Abstract
Objectives
The aim of this study was to assess the effects of concomitant administration of erenumab and sumatriptan on resting blood pressure, pharmacokinetics, safety, and tolerability in healthy subjects.
Methods
In this phase 1, parallel-group, one-way crossover, double-blind, placebo-controlled study, healthy adult subjects were randomized (1:2) to receive either intravenous placebo and subcutaneous sumatriptan 12 mg (i.e. two 6-mg injections separated by 1 hour) or intravenous erenumab 140 mg and subcutaneous sumatriptan 12 mg. Blood pressure was measured pre-dose and at prespecified times post-dose. The primary endpoint was individual time-weighted averages of mean arterial pressure, measured from 0 hours to 2.5 hours after the first dose of sumatriptan. Pharmacokinetic parameters for sumatriptan were evaluated by calculating geometric mean ratios (erenumab and sumatriptan/placebo and sumatriptan). Adverse events and anti-erenumab antibodies were also evaluated.
Results
A total of 34 subjects were randomized and included in the analysis. Least squares mean (standard error) time-weighted averages of mean arterial pressure were 87.4 (1.0) mmHg for the placebo and sumatriptan group and 87.4 (1.2) mmHg for the erenumab and sumatriptan group. Mean difference in mean arterial pressure between groups was −0.04 mmHg (90% confidence interval: −2.2, 2.1). Geometric mean ratio estimates for maximum plasma concentration of sumatriptan was 0.95 (90% confidence interval: 0.82, 1.09), area under the plasma concentration–time curve (AUC) from time 0 to 6 hours was 0.98 (90% confidence interval: 0.93, 1.03), and AUC from time 0 to infinity was 1.00 (90% confidence interval: 0.96, 1.05). No clinically relevant safety findings for co-administration of sumatriptan and erenumab were identified.
Conclusion
Co-administration of erenumab and sumatriptan had no additional effect on resting blood pressure or on pharmacokinetics of sumatriptan. Trial registration: ClinicalTrials.gov, NCT02741310.
Keywords: Erenumab, blood pressure, migraine, sumatriptan, clinical trial, pharmacokinetics, safety
Introduction
Migraine is a disabling neurological disease, the symptoms of which impact work productivity (1) and increase healthcare expenditures (2). Pharmacological therapy for migraine traditionally includes acute and preventive treatments. The goal of acute migraine treatment is to relieve the symptoms associated with migraine attacks, particularly headache pain, whereas the goal of preventive treatment is to reduce migraine frequency/severity (3). Nonsteroidal anti-inflammatory drugs and migraine-specific drugs, such as triptans, are frequently used as acute migraine medications (4,5). Triptans are selective serotonin 5-HT1B/1D receptor agonists that have vasoconstrictive effects on human blood vessels, including cerebral and coronary arteries, mediated through activation of 5-HT1B receptors situated on vascular smooth muscle cells (6–8). Triptans are active mainly at the cranial vasculature but can also cause peripheral vasoconstriction (9) and have been shown to slightly increase blood pressure (BP) (10); consequently, they are contraindicated in patients with uncontrolled hypertension. However, these medications are generally well tolerated among patients with migraine (11), including those with controlled hypertension (9,12).
In addition to the treatment of migraine attacks with acute medications, many patients may benefit from preventive therapy. Current standard preventive treatments include orally administered medications such as topiramate, propranolol, and amitriptyline, which are underused (13) mostly because of safety and tolerability issues (14). Calcitonin gene-related peptide (CGRP) is a neuropeptide that is involved in the pathophysiology of migraine through nociceptive modulation in the trigeminal vascular system (15–18) and has been shown to mediate vasodilation (19). Clinical trials of monoclonal antibodies that target the CGRP pathway suggest that CGRP inhibition is a promising approach in migraine prevention (20–26). Erenumab is a fully human monoclonal antibody that binds to and inhibits the CGRP receptor (27) and has shown efficacy in the prevention of migraine (24–26). Patients who suffer from frequent and moderate to severe migraine attacks require both acute and preventive therapies to manage their symptoms (28). Due to the well-known vasoconstrictive effects of triptans, and the theoretical risk of BP increase with CGRP inhibition, it is important to assess the effect of concomitant administration of erenumab and sumatriptan on BP. To date, clinical trials with erenumab have shown no evidence of increased BP following erenumab administration (24–26), including an analysis of BP using 24-hour ambulatory BP monitoring (29).
The objectives of this study were to assess the effects of combination treatment of erenumab and sumatriptan compared with sumatriptan and placebo on resting BP, pharmacokinetics (PK), safety, and tolerability in healthy subjects. The clinical hypothesis for the study was that there would be no clinically meaningful difference between the effects of placebo and sumatriptan compared with erenumab and sumatriptan on resting BP in healthy subjects.
Methods
Subjects
Healthy adults aged 18 to 55 years, with a body mass index between 18 and 32 kg/m2, and in good general health at screening were eligible. The general health of subjects was confirmed by medical history evaluation, BP assessment, physical examination, 12-lead electrocardiogram, neurological examination, and clinical laboratory assessment (serum chemistry, hematology, or urinalysis). Women were eligible to participate if they were not pregnant or breastfeeding and if they were either using an acceptable method of contraception or were physiologically incapable of becoming pregnant. Subjects with elevated BP (systolic BP [SBP] ≥140 mmHg or diastolic BP [DBP] ≥90 mmHg), elevated heart rate (>100 beats/min), a prolonged corrected QT interval (obtained using Fridericia's formula; ≥450 msec for men and ≥470 msec for women), or history of prolonged QT syndrome were excluded. Subjects were also excluded if they had an unstable medical condition (resulting in hospitalization within 28 days of study day 1, major surgery within 6 months of study day 1, or in the judgement of the study investigator) or had suffered a major cardiovascular event or been treated with a percutaneous coronary intervention or coronary artery bypass graft within 6 months of study day 1. Subjects with a history of malignancy or impaired renal or hepatic function were also excluded. In addition, subjects who had previously received erenumab or any other large molecule that targets CGRP, had a history or evidence of substance or alcohol abuse, or those who had a contraindication to sumatriptan were excluded.
Subjects were asked to avoid strenuous exercise during screening and 48 hours before endpoint assessments (days 1, 2, 4, and 5) and any exercise or consumption of caffeinated food and beverages ≥ 30 minutes before endpoint assessment (days 1, 2, 4, and 5). Smoking was prohibited 30 days prior to the beginning of the treatment period and during the study, as was the consumption of ≥ 2 alcoholic drinks daily during the study or any alcohol within 24 hours of a clinic visit. Use of any over-the-counter or prescription medication within 14 days or 5 half-lives (whichever was longer), or any investigational product within 30 days or 5 half-lives (whichever was longer), of receiving the first dose of erenumab or placebo was prohibited. Use of simple analgesics was permitted during the study, except within the 48 hours before receiving sumatriptan.
Study design and procedures
We performed a phase 1, parallel-group, one-way crossover, double-blind, placebo-controlled, concomitant therapy study in healthy subjects conducted at one center in Belgium from 22 February 2016 (first subject enrolled) to 11 August 2016 (last subject completed follow-up). The study comprised the following three phases: Screening (21 days), treatment (6 days), and safety follow-up (83 days). The trial protocol was approved by an independent ethics committee of the University Hospitals KU Leuven, all participants provided written informed consent, and the study was conducted in accordance with the International Conference on Harmonisation Tripartite Guideline on Good Clinical Practice.
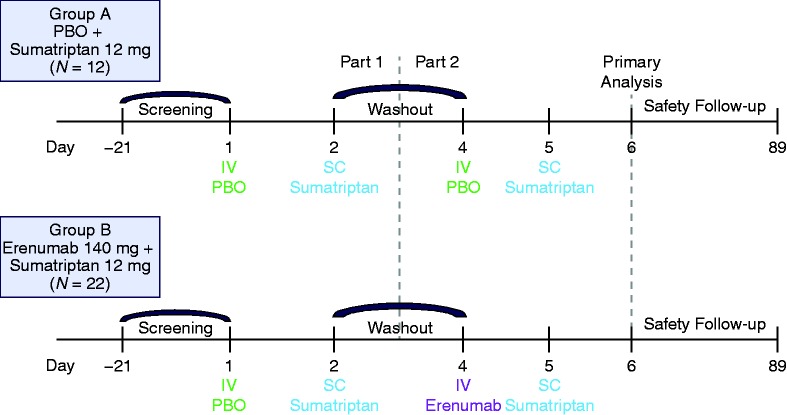
All subjects attended a screening visit within 21 days prior to receiving the first dose of the study drug. At day 1, after the screening phase, eligible subjects were randomly assigned in a 1:2 ratio to intravenous (IV) placebo and subcutaneous (SC) sumatriptan (group A) or IV erenumab and SC sumatriptan (group B) (Figure 1). The 6-day treatment period was composed of two parts. Part 1 included subjects in groups A and B that received IV placebo on day 1 and sumatriptan 12 mg (two 6-mg SC injections separated by 1 hour) on day 2. There was a 2-day washout period between parts 1 and 2. In part 2, subjects in group A received IV placebo on day 4 and sumatriptan 12 mg (two 6-mg SC injections separated by 1 hour) on day 5, while subjects in group B received IV erenumab 140 mg on day 4 and sumatriptan 12 mg (on day 5 6-mg SC injections separated by 1 hour). Sumatriptan injections were administered and subsequent BP measurements were taken following the same schedule on days 2 and 5. The subjects returned for a follow-up visit at day 89.
Figure 1.
Study design.
IV: intravenous; PBO: placebo; SC: subcutaneous.
The study utilized a simple fixed permuted block randomization without stratification. Treatment allocations were made sequentially based on the fixed randomization schedule as subjects were enrolled in the trial. Participants and all study site personnel, except for the pharmacist, were blinded to the assigned treatment groups during the study. Per Amgen standard procedure, unblinded subject enrollment logs were delivered directly to the unblinded site pharmacist in a sealed tamper-evident envelope. Erenumab and sumatriptan were administered at the center by a qualified person. Erenumab was supplied in 5-ml clear glass vials, and placebo was formulated and packaged to match the vials containing erenumab. Sumatriptan was supplied as 6-mg prefilled auto injectors containing 6 mg of sumatriptan succinate. The first 6-mg dose of sumatriptan was administered to the right thigh and the second dose (given 1 hour later) was administered to the left thigh. Individual subject treatment assignments were unblinded to personnel involved in assessing sumatriptan PK and anti-erenumab antibody laboratory values.
Measures
Safety and tolerability: BP, adverse events (AEs), physical examinations, 12-lead electrocardiograms, laboratory assessments, vital sign measurements, and anti-erenumab antibodies were evaluated.
Vital signs: Baseline measures of BP and heart rate were recorded prior to dosing and calculated as the average of the last recording during the screening phase and the recording immediately before the first dose on day 1. During the 6-day treatment period, BP and heart rate were measured 2 hours after administration of placebo on day 1 and after randomized treatment (placebo or erenumab) on day 4; after the first dose of sumatriptan on days 2 and 5, measurements were taken at hours 1 (immediately after the second dose of sumatriptan), 1.25, 1.5, 2, 2.5, 3, 3.5, 4.5, 7, 13, and 25. BP measurements were taken using an Omron 705IT (Omron Healthcare Inc., Kyoto, Japan) with the subject in the semi-recumbent position, in duplicate, and averaged.
Assessment of AEs: Subjects were monitored regularly during the study for AEs, which were coded using the Medical Dictionary for Regulatory Activities, version 18.0, and graded using the Common Terminology Criteria for Adverse Events, version 4.0.
Electrocardiograms, physical examinations, and laboratory safety assessments: 12-lead electrocardiograms were recorded in triplicate before dosing; at 1, 1.5, 2, 3, 7, and 25 hours post-sumatriptan dose (days 2 and 5); and 2 hours post-dose of randomized treatment (day 4). Physical examinations and laboratory safety assessments were performed at multiple time points during the study.
Cohorts of subjects were enrolled in a staggered fashion after review of blinded safety data from six subjects (four subjects in the erenumab and sumatriptan group and two subjects in the placebo and sumatriptan group), so that no more than four subjects received erenumab on day 4, followed by sumatriptan on day 5. The next six subjects were not dosed until the safety review had been conducted.
Antibody analysis: Blood samples were collected prior to dosing on day 1 and at the end of study (day 89) for anti-erenumab antibody testing. Blood samples were collected in 5-ml serum separation tubes and a validated, two-tiered, Meso Scale Discovery electrochemiluminescence bridging immunoassay (Meso Scale Diagnostics, Rockville, MD, USA) was used to detect anti–erenumab-binding antibodies in the serum. The sensitivity of the assay was 24.73 ng/ml and the lower limit of reliable detection (LLRD) was 100 ng/ml. Serum samples that were confirmed to be anti-erenumab antibody positive were analyzed for the presence of neutralizing antibodies using a validated cell-based bioassay. The assay sensitivity was 0.553 μg/ml and LLRD was 1.14 μg/ml.
PK analysis: Blood samples for measuring sumatriptan concentrations were collected before and at 1 hour after the first dose, and at 0, 10, 15, and 30 minutes, and 1, 2, 3.5, and 6 hours after the second dose of sumatriptan on days 2 and 5. Blood samples were collected in 4-ml K3 EDTA collection tubes. Sumatriptan was isolated from human plasma through solid-phase extraction before injection on a Zorbax SB-C18 column (50 × 4.6 mm, 5 mm) (Agilent, Santa Clara, CA, USA) and analysis by liquid chromatography–tandem mass spectrometry using an AB Sciex API 4000 system (SCIEX, Framingham, MA, USA). The lower limit of quantification was 0.100 ng/ml and the nominal assay range was 0.100 ng/ml to 150 ng/ml.
Trial outcomes: The primary endpoint was individual time-weighted averages of mean arterial pressure (MAP), measured from 0 hours pre-dose to 2.5 hours post-dose of the first dose of sumatriptan. To determine if time-weighted average of MAP was similar between subjects who received placebo and sumatriptan and those who received erenumab and sumatriptan, a two-sided 90% confidence interval (CI) for the between-group treatment difference, selected a priori to study initiation, was calculated. If the upper bound of the 90% CI was < 5 mmHg, the hypothesis that the effect on time-weighted MAP of placebo and sumatriptan was similar to that of erenumab and sumatriptan was deemed to have been supported. The rationale for this decision was based on the previous publication by Depré et al. (30). Time-weighted SBP and DBP were analyzed in a similar fashion as time-weighted MAP.
Secondary endpoints included PK parameters for sumatriptan. Geometric mean ratios were calculated for sumatriptan when administered with placebo or with erenumab: The time to the maximum observed plasma concentration (tmax), maximum observed plasma concentration (Cmax), the area under the plasma concentration–time curve (AUC) from time 0 to 6 hours (AUC6hr) after the second dose of sumatriptan, and AUC from time 0 to infinity (AUC∞). Other secondary endpoints evaluated were vital signs, electrocardiograms, physical examinations, laboratory safety tests, AEs, and anti-erenumab antibodies.
A post-hoc analysis evaluated the change in MAP in all subjects after receiving sumatriptan alone, after receiving placebo alone, and after receiving erenumab alone (calculated using measurements from 0 hours pre-dose [baseline] to 2 hours post-dose on day 5 for sumatriptan and placebo, and day 4 for erenumab).
Statistical analyses
At least 20 subjects needed to be enrolled and randomized in the study to achieve a > 99% probability that the upper limit of the 90% CI for the treatment difference would lie below 5 mmHg, if the true difference was 0. The power calculations were based on an assumed within-subject standard deviation (SD) of 3.2 mmHg and using an alpha of 0.05 and one-sided tests. These assumptions were based on results from an earlier phase 1 trial of erenumab in healthy volunteers (29). The true differences could be as high as 2.5 mmHg and would still have had 76.9% power to support the hypothesis. To assess safety, a total sample size of approximately 30 adult subjects was planned to be recruited, for an allocation of 20 subjects to the erenumab group and 10 subjects to placebo.
MAP was calculated using the following formula: MAP = DBP + 0.33 * (SBP−DBP). Individual time weighted averages in MAP were calculated as the measurement–time curve from pre-dose (0 hours) to 2.5 hours of MAP divided by the time period over which the measurements were made. An average of duplicate BP measurements at each time point were used to calculate the time-weighted averages. A two-sided 90% CI (equivalent to a one-sided upper 95% CI) for the mean treatment difference between treatment groups was calculated using a linear mixed-effects model with fixed effects for treatment group, study period, and random effects for subjects. Statistical analyses were conducted using SAS® (version 9.4; SAS Institute, Inc., Cary, NC, USA).
The plasma PK parameters for sumatriptan were determined using non-compartmental analysis of the plasma concentration–time data using Phoenix WinNonlin, version 6.4. AUC6hr was estimated using the linear trapezoidal method and AUC∞ was estimated as the sum of AUClast and Clast/λz (Clast is the last observed concentration, AUClast is the AUC from time 0 up to the last observed concentration, and λz is the first-order terminal rate constant). Cmax was determined by inspection of the data. Log-transformed PK parameters for sumatriptan were analyzed using a linear mixed-effects model with treatment group as a fixed effect and subjects as a random effect. The 90% CIs for the mean differences (test/reference) between erenumab and sumatriptan (test) and placebo and sumatriptan (reference) for log-transformed Cmax, AUC6hr, and AUC∞ were calculated. Summary statistics were provided for tmax. The safety analysis set was used for the primary and secondary endpoint and included all subjects who received at least one injection, and the PK analysis set included all subjects for whom at least one PK parameter or endpoint could be adequately estimated.
Results
Demographics
Thirty-four subjects were enrolled and randomized in this study and 30 subjects completed the study. Mean (SD) MAP was 87.5 (5.0) mmHg and age was 28.5 (9.6) years at baseline. A total of 24/34 subjects were male and 33/34 subjects were Caucasian (Table 1).
Table 1.
Baseline demographics and characteristics.
| Group A (placebo and sumatriptan) n = 12 | Group B (erenumab and sumatriptan) n = 22 | |
|---|---|---|
| Age, years | 27.3 (8.6) | 29.1 (10.3) |
| Sex, n (%) | ||
| Female | 4 (33.3) | 6 (27.3) |
| Male | 8 (66.7) | 16 (72.7) |
| Race, n (%) | ||
| Caucasian | 12 (100) | 21 (95.5) |
| Black or African American | 0 (0.0) | 1 (4.5) |
| BMI, kg/m2 | 23.7 (2.8) | 23.7 (2.7) |
| Blood pressure, mmHg | ||
| Mean arterial pressure | 88.3 (5.3) | 87.0 (4.8) |
| Systolic blood pressure | 122.6 (6.3) | 122.8 (6.8) |
| Diastolic blood pressure | 71.4 (6.3) | 69.4 (5.1) |
BMI: body mass index.
Figures in brackets in Age, BMI and Blood pressure rows unless otherwise stated, data are mean (standard deviation).
Primary endpoint
Least squares mean (standard error) time-weighted averages of MAP were 87.4 (1.0) mmHg for the placebo and sumatriptan group and 87.4 (1.2) mmHg for the concomitant erenumab and sumatriptan group (Table 2). The mean difference in MAP between the groups was −0.04 mmHg (90% CI: −2.2, 2.1). Given that the upper bound of the 90% CI was below the predefined upper bound of 5 mmHg, the difference in time-weighted averages of MAP between treatment groups was not considered clinically significant.
Table 2.
Time-weighted average mean arterial pressure, systolic blood pressure, and diastolic blood pressure.
| Placebo and sumatriptana n = 34 | Erenumab and sumatriptan n = 20 | |
|---|---|---|
| Mean arterial pressure, mmHg | ||
| LS mean (SE) | 87.4 (1.0) | 87.4 (1.2) |
| Difference in LS mean (90% CI) | −0.04 (−2.2, 2.1) | |
| Systolic blood pressure, mmHg | ||
| LS mean (SE) | 120.9 (1.3) | 119.0 (1.6) |
| Difference in LS mean (90% CI) | −1.9 (−4.8, 0.9) | |
| Diastolic blood pressure, mmHg | ||
| LS mean (SE) | 70.8 (1.0) | 71.9 (1.2) |
| Difference in LS mean (90% CI) | 1.01 (−1.0, 3.0) | |
CI: confidence interval; LS: least squares; SE: standard error.
Analysis adjusted for treatment and time as covariates and subject as random effect in a linear mixed-effects model.
Subjects receiving sumatriptan are from group A and group B; subjects in group B received erenumab on day 5 in part 2.
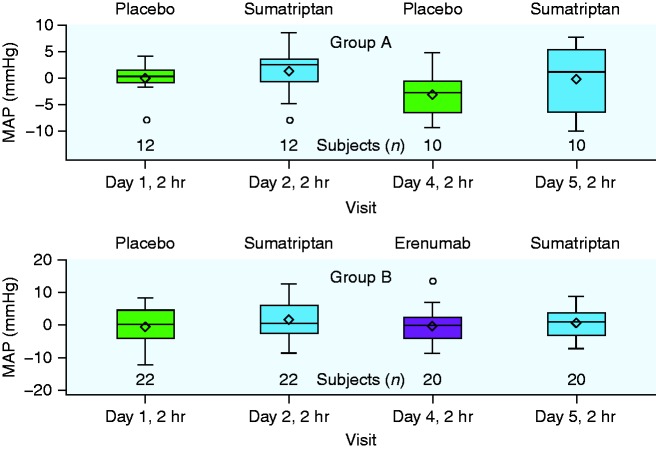
Additionally, changes in SBP and DBP were similar between treatment groups. The mean difference in SBP was −1.93 mmHg (90% CI: −4.8, 0.9) and in DBP was 1.0 mmHg (90% CI: −1.0, 3.0) between groups (Table 2). In post-hoc analyses, mean (SD) change in MAP from pre-dose to 2 hours post-dose was –0.3 (5.2) mmHg for erenumab alone, 0.7 (4.8) mmHg for sumatriptan alone, and −0.5 (5.7) mmHg for placebo (Figure 2).
Figure 2.
Change in MAP from pre-dose (0 hours) to 2 hours post-dose. Horizontal inner line represents the median, diamond symbol represents the mean, width of the box represents the interquartile range, and whiskers represent 1.5 times above or below the interquartile range.
MAP: mean arterial pressure.
PK
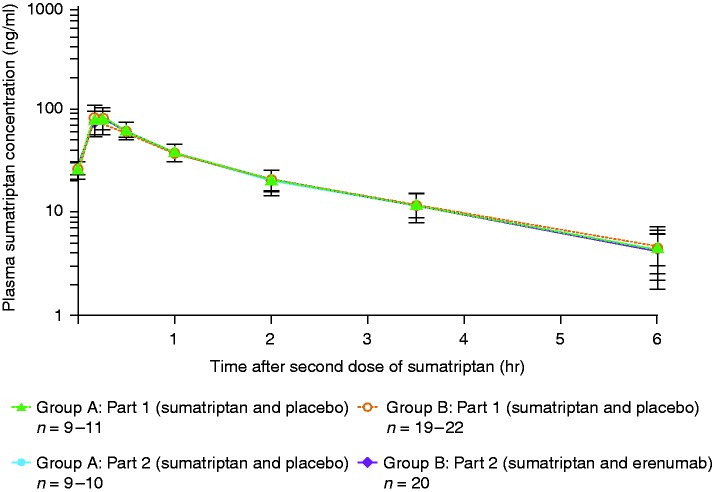
The PK analysis set comprised samples from all 34 subjects. The statistical summary of sumatriptan PK parameters is provided in Table 3. Geometric mean ratio estimate for Cmax was 0.95 (90% CI: 0.82, 1.09), for AUC6hr was 0.98 (90% CI: 0.93, 1.03), and for AUC∞ was 1.00 (90% CI: 0.96, 1.05). Additionally, similar mean sumatriptan plasma concentration–time profiles and median tmax values for sumatriptan were observed for both treatment groups (Figure 3, Supplementary Table 1).
Table 3.
Pharmacokinetic measures of sumatriptan.
| Placebo and sumatriptana (reference) n = 34 | Erenumab and sumatriptan (test) n = 20 | |
|---|---|---|
| Cmax (ng/ml) | ||
| LS meanb | 83.50 | 79.00 |
| Ratioc,d, (90% CI) | 0.95 (0.82, 1.09) | |
| AUC6hr (hr*ng/ml) | ||
| LS meanb | 133.33 | 130.59 |
| Ratioc,d, (90% CI) | 0.98 (0.93, 1.03) | |
| AUC∞ (hr*ng/ml) | ||
| LS meanb | 144.32 | 144.81 |
| Ratioc,d, (90% CI) | 1.00 (0.96, 1.05) | |
AUC: area under the plasma concentration–time curve; AUC6hr: AUC from time 0 to 6 hours; AUC∞: AUC from time 0 to infinity; Cmax: maximum observed plasma concentration; CI: confidence interval; LS: least squares.
Table is based on the safety analysis set.
Subjects receiving sumatriptan are from group A and group B; subjects in group B received erenumab on day 5 in part 2.
LS geometric mean using the SAS PROC MIXED procedure, version 9.3 (SAS Institute Inc., Cary, NC, USA). cRatios are test/reference.
The ratio and CI are based on natural log scale data converted back to the original scale.
Figure 3.
Plasma sumatriptan concentration over time. Data are mean ± standard deviation.
AEs
All 34 subjects were included in the safety analysis set. No serious AEs were reported. During part 1 of the study (when all subjects received placebo and sumatriptan), two subjects in each randomized treatment group experienced sumatriptan-related injection site reactions that led to treatment withdrawal. The events that led to subject withdrawal were injection site erythema, injection site swelling, and injection site urticaria (one AE each) in group A and injection site erythema and injection site swelling (two AEs each) in group B. No subject experienced AEs that led to treatment withdrawal during part 2 of the study (Table 4). All AEs were mild to moderate in severity.
Table 4.
Subject incidence of adverse events.
| Part 1 | Part 2 | |||
|---|---|---|---|---|
| Group A |
Group B |
Group A |
Group B |
|
| n (%) | Placebo and sumatriptan n = 12 | Placebo and sumatriptan n = 22 | Placebo and sumatriptan n = 12 | Erenumab and sumatriptan n = 22 |
| Adverse events | 11 (91.7) | 19 (86.4) | 9 (75.0) | 17 (77.3) |
| Adverse events leading to treatment discontinuationa | 2 (16.7) | 2 (9.1) | 0 (0.0) | 0 (0.0) |
| Adverse events reported by ≥10% of subjects in any treatment arm | ||||
| Head discomfort | 4 (33.3) | 8 (36.4) | 3 (25.0) | 8 (36.4) |
| Paresthesia | 4 (33.3) | 3 (13.6) | 4 (33.3) | 4 (18.2) |
| Injection site erythema | 2 (16.7) | 4 (18.2) | 1 (8.3) | 2 (9.1) |
| Injection site swelling | 2 (16.7) | 4 (18.2) | 1 (8.3) | 2 (9.1) |
| Discomfort | 4 (33.3) | 0 (0.0) | 1 (8.3) | 0 (0.0) |
| Headache | 1 (8.3) | 3 (13.6) | 0 (0.0) | 4 (18.2) |
| Throat tightness | 2 (16.7) | 1 (4.5) | 2 (16.7) | 1 (4.5) |
| Musculoskeletal discomfort | 0 (0.0) | 1 (4.5) | 0 (0.0) | 4 (18.2) |
| Hematuria | – | – | 1 (8.3) | 3 (13.6) |
Table based on safety analysis set.
From group A, the following events led to withdrawal: Injection site erythema, injection site swelling, and injection site urticaria (one adverse event each). From group B, the following events led to withdrawal: Injection site erythema and injection site swelling (two adverse events each).
In part 2, nine subjects (75.0%) in the placebo and sumatriptan group and 17 subjects (77.3%) in the erenumab and sumatriptan group reported AEs, and all were mild to moderate in severity. The most frequent AEs (occurring in ≥10% of subjects in either group) were head discomfort (three subjects [25.0%] in the placebo and sumatriptan group and eight subjects [36.4%] in the erenumab and sumatriptan group), paresthesia (four subjects [33.3%] in the placebo and sumatriptan group and four subjects [18.2%] in the erenumab and sumatriptan group), musculoskeletal discomfort (no subjects in the placebo and sumatriptan group and four subjects [18.2%] in the erenumab and sumatriptan group), and headache (no subjects in the placebo and sumatriptan group and four subjects [18.2%] in the erenumab and sumatriptan group) (Table 4). No clinically significant changes in serum chemistry, hematology laboratory values, vital signs, or 12-lead electrocardiograms were observed in either treatment group.
Anti-erenumab antibodies
One subject in the erenumab and sumatriptan group developed anti–erenumab-binding antibodies during part 2 of the study, which were shown to be neutralizing at the end of the study. The subject was tested at a 3-month follow-up visit and was negative for anti–erenumab-neutralizing antibodies.
Discussion
This study demonstrated that co-administration of erenumab and sumatriptan had no additional effect on resting BP beyond the effects of sumatriptan monotherapy and did not affect the PK of sumatriptan in healthy subjects. Additionally, a post-hoc analysis showed that administration of erenumab alone did not affect resting BP. As migraine treatment involves acute and preventive therapy, and patients with frequent attacks may need both to manage their symptoms (29), the results of this study suggest that combining erenumab and sumatriptan is unlikely to alter BP in a real-world setting.
Therapies like erenumab that target the CGRP pathway represent a promising new class of preventive treatment for patients with migraine (20–26,31). Monoclonal antibodies that target the CGRP pathway have been shown to be efficacious in migraine prevention (20–26,32). Although CGRP is a potent vasodilator, small molecule or biologic CGRP antagonists appear to prevent CGRP-dependent vasodilation without eliciting vasoconstriction (33,34). The absence of vasoconstriction with CGRP antagonism was confirmed in an experimental study with a peptide CGRP receptor antagonist (CGRP8-37). Intra-arterial infusion of CGRP8-37 did not affect resting blood flow, suggesting that CGRP does not play a role in the regulation of peripheral vascular tone under normal, resting conditions (35,36).
Although the findings of this study were observed in healthy subjects, they are consistent with observations from a study of the co-administration of sumatriptan and telcagepant, an orally administered small molecule CGRP receptor inhibitor, in patients with migraine (30). Depré et al. found that administration of telcagepant alone did not elevate BP and elevations in BP following co-administration of sumatriptan and telcagepant were similar to those observed following administration of sumatriptan alone (30). The use of triptan-based medications was permitted in trials of erenumab for the treatment of chronic and episodic migraine (24–26). In a phase 3 trial of erenumab in patients with episodic migraine, 59% of patients used triptans (24), and in a phase 2 trial of erenumab in patients with chronic migraine, 78% of patients used triptans (26). In support of the combinatorial use of erenumab and triptans, no cardiovascular safety signal has been identified in any trials of erenumab (24–26). Additionally, an association between serum erenumab concentration and BP was not observed using 24-hour ambulatory BP monitoring in a phase 1 study of erenumab in healthy subjects and patients with migraine (29).
Both CGRP and serotonin are thought to be involved in the pathophysiological mechanisms underlying migraine through nociceptive mechanisms in the trigeminovascular system. Activation of the trigeminovascular system in migraine has been shown to cause the diminution of serotonin and release of CGRP (37). It has been observed that agonists of serotonin selectively normalize elevated levels of CGRP and that triptans reduce release of CGRP (38). The interplay of these neurotransmitters, and others, are thought to be key in providing nociceptive information to higher brain centers (38). The use of triptans in combination with CGRP pathway inhibitors, such as erenumab, may result in enhanced management of migraine.
The 12-mg dose of SC sumatriptan used in this study was selected because it is the maximum dose recommended for use within a 24-hour period and the 140-mg dose of erenumab is the highest dose planned for clinical use. Erenumab is intended to be administered SC in clinic. However, in this study, erenumab was administered IV and sumatriptan SC, as the highest plasma concentrations for both these medications have been observed following single-dose administration through these routes (29,39). Exposures (Cmax) following IV administration of erenumab 140 mg have been shown to be two-fold greater than those obtained during repeat administration of monthly erenumab 140 mg SC (29). Plasma PK samples were collected through to 6 hours after the administration of the second dose of sumatriptan to establish subject exposure to sumatriptan. The bounds of the 90% CI for the geometric mean ratios for all sumatriptan PK parameters in the presence and absence of erenumab were completely within 0.80 to 1.25, indicating there were no clinically relevant PK drug–drug interactions between sumatriptan and erenumab.
Erenumab, administered in combination with sumatriptan or with placebo, was well tolerated. The safety profile in both parts of the study and in both treatment groups was similar. All AEs were mild to moderate in severity and there were no serious AEs reported. Many of the reported AEs were consistent with the use of SC sumatriptan (40) and were considered to be related to sumatriptan and not to erenumab. Additionally, the AEs that led to treatment withdrawal were all sumatriptan-related injection site reactions that were reported in part 1 of the study when subjects had not yet received erenumab. In accordance with the favorable safety and tolerability profile of erenumab observed in larger clinical trials (24–26), there were no clinically relevant safety concerns relating to erenumab identified in our study. Overall, no safety concerns were identified when erenumab was co-administered with sumatriptan.
Limitations of this study were that the majority of the subjects were male (while migraine is a disease that primarily affects females (41)), and the effects on BP were assessed in healthy subjects who did not suffer from migraine headaches. As changes in arterial function have previously been reported in patients with migraine (42,43), caution should be exercised when extrapolating these results to patients with migraine in a clinical setting. In addition, CGRP inhibition may in theory increase BP or aggravate ischemia (44,45). However, the results presented here, and in a phase 1 study of erenumab (29), showed no increase in BP with erenumab. Furthermore, in an exercise treadmill test, patients with stable angina treated with erenumab did not experience a reduction in exercise capacity versus placebo (46).
In conclusion, this study did not show evidence of pharmacodynamic (no increase in BP with erenumab with or without sumatriptan above that caused by sumatriptan alone) or PK interaction between erenumab and sumatriptan, and therefore, the results support the concomitant use of erenumab and triptans. Additionally, this study provides evidence that inhibition of the CGRP receptor with erenumab does not increase resting BP.
Supplemental Material
Supplemental material, Supplementary material for Phase 1, randomized, parallel-group, double-blind, placebo-controlled trial to evaluate the effects of erenumab (AMG 334) and concomitant sumatriptan on blood pressure in healthy volunteers by Jan de Hoon, Anne Van Hecken, Corinne Vandermeulen, Marissa Herbots, Yumi Kubo, Ed Lee, Osa Eisele, Gabriel Vargas and Kristin Gabriel in Cephalalgia
Acknowledgements
The authors thank the staff of the Center for Clinical Pharmacology, most importantly Marissa Herbots, for assisting in the study conduct and data collection. Medical writing support was provided by Devon Allen, BSc (Hons), of Fishawack Communications Ltd, funded by Amgen, and by Lori Smette, PhD, of Amgen. Erenumab is co-developed in partnership with Amgen and Novartis.
Clinical implications
Patients who suffer from frequent and moderate to severe migraines require both acute and preventative therapies.
Co-administration of erenumab and sumatriptan was well tolerated, with no additional effects on resting blood pressure or on the pharmacokinetics of sumatriptan.
Furthermore, administration of erenumab alone did not affect resting blood pressure.
The results from this study support the concomitant use of erenumab and triptans.
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JdH acted as Principal Investigator, for which the university received research grants from Abide, Amgen, Galderma, Genentech, GlaxoSmithKline, Janssen Research & Development, Lilly Chorus, MSD, Novartis, Sanofi Pasteur, UCB, and Vertex; and acted as a consultant for Ablynx, Amgen, Eli Lilly, Genentech, Novartis, and UCB; AVH, MH have nothing to disclose; CV acted as sub-investigator for this study, for which the university received a research grant (Amgen); EL, OE, and GV are employees and stockholders of Amgen; YK and KG were employed by Amgen at the time of the study.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Amgen Inc.
References
- 1.Serrano D, Manack AN, Reed ML, et al. Cost and predictors of lost productive time in chronic migraine and episodic migraine: Results from the American Migraine Prevalence and Prevention (AMPP) Study. Value Health 2013; 16: 31–38. [DOI] [PubMed] [Google Scholar]
- 2.Raval AD, Shah A. National trends in direct health care expenditures among US adults with migraine: 2004 to 2013. J Pain 2017; 18: 96–107. [DOI] [PubMed] [Google Scholar]
- 3.Antonaci F, Ghiotto N, Wu S, et al. Recent advances in migraine therapy. Springerplus 2016; 5: 637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cameron C, Kelly S, Hsieh SC, et al. Triptans in the acute treatment of migraine: A systematic review and network meta-analysis. Headache 2015; 55 Suppl 4: S221–S235. [DOI] [PubMed] [Google Scholar]
- 5.Evers S, Afra J, Frese A, et al. EFNS guideline on the drug treatment of migraine – revised report of an EFNS task force. Eur J Neurol 2009; 16: 968–981. [DOI] [PubMed] [Google Scholar]
- 6.Connor HE, Feniuk W, Humphrey PP. 5-Hydroxytryptamine contracts human coronary arteries predominantly via 5-HT2 receptor activation. Eur J Pharmacol 1989; 161: 91–94. [DOI] [PubMed] [Google Scholar]
- 7.Maassen Van Den Brink A, Saxena PR. Coronary vasoconstrictor potential of triptans: A review of in vitro pharmacologic data. Headache 2004; 44 Suppl 1: S13–S19. [DOI] [PubMed] [Google Scholar]
- 8.Parsons AA, Whalley ET, Feniuk W, et al. 5-HT1-like receptors mediate 5-hydroxytryptamine-induced contraction of human isolated basilar artery. Br J Pharmacol 1989; 96: 434–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tullo V, Bussone G, Omboni S, et al. Efficacy of frovatriptan and other triptans in the treatment of acute migraine of hypertensive and normotensive subjects: A review of randomized studies. Neurol Sci 2013; 34 Suppl 1: S87–S91. [DOI] [PubMed] [Google Scholar]
- 10.MacIntyre PD, Bhargava B, Hogg KJ, et al. Effect of subcutaneous sumatriptan, a selective 5HT1 agonist, on the systemic, pulmonary, and coronary circulation. Circulation 1993; 87: 401–405. [DOI] [PubMed] [Google Scholar]
- 11.Dodick DW, Martin VT, Smith T, et al. Cardiovascular tolerability and safety of triptans: A review of clinical data. Headache 2004; 44 Suppl 1: S20–S30. [DOI] [PubMed] [Google Scholar]
- 12.O'Quinn S, Davis RL, Gutterman DL, et al. Prospective large-scale study of the tolerability of subcutaneous sumatriptan injection for acute treatment of migraine. Cephalalgia 1999; 19: 223–331. [DOI] [PubMed] [Google Scholar]
- 13.Hepp Z, Dodick DW, Varon SF, et al. Adherence to oral migraine-preventive medications among patients with chronic migraine. Cephalalgia 2015; 35: 478–488. [DOI] [PubMed] [Google Scholar]
- 14.Hepp Z, Bloudek LM, Varon SF. Systematic review of migraine prophylaxis adherence and persistence. J Manag Care Pharm 2014; 20: 22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: Studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol 1993; 33: 48–56. [DOI] [PubMed] [Google Scholar]
- 16.Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol 1990; 28: 183–187. [DOI] [PubMed] [Google Scholar]
- 17.Ho TW, Edvinsson L, Goadsby PJ. CGRP and its receptors provide new insights into migraine pathophysiology. Nat Rev Neurol 2010; 6: 573–582. [DOI] [PubMed] [Google Scholar]
- 18.Lassen LH, Haderslev PA, Jacobsen VB, et al. CGRP may play a causative role in migraine. Cephalalgia 2002; 22: 54–61. [DOI] [PubMed] [Google Scholar]
- 19.Brain SD, Williams TJ, Tippins JR, et al. Calcitonin gene-related peptide is a potent vasodilator. Nature 1985; 313: 54–56. [DOI] [PubMed] [Google Scholar]
- 20.Bigal ME, Dodick DW, Rapoport AM, et al. Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of high-frequency episodic migraine: A multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol 2015; 14: 1081–1090. [DOI] [PubMed] [Google Scholar]
- 21.Bigal ME, Edvinsson L, Rapoport AM, et al. Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of chronic migraine: A multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol 2015; 14: 1091–1100. [DOI] [PubMed] [Google Scholar]
- 22.Dodick DW, Goadsby PJ, Silberstein SD, et al. Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: A randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol 2014; 13: 1100–1107. [DOI] [PubMed] [Google Scholar]
- 23.Dodick DW, Goadsby PJ, Spierings EL, et al. Safety and efficacy of LY2951742, a monoclonal antibody to calcitonin gene-related peptide, for the prevention of migraine: A phase 2, randomised, double-blind, placebo-controlled study. Lancet Neurol 2014; 13: 885–892. [DOI] [PubMed] [Google Scholar]
- 24.Goadsby PJ, Reuter U, Hallström Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med 2017; 377: 2123–2132. [DOI] [PubMed] [Google Scholar]
- 25.Sun H, Dodick DW, Silberstein S, et al. Safety and efficacy of AMG 334 for prevention of episodic migraine: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol 2016; 15: 382–390. [DOI] [PubMed] [Google Scholar]
- 26.Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: A randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol 2017; 16: 425–434. [DOI] [PubMed] [Google Scholar]
- 27.Shi L, Lehto SG, Zhu DX, et al. Pharmacologic characterization of AMG 334, a potent and selective human monoclonal antibody against the calcitonin gene-related peptide receptor. J Pharmacol Exp Ther 2016; 356: 223–231. [DOI] [PubMed] [Google Scholar]
- 28.Silberstein SD. Preventive migraine treatment. Continuum (Minneap Minn) 2015; 21: 973–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Hoon J, Van Hecken A, Vandermeulen C, et al. Phase 1, randomized, double-blind, placebo-controlled, single-dose and multiple-dose studies of erenumab in healthy subjects and patients with migraine. Clin Pharmacol Ther. Epub ahead of print 24 July 2017. DOI: 10.1002/cpt.799. [DOI] [PubMed] [Google Scholar]
- 30.Depré M, Macleod C, Palcza J, et al. Lack of hemodynamic interaction between CGRP-receptor antagonist telcagepant (MK-0974) and sumatriptan: Results from a randomized study in patients with migraine. Cephalalgia 2013; 33: 1292–1301. [DOI] [PubMed] [Google Scholar]
- 31.Ho TW, Connor KM, Zhang Y, et al. Randomized controlled trial of the CGRP receptor antagonist telcagepant for migraine prevention. Neurology 2014; 83: 958–966. [DOI] [PubMed] [Google Scholar]
- 32.Olesen J, Diener HC, Husstedt IW, et al. Calcitonin gene–related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med 2004; 350: 1104–1110. [DOI] [PubMed] [Google Scholar]
- 33.Petersen KA, Birk S, Lassen LH, et al. The CGRP-antagonist, BIBN4096BS does not affect cerebral or systemic haemodynamics in healthy volunteers. Cephalalgia 2005; 25: 139–147. [DOI] [PubMed] [Google Scholar]
- 34.Van der Schueren BJ, Blanchard R, Murphy MG, et al. The potent calcitonin gene-related peptide receptor antagonist, telcagepant, does not affect nitroglycerin-induced vasodilation in healthy men. Br J Clin Pharmacol 2011; 71: 708–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubio-Beltran E, Labastida A, de Vries R, et al. Effects of AMG 334 on human isolated coronary artery. Cephalalgia 2016; 36 Suppl 1: 41. . [Google Scholar]
- 36.Vanmolkot FH, Van der Schueren BJ, de Hoon JN. Calcitonin gene-related peptide-induced vasodilation in the human forearm is antagonized by CGRP8-37: Evaluation of a human in vivo pharmacodynamic model. Clin Pharmacol Ther 2006; 79: 263–273. [DOI] [PubMed] [Google Scholar]
- 37.Goadsby PJ, Holland PR, Martins-Oliveira M, et al. Pathophysiology of migraine: A disorder of sensory processing. Physiol Rev 2017; 97: 553–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aggarwal M, Puri V, Puri S. Serotonin and CGRP in migraine. Ann Neurosci 2012; 19: 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Obaidi M, Offman E, Messina J, et al. Improved pharmacokinetics of sumatriptan with Breath Powered™ nasal delivery of sumatriptan powder. Headache 2013; 53: 1323–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.GlaxoSmithKline. Imitrex® (sumatriptan succinate) injection for subcutaneous use prescribing information. Research Triangle Park, NC: GlaxoSmithKline, 2015.
- 41.Stewart WF, Wood C, Reed ML, et al. Cumulative lifetime migraine incidence in women and men. Cephalalgia 2008; 28: 1170–1178. [DOI] [PubMed] [Google Scholar]
- 42.de Hoon JN, Willigers JM, Troost J, et al. Cranial and peripheral interictal vascular changes in migraine patients. Cephalalgia 2003; 23: 96–104. [DOI] [PubMed] [Google Scholar]
- 43.Vanmolkot FH, Van Bortel LM, de Hoon JN. Altered arterial function in migraine of recent onset. Neurology 2007; 68: 1563–1570. [DOI] [PubMed] [Google Scholar]
- 44.Keith IM. The role of endogenous lung neuropeptides in regulation of the pulmonary circulation. Physiol Res 2000; 49: 519–537. [PubMed] [Google Scholar]
- 45.Mair J, Lechleitner P, Längle T, et al. Plasma CGRP in acute myocardial infarction. Lancet 1990; 335: 168. [DOI] [PubMed] [Google Scholar]
- 46.Depre C, Antalik L, Starling A, et al. A randomized, double-blind, placebo-controlled study to evaluate the effect of erenumab on exercise time during a treadmill test in patients with stable angina. Abstract presented at the 18th Congress of the International Headache Society (IHC), 7–10 September 2017, Vancouver, Canada. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary material for Phase 1, randomized, parallel-group, double-blind, placebo-controlled trial to evaluate the effects of erenumab (AMG 334) and concomitant sumatriptan on blood pressure in healthy volunteers by Jan de Hoon, Anne Van Hecken, Corinne Vandermeulen, Marissa Herbots, Yumi Kubo, Ed Lee, Osa Eisele, Gabriel Vargas and Kristin Gabriel in Cephalalgia