Abstract
Reactions of tethered, tertiary sulfonamides with thermally generated benzynes are reported. Typically, the N-S bonds in the substrates cleave and saturated heterocycles [tetrahydroquinolines (n = 2) and indolines (n = 1)] are formed. The process is accompanied by either sulfonyl transfer or desulfonylation from a zwitterionic intermediate, with the favored pathway being largely dependent upon the size (5- vs. 6-membered) of the N-containing ring in the zwitterion.
Graphical Abstract
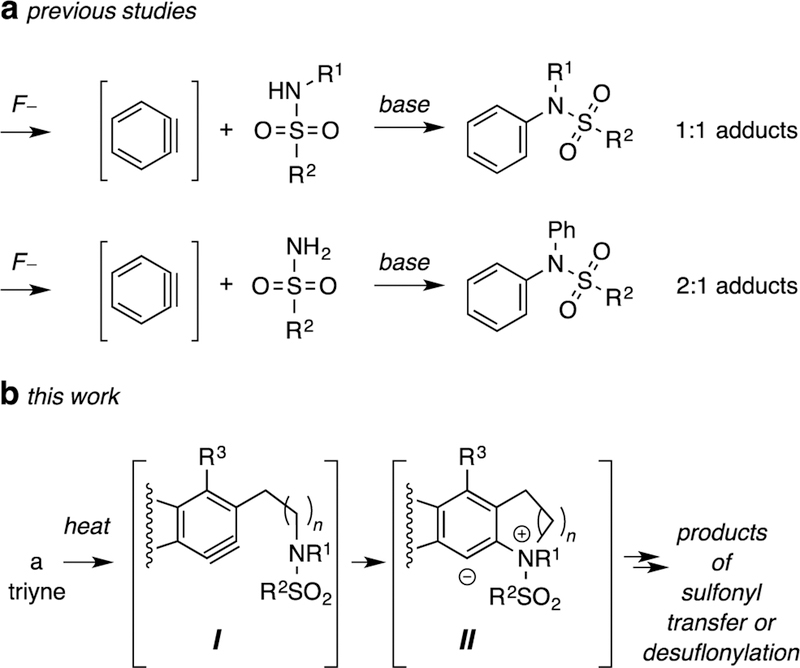
Sulfonamides are known to react, in situ, with benzyne intermediates1 made by the Kobayashi protocol2 (fluoride ion + o-silylaryltriflate). Under these basic conditions, primary or secondary sulfonamides trap the benzyne intermediates (Figure 1a). Aryl-substituted sulfonamides are produced by this formal insertion reaction of the aryne triple bond into an N-H bond of the sulfonamide; the N-S bonds in the substrates remain intact in the product. We now disclose an alternative e mode of reaction that emerges when benzynes (cf. I) derived from thermal cyclization of triyne precursors3 are trapped by fully substituted (i.e., tertiary) sulfonamides (Figure 1b). The evidence in hand suggests that (the intramolecular) reaction of various tertiary sulfonamides with HDDA-benzynes gives zwitterions II, from which different products are obtained via distinct reaction pathways. These mostly involve either sulfonyl transfer or desulfonylation. In these reactions, the sulfonyl group from the sulfonamide substrates are replaced by aryl groups, which, to our knowledge, is a unique feature of the reaction outcomes reported here.4 The products contain either a tetrahydroquinoline (THQ) or indoline core structure depending on whether the diyne terminus of the triyne substrate and the sulfonamide nitrogen are connected by three or two atoms, respectively.1
Figure 1.
a. Known modes of reaction of arynes with sulfonamides. b. Studies reported here show complementary reactions in which sulfonyl migration or desulfonylation occurs.
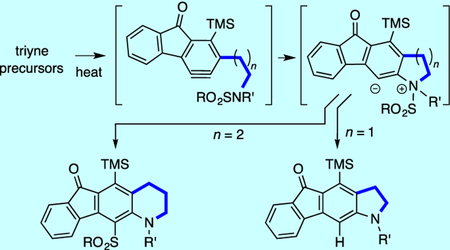
Shown in Table 1 is a series of reactions of hexadehydro-Diels-Alder (HDDA) substrates that serve as precursors to products having a newly fused piperidine ring. That is, the amide (always a p-toluenesulfonamide) and alkyne are connected by a trimethylene linker. When each of the triynes 1a–f was heated in a relatively inert5 solvent, the corresponding THQs 2a-f were isolated in excellent yield. We infer that within a THQ zwitterion such as II (n = 2) there is ample orbital overlap to permit 1,3-migration of the sulfonyl moiety (see further discussion below). It is worth mentioning that none of the product arising from a potential aza-Claisen rearrangement of the intermediate zwitterion arising from 1c and leading to 2c was observed.
Table 1.
Reactions of Ts-amide-containing triynes 1a-f having various linkers (ABC) give sulfonyl-transfer product tetrahydroquinolines 2a-f via benzyne intermediates I.
 |
% yield is of chromatographically purified material.
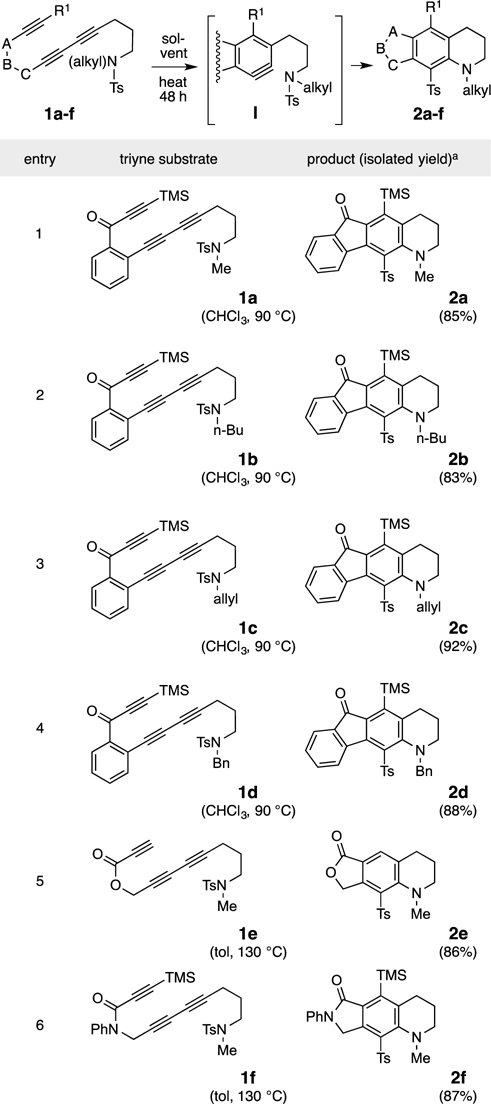
We studied several related substrates that also contained a trimethylene linker between the terminal alkyne and trapping sulfonamide. The results are summarized in Table 2. Each substrate showed behavior different from those in Table 1. Substrate 1g (entry 1) is an N-aryl sulfonamide; it gave rise to a complicated product array (tlc and 1H NMR) with no clear evidence for the presence of any of 2g. Triynes 1h and 1i (entries 2 and 3) are both methane- rather than toluenesulfonamides. They provided products (2h and 2i, respectively) in which the sulfonyl group was absent. Elimination of sulfene (IV) from within the zwitterion III would account for these outcomes (see “mechanistic rationales,” at the bottom of Table 2). Finally, the nosylamide 1j gave two products. Sulfone 2j arises by the same path as that to the tosylamide products (Table 1), but its formation was accompanied by that of the p-nitrophenyl-substituted biaryl compound 2j’ in which SO2 has been ejected. This variant of the Truce-Smiles rearrangement6 can be viewed as proceeding by ipso-attack para to the nitro substituent in V. Loss of SO2 from the delocalized zwitterion VI would lead to 2j’. Similar transformations of classic benzynes have been demonstrated.1c
Table 2.
Reactions of triynes 1g-j, having various sulfonamide groups (R2), leading to tetrahydroquinoline derivatives 2g-j via benzyne intermediates I.
 |
% yield is of chromatographically purified material.
We also studied several lower homolog substrates (1k-n, Table 3) containing a dimethylene link between the diyne terminus and sulfonamide nitrogen atom. These led to the indoline derivatives 2k-n (entries 1–4), each containing a newly formed 5-membered heterocycle. In this series we did not observe products of 1,3-sulfonyl migration analogous to those in reactions of the tosyl-sulfonamide leading to THQs (cf. Table 1). Rather, the isolated products were all devoid of the sulfonyl group. We attribute this (bottom of Table 3) to the higher activation energy (more strained transition state structure) that would be required for the conversion of the 5-membered zwitterion VII, which contains a retracted sulfonyl moiety, to the non-observed product VIII. Desulfonylation within VII could proceed by the sulfene ejection (cf. III to IV) for the Ms-substrates (entries 3 and 4) or by intervention of trace levels of a protic species such as water (IX to X, Table 3, bottom). Protonation of the carbon and desulfonylation by the resulting hydroxide would account for product formation. Given the relatively low concentration at which these experiments were performed (initial substrate concentration of 0.01 M), we cannot confidently judge which is the more likely scenario. The N-arylated, one methylene-lower homolog 1n gave an interesting result in contrast to the behavior of substrate 1g (Table 2, entry 1). The methanesulfonamide 1n smoothly provided the N-phenylindoline 2n, again by loss of sulfene.
Table 3.
Reactions of triynes 1k-n, having tosyl or mesyl sulfonamide groups (R2), give desulfonylated indoline derivatives 2k-n.
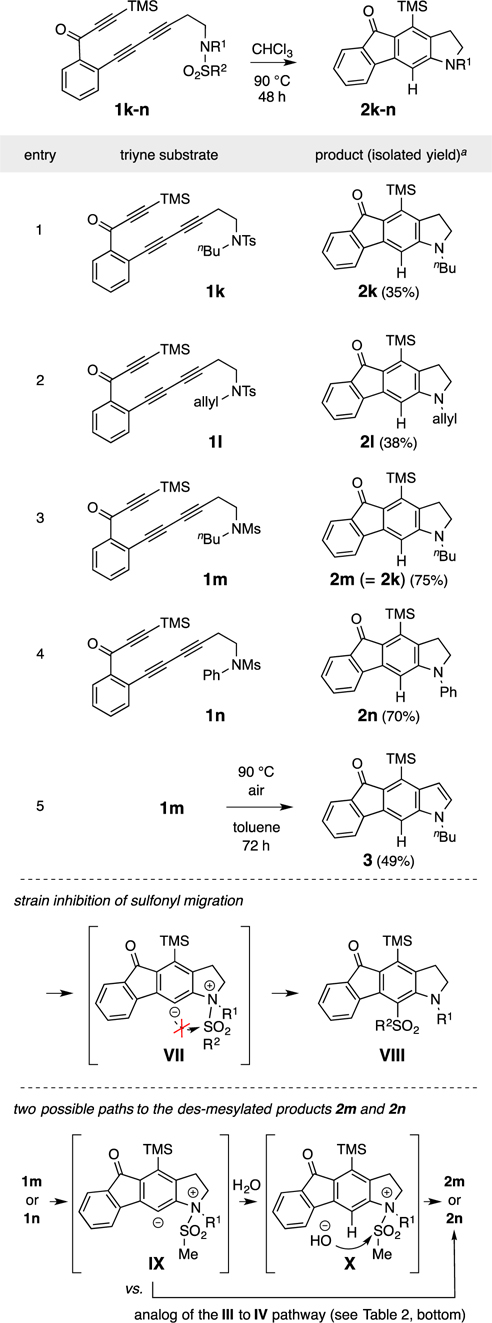 |
% yield is of chromatographically purified material.
Finally, we observed that each of these indoline products was susceptible to air autoxidation7 to the corresponding indole. Performing the HDDA reaction under a nitrogen atmosphere substantially improved the cleanliness of the product mixture. In the case of 1m, we heated the reaction mixture for an extended period of time (90 °C, 72 h) under a headspace of air and isolated the indole 3 as the main product; no indoline remained in the crude reaction mixture.
In conclusion, we have demonstrated that the nitrogen in tertiary sulfonamide groups can react with HDDA-generated benzynes to produce zwitterion intermediates. Various reaction pathways ensue from these species, depending on the size of the newly formed nitrogen heterocycle and the nature of the substituent (alkyl vs. aryl) present on the zwitterionic nitrogen atom. The processes include sulfonyl transfer to the vicinal, benzyne-derived carbon atom or desulfonylation events. Each results in the formation of a new, saturated, benzo-fused piperidine (i.e., a tetrahydroquinoline) or pyrrolidine (i.e., an indoline) ring. Among other things, each of these transformations results in the replacement of a robust N-SO2R bond8 by a N-C bond, potentiated by the high energy of the reactive benzyne intermediate.
Supplementary Material
ACKNOWLEDGMENT
Support for this research was provided by the National Institutes of General Medical Sciences of the U.S. Department of Health and Human Services (R01 GM65597, then R35 GM127097). L.Z. received support from the China Scholarship Council Program (201706175084). A portion of the NMR spectral data were acquired with an instrument purchased with funds provided by the NIH Shared Instrumentation Grant program (S10OD011952). The X-ray diffraction analysis was performed by Dr. Victor G. Young (University of Minnesota).
Footnotes
ASSOCIATED CONTENT
Supporting Information. The Supporting Information is available free of charge on the ACS Publications website.
Experimental details for the preparation of new compounds; and spectroscopic data (including copies of 1H and 13C NMR spectra) for their characterization.
Notes
The authors have no competing financial interests to declare.
Throughout this manuscript we have used Roman numerals (I–X) to label structures of intermediate species that were not isolated and Arabic numerals to designate the structures of isolated (and newly characterized) compounds.
REFERENCES
- 1.(a) Liu Z; Larock RC Intermolecular C-N Addition of Amides and S-N Addition of Sulfinamides to Arynes. J. Am. Chem. Soc 2005, 127, 13112–13113. [DOI] [PubMed] [Google Scholar]; (b) Liu Z; Larock RC Facile N-Arylation of Amines and Sulfonamides and O-Arylation of Phenols and Arenecarboxylic Acids. J. Org. Chem 2006, 71, 3198–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Holden CM; Sohel SMA; Greaney MF Metal Free Bi(hetero)aryl Synthesis: A Benzyne Truce–Smiles Rearrangement. Angew. Chem. Int. Ed 2016, 55, 2450–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Qiu D; He J; Yue X; Shi J; Li Yang. Diamination of Domino Aryne Precursor with Sulfonamides. Org. Lett 2016, 18, 3130–3133. [DOI] [PubMed] [Google Scholar]; (e) Li L; Qiu D; Shi J; Li Yang. Vicinal Diamination of Arenes with Domino Aryne Precursors. Org. Lett 2016, 18, 3726–3729. [DOI] [PubMed] [Google Scholar]; (f) Ikawa T; Sumil Y; Masuda S; Wang D; Emi Y; Takagi A; Akai S Synthesis of Optically Active 2,3-Disubstituted Indoline Derivatives through Cycloaddition Reactions between Benzynes and α,β-Unsaturated γ-Aminobutyronitriles. Synlett 2018, 29, 530–536. [Google Scholar]
- 2.Himeshima Y; Sonoda T; Kobayashi H Fluoride-induced 1,2-Elimination of o-Trimethylsilylphenyl Triflate to Benzyne under Mild Conditions. Chem. Lett 1983, 1211–1214.
- 3.(a) Miyawaki K; Suzuki R; Kawano T; Ueda I Cycloaromatization of a Non-Conjugated Polyenyne System. Tet. Lett 1997, 38, 3943–3946. [Google Scholar]; (b) Bradley AZ; Johnson RP Thermolysis of 1,3,8-Nonatriyne: Evidence for Intramolecular [2 + 4] Cycloaromatization to a Benzyne Intermediate. J. Am. Chem. Soc 1997, 119, 9917–9918. [Google Scholar]; (c) Tsui JA, and Sterenberg BT A Metal-Templated 4 + 2 Cycloaddition Reaction of an Alkyne and a Diyne To Form a 1,2-Aryne. Organometallics 2009, 28, 4906–4908. [Google Scholar]; (d) Yun SY; Wang K-P; Lee N-K; Mamidipalli P; Lee D Alkane C–H Insertion by Aryne Intermediates with a Silver Catalyst. J. Am. Chem. Soc 2013, 135, 4668–4671. [DOI] [PubMed] [Google Scholar]; (e) Hoye TR; Baire B; Niu D; Willoughby PH; Woods BP The Hexadehydro-Diels-Alder reaction. Nature 2012, 490, 208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Searles S; Nukina S Cleavage and Rearrangement of Sulfonamides. Chem. Rev 1959, 59, 1077–1103. [Google Scholar]
- 5. Although solvents like toluene have been observed to trap benzynes in Diels-Alder and or ene type reactions, those processes are sufficiently slow that they do not interfere with the more facile trapping by the nucleophilic (and internally linked) nitrogen atom of the sulfonamide.
- 6.Henderson ARP; Kosowan JR; Wood TE The Truce–Smiles Rearrangement and Related Reactions: A Review Can. J. Chem. 2017, 95, 483–504. [Google Scholar]
- 7.Padwa A; Kappe CO; Cochran JE; Snyder JP Studies Dealing with the Cycloaddition/Ring Opening/Elimination Sequence of 2-Amino-Substituted Isobenzofurans. J. Org. Chem 1997, 62, 2786–2797. [DOI] [PubMed] [Google Scholar]
- 8.For a discussion that reflects an early appreciation of the robustness of sulfonamides and their stability, see: Searles S; Nukina S Cleavage and Rearrangement of Sulfonamides. Chem. Rev 1959, 59, 1077–1103. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.