15. Garcia-Palomares S, Navarro S, Pertusa JF, et al. Delayed fatherhood in mice decreases reproductive fitness and longevity of offspring. Biol Reprod. 2009;80(2):343–349. [DOI] [PubMed] [Google Scholar]
- 16. Garcia-Palomares S, Pertusa JF, Miñarro J, et al. Long-term effects of delayed fatherhood in mice on postnatal development and behavioral traits of offspring. Biol Reprod. 2009;80(2):337–342. [DOI] [PubMed] [Google Scholar]
- 17. Serre V, Robaire B. Paternal age affects fertility and progeny outcome in the Brown Norway rat. Fertil Steril. 1998;70(4):625–631. [DOI] [PubMed] [Google Scholar]
- 18. Katz-Jaffe MG, Parks J, McCallie B, Schoolcraft WB. Aging sperm negatively impacts in vivo and in vitro reproduction: a longitudinal murine study. Fertil Steril. 2013;100(1):262–268.e1-e2. [DOI] [PubMed] [Google Scholar]
- 19. Barker DJ. Mothers, Babies And Health In Later Life. 2nd ed Glasgow, Scotland: Churchill Livingstone; 1998. [Google Scholar]
- 20. Agnish ND, Keller KA. The rationale for culling of rodent litters. Fundam Appl Toxicol. 1997;38(1):2–6. [DOI] [PubMed] [Google Scholar]
- 21. Donjacour A, Liu X, Lin W, Simbulan R, Rinaudo PF. In vitro fertilization affects growth and glucose metabolism in a sex-specific manner in an outbred mouse model. Biol Reprod. 2014;90(4):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Feuer SK, Liu X, Donjacour A, et al. Use of a mouse in vitro fertilization model to understand the developmental origins of health and disease hypothesis. Endocrinology. 2014;155(5):1956–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Caballero-Campo P, Buffone MG, Benencia F, et al. A role for the chemokine receptor CCR6 in mammalian sperm motility and chemotaxis. J Cell Physiol. 2014;229(1):68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Travis AJ, Foster JA, Rosenbaum NA, et al. Targeting of a germ cell-specific type 1 hexokinase lacking a porin-binding domain to the mitochondria as well as to the head and fibrous sheath of murine spermatozoa. Mol Biol Cell. 1998;9(2):263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bloise E, Lin W, Liu X, et al. Impaired placental nutrient transport in mice generated by in vitro fertilization. Endocrinology. 2012;153(7):3457–3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Estomba H, Muñoa-Hoyos I, Gianzo M, et al. Expression and localization of opioid receptors in male germ cells and the implication for mouse spermatogenesis. PLoS One. 2016;11(3):e0152162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maheshwari A, Porter M, Shetty A, Bhattacharya S. Women’s awareness and perceptions of delay in childbearing. Fertil Steril. 2008;90(4):1036–1042. [DOI] [PubMed] [Google Scholar]
- 28. Rinaudo P, Wang E. Fetal programming and metabolic syndrome. Annu Rev Physiol. 2012;74:107–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dewailly D, Robert Y, Helin I, et al. Ovarian stromal hypertrophy in hyperandrogenic women. Clin Endocrinol (Oxf). 1994;41(5):557–562. [DOI] [PubMed] [Google Scholar]
- 30. Eisenberg ML, Hsieh MH, Walters RC, Krasnow R, Lipshultz LI. The relationship between anogenital distance, fatherhood, and fertility in adult men. PLoS One. 2011;6(5):e18973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van den Driesche S, Scott HM, MacLeod DJ, Fisken M, Walker M, Sharpe RM. Relative importance of prenatal and postnatal androgen action in determining growth of the penis and anogenital distance in the rat before, during and after puberty. Int J Androl. 2011;34(6 pt 2):e578–e586. [DOI] [PubMed] [Google Scholar]
- 32. Moody S, Goh H, Bielanowicz A, Rippon P, Loveland KL, Itman C. Prepubertal mouse testis growth and maturation and androgen production are acutely sensitive to di-n-butyl phthalate. Endocrinology. 2013;154(9):3460–3475. [DOI] [PubMed] [Google Scholar]
- 33. Mitchell RT, Mungall W, McKinnell C, et al. Anogenital distance plasticity in adulthood: implications for its use as a biomarker of fetal androgen action. Endocrinology. 2015;156(1):24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhu JL, Madsen KM, Vestergaard M, Basso O, Olsen J. Paternal age and preterm birth. Epidemiology. 2005;16(2):259–262. [DOI] [PubMed] [Google Scholar]
- 35. Astolfi P, De Pasquale A, Zonta L. Late childbearing and its impact on adverse pregnancy outcome: stillbirth, preterm delivery and low birth weight. Rev Epidemiol Sante Publique. 2005;53(spec no 2):2S97–2S105. [PubMed] [Google Scholar]
- 36. Dain L, Auslander R, Dirnfeld M. The effect of paternal age on assisted reproduction outcome. Fertil Steril. 2011;95(1):1–8. [DOI] [PubMed] [Google Scholar]
- 37. Bellver J, Garrido N, Remohí J, Pellicer A, Meseguer M. Influence of paternal age on assisted reproduction outcome. Reprod Biomed Online. 2008;17(5):595–604. [DOI] [PubMed] [Google Scholar]
- 38. Frattarelli JL, Miller KA, Miller BT, Elkind-Hirsch K, Scott RT., Jr Male age negatively impacts embryo development and reproductive outcome in donor oocyte assisted reproductive technology cycles. Fertil Steril. 2008;90(1):97–103. [DOI] [PubMed] [Google Scholar]
- 39. Klonoff-Cohen HS, Natarajan L. The effect of advancing paternal age on pregnancy and live birth rates in couples undergoing in vitro fertilization or gamete intrafallopian transfer. Am J Obstet Gynecol. 2004;191(2):507–514. [DOI] [PubMed] [Google Scholar]
- 40. Robertshaw I, Khoury J, Abdallah ME, Warikoo P, Hofmann GE. The effect of paternal age on outcome in assisted reproductive technology using the ovum donation model. Reprod Sci. 2014;21(5):590–593. [DOI] [PubMed] [Google Scholar]
- 41. Luna M, Finkler E, Barritt J, et al. Paternal age and assisted reproductive technology outcome in ovum recipients. Fertil Steril. 2009;92(5):1772–1775. [DOI] [PubMed] [Google Scholar]
- 42. Gunes S, Hekim GN, Arslan MA, Asci R. Effects of aging on the male reproductive system. J Assist Reprod Genet. 2016;33(4):441–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jodar M, Oriola J, Mestre G, et al. Polymorphisms, haplotypes and mutations in the protamine 1 and 2 genes. Int J Androl. 2011;34(5 pt 1):470–485. [DOI] [PubMed] [Google Scholar]
- 44. Hagaman JR, Moyer JS, Bachman ES, et al. Angiotensin-converting enzyme and male fertility. Proc Natl Acad Sci U S A. 1998;95(5):2552–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nayernia K, Adham IM, Burkhardt-Göttges E, et al. Asthenozoospermia in mice with targeted deletion of the sperm mitochondrion-associated cysteine-rich protein (Smcp) gene. Mol Cell Biol. 2002;22(9):3046–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kim ST, Moley KH. Paternal effect on embryo quality in diabetic mice is related to poor sperm quality and associated with decreased glucose transporter expression. Reproduction. 2008;136(3):313–322. [DOI] [PubMed] [Google Scholar]
- 47. Singh NP, Muller CH, Berger RE. Effects of age on DNA double-strand breaks and apoptosis in human sperm. Fertil Steril. 2003;80(6):1420–1430. [DOI] [PubMed] [Google Scholar]
- 48. Yuen RK, Merico D, Cao H, et al. Genome-wide characteristics of de novo mutations in autism. NPJ Genom Med. 2016;1:160271–1602710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ozkosem B, Feinstein SI, Fisher AB, O’Flaherty C. Advancing age increases sperm chromatin damage and impairs fertility in peroxiredoxin 6 null mice. Redox Biol. 2015;5:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shea JM, Serra RW, Carone BR, et al. Genetic and epigenetic variation, but not diet, shape the sperm methylome. Dev Cell. 2015;35(6):750–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Morgan CP, Bale TL. Early prenatal stress epigenetically programs dysmasculinization in second-generation offspring via the paternal lineage. J Neurosci. 2011;31(33):11748–11755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rodgers AB, Morgan CP, Leu NA, Bale TL. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc Natl Acad Sci U S A. 2015;112(44):13699–13704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Supplementary_Table_S1A,B_Fig_S1 for Advanced Paternal Age Affects Sperm Count and Anogenital Distance in Mouse Offspring by Pedro Caballero-Campo, Wingka Lin, Rhodel Simbulan, Xiaowei Liu, Sky Feuer, Annemarie Donjacour, and Paolo F. Rinaudo in Reproductive Sciences