Abstract
Herein, the synthesis and pharmacological characterization of an extended library of differently substituted N-methyl-14-O-methylmorphinans with natural and unnatural amino acids and three dipeptides at position 6 that emerged as potent μ/δ opioid receptor (MOR/DOR) agonists with peripheral antinociceptive efficacy is reported. The current study adds significant value to our initial structure–activity relationships on a series of zwitterionic analogues of 1 (14-O-methyloxymorphone) by targeting additional amino acid residues. The new derivatives showed high binding and potent agonism at MOR and DOR in vitro. In vivo, the new 6-amino acid- and 6-dipeptide-substituted derivatives of 1 were highly effective in inducing antinociception in the writhing test in mice after subcutaneous administration, which was antagonized by naloxone methiodide demonstrating activation of peripheral opioid receptors. Such peripheral opioid analgesics may represent alternatives to presently available drugs for a safer pain therapy.
Introduction
Adequate treatment of acute and chronic severe pain remains a global medical and socioeconomical challenge at the beginning of the third millennium.1 Current analgesics are either ineffective in a large proportion of patients or associated with significant adverse effects.1,2 Effective pain relief can be achieved with opioid analgesics, but undesirable side effects following acute administration (i.e., respiratory depression, constipation, sedation, dizziness, nausea) and prolonged use (i.e., tolerance, dependence, abuse liability) limit their clinical usefulness.3−5 In the last years, the huge rise in prescription opioids resulted in increased opioid-related deaths, and consequently a substantial public concern.5,6
Opioid receptors have key functions in modulating pain and other behavioral responses.7−14 They are G protein-coupled receptors with seven transmembrane helixes7−9 and are localized in the central and peripheral nervous systems (CNS and PNS).10−12 Activation of μ (MOR), δ (DOR), and κ (KOR) opioid receptors mediates analgesic effects of opioids.7,8 Currently, most opioids used in clinical practice are agonists at MOR causing severe CNS (i.e., respiratory depression, sedation, tolerance) and intestinal (i.e., constipation) side effects, and are mostly misused and abused.3−5,14 The imperative need for safer pain medications continues to drive the search for new lead molecules. Furthermore, the complexity of pain syndromes requires tailored pharmacological interventions and efficient drugs to fully control pain.15,16 Diverse strategies in designing better opioid analgesics are considered, including targeting peripheral opioid receptors,1,7,17−19 ligands acting at multiple opioid receptors,20,21 G protein-biased agonism,1,22−24 and abuse-deterrent formulations of existing opioids.1,25,26
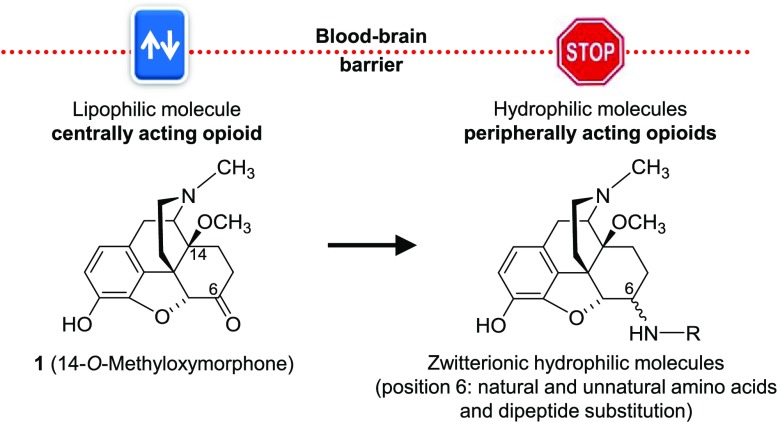
Targeting peripheral opioid receptors as effective means of treating pain and avoiding the CNS-mediated side effects has been a research area of substantial and continuous attention in the past years.1,7,17,18 Preclinical and clinical studies have established that opioid agonists that are not able to enter the CNS produce analgesia by activating opioid receptors in the periphery in a variety of pain conditions with a more favorable side effect profile. Increasing the hydrophilicity of opioids to limit their access to the CNS and thus to minimize the incidence of undesirable CNS effects includes diverse chemical alterations.17,18,27−30 Earlier works to generate peripheral opioids targeted the quaternization of the nitrogen in morphine, oxymorphone, and naloxone.27,31 Such quaternary compounds have decreased BBB permeability, while they have low binding affinity to opioid receptors. Quaternization also diminishes in vivo activity; therefore, alternative strategies were pursued. Polar or ionizable substituents are able to increase polarity and inhibit the cross of the blood–brain barrier (BBB). Morphinans having hydrophilic groups attached to the C6 position were prepared from β-oxymorphamine,32 β-naltrexamine,32 β-funaltrexamine,33 and 14-O-methylmorphine.34,35 The emerged molecules had reduced ability to enter the CNS, without substantially decreased in vitro and in vivo opioid activity. Small molecules, such as 5-pyrrolidinylquinoxalines, were also designed with limited BBB penetration and peripheral antinociceptive effects in animal models.36 Peripheral restriction was also achieved with peptidic agonists that produce analgesia by activating MOR or KOR in the periphery.17,18,37,38 By computer simulations at low pH, a fluorinated fentanyl analogue was designed, and it has been recently reported that, in contrast to fentanyl, it activates specifically MOR in acidified peripheral tissues to produce antinociception and lacks the typical opioid side effects in animals.39,40 Another approach to prevent BBB penetration includes a polyglycerol–morphine conjugate eliciting analgesia in inflamed tissues via selective activation of peripheral opioid receptors.41
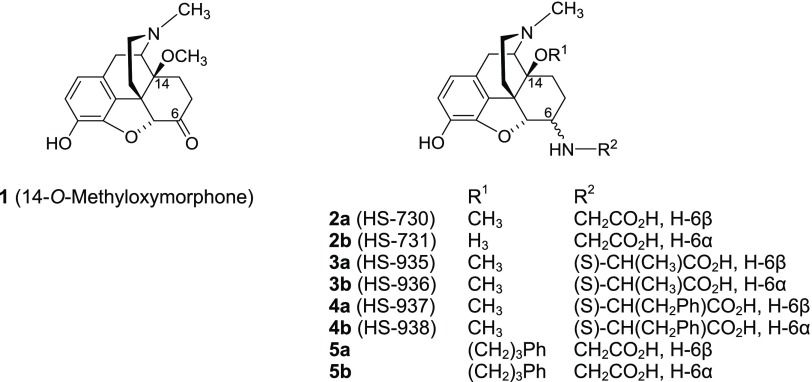
Previous studies from our laboratory in the field of peripheral opioid agonists targeted the attachment of amino acid residues at C6 position to the centrally acting MOR agonist 14-O-methyloxymorphone (1, Figure 1).42 The first series of 6-amino acid-substituted derivatives (i.e., Gly, l-Ala, and l-Phe, compounds 2–5, Figure 1),43−50 as zwitterionic molecules, displayed high affinities at MOR and potent agonism, were more hydrophilic than 1, and therefore restricted BBB penetration. They were very effective as antinociceptive agents in several pain models in rodents after systemic subcutaneous (sc), intraperitoneal (ip), oral, and local (intraplantar) administration by activating peripheral opioid receptors.45−50
Figure 1.
Structures of compound 1 and previous 6-amino acid-substituted derivatives 2–5. Ph, phenyl.
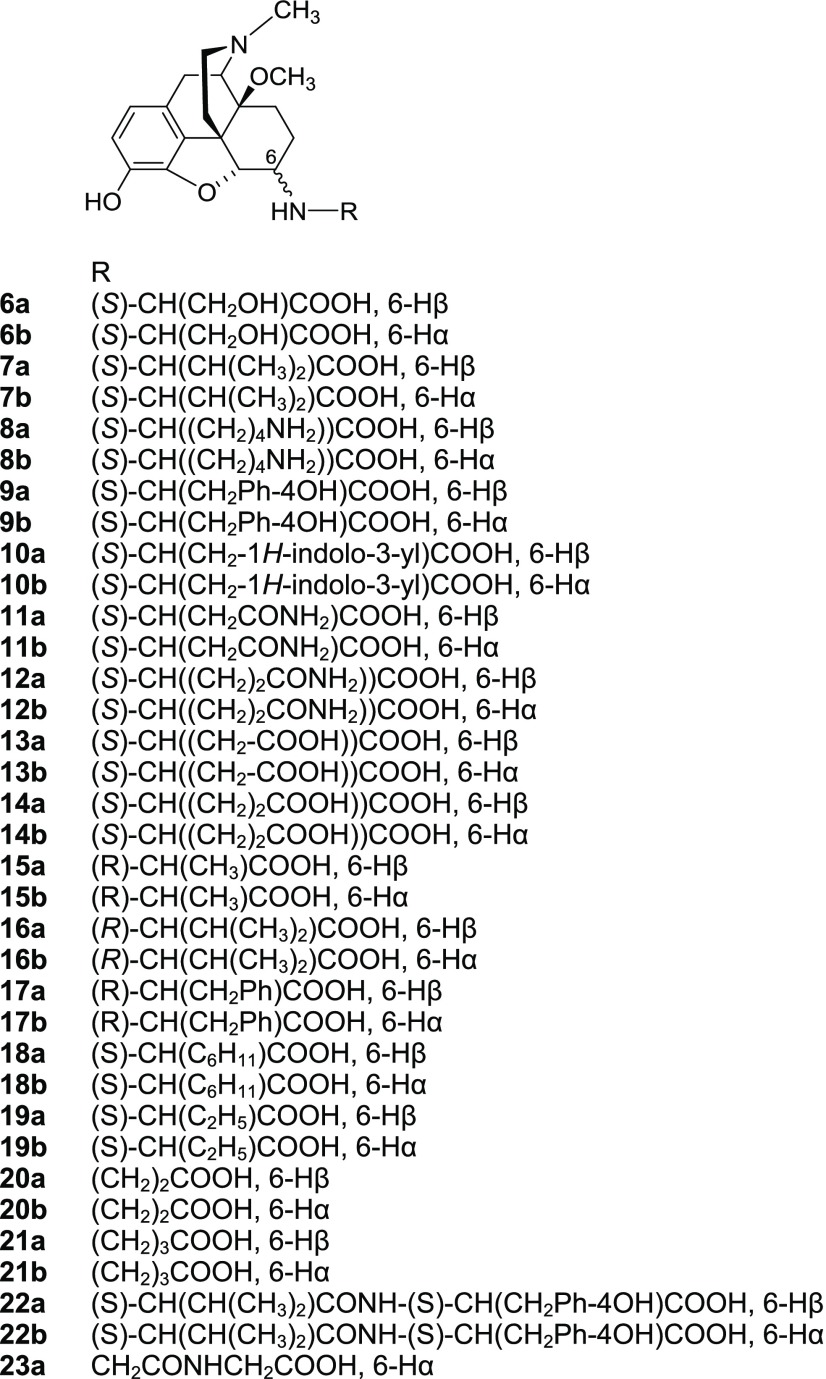
In the present study, we targeted additional derivatization of compound 1 through introduction of a number of amino acid residues of the l- and/or d-series at position 6, including natural amino acids, i.e., Ser, Val, Lys, Tyr, Trp, Asn, Gln, Asp, and Glu (6–14, Figure 2), unnatural amino acids, i.e., d-Ala, d-Val, d-Phe, l-Chg (l-cyclohexylglycine), l-Abu (l-2-aminobutyric acid), β-Ala, and GABA (γ-aminobutyric acid) (15–21, Figure 2), as well as three dipeptides (22a/b and 23a, Figure 2). Herein, we report on the synthesis, pharmacological characterization, and peripheral antinociceptive efficacy of novel 6-amino acid- and 6-dipeptide-substituted derivatives of 1, and emerged structure–activity relationships (SAR).
Figure 2.
Structures of new 6-amino acid (6–21)- and 6-dipeptide-substituted derivatives (22a/b and 23a). Ph, phenyl.
Results and Discussion
Chemistry
Compound 1 was prepared as described earlier.42 Reductive amination of 1·HBr was performed using amino acid tert-butyl ester hydrochlorides or dipeptide benzyl ester hydrochlorides and NaBH3CN in CH3OH at 21 °C. Medium-pressure liquid chromatography (MPLC) was used to separate the diastereoisomers providing ester derivatives 24a/b–41a/b (Figure 3). Typically, the ratio of 6β-amino to 6α-amino epimers was found to be between 4:1 and 2:1. The coupling constants (J(5,6)) between H-C(5) and H-C(6) were used for assignment of configuration at C(6). The 6α-amino epimers have smaller J(5,6) values (i.e., 3–4 Hz) than the 6β-amino epimers (6.5–7.8 Hz).43,49,51,52 The results for compounds 24a/b–41a/b are in agreement with the earlier findings. The amino acid derivatives 6–21 were obtained through ester cleavage of the tert-butyl derivatives in dioxane/HCl. Catalytic hydrogenation of the benzyl esters 40 and 41 in CH3OH using 10% Pd/C catalyst provided the dipeptides 22a/b and 23, respectively (see Supporting Information for details).
Figure 3.
Structures of new 6-amino acid (24–39)- and 6-dipeptide-substituted esters (40a/b and 41a/b). t-Bu, tert-butyl; Ph, phenyl.
Pharmacology
In vitro binding affinity and functional activity of the new 6-amino acid (6–21)- and 6-dipeptide-substituted derivatives (22a/b and 23a) were evaluated at MOR, DOR, and KOR (Tables 1 and 2). Competition binding assays using rat brain (MOR and DOR) and guinea-pig brain (KOR) membranes (Table 1) were performed according to published procedures.44,49 Opioid-binding profiles of 6–23 were compared to those of compound 1 and earlier reported 6-amino acid-substituted analogues 2–5.44,49 In addition, we have assessed binding at the human opioid receptors stably transfected in Chinese hamster ovary (CHO) cells (Table S1).
Table 1. Binding Affinities at the Opioid Receptors and Calculated Physicochemical Properties of 6-Amino Acid (2–21)- and 6-Dipeptide-Substituted Derivatives (22a/b and 23a), and Reference Compound 1.
| opioid
receptor binding Ki (nM)a |
physicochemical propertiesb | |||||
|---|---|---|---|---|---|---|
| compd | amino acid substitution at position 6 | MOR | DOR | KOR | Ki ratio MOR/DOR/KOR | c log D7.4 |
| 1c | 0.10 ± 0.01 | 4.80 ± 0.22 | 10.2 ± 2.0 | 1/48/102 | 0.48 | |
| 2ac | α-Gly | 0.89 ± 0.09 | 15.4 ± 1.4 | 43.2 ± 7.0 | 1/7/49 | –3.35 |
| 2bc | β-Gly | 0.83 ± 0.02 | 7.86 ± 0.64 | 44.8 ± 0.1 | 1/9.5/54 | –3.35 |
| 3ac | α-l-Ala | 0.77 ± 0.09 | 26.9 ± 0.8 | 142 ± 43 | 1/35/184 | –2.81 |
| 3bc | β-l-Ala | 1.90 ± 0.08 | 7.71 ± 0.94 | 63.7 ± 7.8 | 1/4.1/34 | –2.81 |
| 4ac | α-l-Phe | 0.95 ± 0.07 | 3.67 ± 0.32 | 28.5 ± 4.2 | 1/3.9/30 | –1.13 |
| 4bc | β-l-Phe | 2.58 ± 0.09 | 1.03 ± 0.13 | 151 ± 17 | 1/0.4/59 | –1.13 |
| 5ad | α-Gly | 0.19 ± 0.02 | 0.22 ± 0.02 | 0.73 ± 0.01 | 1/1.2/3.8 | –0.85 |
| 5bd | β-Gly | 0.16 ± 0.02 | 0.19 ± 0.01 | 0.81 ± 0.03 | 1/1.2/5.1 | –0.85 |
| 6a | α-l-Ser | 2.21 ± 0.02 | 5.32 ± 0.39 | 196 ± 24 | 1/2.4/89 | –3.89 |
| 6b | β-l-Ser | 2.14 ± 0.03 | 5.29 ± 0.31 | 152 ± 15 | 1/2.5/71 | –3.89 |
| 7a | α-l-Val | 3.16 ± 0.25 | 3.91 ± 0.30 | 325 ± 19 | 1/1.2/103 | –1.94 |
| 7b | β-l-Val | 3.04 ± 0.12 | 3.52 ± 0.04 | 305 ± 39 | 1/1.2/100 | –1.94 |
| 8a | α-l-Lys | 0.19 ± 0.04 | 1.27 ± 0.42 | 12.6 ± 1.3 | 1/6.7/66 | –5.57 |
| 8b | β-l-Lys | 0.53 ± 0.09 | 3.34 ± 0.46 | 33.7 ± 4.1 | 1/6.3/64 | –5.57 |
| 9a | α-l-Tyr | 0.83 ± 0.04 | 2.18 ± 0.98 | 39.5 ± 0.2 | 1/2.6/48 | –1.41 |
| 9b | β-l-Tyr | 3.20 ± 0.55 | 3.89 ± 0.64 | 186 ± 33 | 1/1.2/58 | –1.41 |
| 10a | α-l-Trp | 0.36 ± 0.01 | 1.02 ± 0.12 | 25.1 ± 9.3 | 1/2.8/70 | –1.03 |
| 10b | β-l-Trp | 0.65 ± 0.09 | 1.19 ± 0.40 | 8.66 ± 0.64 | 1/1.8/13 | –1.03 |
| 11a | α-l-Asn | 1.17 ± 0.07 | 3.37 ± 0.21 | 74.0 ± 9.7 | 1/2.9/63 | –4.29 |
| 11b | β-l-Asn | 1.26 ± 0.05 | 2.25 ± 0.11 | 103 ± 10 | 1/1.8/82 | –4.29 |
| 12a | α-l-Gln | 3.24 ± 0.14 | 5.13 ± 0.52 | 351 ± 66 | 1/1.6/108 | –4.04 |
| 12b | β-l-Gln | 2.48 ± 0.30 | 4.87 ± 0.76 | 290 ± 3 | 1/2.0/117 | –4.04 |
| 13a | α-l-Asp | 1.36 ± 0.05 | 14.6 ± 3.6 | 50.2 ± 9.2 | 1/11/37 | –5.64 |
| 13b | β-l-Asp | 3.42 ± 0.05 | 22.6 ± 2.2 | 351 ± 43 | 1/6.6/103 | –5.64 |
| 14a | α-l-Glu | 1.45 ± 0.09 | 9.03 ± 0.70 | 87.2 ± 4.2 | 1/6.2/60 | –5.39 |
| 14b | β-l-Glu | 11.6 ± 0.7 | 7.64 ± 0.64 | 1252 ± 70 | 1/0.7/108 | –5.39 |
| 15a | α-d-Ala | 0.69 ± 0.11 | 10.4 ± 0.01 | 71.5 ± 9.6 | 1/15/104 | –2.81 |
| 15b | β-d-Ala | 1.48 ± 0.08 | 11.3 ± 0.9 | 142 ± 33 | 1/7.6/96 | –2.81 |
| 16a | α-d-Val | 1.70 ± 0.09 | 1.93 ± 0.24 | 202 ± 3 | 1/1.1/119 | –1.94 |
| 16b | β-d-Val | 1.02 ± 0.07 | 1.68 ± 0.16 | 159 ± 17 | 1/1.6/156 | –1.94 |
| 17a | α-d-Phe | 0.61 ± 0.01 | 3.69 ± 0.59 | 76.4 ± 9.3 | 1/6.0/125 | –1.13 |
| 17b | β-d-Phe | 1.28 ± 0.38 | 1.19 ± 0.13 | 139 ± 23 | 1/0.9/109 | –1.13 |
| 18a | α-l-Chg | 1.23 ± 0.20 | 14.3 ± 0.09 | 177 ± 30 | 1/12/144 | –1.26 |
| 18b | β-l-Chg | 1.66 ± 1.04 | 1.30 ± 0.16 | 118 ± 23 | 1/0.8/71 | –1.26 |
| 19a | α-l-Abu | 0.76 ± 0.19 | 37.5 ± 3.4 | 144 ± 7 | 1/49/189 | –2.34 |
| 19b | β-l-Abu | 1.83 ± 0.04 | 1.30 ± 0.24 | 201 ± 10 | 1/0.7/110 | –2.34 |
| 20a | α-β-Ala | 1.30 ± 0.08 | 60.0 ± 1.9 | 182 ± 8 | 1/46/140 | –3.18 |
| 20b | β-β-Ala | 1.04 ± 0.04 | 13.9 ± 1.7 | 71.4 ± 4.6 | 1/13/69 | –3.18 |
| 21a | α-GABA | 0.77 ± 0.07 | 12.5 ± 0.7 | 45.6 ± 4.4 | 1/16/59 | –2.93 |
| 21b | β-GABA | 1.41 ± 0.13 | 6.61 ± 0.67 | 147 ± 13 | 1/4.7/104 | –2.93 |
| 22a | α-l-Val-l-Tyr | 0.82 ± 0.13 | 1.19 ± 0.42 | 69.0 ± 2.5 | 1/1.5/84 | –1.02 |
| 22b | β-l-Val-l-Tyr | 0.44 ± 0.01 | 1.38 ± 0.22 | 390 ± 49 | 1/3.1/886 | –1.02 |
| 23a | β-Gly-Gly | 4.62 ± 0.09 | 7.52 ± 0.43 | 203 ± 34 | 1/1.6/44 | –4.27 |
Determined in competition radioligand binding assays using rat brain membranes (MOR and DOR) and guinea-pig brain membranes (KOR). Values represent the mean ± SEM of three to four independent experiments each performed in duplicate.
Calculated using the MarvinSketch 18.8 (ChemAxon).
Data from ref (44).
Data from ref (49).
Table 2. In Vitro Agonist Activity at the Opioid Receptors of 6-Amino Acid (2–21)- and 6-Dipeptide-Substituted Derivatives (22a/b and 23a), and Reference Compound 1a.
| MOR |
DOR |
KOR |
|||||
|---|---|---|---|---|---|---|---|
| compd | amino acid substitution at position 6 | EC50 (nM) | % stim. | EC50 (nM) | % stim. | EC50 (nM) | % stim. |
| 1 | 3.83 ± 0.95 | 97 ± 3 | 37.3 ± 8.5 | 106 ± 1 | 116 ± 31 | 77 ± 5 | |
| 2a | α-Gly | 1.16 ± 0.59 | 99 ± 4 | 9.61 ± 4.14 | 103 ± 1 | 399 ± 119 | 87 ± 4 |
| 2b | β-Gly | 3.78 ± 0.73 | 98 ± 9 | 7.92 ± 1.63 | 103 ± 7 | 361 ± 154 | 82 ± 9 |
| 3a | α-l-Ala | 1.34 ± 0.17 | 97 ± 5 | 9.55 ± 4.47 | 93 ± 9 | 214 ± 71 | 51 ± 3 |
| 3b | β-l-Ala | 6.24 ± 0.79 | 87 ± 6 | 5.20 ± 1.70 | 104 ± 6 | 392 ± 160 | 64 ± 9 |
| 4a | α-l-Phe | 0.38 ± 0.19 | 93 ± 8 | 0.39 ± 0.19 | 102 ± 2 | 219 ± 2 | 39 ± 12 |
| 4b | β-l-Phe | 6.76 ± 3.16 | 99 ± 8 | 0.48 ± 0.16 | 94 ± 8 | 1172 ± 505 | 81 ± 9 |
| 6a | α-l-Ser | 1.60 ± 0.59 | 87 ± 5 | 13.9 ± 1.5 | 101 ± 2 | 1213 ± 200 | 44 ± 5.2 |
| 6b | β-l-Ser | 3.56 ± 0.76 | 101 ± 9 | 6.98 ± 4.24 | 98 ± 1 | 201 ± 116 | 88 ± 2 |
| 7a | α-l-Val | 10.5 ± 4.0 | 95 ± 8 | 33.8 ± 8.9 | 91 ± 2 | 462 ± 126 | 51 ± 0.5 |
| 7b | β-l-Val | 11.7 ± 5.7 | 84 ± 4 | 5.73 ± 2.48 | 96 ± 9 | 1117 ± 411 | 68 ± 10 |
| 8a | α-l-Lys | 2.25 ± 0.07 | 90 ± 8 | 152 ± 2 | 106 ± 4 | 118 ± 26 | 79 ± 13 |
| 8b | β-l-Lys | 6.85 ± 1.39 | 90 ± 7 | 45.1 ± 22.7 | 93 ± 8 | 525 ± 24 | 62 ± 7 |
| 9a | α-l-Tyr | 1.87 ± 0.44 | 92 ± 2 | 1.76 ± 0.22 | 88 ± 6 | 100 ± 6 | 62 ± 8 |
| 9b | β-l-Tyr | 24.7 ± 9.8 | 93 ± 7 | 6.23 ± 1.03 | 95 ± 0.8 | 774 ± 57 | 60 ± 3 |
| 10a | α-l-Trp | 0.51 ± 0.34 | 93 ± 9 | 2.52 ± 1.41 | 102 ± 8 | 70.1 ± 20.5 | 61 ± 8 |
| 10b | β-l-Trp | 1.64 ± 0.43 | 101 ± 11 | 2.18 ± 0.72 | 96 ± 5 | 181 ± 49 | 87 ± 8 |
| 11a | α-l-Asn | 0.83 ± 0.17 | 99 ± 0.2 | 9.78 ± 3.32 | 106 ± 6 | 81.7 ± 12.3 | 67 ± 12 |
| 11b | β-l-Asn | 2.04 ± 0.44 | 96 ± 14 | 3.18 ± 0.93 | 88 ± 5 | 923 ± 11 | 71 ± 9 |
| 12a | α-l-Gln | 2.27 ± 0.11 | 90 ± 8 | 7.80 ± 3.61 | 104 ± 10 | 185 ± 30 | 70 ± 3 |
| 12b | β-l-Gln | 9.54 ± 1.15 | 98 ± 4 | 3.96 ± 0.07 | 103 ± 9 | 1410 ± 418 | 63 ± 17 |
| 13a | α-l-Asp | 4.10 ± 1.29 | 90 ± 7 | 10.1 ± 3.8 | 97 ± 3 | 2991 ± 659 | 83 ± 17 |
| 13b | β-l-Asp | 1.45 ± 0.01 | 74 ± 3 | 11.8 ± 2.2 | 101 ± 1 | 753 ± 358 | 49 ± 7 |
| 14a | α-l-Glu | 3.11 ± 1.32 | 105 ± 3 | 10.8 ± 2.1 | 98 ± 3 | 1167 ± 448 | 68 ± 5 |
| 14b | β-l-Glu | 12.7 ± 4.7 | 98 ± 4 | 4.60 ± 1.79 | 101 ± 3 | 2233 ± 238 | 76 ± 0.7 |
| 15a | α-d-Ala | 1.44 ± 0.49 | 100 ± 8 | 24.3 ± 5.3 | 106 ± 7 | 254 ± 118 | 67 ± 13 |
| 15b | β-d-Ala | 15.4 ± 8.4 | 102 ± 9 | 5.46 ± 2.68 | 106 ± 6 | 1001 ± 192 | 86 ± 2 |
| 16a | α-d-Val | 4.51 ± 2.35 | 105 ± 4 | 1.12 ± 0.69 | 93 ± 4 | 2218 ± 818 | 96 ± 16 |
| 16b | β-d-Val | 2.38 ± 0.77 | 101 ± 5 | 1.30 ± 0.64 | 99 ± 0.2 | 1278 ± 283 | 98 ± 16 |
| 17a | α-d-Phe | 0.77 ± 0.24 | 96 ± 4 | 8.36 ± 0.28 | 95 ± 10 | 215 ± 32 | 80 ± 11 |
| 17b | β-d-Phe | 0.68 ± 0.29 | 78 ± 5 | 1.71 ± 0.78 | 96 ± 1 | 611 ± 350 | 92 ± 10 |
| 18a | α-l-Chg | 2.88 ± 1.21 | 86 ± 8 | 20.5 ± 8.0 | 101 ± 1 | 250 ± 80 | 52 ± 6 |
| 18b | β-l-Chg | 5.33 ± 0.89 | 86 ± 9 | 3.57 ± 1.63 | 96 ± 10 | 282 ± 58 | 59 ± 7 |
| 19a | α-l-Abu | 5.12 ± 0.09 | 88 ± 2 | 98.2 ± 5.1 | 104 ± 9 | 942 ± 235 | 61 ± 2 |
| 19b | β-l-Abu | 5.47 ± 1.18 | 83 ± 2 | 2.22 ± 1.64 | 100 ± 3 | 572 ± 18 | 72 ± 0.5 |
| 20a | α-β-Ala | 3.52 ± 1.65 | 99 ± 7 | 96.4 ± 8.7 | 97 ± 2 | 186 ± 15 | 78 ± 9 |
| 20b | β-β-Ala | 5.74 ± 0.47 | 78 ± 6 | 20.1 ± 10.7 | 99 ± 3 | 622 ± 311 | 67 ± 12 |
| 21a | α-GABA | 2.88 ± 0.82 | 103 ± 4 | 34.4 ± 6.5 | 103 ± 1 | 2034 ± 253 | 104 ± 9 |
| 21b | β-GABA | 12.3 ± 6.8 | 86 ± 7 | 6.06 ± 1.07 | 103 ± 6 | 3396 ± 1982 | 74 ± 5 |
| 22a | α-l-Val-l-Tyr | 0.89 ± 0.04 | 84 ± 7 | 1.16 ± 0.60 | 88 ± 5 | 330 ± 179 | 50 ± 2 |
| 22b | β-l-Val-l-Tyr | 0.16 ± 0.06 | 73 ± 5 | 1.56 ± 0.83 | 89 ± 7 | 1884 ± 594 | 63 ± 0.1 |
| 23a | β-Gly-Gly | 4.39 ± 0.54 | 85 ± 9 | 2.84 ± 1.63 | 102 ± 7 | 885 ± 298 | 75 ± 0.7 |
[35S]GTPγS binding assays were performed with membranes from CHO stably expressing the human opioid receptors. Percentage stimulation (% stim.) is presented relative to the reference full agonists DAMGO (MOR), DPDPE (DOR), and U69,593 (KOR). Values represent the mean ± SEM of three to four independent experiments each performed in duplicate.
As shown in Table 1, all compounds, except 14b, have high binding affinity in the subnanomolar or low nanomolar range (Ki = 0.19–4.62 nM) at MOR in rat brain membranes. Similar observations were made at the cloned human MOR expressed in CHO cells. Compared to 1, the 6α-l-Lys derivative 8a had similar MOR affinity (Ki of 0.10 nM for 1 vs 0.19 nM for 8a), whereas the next best compound was the 6α-l-Trp-substituted 10a, having a Ki value of 0.36 nM at MOR. Moreover, 8a and 10a also exhibited increased affinity at MOR than the earlier described derivatives 2–444 (P < 0.05, analysis of variance (ANOVA)) and comparable MOR affinity to 5a and 5b(49) (P > 0.05, ANOVA). Most 6-amino acid-substituted derivatives (exception being 13a/b, 15a/b, 18a, 19a, 20a/b, and 21a) of the new series have binding affinities in the low nanomolar range (Ki = 1.02–9.03 nM) at DOR in rat brain membranes, being augmented or comparable to DOR affinity of 1 (Ki = 4.80 nM), consequently resulting in decreased or even a complete loss of MOR vs DOR selectivity (Table 1). Binding affinities at the DOR in the rat brain correlate with affinity data obtained at the human DOR expressed in CHO cells (Table S1). Generally, the attachment of amino acid residues at C6 position in 1 caused an increased binding at DOR. The 6α-l-Abu derivative 19a was found to be the most MOR-selective with respect to DOR, with a DOR/MOR selectivity ratio of 49 that was similar to the ratio of 48 calculated for 1 (Table 1). Binding at KOR in guinea-pig brain membranes was generally decreased for the 6-amino acid (6–21)- and 6-dipeptide-substituted derivatives (22a/b and 23a) compared to 1 (Ki = 10.2 nM), thus causing an increase in MOR vs KOR selectivity. Among them, the 6β-l-Val-l-Tyr-substituted 22b was the most MOR-selective with respect to KOR, with a KOR/MOR selectivity ratio of 886 (Table 1).
Introduction of unnatural amino acids at position 6, d-Ala, d-Val, d-Phe, l-Chg, l-Abu, β-Ala, and GABA, in 1 led to 15a/b, 16a/b, 17a/b, 18a/b, 19a/b, 20a/b, and 21a/b, respectively, all generally showing high binding MOR affinities equivalent to compounds with natural amino acids (Tables 1 and S1). We also compared the 6-d-amino acid-substituted derivatives with their corresponding 6-l-amino acid analogues. No major changes in the rat and human MOR affinity were observed upon substitution of 6-l-Ala in the previously reported 3a/b44 with the 6-d-Ala residue in 15a/b, and the replacement of the 6-l-Phe in 4a/b44 increased ca. 2 times MOR affinity only for the 6β-d-Phe analogue 17b (P < 0.05, ANOVA). An increase (2–3 times) in MOR affinity was also produced when the 6-l-Val residue in 7a/b was replaced by 6-d-Val in 16a/b (P < 0.05, ANOVA), while MOR selectivity remained unaffected (Table 1).
In this study, we also report on the high affinities at MOR of 6-dipeptide-substituted derivatives 22a/b and 23a. The 6-l-Val-l-Tyr-substituted derivatives, 22a and 22b, showed 4 to 7 times increased MOR binding affinity in the rat brain than their single 6-amino acid-substituted derivatives with 6-l-Val (7a and 7b) and 6β-l-Tyr residues (9b) (Table 1). Very good binding was also displayed by 22a and 22b at the human MOR (Table S1). Compared to the 6α-Gly-substituted derivative 2a, the 6α-Gly-Gly-substituted analogue (23a) of 1 displayed about 5 times lower affinity at MOR, and thus decreased MOR selectivity (Table 1).
When evaluating the effect of α/β orientation of the amino acid residue at C6 position on the interaction with MOR and DOR, it was observed that binding affinities of α- vs β-epimers were largely comparable (Table 1), with few exceptions, where the 6α-l-Glu-substituted 14a (Ki = 1.45 nM) showed 8 times increased rat MOR affinity than the 6β-l-Glu analogue 14b (Ki = 11.6 nM), the 6α-l-Chg-substituted 18a (Ki = 14.3 nM) showed 11 times decreased rat DOR affinity than the 6β-l-Chg analogue 18b (Ki = 1.30 nM), and the 6α-l-Abu-substituted 19a (Ki = 37.5 nM) showed 29 times decreased DOR affinity than the 6β-l-Abu analogue 19b (Ki = 1.30 nM) (all P < 0.05 ANOVA). Similar observations were made at the human MOR and DOR expressed in CHO cells (Table S1). Concerning the influence of α/β orientation on the interaction with KOR in the guinea-pig brain, only the 6α-l-Glu-substituted 14a (Ki = 87.2 nM) showed 14 times increased KOR affinity than the 6β-l-Glu analogue 14b (Ki = 1252 nM), and in the case of the 6-l-Val-l-Tyr-substituted derivatives 22a and 22b, the 6β analogue displayed about 6 times lower KOR affinity (Table 1).
In vitro functional activity at the opioid receptors was assessed for 6-amino acid (6–21)- and 6-dipeptide-substituted derivatives (22a/b and 23a) in the guanosine-5′-O-(3-[35S]thio)-triphosphate ([35S]GTPγS) binding assays with membrane preparations from CHO cells expressing the human opioid receptors as described previously.49 Potencies (ED50) and efficacies (% stimulation) are presented in Table 2. We have reported earlier on the potent agonistic activity of 2–4 in the mouse vas deferens bioassay44 and for 2b also in the rat vas deferens bioassay.48 In this study, SAR outcomes of opioid receptor-induced G protein activation upon ligand binding are described. Efficacies are presented as percentage stimulation relative to the standard full agonists, [d-Ala2,N-Me-Phe4,Gly-ol5]enkephalin (DAMGO for MOR),53 [d-Pen2,d-Pen5]enkephalin (DPDPE for DOR)54 and N-methyl-2-phenyl-N-[(5R,7S,8S)-7-(pyrrolidin-1-yl)-1-oxaspiro[4.5]dec-8-yl]acetamide (U69,593 for KOR).55
Based on the functional activities at MOR, most derivatives were very potent agonists (EC50 = 0.16–9.54 nM) with high efficacy behaving as full agonists (≥85% of the response to DAMGO), with the most potent agonists being 4a, 10a, 11a, 17a/b, and 22a/b (EC50 < 1 nM) (Table 2). Most of the new 6-amino acid- and 6-dipeptide-substituted derivatives mainly maintained the high agonist potency and full efficacy of 1 at MOR. Only compounds 7a/b, 9b, 14b, 15b, and 21b showed lower potencies at MOR (EC50 = 12.3–24.7 nM). Derivatives 7b, 17b, 19b, 20b, and 22a exhibited high efficacies at MOR of around 80%, whereas 13b and 22b were highly potent partial agonists at MOR with efficacies of around 70%. The most potent agonist was the 6β-l-Val-l-Tyr-substituted 22b with a 24 times increased MOR potency than 1 (EC50 of 3.83 nM for 1 vs 0.16 nM for 22b), while 22b also displayed a distinct functional activity profile as a partial agonist vs full MOR agonism of 1 (Table 2).
All compounds 2–23 were full agonists at DOR (≥85% of the response to DPDPE) similar to 1, with EC50 values ranging between 0.39 and 152 nM (Table 2). All, except 8a, showed much increased DOR agonist potencies than 1 (EC50 = 37.3 nM); hence, the functional MOR selectivity was greatly decreased or even shifted to DOR selectivity in several cases. Particularly, the 6β-l-Phe-substituted 4b was a very potent, selective, and full agonist at DOR, with a 14 times increased potency at DOR vs MOR. In our earlier report on competition binding studies in rat brain preparations44 and current data at the human opioid receptors, 4b exhibits preference for DOR over MOR (P < 0.05, ANOVA) (Tables 1 and S1). Moreover, the functional profile of 4b at human MOR and DOR established in the present study using [35S]GTPγS binding assays supports and extends our previous findings in the mouse vas deferens bioassay.44 An increase, although less pronounced (2–4 times), in agonist potency at DOR vs MOR was also observed for other 6-amino acid-substituted derivatives, mainly for the 6β-epimers, 6β-l-Val (7b), 6β-l-Tyr (9b), 6β-l-Gln (12b), 6β-l-Glu (14b), 6β-d-Ala (15b), 6β-l-Abu (19b), and 6β-GABA (21b) (P > 0.05, ANOVA, except for 9b vs 9a, P < 0.05). Only the 6α-epimer 6α-d-Val-substituted 16a showed ca. 4 times increased potency, although not statistically significant (P > 0.05, ANOVA), at DOR over MOR (Table 2). At KOR, most derivatives were partial agonists (<85% of the response to U69,593) similar to 1, but with reduced potencies than 1 (Table 2). Only compounds 2a, 6b, 10b, 15b, 16a/b, 17b, and 21b were full agonists at KOR, while they displayed lower potencies compared to 1. Generally, the functional MOR vs KOR selectivity was notably increased.
We also examined in vitro functional activities at the opioid receptors of 6-l-Ala (3a/b), 6-l-Phe (4a/b), and 6-l-Val (7a/b)-substituted derivatives in correlation to their corresponding 6-d-amino acid analogues, 15a/b, 17a/b, and 16a/b, respectively. Similar to our observations from binding studies (Tables 1 and S1), replacement of l-amino acids with d-amino acids at position 6 left MOR agonist potencies unchanged or caused an increase, but largely retained with some exceptions MOR full agonism (Table 2). Exchange of the 6β-l-Phe with the 6β-d-Phe residue converted a full agonist to a partial agonist at MOR (99% for 4b vs 78% for 17b, P < 0.05, ANOVA), while substitution of 6β-l-Val with a 6β-d-Val residue converted a partial agonist to a full agonist (84% for 7b vs 101% for 16b, P < 0.05, ANOVA). The full agonism at DOR was not affected by the replacement of l- with d-amino acids, while some alterations in the functional activity at KOR were noted, specifically changing the partial agonist profile of 3b, 4a, 7a, and 7b to full agonists 15b, 17a, 16a, and 16b, respectively (all P < 0.05, ANOVA) (Table 2).
The two 6-l-Val-l-Tyr-substituted derivatives, 22a and 22b, were highly potent MOR partial agonists and very potent DOR full agonists, with 22a lacking selectivity, while 22b was 10 times more selective for MOR over DOR. The 6β-Gly-Gly-substituted 23a was less potent and a full agonist at MOR and DOR, with slight preference for DOR, although not statistically significant (P > 0.05, ANOVA). All three 6-dipeptide-substituted derivatives showed much reduced potencies at KOR and were partial agonists at this receptor (Table 2).
Following the pattern of SAR, we evaluated the effect of α/β orientation of the amino acid residue at C6 position on the in vitro functional profile at the opioid receptors. While the α-epimers were frequently favored for MOR by strongly activating this receptor, the β-epimers showed augmented interaction with potent activation of DOR. Few exceptions were detected with lower potencies at MOR for α-epimers over β-epimers, although not statistically significant, specifically for 6α/β-l-Asp analogues 13a and 13b (P > 0.05, ANOVA), 6α/β-l-Val analogues 16a and 16b (P > 0.05, ANOVA), and 6α/β-l-Val-l-Tyr analogues 22a and 22b (P < 0.05, ANOVA). A decrease in potency at DOR was noted for the 6β-l-Tyr-substituted 9b when comparing to its 6β counterpart 9a (P < 0.05, ANOVA). While efficacies at MOR were altered in some cases by the α/β orientation of the 6-amino acid residue, with β-epimers becoming partial agonists, efficacies at DOR remained unchanged. Potencies at KOR were significantly lowered independent of the α- or β-substitution, whereas efficacies were mostly unaffected with only few exceptions (Table 2).
Evaluation of pharmacokinetic properties represents a key feature in today’s drug discovery and development, particularly in predicting response profiles in vivo of bioactive molecules.56,57 In this study, we have targeted opioid agonists from the class of N-methylmorphinans substituted with different amino acids and dipeptides at position 6 as analgesics activating peripheral opioid receptors. We have used the coefficient of distribution, log D at the physiological pH of 7.4 (log D7.4) as a major physicochemical factor describing the capability of a drug to pass lipophilic membranes.58,59 The calculation of the log D7.4 (c log D7.4) of 6-amino acid (2–21)- and 6-dipeptide-substituted derivatives (22a/b and 23a) (Figures 1 and 2) was performed with the software MarvinSketch 18.8 (ChemAxon), and the values are included in Table 1. The c log D7.4 values were ranging between −5.64 and −0.85, indicating the poor capability to enter the CNS. In contrast, the c log D7.4 of compound 1 is 0.48. Based on the c log D7.4 values, all compounds (2–23) showed much higher hydrophilicity than 1. The presence of amino acid residues as ionizable functional groups increases the polarity and therefore limits the BBB penetration.
The restricted capability to enter the CNS of compounds 2–5 was earlier demonstrated experimentally employing a commonly used pharmacological approach.35,39,60,61 Antinociceptive effects of 2–5 in pain models of thermal nociception (tail-flick test)45 and inflammatory hyperalgesia in the rat (the formalin test and carrageenan-induced hyperalgesia)46,49 after systemic sc administration were completely blocked by sc naloxone methiodide, an opioid antagonist that does not cross the BBB.27 Furthermore, naloxone methiodide completely antagonized antinociceptive effects of 6β-Gly-substituted 2b in the mouse eye wiping behavioral test for trigeminal nociception following systemic ip administration.50 We also reported that antihyperalgesic effects of 2b given orally to rats with inflammatory hyperalgesia was reversed by sc administered naloxone methiodide.46 In the current study, as mentioned below, we also established the limited ability to pass the BBB for the new compounds 6–23.
Based on in vitro opioid activity and polarity profiles (Tables 1 and 2), a number of 6-amino acid-substituted derivatives, 2a/b, 3a/b, 4a/b, 5a/b, 6a, 7b, 8a, 9a, 10b, 11a, 13a, 14a, 16b, 17a/b, 18a, 19a, 20a, and 21a, and two 6-dipeptide-substituted 22a and 23a were evaluated in vivo for antinociceptive activity after sc administration in mice in a model of visceral pain, the acetic acid-induced writhing assay.48,62 All compounds were effective in inhibiting the writhing behavior. Antinociceptive potencies as ED50 values with 95% confidence intervals (95% CI) are shown in Table 3, and were compared to those of morphine and compound 1. We present first antinociceptive data in the acetic acid-induced writhing assay for the previously developed 6-amino acid-substituted 2a, 3a/b, 4a/b, and 5a/b (Figure 1 and Table 3),43,44 as well as for the new series of compounds (Figure 2 and Table 3). We have earlier reported on the high efficacy in inhibiting the writhing response of 6β-Gly-substituted 2b after sc administration, being 24 times more potent than morphine.48 Our present results are in line with these findings.
Table 3. Antinociceptive Potencies of 6-Amino Acid (2–21)- and 6-Dipeptide-Substituted Derivatives (22a and 23a), Reference Compound 1 and Morphine in the Writhing Assay in Mice after sc Administrationa.
| compd | amino acid substitution at position 6 | ED50 (μg/kg, sc) (95% CI) |
|---|---|---|
| morphine | 437 (249–768) | |
| 1 | 3.26 (1.24–8.52) | |
| 2a | α-Gly | 35.7 (15.2–84.0) |
| 2b | β-Gly | 27.5 (11.0–68.7) |
| 3a | α-l-Ala | 16.0 (5.45–47.0) |
| 3b | β-l-Ala | 86.3 (49.0–152) |
| 4a | α-l-Phe | 31.1 (14.3–67.7) |
| 4b | β-l-Phe | 579 (268–1252) |
| 5a | α-Gly | 36.1 (15.1–84.4) |
| 5b | β-Gly | 41.6 (15.5–112) |
| 6a | α-l-Ser | 32.1 (15.8–65.1) |
| 7b | β-l-Val | 117 (44.1–312) |
| 8a | α-l-Lys | 20.6 (10.1–41.8) |
| 9a | α-l-Tyr | 14.6 (5.15–41.7) |
| 10b | β-l-Trp | 92.7 (37.5–229) |
| 11a | α-l-Asn | 15.2 (6.89–33.3) |
| 13a | α-l-Asp | 38.2 (16.4–88.9) |
| 14a | α-l-Glu | 36.1 (16.3–79.6) |
| 16b | β-d-Val | 14.0 (5.90–33.3) |
| 17a | α-d-Phe | 18.1 (8.37–39.2) |
| 17b | β-d-Phe | 250 (120–517) |
| 18a | α-l-Chg | 20.6 (4.33–98.2) |
| 19a | α-l-Abu | 17.5 (5.13–59.6) |
| 20a | α-β-Ala | 31.2 (13.2–73.9) |
| 21a | α-GABA | 41.9 (22.6–77.8) |
| 22a | β-l-Val-l-Tyr | 178 (58.0–545) |
| 23a | β-Gly-Gly | 104 (58.5–185) |
Groups of mice were administered sc test compound or saline (control), and evaluated in the acetic acid-induced writhing assay. Each compound was tested in at least three doses (n = 6–7 mice per dose). Inhibition of the writhing response was assessed 30 min after drug administration, and ED50 values and 95% confidence intervals (CI in parentheses) were calculated from dose–response curves.
As shown in Table 3, all compounds, except 4b, were more effective in inducing an antinociceptive response than morphine (ED50 = 437 μg/kg), while they showed generally lower potencies compared to 1 (ED50 = 3.26 μg/kg) in the writhing assay. The most potent compounds were 3a, 9a, 11a, 16b, 17a, and 19a with ED50 < 20 μg/kg. The 6β-Gly-substituted 2a displayed comparable antinociceptive potency to its 6β counterpart 2b, while the 6α-epimers with an l-Ala and l-Phe residue (3a and 4a, respectively) had 5 and 19 times increased potencies than their corresponding 6β-epimers, 3b and 4b, a finding that correlates to the lower in vitro agonist potency of 3b and 4b (Table 2). The same pattern on significantly reduced potency for the 6β-epimer was observed when evaluating 6α and 6β derivatives substituted with the unnatural amino acid d-Phe, 17a and 17b, respectively, likely as a result of diminished efficacy at MOR of 17b (Table 2). We also examined antinociceptive profiles of 6-l-Phe (4a/b)- and 6-l-Val (7a/b)-substituted derivatives in association with their 6-d-amino acid analogues, 17a/b and 16a/b, respectively. Replacement of 6-l-Phe (4a/b) and 6β-l-Val residues (7b) with d-amino acids at position 6, 17a/b and 16b, respectively, produced an increase in antinociceptive potency, particularly for 16b (8 times vs 7a, Table 3), a profile that supports the results from in vitro binding and agonist activity (Tables 1 and 2). The 6-dipeptide-substituted derivatives 22a (6α-l-Val-l-Tyr-substituted) and 23a (6α-l-Val-l-Tyr-substituted) were also effective in inducing an antiwrithing response, with 2.5 and 4 times, respectively, higher potency than morphine (Table 3).
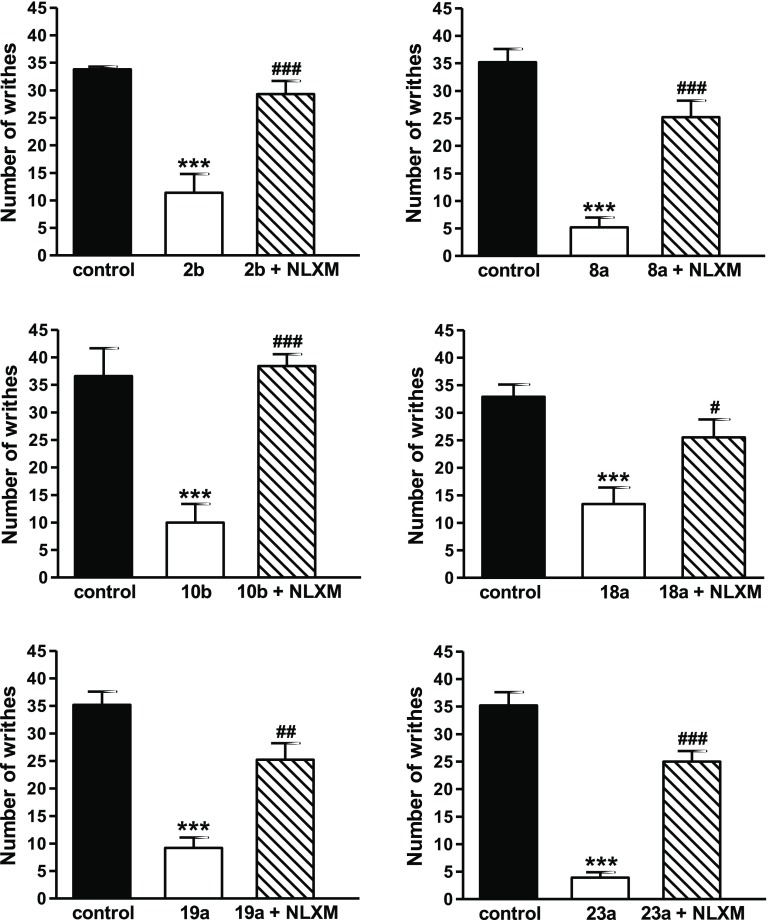
To determine whether targeted 6-amino acid and 6-dipeptide analogues inhibit writhing behavior in mice through peripheral opioid receptors activation, we used naloxone methiodide.27 Six 6-amino acid-substituted derivatives were selected, including three derivatives with a natural amino acid, 2b (6β-Gly), 8a (6β-l-Lys), and 10b (6β-l-Trp), two with an unnatural amino acid, 18a (6α-l-Chg) and 19a (6α-l-Abu), and two dipeptides, 22a (6β-l-Val-l-Tyr) and 23b (6β-Gly-Gly) (Figure 4). Subcutaneous co-administration of these opioid agonists with naloxone methiodide reversed their antinociceptive effect in the writhing assay indicative of activation of peripheral opioid receptors, due to increased polarity, and therefore restricted BBB penetration (Table 1). The results on the peripheral site of action of 2b corroborate and extend our earlier observations in nociceptive and inflammatory pain models in rats.45,46 Using the writhing assay in mice, we have demonstrated previously the lack of 2b to enter the CNS as intracerebroventricular administration of d-Phe-Cys-Tyr-d-Trp-Arg-Thr-Pen-Thr-NH2 (CTAP),63 an MOR-selective antagonist, did not reverse the antinociceptive effects of systemic sc 2b.48
Figure 4.
Systemic sc administration of 6-amino acid- and 6-dipeptide-substituted derivatives produces antinociception via activation peripheral receptors in the writhing assay in mice. Antinociceptive effects of 2b (50 μg/kg), 8b (50 μg/kg), 10b (200 μg/kg), 18b (50 μg/kg), 19b (50 μg/kg), and 23a (200 μg/kg) were antagonized by sc co-administration with naloxone methiodide (NLXM, 1 mg/kg) in the acetic acid-induced writhing assay in mice at 30 min after drug administration. Values are shown as mean of writhes ± SEM (n = 6–7 mice per group). ***P < 0.001 vs control group; #P < 0.05, ##P < 0.01 and ###P < 0.001 vs agonist treated group, ANOVA with Tukey’s post hoc test.
Conclusions
In summary, we have extended the SAR on our earlier 6-amino acid-substituted derivatives of 1 by targeting additional amino acid residues of the l- and/or d-series at position 6. In this study, we reported on the synthesis and pharmacological characterization of a library of differently substituted N-methyl-14-O-methylmorphinans with natural amino acids, i.e., Ser, Val, Lys, Tyr, Trp, Asn, Gln, Asp, and Glu (6–14), unnatural amino acids, i.e., d-Ala, d-Val, d-Phe, l-Chg, l-Abu, β-Ala, and GABA (15–21), and three dipeptides (22a/b and 23) at C6 position, which emerged as potent MOR/DOR agonists producing antinociception via activation of peripheral opioid receptors. On the basis of the SAR studies comprising results on in vitro binding and functional profiles and antinociceptive activities, the potent MOR/DOR agonist profile and reduced interaction and activation of KOR for most compounds was established. Derivatives with unnatural amino acids at position 6 generally showed high MOR and DOR binding affinities and agonism, similar to compounds with natural amino acids. Substituting l-amino acids by d-amino acids left MOR binding affinities and agonist potencies unchanged or caused an increase, but largely retained MOR full agonism. The full agonism at DOR was not affected by the replacement of l- with d-amino acids, while conversion from partial agonists to full agonists at KOR was observed. While the α-epimers were often favored for MOR by strongly activating this receptor, the β-epimers showed increased binding and potent activation of DOR. We also presented opioid activities of 6-dipeptide-substituted analogues 22a and 22b as highly potent MOR partial agonists and very potent DOR full agonists, while 23a was less potent and an MOR/DOR full agonist. In vivo, the new 6-amino acid- and 6-dipeptide-substituted derivatives of 1 were highly effective in inducing antinociception in the acetic acid-induced writhing assay after sc administration in mice, which was antagonized by naloxone methiodide demonstrating activation of peripheral opioid receptors. The presence of amino acid residues as ionizable functional groups increased polarity and therefore restricted the ability to pass the BBB. Future studies remain to establish efficacy in models of chronic pain and the potential for CNS side effects. Such peripherally selective opioid analgesics may represent alternatives to presently used drugs for an efficient and safer pain therapy, in light of the current opioid epidemic.
Experimental Section
Chemistry
General Methods
The starting material thebaine was obtained from Tasmanian Alkaloids Ltd., Westbury, Tasmania, Australia. All other chemicals used were of reagent grade and obtained from standard commercial sources. Melting points were determined on a Kofler melting point microscope and are uncorrected. 1H NMR spectra were obtained on a Varian Gemini 200 (200 MHz) spectrometer using tetramethylsilane (TMS) as internal standard for CDCl3 and 3-(trimethylsilyl)-1-propanesulfonic acid sodium salt (DSS) for D2O. Coupling constants (J) are given in Hz. IR spectra were taken on a Mattson Galaxy FTIR series 3000 in KBr pellets (in cm–1). Mass spectra were recorded on a Bruker-Esquire 3000+ apparatus. Elemental analyses were performed at the Microanalytic Laboratory of the University of Vienna, Austria. For column chromatography (MPLC), silica gel 60 (0.040–0.063 mm, Fluka, Switzerland) was used. TLC was performed on silica gel plates Polygram SIL G/UV254 (Macherey-Nagel, Germany) with CH2Cl2/CH3OH/NH4OH 90:9:1 as eluent. The elemental analysis values were found to be within ±0.4% of the calculated values, indicating a purity of the tested compounds of >95%.
General Procedure for the Synthesis of 6-Amino Acid Derivatives 6–21 from the Corresponding Ester Precursors 24–39
A mixture of 0.25 mmol of the corresponding ester and 4 M hydrogen chloride solution in 1,4-dioxane (1 mL) was refluxed until the reaction was complete (usually 3 h). The end of the reaction was monitored by TLC. The product was filtered off under inert conditions, washed with diethyl ether, dried (when the product was pure enough, it was used as such), and recrystallized from ethanol, methanol or isopropanol, or the residue was dissolved in water and freeze-dried to afford a lyophilisate.
General Procedure for the Synthesis of 6-Dipeptide Derivatives 22 and 23 from the Corresponding Ester Precursors 40 and 41
A mixture of the respective ester derivative (0.15 mmol), CH3OH (10 mL), and 10% Pd/C catalyst was hydrogenated at 30 psi and room temperature for 2 h. The catalyst was filtered off and the filtrate evaporated to give a colorless foam.
(2S)-2-[(4,5α-Epoxy-3-hydroxy-14β-methoxy-17-methylmorphinan-6α-yl)amino]-3-hydroxypropionic Acid Dihydrochloride (6a·2HCl)
Colorless crystals from EtOH (69%); Mp >290 °C (dec.); IR (KBr): 1738 (C=O). 1H NMR (D2O): δ 6.92 (d, J = 8.4, 1 ar. H), 6.83 (d, J = 8.4, 1 ar. H), 5.07 (d, J = 3.0, H-C(5)), 3.37 (s, MeO), 2.95 (s, MeN); MS (ESI) m/z 404.7 (M+ + 1). Anal. (C21H28N2O6·2HCl·2.1H2O·0.4C4H8O2) C, H, N.
(2S)-2-[(4,5α-Epoxy-3-hydroxy-14β-methoxy-17-methylmorphinan-6β-yl)amino]-3-hydroxypropionic Acid Dihydrochloride (6b·2HCl)
Colorless crystals from EtOH (88%); Mp 310–324 °C (dec.); IR (KBr): 1738 (C=O). 1H NMR (D2O): δ 6.93 (d, J = 8.2, 1 ar. H), 6.84 (d, J = 8.2, 1 ar. H), 4.94 (d, J = 7.8, H-C(5)), 3.34 (s, MeO), 2.93 (s, MeN); MS (ESI) m/z 404.7 (M+ + 1). Anal. (C21H28N2O6·2HCl·2.1H2O) C, H, N.
(2S)-2-[(4,5α-Epoxy-3-hydroxy-14β-methoxy-17-methylmorphinan-6α-yl)amino]-3-methylbutyric Acid Dihydrochloride (7a·2HCl)
Colorless crystals from CH3OH (84%); Mp >298 °C (dec.); IR (KBr): 1734 (C=O) cm–1; 1H NMR (D2O): δ 6.83 (d, J = 8.0, 1 ar. H), 6.74 (d, J = 8.0, 1 ar. H), 4.99 (d, J = 2.8, H-C(5)), 3.26 (s, MeO), 2.86 (s, MeN), 1.01 (d, J = 6.8, CHMe), 1.00 (d, J = 6.8, CHMe); MS (ESI): m/z 416.8 (M+ + 1). Anal. (C23H32N2O5·2HCl·1.5H2O·1.0CH3OH) C, H, N.
(2S)-2-[(4,5α-Epoxy-3-hydroxy-14β-methoxy-17-methylmorphinan-6β-yl)amino]-3-methylbutyric Acid Dihydrochloride (7b·2HCl)
Colorless crystals from EtOH (97%); Mp 279–292 °C; IR (KBr): 1734 (C=O) cm–1; 1H NMR (D2O): δ 6.80 (d, J = 8.1, 1 ar. H), 6.76 (d, J = 8.1, 1 ar. H), 4.82 (d, J = 7.8, H-C(5)), 3.24 (s, MeO), 2.84 (s, MeN), 0.99 (d, J = 7.0, CHMe), 0.93 (d, J = 7.0, CHMe); MS (ESI): m/z 416.8 (M+ + 1). Anal. (C23H32N2O5·2HCl·2.0H2O·1.8C2H5OH) C, H, N.
(2S)-6-Amino-2-[(4,5α-epoxy-3-hydroxy-14β-methoxy-17-methylmorphinan-6α-yl)amino]hexanoic Acid Trihydrochloride (8a·3HCl)
Colorless crystals from CH3OH (86%); 279–287 °C (dec.); IR (KBr): 1718 (C=O). 1H NMR (D2O): δ 6.92 (d, J = 8.4, 1 ar. H), 6.82 (d, J = 8.4, 1 ar. H), 5.06 (s, br, H-C(5)), 3.34 (s, MeO), 2.93 (s, MeN); MS (ESI) m/z 445.7 (M+ + 1). Anal. (C24H35N3O5·3HCl·2.8H2O) C, H, N.
(2S)-6-Amino-2-[(4,5α-epoxy-3-hydroxy-14β-methoxy-17-methylmorphinan-6β-yl)amino]hexanoic Acid Trihydrochloride (8b·3HCl)
Colorless crystals from CH3OH (91%); 269–274 °C (dec.); IR (KBr): 1721 (C=O). 1H NMR (D2O): δ 6.91 (d, J = 8.1, 1 ar. H), 6.86 (d, J = 8.1, 1 ar. H), 4.89 (d, J = 7.6, H-C(5)), 3.33 (s, MeO), 2.92 (s, MeN); MS (ESI) m/z 445.8 (M+ + 1). Anal. (C24H35N3O5·3HCl·1.0H2O·0.9C4H8O2) C, H, N.
(2S)-2-[(4,5α-Epoxy-3-hydroxy-14β-methoxy-17-methylmorphinan-6α-yl)amino]-3-(4-hydroxyphenyl)propionic Acid Dihydrochloride (9a·2HCl)
Colorless crystals from EtOH (38%); Mp >229 °C (dec.); IR (KBr): 1734 (C=O) cm–1; 1H NMR (D2O): δ 7.25 J = (d, J = 8.4 Hz, 2 ar. H), 6.90 (d, J = 8.4 Hz, 2 ar. H), 6.87 (d, J = 8.5 Hz, H-C(1)), 6.78 (d, J = 8.5 Hz, H-C(2)), 4,91 (s, br, OH), 4.87 (d, J = 2.4, H-C(5)), 3.25 (s, CH3O), 2.91 (s, CH3N); MS (ESI) m/z 481.3 [M+ + 1]; Anal. (C27H36N2O6·2HCl·2.1H2O) C, H, N.
(2S)-2-[(4,5α-Epoxy-3-hydroxy-14β-methoxy-17-methylmorphinan-6β-yl)amino]-3-(4-hydroxyphenyl)propionic Acid Dihydrochloride (9b·2HCl)
Colorless crystals from EtOH (54%); Mp >245 °C (dec.); IR (KBr): 1732 (C=O) cm–1; 1H NMR (D2O): δ 7.18 (d, J = 8.4 Hz, 2 ar. H), 6.92 (d, J = 8.5 Hz, 1 ar. H), 6.85 (d, J = 8.5 Hz, 1 ar. H), 6.75 (d, J = 8.4 Hz, 2 ar. H), 4.87 (d, J = 2.4, H-C(5)), 3.28 (s, CH3O), 2.90 (s, CH3N); MS (ESI) m/z 481.3 [M+ + 1]; Anal. (C27H36N2O6·2HCl·1.0H2O) C, H, N.
(2S)-2-[(4,5α-Epoxy-3-hydroxy-14β-methoxy-17-methylmorphinan-6α-yl)-amino]-3-(1H-indolo-3yl)propionic Acid Dihydrochloride (10a·2HCl)
Colorless solid (83%); Mp >220 °C (dec.); IR (KBr): 1730 (C=O) cm–1; 1H NMR (DMSO-d6): δ 7.66 (d, J = 7.6, 1 ar. H), 7.40 (d, J = 8.0, 2 ar. H), 7.14–7.03 (m, 2 ar. H), 6.83 (d, J = 8.2, 1 ar. H), 6.66 (d, J = 8.2, 1 ar. H), 4,91 (s, br, OH), 4.29 (m, H-C(5)), 4.10 (m, NHCHCH2), 3.12 (s, CH3O), 2.86 (s, CH3N); MS (ESI) m/z 504.3 [M+ + 1]; Anal. (C29H33N3O5·3HCl·1.1C4H8O2) C, H, N.
(2S)-2-[(4,5α-Epoxy-3-hydroxy-14β-methoxy-17-methylmorphinan-6β-yl)-amino]-3-(1H-indolo-3yl)propionic Acid Dihydrochloride ·(10b·2HCl)
Colorless solid from EtOH (83%); Mp >220 °C (dec.); IR (KBr): 1736 (C=O) cm–1; 1H NMR (DMSO-d6): δ 7.66 (d, J = 7.6 Hz, 1 ar. H), 7.40 (d, J = 8.0 Hz, 2 ar. H), 7.14-7.03 (m, 2 ar. H), 6.83 (d, J = 7.6 Hz, H-C(1)), 6.66 (d, J = 8.2 Hz, H-C(2)), 4,91 (s, br, OH), 4.29 (m, H-C(5)), 4.10 (m, NHCHCH2), 3.12 (s, CH3O), 2.86 (s, CH3N); MS (ESI) m/z 504.3 [M+ + 1]; Anal. (C29H33N3O5·3HCl·1.1C4H8O2) C, H, N.
(2S)-3-Carbamoyl-2-[(4,5α-epoxy-3-hydroxy-14β-methoxy-17-methylmorphinan-6α-yl)amino]propionic Acid Dihydrochloride (11a·2HCl)
Amorphous solid (79%); Mp >230 °C (dec.); IR (KBr): 1735 (C=O). 1H NMR (D2O): δ 6.78 (d, J = 8.4, ar. H,), 6.69 (d, J = 8.4, ar. H), 4.90 (d, J = 3.4 Hz, H-C(5)), 3.63 (s, MeO), 2.82 (s, MeN); MS (ESI): m/z 431.7 (M+ + 1). Anal. (C22H29N3O6·2HCl·2.7H2O·3.2C4H8O2) C, H, N.
(2S)-3-Carbamoyl-2-[(4,5α-epoxy-3-hydroxy-14β-methoxy-17-methylmorphinan-6β-yl)amino]propionic Acid Dihydrochloride (11b·2HCl)
Amorphous solid (84%); Mp >230 °C (dec.); IR (KBr): 1734 (C=O); 1H NMR (D2O): δ 6.77 (d, J = 8.4, ar. H,), 6.72 (d, J = 8.4, ar. H), 4.84 (d, J = 7.2 Hz, H-C(5)), 3.61 (s, MeO), 2.81 (s, MeN); MS (ESI): m/z 431.8 (M+ + 1). Anal. (C22H29N3O6·2HCl·3.6H2O·1.4C2H5OH) C, H, N.
(2S)-4-Carbamoyl-2-[(4,5α-epoxy-3-hydroxy-14β-methoxy-17-methylmorphinan-6α-yl)amino]butanoic Acid Dihydrochloride (12a·2HCl)
Amorphous solid (75%); Mp >230 °C (dec.); IR (KBr): 1653 (C=O). 1H NMR (D2O): δ 6.82 (d, J = 8.4, 1 ar. H), 6.73 (d, J = 8.4, 1 ar. H), 4.95 (d, J = 3.6, H-C(5)), 3.27 (s, MeO), 2.86 (s, MeN); MS (ESI) m/z 445.7 (M+ + 1). Anal. (C23H31N3O6·2HCl·0.2H2O·1.0CH2Cl2) C, H, N.
(2S)-4-Carbamoyl-2-[(4,5α-epoxy-3-hydroxy-14β-methoxy-17-methylmorphinan-6β-yl)amino]butanoic Acid Dihydrochloride (12b·2HCl)
Amorphous solid (81%); Mp >230 °C (dec.); IR (KBr): 1653 (C=O). 1H NMR (D2O): δ 6.78 (d, J = 8.4, 1 ar. H), 6.73 (d, J = 8.4, 1 ar. H), 4.82 (d, J = 7.6, H-C(5)), 3.21 (s, MeO), 2.80 (s, MeN); MS (ESI) m/z 445.7 (M+ + 1). Anal. (C23H31N3O6·2HCl·2.6H2O·1.1C4H8O2) C, H, N.
(2S)-2-[(4,5α-Epoxy-3-hydroxy-14β-methoxy-17-methylmorphinan-6α-yl)-amino]butanedioic Acid Dihydrochloride (13a·2HCl)
Beige lyophilisate (76%); IR (KBr): 1722 (C=O) cm–1; 1H NMR (D2O): δ 6.83 (d, J = 8.2, 1 ar. H), 6.70 (d, J = 8.2, 1 ar. H), 5.00 (s, br, H-C(5)), 3.25 (s, MeO), 2.84 (s MeN+); MS (ESI): m/z 433 (M+ + 1). Anal. (C22H28N2O7·2HCl·2.0H2O) C, H, N.
(2S)-2-[(4,5α-Epoxy-3-hydroxy-14β-methoxy-17-methylmorphinan-6β-yl)amino]butanedioic Acid Dihydrochloride (13b·2HCl)
Colorless crystals from EtOH (83%); IR (KBr): 1729 (C=O) cm–1; 1H NMR (D2O): δ 6.82 (d, J = 8.1, 1 ar. H), 6.70 (d, J = 8.1, 1 ar. H), 4.84 (d, J = 7.2, H-C(5)), 3.26 (s, MeO), 2.85 (d, J = 3.4, MeN); MS (ESI): m/z 433 (M+ + 1). Anal. (C22H28N2O7·2HCl·2.0H2O) C, H, N.
(2S)-2-[(4,5α-Epoxy-3-hydroxy-14β-methoxy-17-methylmorphinan-6β-yl)-amino]pentanedioic Acid Dihydrochloride (14a·2HCl)
Colorless lyophilisate (40%); IR (KBr): 1731 (C=O) cm–1; 1H NMR (D2O): δ 6.82 (d, J = 8.4, 1 ar. H), 6.73 (d, J = 8.4, 1 ar. H), 4.94 (d, J = 3.0, H-C(5)), 3.27 (s, MeO), 2.86 (s MeN+); MS (ESI): m/z 446.6 (M+ + 1). Anal. (C23H30N2O7·2HCl·2.7H2O) C, H, N.
(2S)-2-[(4,5α-Epoxy-3-hydroxy-14β-methoxy-17-methylmorphinan-6β-yl)amino]pentanedioic Acid Dihydrochloride (14b·2HCl)
Colorless lyophilisate (97%); Mp >265 °C (dec.); IR (KBr): 1735 (C=O) cm–1; 1H NMR (D2O): δ 6.82 (d, J = 8.1, 1 ar. H), 6.70 (d, J = 8.1, 1 ar. H), 4.81 (d, J = 7.8, H-C(5)), 3.25 (s, MeO), 2.84 (s, MeN+); MS (ESI): m/z 446.7 (M+ + 1). Anal. (C23H30N2O7·2HCl·2.0H2O) C, H, N.
(2S)-2-[(4,5α-Epoxy-3-hydroxy-14β-methoxy-17-methylmorphinan-6α-yl)amino]propanoic Acid Dihydrochloride (15a·2HCl)
Off-white crystals (81%); Mp >230 °C (dec.); IR (KBr): 1736 (C=O); 1H NMR (DMSO-d6): δ 6.80 (d, J = 8.2, 1 ar. H), 6.64 (d, J = 8.2, 1 ar. H), 4.82 (d, J = 4.0, H-C(5)), 2.50 (s, MeN); MS (ESI): m/z 389.3 (M+ + 1). Anal. (C21H28N2O5·2HCl·2.4H2O·2.9C4H8O2) C, H, N.
(2S)-2-[(4,5α-Epoxy-3-hydroxy-14β-methoxy-17-methylmorphinan-6β-yl)amino]propanoic Acid Dihydrochloride (15b·2HCl)
Off-white crystals (66%); Mp >230 °C (dec.); IR (KBr): 1749 (C=O); 1H NMR (DMSO-d6): δ 6.77 (d, J = 8.1, 1 ar. H), 6.69 (d, J = 8.1, 1 ar. H), 5.02 (d, J = 7.0, H-C(5)), 2.50 (s, MeN); MS (ESI): m/z 389.2 (M+ + 1). Anal. (C21H28N2O5·2HCl·1.0H2O·0.1C4H8O2) C, H, N.
(2R)-2-[(4,5α-Epoxy-3,14β-dihydroxy-17-methylmorphinan-6α-yl)amino]-3-methylbutyric Acid Dihydrochloride (16a·2HCl)
Colorless crystals from EtOH (95%); Mp >291 °C (dec.); IR (KBr): 1729 (C=O) cm–1; 1H NMR (D2O): δ 6.82 (d, J = 8.4, 1 ar. H), 6.73 (d, J = 8.4, 1 ar. H), 4.95 (d, J = 2.8, H-C(5)), 3.25 (s, MeO), 2.86 (s, MeN), 1.02 (d, J = 7.0, CHMe), 0.95 (d, J = 7.0, CHMe); MS (ESI): m/z 416.7 (M+ + 1). Anal. (C23H32N2O5·2HCl·1.8H2O) C, H, N.
(2R)-2-[(4,5α-Epoxy-3,14β-dihydroxy-17-methylmorphinan-6β-yl)amino]-3-methylbutyric Acid Dihydrochloride (16b·2HCl)
Colorless crystals from EtOH (98%); Mp >322 °C (dec.); IR (KBr): 1729 (C=O) cm–1; 1H NMR (D2O): δ 6.81 (d, J = 8.6, 1 ar. H), 6.76 (d, J = 8.6, 1 ar. J = H), 4.83 (d, J = 8.0, H-C(5)), 3.24 (s, MeO), 2.84 (s, MeN), 1.00 (d, J = 7.5, CHMe), 0.96 (d, J = 7.5, CHMe); MS (ESI): m/z 416.7 (M+ + 1). Anal. (C23H32N2O5·2HCl·1.5H2O·0.5C2H5OH) C, H, N.
(2R)-2-[(4,5α-Epoxy-3-hydroxy-14β-methoxy-17-methyl-morphinan-6α-yl)-amino]-3-phenylpropionic Acid Dihydrochloride (17a·2HCl)
Beige crystals (70%); Mp >230 °C (dec.); IR (KBr): 1736 (C=O); 1H NMR (DMSO-d6): δ 9.37 and 9.10 (2s, 2H, CO2H, +NH), 7.30–7.27 (m, 5 ar. H), 6.79 (d, J = 8.2, 1 ar. H), 6.68 (d, J = 8.2, 1 ar. H), 4.73 (d, J = 3.0, H-C(5)), 2.50 (s, MeN); MS (ESI): m/z 465.4 (M+ + 1). Anal. (C27H32N2O5·2HCl·1.4H2O·0.3C4H8O2) C, H, N.
(2R)-2-[(4,5α-Epoxy-3-hydroxy-14β-methoxy-17-methyl-morphinan-6β-yl)-amino]-3-phenylpropionic Acid Dihydrochloride (17b·2HCl)
Beige crystals (85%); Mp >230 °C (dec.); IR (KBr): 1735 (C=O); 1H NMR (DMSO-d6): δ 9.46 and 9.12 (2s, 2H, CO2H, +NH), 7.19–7.45 (m, 5 ar. H), 6.76 (d, J = 7.8, 1 ar. H), 6.68 (d, J = 7.8, 1 ar. H), 4.50 (d, J = 6.6, H-C(5)), 2.50 (s, MeN); MS (ESI): m/z 465.4 (M+ + 1). Anal. (C27H32N2O5·2HCl·3.6H2O·0.2C4H8O2) C, H, N.
(2S)-2-[(4,5α-Epoxy-3-hydroxy-14β-methoxy-17-methyl-morphinan-6α-yl)-amino]-2-cyclohexylacetic Acid Dihydrochloride (18a·2HCl)
Beige crystals (89%); Mp >230 °C (dec.); IR (KBr): 1728 (C=O); 1H NMR (DMSO-d6): δ 9.28 and 9.05 (2s, 2H, CO2H, +NH), 6.77 (d, J = 8.0, 1 ar. H), 6.63 (d, J = 8.0, 1 ar. H), 4.79 (d, J = 3.2, H-C(5)), 2.50 (s, MeN); MS (ESI): m/z 457.4 (M+ + 1). Anal. (C26H37N2O5·2HCl·3.2H2O·0.6C4H8O2) C, H, N.
(2S)-2-[(4,5α-Epoxy-3-hydroxy-14β-methoxy-17-methyl-morphinan-6β-yl)-amino]-2-cyclohexylacetic Acid Dihydrochloride (18b·2HCl)
Beige crystals (81%); Mp >230 °C (dec.); IR (KBr): 1741 (C=O); 1H NMR (DMSO-d6): δ 9.56 and 9.19 (2s, 2H, CO2H, +NH), 6.80 (d, J = 8.0, 1 ar. H), 6.69 (d, J = 8.0, 1 ar. H), 4.78 (d, J = 6.6, H-C(5)), 2.50 (s, MeN); MS (ESI): m/z 457.4 (M+ + 1). Anal. (C26H37N2O5·2HCl·3.2H2O·0.3C4H8O2) C, H, N.
(2S)-2-[(4,5α-Epoxy-3-hydroxy-14β-methoxy-17-methylmorphinan-6α-yl)amino]-2-ethylacetic Acid Dihydrochloride (19a·2HCl)
Colorless crystals (95%); Mp >230 °C (dec.); IR (KBr): 1740 (C=O); 1H NMR (DMSO-d6): δ 9.31 and 9.14 (2s, 2H, CO2H, +NH), 6.78 (d, J = 8.3, 1 ar. H), 6.64 (d, J = 8.3, 1 ar. H), 4.96 (d, J = 4.0, H-C(5)), 2.50 (s, MeN); MS (ESI): m/z 403.4 (M+ + 1). Anal. (C22H30N2O5·2HCl·3.9H2O) C, H, N.
(2S)-2-[(4,5α-Epoxy-3-hydroxy-14β-methoxy-17-methylmorphinan-6β-yl)amino]-2-ethylacetic Acid Dihydrochloride (19b·2HCl)
Beige crystals (81%); Mp >230 °C (dec.); IR (KBr): 1741 (C=O); 1H NMR (DMSO-d6): δ 9.31 and 9.14 (2s, 2H, CO2H, +NH), 6.79 (d, J = 8.1, 1 ar. H), 6.69 (d, J = 8.1, 1 ar. H), 4.83 (d, J = 7.2, H-C(5)), 2.56 (s, MeN); MS (ESI): m/z 403.4 (M+ + 1). Anal. (C22H30N2O5·2HCl·3.5H2O·1.4C4H8O2) C, H, N.
3-[(4,5α-Epoxy-3-hydroxy-14β-methoxy-17-methylmorphinan-6α-yl)amino]propionic Acid Dihydrochloride (20a·2HCl)
Colorless crystals from EtOH (82%). Mp >290 °C (dec.); IR (KBr): 1724 (C=O) cm–1; 1H NMR (D2O): δ 6.82 (d, J = 8.2, 1 ar. H), 6.73 (d, J = 8.2, 1 ar. H), 5.01 (d, J = 3.4, H-C(5)), 3.28 (s, MeO), 2.87 (s, MeN); MS (ESI): m/z 388.8 (M+ + 1). Anal. (C21H28N2O5·2HCl·2.7H2O·1.0C2H5OH) C, H, N.
3-[(4,5α-Epoxy-3-hydroxy-14β-methoxy-17-methylmorphinan-6β-yl)amino]propionic Acid Dihydrochloride (20b·2HCl)
Colorless crystals (78%). Mp >270 °C (dec.); IR (KBr): 1724 (C=O) cm–1; 1H NMR (D2O): δ 6.83 (d, J = 8.4, 1 ar. H), 6.78 (d, J = 8.4, 1 ar. H), 5.01 (d, J = 7.8, H-C(5)), 3.25 (s, MeO), 2.85 (s, MeN); MS (ESI): m/z 388.8 (M+ + 1). Anal. (C21H28N2O5·2HCl·3.0H2O·) C, H, N.
3-[(4,5α-Epoxy-3-hydroxy-14β-methoxy-17-methylmorphinan-6α-yl)amino]butyric Acid Dihydrochloride (21a·2HCl)
Colorless crystals from EtOH (84%). Mp 267–269 °C (dec.); IR (KBr): 1719 (C=O) cm–1; 1H NMR (D2O): δ 6.82 (d, J = 8.3, 1 ar. H), 6.74 (d, J = 8.3, 1 ar. H), 5.01 (d, J = 3.8, H-C(5)), 3.28 (s, MeO), 2.87 (s, MeN); MS (ESI): m/z 402.7 (M+ + 1). Anal. (C22H30N2O5·2HCl·0.6H2O) C, H, N.
3-[(4,5α-Epoxy-3-hydroxy-14β-methoxy-17-methylmorphinan-6β-yl)amino]butyric Acid Dihydrochloride (21b·2HCl)
Colorless crystals from EtOH (84%); Mp 266–270 °C (dec.); IR (KBr): 1712 (C=O) cm–1; 1H NMR (D2O): δ 6.83 (d, J = 8.4, 1 ar. H), 6.78 (d, J = 8.4, 1 ar. H), 4.81 (d, J = 7.8, H-C(5)), 3.25 (s, MeO), 2.85 (s, MeN); MS (ESI): m/z 402.7 (M+ + 1). Anal. (C22H30N2O5·2HCl·2.3H2O) C, H, N.
(2S)-2-[[(4,5α-Epoxy-3-hydroxy-14β-methoxy-17-methylmorphinan-6α-yl)amino]-(2S)-3-methylbutyrylamino]-3-(4-hydroxyphenyl)propionic Acid (22a)
Colorless foam (91%); IR (KBr): 1720 (C=O); 1H NMR (D2O): δ 7.06–6.98 (m, 2 ar. H), 6.80–6.60 (m, 4 ar. H), 4.39 (m, H-C(5)), 3.25 (s, MeO), 2.40 (s, br, MeN), 0.80 (s, 3 H, isopropyl), 0.77 (s, 3 H, isopropyl); MS (ESI): m/z 579.7 (M+ + 1). Anal. (C32H41N3O7·3.9H2O) C, H, N.
(2S)-2-[[(4,5α-Epoxy-3-hydroxy-14β-methoxy-17-methylmorphinan-6β-yl)amino]-(2S)-3-methylbutyrylamino]-3-(4-hydroxyphenyl)propionic Acid (22b)
Colorless foam (95%); IR (KBr): 1734 (C=O); 1H NMR (DMSO-d6): δ 6.93 (d, J = 8.4, 1 ar. H), 6.84 (d, J = 8.4, 1 ar. H), 6.62–6.50 (m, 4 ar. H), 4.24 (d, J = 6.6, H-C(5)), 3.14 (s, MeO), 2.48 (s, MeN), 0.83 (s, J = 6.6, 3 H, isopropyl), 0.73 (s, J = 6.6, 3 H, isopropyl); MS (ESI): m/z 579.8 (M+ + 1). Anal. (C32H41N3O7·3.2H2O·0.1CH3OH) C, H, N.
2-[2-[(4,5-Epoxy-3-hydroxy-14β-methoxy-17-methylmorphinan-6β-yl)amino]acetylamino]acetic Acid (23a)
Colorless foam (77%); IR (KBr): 1752 (C=O). 1H NMR (DMSO-d6): δ 6.63 (d, J = 8.0, 1 ar. H), 6.51 (d, J = 8.0, 1 ar. H), 4.26 (d, J = 7.2, H-C(5)), 3.20 (s, MeO), 2.37 (s, MeN); MS (ESI): m/z 431.7 (M+ + 1). Anal. (C21H28N2O6·2.0H2O·1.0CH3OH) C, H, N.
Calculation of Physicochemical Properties
Coefficient of distribution, log D7.4, as log D at pH 7.4, was calculated (c log D7.4) with MarvinSketch 18.8 (ChemAxon, www.chemaxon.com).
Pharmacology: Drugs and Chemicals
Cell culture media and supplements were obtained from Sigma-Aldrich Chemicals (St. Louis, MO) or Life Technologies (Carlsbad, CA). Radioligands [3H]DAMGO, [3H]diprenorphine, [3H]U69,593, and [35S]GTPγS were purchased from PerkinElmer (Boston). [3H][Ile5,6]deltorphin II was obtained from the Institute of Isotopes Co. Ltd. (Budapest, Hungary). DAMGO, [d-Pen2,d-Pen5]enkephalin (DPDPE), diprenorphine, U69,593, naloxone, naloxone methiodide, tris(hydroxymethyl) aminomethane (Tris), 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid (HEPES), unlabeled GTPγS, and guanosine diphosphate (GDP) were obtained from Sigma-Aldrich Chemicals (St. Louis, MO). Morphine hydrochloride was obtained from Gatt-Koller GmbH (Innsbruck, Austria). All other chemicals were of analytical grade and obtained from standard commercial sources. Test compounds were prepared as 1 mM stocks in water and further diluted to working concentrations in the appropriate medium.
Animals
Sprague-Dawley rat brains and guinea-pig brains were obtained frozen from Labortierkunde und Laborgenetik, Medizinische Universität Wien (Himberg, Austria). Male CD1 mice (30–35 g) were obtained from the Center of Biomodels and Experimental Medicine (CBEM) (Innsbruck, Austria). Mice were group-housed in a temperature-controlled room with a 12 h light/dark cycle and with free access to food and water. All animal studies were conducted in accordance with ethical guidelines and animal welfare standards according to Austrian regulations for animal research and were approved by the Committee of Animal Care of the Austrian Federal Ministry of Science and Research.
Cell Culture
CHO cells stably expressing human opioid receptors, MOR, DOR, or KOR (CHO-hMOR, CHO-hDOR, and CHO-hKOR cell lines), were kindly provided by Dr. Lawrence Toll (SRI International, Menlo Park, CA). The CHO-hMOR and CHO-hDOR cell lines were maintained in Dulbecco’s Minimal Essential Medium (DMEM)/Ham’s F-12 medium supplemented with fetal bovine serum (FBS, 10%), penicillin/streptomycin (0.1%), l-glutamine (2 mM), and geneticin (400 μg/mL). The CHO-hKOR cell line was maintained in DMEM supplemented with FBS (10%), penicillin/streptomycin (0.1%), l-glutamine (2 mM), and geneticin (400 μg/ml). Cell cultures were maintained at 37 °C in 5% CO2 humidified air.
Membrane Preparation
Membranes were prepared from Sprague-Dawley rat brains or guinea-pig brains according to the described procedures.44,45 Brains without cerebella were homogenized on ice in 5 vol/wt of ice-cold 50 mM Tris-HCl buffer (pH 7.4) and diluted in 30 vol/wt of the same buffer. After centrifugation at 40 000g for 20 min at 4 °C, the pellets were resuspended in 30 vol/wt of 50 mM Tris-HCl buffer (pH 7.4) and incubated at 37 °C for 30 min. The centrifugation step described above was repeated, and the final pellets were resuspended in 5 vol/wt of 50 mM Tris-HCl buffer (pH 7.4) containing 0.32 M sucrose and stored at −80 °C until use.
Membranes from CHO cells expressing human opioid receptors were prepared in 50 mM Tris-HCl buffer (pH 7.7) according to a previously described procedure.64 Cells expressing human opioid receptors grown at confluence were removed from the culture plates by scraping, homogenized in 50 mM Tris buffer (pH 7.7), using a Polytron homogenizer, then centrifuged once and washed by an additional centrifugation at 27 000g for 15 min, at 4 °C. The final pellet was resuspended in Tris buffer and stored at −80 °C until use. Protein content of brain and cell membrane preparation homogenates was determined by the method of Bradford using bovine serum albumin as the standard.65
Radioligand Binding Assays for Opioid Receptors
Binding assays were performed in 50 mM Tris-HCl buffer (pH 7.4) in a final volume of 1 mL, with rodent brain preparations (0.3–0.5 mg protein) or membranes from CHO cells expressing the human opioid receptors (15–20 μg) and various concentrations of test compound as described previously.44,49,64 Rat brain membranes were incubated with either [3H]DAMGO (1 nM, 45 min, 35 °C) or [3H][Ile5,6]deltorphin II (0.5 nM, 45 min, 35 °C) for labeling MOR or DOR receptors, respectively. Guinea-pig brain membranes were incubated with [3H]U69,593 (1 nM, 30 min, 30 °C) for labeling KOR. Binding assays with CHO cell membranes were conducted at 25 °C for 60 min using [3H]DAMGO (1 nM) or [3H]diprenorphine (0.2 nM) for labeling MOR or DOR, respectively. Nonspecific binding was determined using 10 μM naloxone (rodent brain) or 1–10 μM of the unlabeled counterpart of each radioligand (CHO cells). Reactions were terminated by rapid filtration through Whatman glass fiber GF/C filters. Filters were washed three times with 5 mL of ice-cold 50 mM Tris-HCl buffer (pH 7.4) using a Brandel M24R cell harvester (Gaithersburg, MD). Radioactivity retained on the filters was counted by liquid scintillation counting using a Beckman Coulter LS6500 (Beckman Coulter Inc., Fullerton, CA). All binding experiments were performed in duplicate and repeated at least three times.
[35S]GTPγS Functional Assays for Opioid Receptors
Binding of [35S]GTPγS to membranes from CHO cells stably expressing the human opioid receptors was conducted according to the published procedures.49,64 Cell membranes (5–10 μg) in 20 mM HEPES, 10 mM MgCl2, and 100 mM NaCl (pH 7.4) were incubated with 0.05 nM [35S]GTPγS, 10 μM GDP, and various concentrations of test compound in a final volume of 1 mL, for 60 min at 25 °C. Nonspecific binding was determined using 10 μM GTPγS, and the basal binding was determined in the absence of test ligand. Samples are filtered over glass Whatman glass GF/B fiber filters and counted as described for binding assays. All experiments were performed in duplicate and repeated at least three times.
Drug Administration
Solutions of test compounds were prepared in sterile physiological 0.9% saline and further diluted to working doses in saline solution. Test compounds or saline (control) were administered by sc route in a volume of 10 μL/1 g of body weight. All doses are expressed in terms of salts. Separate groups of mice received the respective dose of compound, and individual mice were only used once for behavioral testing.
Acetic Acid-Induced Writhing Assay
Writhing was induced in male CD1 mice by ip injection of a 0.6% acetic acid aqueous solution as described previously.48,62 Groups of mice were administered sc different doses of test compound or saline (control), and 5 min prior to testing (25 min after drug or saline), each animal received an ip injection of acetic acid solution. Each mouse was placed in individual transparent Plexiglas chambers, and the number of writhes was counted during a 10 min observation period. Antinociceptive activity, as percentage decrease in number of writhes compared to the control group, was calculated according to the following formula: % inhibition of writhing = 100 × [(C – T)/C], where C is the mean number of writhes in control animals and T is the number of writhes in drug-treated mice. For the antagonism study, naloxone methiodide was sc co-administered with the respective opioid agonist, and writhing was assessed as described above.
Data and Statistical Analysis
Data were analyzed and graphically processed using the GraphPad Prism 5.0 Software (GraphPad Prism Software Inc., San Diego, CA). For in vitro assays, inhibition constant (Ki in nM), potency (EC50 in nM), and efficacy (% stimulation) values were determined from concentration–response curves by nonlinear regression analysis. The Ki values were determined by the method of Cheng and Prusoff.66 In the [35S]GTPγS binding assays, efficacy was determined relative to the reference full opioid agonists, DAMGO (MOR), DPDPE (DOR), and U69,593 (KOR). In vitro data are presented as mean ± SEM of three to four independent experiments each performed in duplicate. For the writhing assay, dose–response relationship of percentage inhibition of writhing was constructed, and the doses necessary to produce a 50% effect (ED50) and 95% confidence intervals (95% CI) were calculated.67 Each experimental group included six to seven mice. Data were statistically evaluated using one-way ANOVA with Tukey’s post hoc test for multiple comparisons with significance set at P < 0.05.
Acknowledgments
The authors acknowledge Tasmanian Alkaloids Pty. Ltd., Westbury, Tasmania, Australia, for the generous gift of thebaine. The authors also thank Andrea Kaletsch and Beatrix Jungwirth for technical assistance. This research was supported by the Austrian Science Fund (FWF: P21350, TRP16-B18 and TRP19-B18), Tyrolean Research Fund (TWF-UNI-0404/949), and the University of Innsbruck.
Glossary
Abbreviations
- BBB
blood–brain barrier
- CHO
Chinese hamster ovary
- CNS
central nervous system
- DAMGO
[d-Ala2,N-Me-Phe4,Gly-ol5]enkephalin
- DOR
δ opioid receptor
- DPDPE
[d-Pen2,d-Pen5]enkephalin
- GABA
γ-aminobutyric acid
- MOR
μ opioid receptor
- ip
intraperitoneal
- KOR
κ opioid receptor
- l-Abu
l-2-aminobutyric acid
- l-Chg
l-cyclohexylglycine
- NLXM
naloxone methodide
- SAR
structure–activity relationships
- sc
subcutaneous
- U69,593
N-methyl-2-phenyl-N-[(5R,7S,8S)-7-(pyrrolidin-1-yl)-1-oxaspiro[4.5]dec-8-yl]acetamide
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jmedchem.8b01327.
Additional chemical and pharmacological information: general procedure for the synthesis of 6-amino acid esters 24–41; binding affinities of 6-amino acid (2a/b, 3a/b, 4a/b, 14a/b, 15a/b, 17a/b, and 19a/b) and 6-dipeptide substituted derivatives (22a/b) at the human opioid receptors (PDF)
Molecular formula strings (CSV)
Author Contributions
§ S.R. and T.B.H. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Yekkirala A. S.; Roberson D. P.; Bean B. P.; Woolf C. J. Breaking Barriers to Novel Analgesic Drug Development. Nat. Rev. Drug Discovery 2017, 16, 545–564. 10.1038/nrd.2017.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur B. M.; Lala P. K.; Shaw J. L. Pharmacogenetics of Chronic Pain Management. Clin. Biochem. 2014, 47, 1169–1187. 10.1016/j.clinbiochem.2014.05.065. [DOI] [PubMed] [Google Scholar]
- Benyamin R.; Trescot A. M.; Datta S.; Buenaventura R.; Adlaka R.; Sehgal N.; Glaser S. E.; Vallejo R. Opioid Complications and Side Effects. Pain Physician 2008, 11, S105–120. [PubMed] [Google Scholar]
- Imam M. Z.; Kuo A.; Ghassabian S.; Smith M. T. Progress in Understanding Mechanisms of Opioid-induced Gastrointestinal Adverse Effects and Respiratory Depression. Neuropharmacology 2018, 131, 238–255. 10.1016/j.neuropharm.2017.12.032. [DOI] [PubMed] [Google Scholar]
- Volkow N.; Benveniste H.; McLellan A. T. Use and Misuse of Opioids in Chronic Pain. Annu. Rev. Med. 2018, 69, 451–465. 10.1146/annurev-med-011817-044739. [DOI] [PubMed] [Google Scholar]
- Severino A. L.; Shadfar A.; Hakimian J. K.; Crane O.; Singh G.; Heinzerling K.; Walwyn W. M. Pain Therapy Guided by Purpose and Perspective in Light of the Opioid Epidemic. Front. Psychiatry 2018, 9, 119. 10.3389/fpsyt.2018.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C. Opioid Receptors. Annu. Rev. Med. 2016, 67, 433–451. 10.1146/annurev-med-062613-093100. [DOI] [PubMed] [Google Scholar]
- Corder G.; Castro D. C.; Bruchas M. R.; Scherrer G. Endogenous and Exogenous Opioids in Pain. Annu. Rev. Neurosci. 2018, 41, 453–473. 10.1146/annurev-neuro-080317-061522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y.; Filizola M. Opioid Receptors: Structural and Mechanistic Insights into Pharmacology and Signaling. Eur. J. Pharmacol. 2015, 763, 206–213. 10.1016/j.ejphar.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A.; Fox C. A.; Akil H.; Watson S. J. Opioid-receptor mRNA Expression in the Rat CNS: Anatomical and Functional Implications. Trends Neurosci. 1995, 18, 22–29. 10.1016/0166-2236(95)93946-U. [DOI] [PubMed] [Google Scholar]
- Stein C. Opioid Receptors on Peripheral Sensory Neurons. Adv. Exp. Med. Biol. 2003, 521, 69–76. [PubMed] [Google Scholar]
- Holzer P. Opioids and Opioid Receptors in the Enteric Nervous System: From a Problem in Opioid Analgesia to a Possible New Prokinetic Therapy in Humans. Neurosci. Lett. 2004, 361, 192–195. 10.1016/j.neulet.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Lutz P. E.; Kieffer B. L. Opioid Receptors: Distinct Roles in Mood Disorders. Trends Neurosci. 2013, 36, 195–206. 10.1016/j.tins.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak G. W.; Pan Y. X. Mu Opioids and Their Receptors: Evolution of a Concept. Pharmacol. Rev. 2013, 65, 1257–1317. 10.1124/pr.112.007138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosser T.; Woolf C. J.; FitzGerald G. A. Time for Nonaddictive Relief of Pain. Science 2017, 355, 1026–1027. 10.1126/science.aan0088. [DOI] [PubMed] [Google Scholar]
- Bannister K.; Kucharczyk M.; Dickenson A. H. Hopes for the Future of Pain Control. Pain Ther. 2017, 6, 117–128. 10.1007/s40122-017-0073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C.; Schäfer M.; Machelska H. Attacking Pain at its Source: New Perspectives on Opioids. Nat. Med. 2003, 9, 1003–1008. 10.1038/nm908. [DOI] [PubMed] [Google Scholar]
- Albert-Vartanian A.; Boyd M. R.; Hall A. L.; Morgado S. J.; Nguyen E.; Nguyen V. P.; Patel S. P.; Russo L. J.; Shao A. J.; Raffa R. B. Will Peripherally Restricted κ Opioid Receptor Agonists (pKORAs) Relieve Pain with Less Opioid Adverse Effects and Abuse Potential?. J. Clin. Pharm. Ther. 2016, 41, 371–382. 10.1111/jcpt.12404. [DOI] [PubMed] [Google Scholar]
- Kivell B.; Prisinzano T. E. Kappa Opioids and the Modulation of Pain. Psychopharmacology 2010, 210, 109–119. 10.1007/s00213-010-1819-6. [DOI] [PubMed] [Google Scholar]
- Kleczkowska P.; Lipkowski A. W.; Tourwé D.; Ballet S. Hybrid Opioid/Non-opioid Ligands in Pain Research. Curr. Pharm. Des. 2013, 19, 7435–7450. 10.2174/138161281942140105165646. [DOI] [PubMed] [Google Scholar]
- Günther T.; Dasgupta P.; Mann A.; Miess E.; Kliewer A.; Fritzwanker S.; Steinborn R.; Schulz S. Targeting Multiple Opioid Receptors - Improved Analgesics with Reduced Side Effects?. Br. J. Pharmacol. 2018, 175, 2857–2868. 10.1111/bph.13809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogra S.; Yadav P. N. Biased Agonism at Kappa Opioid Receptors: Implication in Pain and Mood Disorders. Eur. J. Pharmacol. 2015, 763, 184–190. 10.1016/j.ejphar.2015.07.018. [DOI] [PubMed] [Google Scholar]
- Manglik A.; Lin H.; Aryal D. K.; McCorvy J. D.; Dengler D.; Corder G.; Levit A.; Kling R. C.; Bernat V.; Hübner H.; Huang X. P.; Sassano M. F.; Giguère P. M.; Löber S.; Duan D.; Scherrer G.; Kobilka B. K.; Gmeiner P.; Roth B. L.; Shoichet B. K. Structure-based Discovery of Opioid Analgesics with Reduced Side Effects. Nature 2016, 537, 185–190. 10.1038/nature19112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid C. L.; Kennedy N. M.; Ross N. C.; Lovell K. M.; Yue Z.; Morgenweck J.; Cameron M. D.; Bannister T. D.; Bohn L. M. Bias Factor and Therapeutic Window Correlate to Predict Safer Opioid Analgesics. Cell 2017, 171, 1165–1175. 10.1016/j.cell.2017.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setnik B.; Schoedel K. A.; Levy-Cooperman N.; Shram M.; Pixton G. C.; Roland C. L. Evaluating the Abuse Potential of Opioids and Abuse-deterrent Opioid Formulations: A Review of clinical Study Methodology. J. Opioid Manage. 2017, 13, 485–523. 10.5055/jom.2017.0412. [DOI] [PubMed] [Google Scholar]
- Pergolizzi J. V. Jr.; Raffa R. B.; Taylor R. Jr.; Vacalis S. Abuse-deterrent Opioids: An Update on Current Approaches and Considerations. Curr. Med. Res. Opin. 2018, 34, 711–723. 10.1080/03007995.2017.1419171. [DOI] [PubMed] [Google Scholar]
- Brown D. R.; Goldberg L. I. The Use of Quaternary Narcotic Antagonists in Opiate Research. Neuropharmacology 1985, 24, 181–191. 10.1016/0028-3908(85)90072-3. [DOI] [PubMed] [Google Scholar]
- DeHaven-Hudkins D. L.; Dolle R. E. Peripherally Restricted Agonists as Novel Analgesic Agents. Curr. Pharm. Des. 2004, 10, 743–757. 10.2174/1381612043453036. [DOI] [PubMed] [Google Scholar]
- Sehgal N.; Smith H. S.; Manchikanti L. Peripherally Acting Opioids and Clinical Implications for Pain Control. Pain Physician 2011, 14, 249–258. [PubMed] [Google Scholar]
- Spetea M.; Asim M. F.; Wolber G.; Schmidhammer H. The μ Opioid Receptor and Ligands Acting at the μ Opioid Receptor, as Therapeutics and Potential Therapeutics. Curr. Pharm. Des. 2013, 19, 7415–7434. 10.2174/13816128113199990362. [DOI] [PubMed] [Google Scholar]
- Iorio M. A.; Frigeni V. Narcotic Agonist/Antagonist Properties of Quaternary Diastereoisomers Derived from Oxymorphone and Naloxone. Eur. J. Med. Chem. 1984, 19, 301–303. [Google Scholar]
- Botros S.; Lipkowski A. W.; Larson D. L.; Stark A. P.; Takemori A. E.; Portoghese P. S. Opioid Agonist and Antagonist Activities of Peripherally Selective Derivatives of Naltrexamine and Oxymorphamine. J. Med. Chem. 1989, 32, 2068–2071. 10.1021/jm00129a009. [DOI] [PubMed] [Google Scholar]
- Larson D. L.; Hua M.; Takemori A. K.; Portoghese P. S. Possible Contribution of a Glutathione Conjugate to the Long-duration Action of β-funaltrexamine. J. Med. Chem. 1993, 36, 3669–3673. 10.1021/jm00075a023. [DOI] [PubMed] [Google Scholar]
- Lacko E.; Varadi A.; Rapavi R.; Zador F.; Riba P.; Benyhe S.; Borsodi A.; Hosztafi S.; Timar J.; Noszal B.; Furst S.; Al-Khrasani M. A Novel μ-opioid Receptor Ligand with High In Vitro and In Vivo Agonist Efficacy. Curr. Med. Chem. 2012, 19, 4699–4707. 10.2174/092986712803306376. [DOI] [PubMed] [Google Scholar]
- Balogh M.; Zádori Z. S.; Lázár B.; Karádi D.; László S.; Mousa S. A.; Hosztafi S.; Zádor F.; Riba P.; Schäfer M.; Fürst S.; Al-Khrasani M. The Peripheral Versus Central Antinociception of a Novel Opioid Agonist: Acute Inflammatory Pain in Rats. Neurochem. Res. 2018, 43, 1250–1257. 10.1007/s11064-018-2542-7. [DOI] [PubMed] [Google Scholar]
- Bourgeois C.; Werfel E.; Galla F.; Lehmkuhl K.; Torres-Gómez H.; Schepmann D.; Kögel B.; Christoph T.; Straßburger W.; Englberger W.; Soeberdt M.; Hüwel S.; Galla H. J.; Wünsch B. Synthesis and Pharmacological Evaluation of 5-pyrrolidinylquinoxalines as a Novel Class of Peripherally Restricted κ-opioid Receptor Agonists. J. Med. Chem. 2014, 57, 6845–6860. 10.1021/jm500940q. [DOI] [PubMed] [Google Scholar]
- Schiller P. W. Opioid Peptide-derived Analgesics. AAPS J. 2005, 7, E560–565. 10.1208/aapsj070356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldrich J. V.; McLaughlin J. P. Peptide Kappa Opioid Receptor Ligands: Potential for Drug Development. AAPS J. 2009, 11, 312–322. 10.1208/s12248-009-9105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spahn V.; Del Vecchio G.; Labuz D.; Rodriguez-Gaztelumendi A.; Massaly N.; Temp J.; Durmaz V.; Sabri P.; Reidelbach M.; Machelska H.; Weber M.; Stein C. A Nontoxic Pain killer Designed by Modeling of Pathological Receptor Conformations. Science 2017, 355, 966–969. 10.1126/science.aai8636. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Gaztelumendi A.; Spahn V.; Labuz D.; Machelska H.; Stein C. Analgesic Effects of a Novel pH-dependent μ-opioid Receptor Agonist in Models of Neuropathic and Abdominal Pain. Pain. 2018, 159, 2277–2284. 10.1097/j.pain.0000000000001328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Rodríguez S.; Quadir M. A.; Gupta S.; Walker K. A.; Zhang X.; Spahn V.; Labuz D.; Rodriguez-Gaztelumendi A.; Schmelz M.; Joseph J.; Parr M. K.; Machelska H.; Haag R.; Stein C. Polyglycerol-opioid Conjugate Produces Analgesia Devoid of Side Effects. Elife 2017, 6, e2708 10.7554/eLife.27081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidhammer H.; Aeppli L.; Atwell L.; Fritsch F.; Jacobson A. E.; Nebuchla M.; Sperk G. Synthesis and Biological Evaluation of 14-Alkoxymorphinans. 1. Highly Potent Opioid Agonists in the Series of (−)-14-Methoxy-N-methylmorphinan-6-ones. J. Med. Chem. 1984, 27, 1575–1579. 10.1021/jm00378a009. [DOI] [PubMed] [Google Scholar]
- Schütz J.; Brandt W.; Spetea M.; Wurst K.; Wunder G.; Schmidhammer H. Synthesis of 6-amino Acid Substituted Derivatives of the Highly Potent Analgesic 14-O-Methyloxymorphone. Helv. Chim. Acta 2003, 86, 2142–2148. 10.1002/hlca.200390171. [DOI] [Google Scholar]
- Spetea M.; Friedmann T.; Riba P.; Schütz J.; Wunder G.; Langer T.; Schmidhammer H.; Fürst S. In Vitro Opioid Activity Profiles of 6-Amino Acid Substituted Derivatives of 14-O-Methyloxymorphone. Eur. J. Pharmacol. 2004, 483, 301–308. 10.1016/j.ejphar.2003.10.049. [DOI] [PubMed] [Google Scholar]
- Fürst S.; Riba P.; Friedmann T.; Timar J.; Al-Khrasani M.; Obara I.; Makuch W.; Spetea M.; Schütz J.; Przewlocki R.; Przewlocka B.; Schmidhammer H. Peripheral Versus Central Antinociceptive Actions of 6-amino Acid-substituted Derivatives of 14-O-Methyloxymorphone in Acute and Inflammatory Pain in the Rat. J. Pharmacol. Exp. Ther. 2005, 312, 609–618. 10.1124/jpet.104.075176. [DOI] [PubMed] [Google Scholar]
- Bileviciute-Ljungar I.; Spetea M.; Guo Y.; Schütz J.; Windisch P.; Schmidhammer H. Peripherally Mediated Antinociception of the μ-opioid Receptor Agonist 2-[(4,5α-Epoxy-3-Hydroxy-14β-Methoxy-17-Methylmorphinan-6β-yl)Amino]Acetic Acid (HS-731) after Subcutaneous and Oral Administration in Rats with Carrageenan-induced Hindpaw Inflammation. J. Pharmacol. Exp. Ther. 2006, 317, 220–227. 10.1124/jpet.105.096032. [DOI] [PubMed] [Google Scholar]
- Obara I.; Makuch W.; Spetea M.; Schütz J.; Schmidhammer H.; Przewlocki R.; Przewlocka B. Local Peripheral Antinociceptive Effects of 14-O-methyloxymorphone Derivatives in Inflammatory and Neuropathic Pain in the Rat. Eur. J. Pharmacol. 2007, 558, 60–67. 10.1016/j.ejphar.2006.11.037. [DOI] [PubMed] [Google Scholar]
- Al-Khrasani M.; Spetea M.; Friedmann T.; Riba P.; Kiraly K.; Schmidhammer H.; Fürst S. DAMGO and 6β-Glycine Substituted 14-O-Methyloxymorphone but not Morphine Show Peripheral, Preemptive Antinociception after Systemic Administration in a Mouse Visceral Pain Model and High Intrinsic Efficacy in the Isolated Rat Vas Deferens. Brain Res. Bull. 2007, 74, 369–375. 10.1016/j.brainresbull.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Spetea M.; Windisch P.; Guo Y.; Bileviciute-Ljungar I.; Schütz J.; Asim M. F.; Berzetei-Gurske I. P.; Riba P.; Kiraly K.; Fürst S.; Al-Khrasani M.; Schmidhammer H. Synthesis and Pharmacological Activities of 6-Glycine Substituted 14-phenylpropoxymorphinans, a Novel Class of Opioids with High Opioid Receptor Affinities and Antinociceptive Potencies. J. Med. Chem. 2011, 54, 980–988. 10.1021/jm101211p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillie L. D.; Schmidhammer H.; Mulligan S. J. Peripheral μ-opioid Receptor Mediated Inhibition of Calcium Signaling and Action Potential-evoked Calcium Fluorescent Transients in Primary Afferent CGRP Nociceptive Terminals. Neuropharmacology 2015, 93, 267–273. 10.1016/j.neuropharm.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Jiang J. B.; Hanson R. N.; Portoghese P. S.; Takemori A. E. Stereochemical Studies on Medicinal Agents. 23. Synthesis and Biological Evaluation of 6-Amino Derivatives of Naloxone and Naltrexone. J. Med. Chem. 1977, 20, 1100–1102. 10.1021/jm00218a023. [DOI] [PubMed] [Google Scholar]
- Sayre L. M.; Portoghese P. S. Stereospecific Synthesis of the 6α- and 6β-amino Derivatives of Naltrexone and Oxymorphone. J. Org. Chem. 1980, 45, 3366–3368. 10.1021/jo01304a051. [DOI] [Google Scholar]
- Handa B. K.; Land A. C.; Lord J. A.; Morgan B. A.; Rance M. J.; Smith C. F. Analogues of β-LPH61-64 Possessing Selective Agonist Activity at Mu-opiate Receptors. Eur. J. Pharmacol. 1981, 70, 531–540. 10.1016/0014-2999(81)90364-2. [DOI] [PubMed] [Google Scholar]
- Mosberg H. I.; Hurst R.; Hruby V. J.; Gee K.; Yamamura H. I.; Galligan J. J.; Burks T. F. Bis-penicillamine Enkephalins Possess Highly Improved Specificity Toward Delta Opioid Receptors. Proc. Natl. Acad. Sci. USA 1983, 80, 5871–5874. 10.1073/pnas.80.19.5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahti R. A.; Mickelson M. M.; McCall J. M.; Von Voigtlander P. F. [3H]U-69,593 a Highly Selective Ligand for the Opioid Kappa Receptor. Eur. J. Pharmacol. 1985, 109, 281–284. 10.1016/0014-2999(85)90431-5. [DOI] [PubMed] [Google Scholar]
- Avdeef A.; Testa B. Physicochemical Profiling in Drug Research: A Brief Survey of the State-of-the-art of Experimental Techniques. Cell. Mol. Life Sci. 2002, 59, 1681–1689. 10.1007/PL00012496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faller B.Physicochemical Profiling in Early Drug Discovery: New Challenges at the Age of High-Throughput Screen and Combinatorial Chemistry. In Chemistry and Molecular Aspects of Drug Design and Action; Rekka E. A., Kourounakis P. N., Eds.; CRC: Boca Raton, FL, 2008; pp 303–312. [Google Scholar]
- Bhal S. K.; Kassam K.; Peirson I. G.; Pearl G. M. The Rule of Five Revisited: Applying log D in Place of log P in Drug-likeness Filters. Mol. Pharm. 2007, 4, 556–660. 10.1021/mp0700209. [DOI] [PubMed] [Google Scholar]
- Kokate A.; Li X.; Jasti B. Effect of Drug Lipophilicity and Ionization on Permeability Across the Buccal Mucosa: A Technical Note. AAPS PharmSciTech 2008, 9, 501–504. 10.1208/s12249-008-9071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L.; Kim J.; Ou C.; McFadden W.; van Rijn R. M.; Whistler J. L. Methadone Antinociception is Dependent on Peripheral Opioid Receptors. J. Pain 2009, 10, 369–379. 10.1016/j.jpain.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery J. J.; Raymond T. J.; Giuvelis D.; Bidlack J. M.; Polt R.; Bilsky E. J. In Vivo Characterization of MMP-2200, a Mixed δ/μ Opioid Agonist, in Mice. J. Pharmacol. Exp. Ther. 2011, 336, 767–778. 10.1124/jpet.110.172866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spetea M.; Berzetei-Gurske I. P.; Guerrieri E.; Schmidhammer H. Discovery and Pharmacological Evaluation of a Diphenethylamine Derivative (HS665), a Highly Potent and Selective κ Opioid Receptor Agonist. J. Med. Chem. 2012, 55, 10302–10306. 10.1021/jm301258w. [DOI] [PubMed] [Google Scholar]
- Kramer T. H.; Shook J. E.; Kazmierski W.; Ayres E. A.; Wire W. S.; Hruby V. J.; Burks T. F. Novel Peptidic Mu-opioid Antagonists: Pharmacological Characterization In Vitro and In Vivo. J. Pharmacol. Exp. Ther. 1989, 249, 544–551. [PubMed] [Google Scholar]
- Dumitrascuta M.; Ben Haddou T.; Guerrieri E.; Noha S. M.; Schläfer L.; Schmidhammer H.; Spetea M. Synthesis, Pharmacology, and Molecular Docking Studies on 6-desoxo-N-methylmorphinans as Potent μ-opioid Receptor Agonists. J. Med. Chem. 2017, 60, 9407–9412. 10.1021/acs.jmedchem.7b01363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A Rapid and Sensitive Method for the Quantification of Microgram Quantities of Protein Utilizing the Principle of Protein-dye Binding. Anal. Biochem. 1976, 72, 248–254. 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cheng Y.; Prusoff W. H. Relationship Between the Inhibition Constant (K1) and the Concentration of Inhibitor Which Causes 50 Per Cent Inhibition (I50) of an Enzymatic Reaction. Biochem. Pharmacol. 1973, 22, 3099–3108. 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Litchfield J. T. Jr.; Wilcoxon F. A Simplified Method of Evaluating Dose-effect Experiments. J. Pharmacol. Exp. Ther. 1949, 96, 99–113. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.