Abstract
There is strong evidence that the immune system changes dramatically during pregnancy in order to prevent the developing fetus from being “attacked” by the maternal immune system. Due to these alterations in peripheral immune function, many women that suffer from autoimmune disorders actually find significant relief from their symptoms throughout pregnancy; however, these changes can also leave the mother more susceptible to infections that would otherwise be mitigated by the inflammatory response (Robinson and Klein, 2012). Only one other study has looked at changes in microglial number and morphology during pregnancy and the postpartum period (Haim et al., 2016), but no one has yet examined the neuroimmune response following an immune challenge during this time. Therefore, in this study, we investigated the impact of an immune challenge during various time-points throughout pregnancy and the postpartum period on the expression of immune molecules in the brain of the mother and fetus. Our results indicate that similar to the peripheral immune suppression measured during pregnancy, we also see significant suppression of the immune response in the maternal brain, particularly during late gestation. In contrast to the peripheral immune system, immune modulation in the maternal brain extends moderately into the postpartum period. Additionally, we found that the fetal immune response in the brain and placenta is also suppressed just before parturition, suggesting that cytokine production in the fetus and placenta are mirroring the peripheral cytokine response of the mother.
Keywords: Pregnancy, Hormones, Microglia, Immune function, Peripartum depression, Mood and anxiety disorders
1. Introduction
It is well known that the peripheral immune system changes dramatically during pregnancy in order to prevent the developing fetus from being attacked by the maternal immune system. Many studies have demonstrated that this is the consequence of a transient suppression of maternal cell-mediated immunity against the semi-allogenic fetus during pregnancy (Mellor et al., 2001; Raghupathy, 1997; Trowsdale and Betz, 2006). For most women, these changes in peripheral immune function go unnoticed; however, when this shift in immune function is coupled with sickness, or a pre-existing health condition, many women experience a significant change in their health. For example, many women find relief from pre-existing autoimmune disorders during pregnancy. In 1938, Hench was the first to report that pregnancy could ameliorate the symptoms of rheumatoid arthritis. In these cases, patients reported significant improvement of symptoms during their pregnancy, particularly later in gestation, and a significant worsening of symptoms in the weeks after giving birth (Hazes et al., 2011; Hench, 1938; Nelson and Ostensen, 1997). Similar findings have also been reported for women diagnosed with the autoimmune disease multiple sclerosis (MS). These women have significantly reduced rates of MS relapse during pregnancy, and a significant increase in relapse rates in the first three months postpartum (Bernardi et al., 1991; Confavreux et al., 1998). Similarly, late pregnancy is also associated with an increased severity and mortality associated with a number of infections, both viral and bacterial (Robinson and Klein, 2012), such that these effects of pregnancy on peripheral immune function directly contribute to an overall female susceptibility to disease. The effects of pregnancy on disease susceptibility are a direct result of suppression or modulation of specific immune molecules that likely underlie the disease’s progression or remission. Taken together, this evidence indicates that pregnancy induces significant modulation of the mother’s peripheral immune system and has substantial consequences for her physical health.
It is also known that immune dysregulation is often linked to the onset of certain mental health disorders, including depression and anxiety (Raison and Miller, 2017; Remus and Dantzer, 2016). The peripartum period is a critical time-point in a woman’s life that is associated with an increased risk of certain mental health disorders, most notably peripartum anxiety and depression, which have also been concurrently linked with altered cytokine production in the periphery (Osborne and Monk, 2013). Despite this evidence, researchers have yet to determine whether the immune changes we see in the periphery also occur in the brain during pregnancy and the postpartum period. Only one other study has looked at changes in microglial number and morphology during pregnancy and the postpartum period (Haim et al., 2016), but no one has yet examined the neuroimmune response following an immune challenge during this time.
Thus, the goal of this study was three fold: first, we wanted to determine the impact of pregnancy on immune function in the brain, then, we sought to determine how long these changes in the brain persisted postpartum, and finally, we examined the impact of immune activation on the expression of immune molecules in the brain of fetal pups, the placenta, and in the brain and periphery of the pregnant dams in order to examine the ontogeny of these immune responses across the maternal-fetal interface. Similar to previous reports, we found that the peripheral immune response is significantly suppressed during pregnancy but returns to baseline levels immediately after parturition. In the maternal brain, we see a similar suppression of the immune response during pregnancy, particularly during late gestation; however, in contrast to the peripheral immune system, this immune modulation extends into the postpartum period. Finally, we found that the immune response in the fetal brain and placenta are also suppressed during late gestation, mirroring the peripheral immune response of the mother, an effect that was not dependent on the sex of the fetus.
2. Materials and methods
2.1. Animals
All experiments used female Sprague-Dawley rats ordered from Envigo Laboratories (Indianapolis, IN). Rats were housed in clear polypropylene cages with ad libitum access to food and water in rooms under a 12:12-h light:dark cycle and maintained temperature and humidity. All experiments were performed in accordance with the Institutional Animal Care and Use Committee of the University of Delaware and under the Guide for the Care and Use of Laboratory Animals of the National Institute of Health.
2.2. Breeding
Ninety-six female rats were individually paired with male rats for breeding. Date of conception, embryonic day (E) 0, was determined via the presence of a vaginal plug and day of birth (typically E23) was assigned as Postnatal (P) Day 0. Rats that did not become pregnant at the time of mating were assigned to the Non-pregnant group. Females were pair-housed in clean cages until one day prior to the administration of Lipopolysaccharide (LPS) or saline (at E11 or E21) when they were separated into individual cages and remained individually housed for the duration of the experiment. Pups that were born were housed with their mothers for the entirety of the experiment. The litters that were born were not culled or treated. Following a two-way ANOVA, we did not find a significant main effect of Treatment or Condition, and no Treatment × Condition interaction for litter size between postpartum groups (P0, P2, and P9).
2.3. LPS treatment
Lipopolysaccharide (LPS) derived from Escherichia coli 0111:B4 was obtained from Sigma-Aldrich® (Cat. No. L2630). LPS was diluted with sterile Dulbecco’s phosphate buffered saline (DPBS) to a concentration of 100 µg/mL. Female rats received either LPS (at a final dose of 100 µg/kg in 1 ml/kg) or vehicle (DPBS, 1 ml/kg) via intraperitoneal injections. Female rats received the injection on one of the five separate time-points either during gestation (E11 or E22) or postpartum (P0, P2, or P9); and non-pregnant rats received the injection randomly time-matched to one of the five pregnant groups (N = 96 rats total, n = 8 rats per treatment group and time-point). LPS is a cell-wall component of gram-negative bacteria and was used in these experiments to stimulate a well-characterized cytokine response, thus allowing us to determine how pregnancy modulates the central and peripheral responses to a systemic immune challenge.
2.4. Euthanasia and perfusion
The female rats were euthanized 4 h after LPS or vehicle administration using an overdose of the barbiturate Euthasol® (ANADA 200–071) via intraperitoneal injection. Once anesthetized, a serum sample was collected via cardiac puncture and then the rats were perfused with 0.9% saline solution to remove circulating peripheral immune cells and immune molecules from the brain tissue. After perfusion, half of each brain was extracted and the whole hippocampus (HP), and Hypothalamus/Preoptic Area (HyPoA) were micro-dissected from all 96 rats, and the medial prefrontal cortex (mPFC) was dissected from 84 of the rats using the guide of a rat atlas and immediately flash frozen in cold isopentane and stored at −80 °C until ready for processing. The hemisphere collected for either analysis was randomized. The spleen from all females and the placenta from pregnant females were also collected following the perfusion. We also collected tissue from E11 and E22 fetuses. This allowed us the opportunity to compare fetal immune response to maternal immune response. Tissue from no more than one male and one female pup from each dam were collected and used in subsequent analysis (E11 male saline, n = 6; E11 female saline, n = 5; E11 male LPS, n = 5; E11 female LPS, n = 7; E22 male saline, n = 8; E22 female saline, n = 8; E22 male LPS, n = 8; E22 female LPS, n = 8). Fetal brain, tail, and spleen (though not present at E11) were collected and immediately flash frozen for analysis. Pups were not collected on P0, P2, or P9, but were euthanized via approved methods.
2.5. Real-time PCR
Messenger RNA (mRNA) was extracted from maternal brain, fetal brain, placenta, and maternal or fetal spleen tissue using Isol-RNA Lysis Reagent (Cat. No. 2302700, 5 PRIME). Extracted RNA (1000 ng) was then subjected to DNase treatment to remove any genomic DNA prior to cDNA synthesis using the QuantiTect® Reverse Transcription Kit (Cat. No. 205314, Qiagen). Relative gene expression was measured using the RealMasterMix™ Fast SYBR Kit (Cat. No. 2200830, 5 PRIME) in 10 µL reactions on a CFX96Touch™ real time PCR machine for the following genes: IL-1β, IL-6, IL-4, IFNγ, BDNF, and TLR4. These particular genes were chosen for analysis due to their involvement in general proinflammatory and anti-inflammatory processes (IL-1β, IL-6, IL-4, BDNF) as well as their specific involvement in LPS-induced inflammation (IFNγ and TLR4). The primers for Il-6 and Rplp1 were QuantiTect® Primer Assays Rn_Il6_1_SG (Cat. No. QT00182896, Qiagen) and Rn_Rplp1_1_SG (Cat. No. QT00365561, Qiagen) and were diluted as per the Qiagen protocol for the real-time PCR reaction. All other primers were ordered through Integrated DNA Technologies and diluted to a final concentration of 0.13 µM for the real-time PCR reaction. The sequences of primers were as follows: CD11b forward: CTGGGAGATGTGAATGGAG, reverse: ACTGATGC TGGCTACTGATG (NM_012711.1); IL-1β forward: GAAGTCAAGACC AAAGTGG, reverse: TGAAGTCAACTATGTCCCG (NM_031512.2); BDNF forward: ATCCCATGGGTTACACGAAGGAAG, reverse: AGTAA GGGCCCGAACATACGATTG (NM_001270638.1); IL-4 forward: AAGGAACACCACGGAGAACG, reverse: CAGACCGCTGACACCTCTAC (NM_201270.1); TLR4 forward: CAGAGGAAGAACAAGAAGC, reverse: CCAGATGAACTGTAGCATTC. Rplp1 was used as the reference/housekeeping gene for all samples as it was not significantly different across experimental conditions (neither pregnancy, postpartum condition, nor LPS treatment). For each reaction, the quantitative threshold amplification cycle number (Cq) was determined, and the 2–∆∆Cq method was used to calculate the relative gene expression of each gene in question.
2.6. Fetal sex determination
In order to determine the sex of the fetuses, tails were collected from all E11 and E22 fetuses. Tail DNA was extracted using the Invitrogen Kit (cat. K1820–01). Relative gene expression was performed for the sex-specific SRY gene (forward primer: TACAGCCTGAGGACAT, reverse primer: CTGCTTGCTGATCTCTGAAT) using quantitative Real-Time PCR. Samples were analyzed using the 2–∆∆Cq method described above, with Rplp1 as the housekeeping gene, and all samples that had 2–∆∆Cq values for SRY expression above 20 were considered male fetuses, while samples that had 2–∆∆Cq values below 5 were considered female fetuses.
2.7. Statistical analysis
Two-way ANOVA tests were used to assess the statistical significance of all data in this study using Treatment (LPS vs. saline) and Condition (non-pregnant, E11, E22, P0, P2, and P9) as factors of analysis. For the analysis of fetal tissue, Two-way ANOVA tests were used to assess the statistical significance of all data using Treatment (LPS vs. saline) and Sex (Male vs. Female) as factors. Significant main effects and significant interactions of these analyses are reported using p < 0.05. Significant main effects were followed up with Tukey’s post hoc test (p < 0.05) to analyze individual group differences. Significant interactions were followed up with pairwise comparisons across all groups using Fisher’s least significant difference (p < 0.05). From the maternal HyPoA samples, two samples were removed due to insufficient levels of RNA. From the maternal hippocampus samples, 2–3 samples (never from the same treatment group) per gene analysis had to be removed as significant outliers (greater than 3 Standard Deviations from the mean). From the medial prefrontal cortex samples that were collected, 4 samples had insufficient RNA levels for processing. In both the fetal brain and placenta samples, one sample had to be removed from each analysis due to insufficient RNA levels for subsequent analyses. All analyses were confirmed to have sufficient power (p = 0.8) to determine significant differences. All graphs represent the group mean ± Standard Error of the Mean. Condition × Treatment interactions: Individual groups that do not share any similar capital letters are considered significantly different from each other (p < 0.05). Main effect of Condition: Groups that do not share any similar lowercase letters are considered significantly different (p < 0.05). Main effect of Treatment: LPS treated groups with an asterisk are considered significantly different from saline treated groups (p < 0.05).
3. Results
An examination of the immune response in the periphery and the brain during pregnancy and the postpartum period.
3.1. Maternal spleen
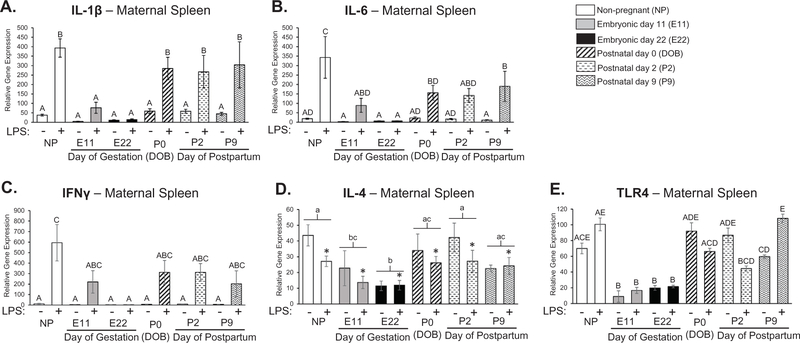
The spleen was analyzed from females in the current experiment as a proxy for peripheral immune activation to confirm previous reports of immunosuppression during pregnancy and extend our understanding of this immunosuppression and its duration into the postpartum period. We found a significant interaction of pregnancy condition and LPS treatment for the expression of IL-1β, IL-6, and IFNγ in the spleen (Treatment × Condition: IL-1β: F5,84 = 3.22; p = 0.010, IL-6: F5,84 = 2.34; p = 0.048, IFNγ: F5,84 = 2.32; p = 0.050; Fig. 1ABC). Post hoc comparisons indicated that the expression of both IL-β and IL-6 were significantly attenuated in LPS-treated females at E11 compared to the expression of these same genes in LPS-treated non-pregnant females (IL-1β: p < 0.001; IL-6: p < 0.001; Fig. 1AB). This attenuation was also apparent at E22 for IL-β, IL-6, and IFNγ (IL-1β: p < 0.001; IL-6: p < 0.001, IFNγ: p < 0.001; Fig. 1ABC). Surprisingly, the expression of IL-1β and IFNγ immediately returned to pre-pregnancy levels on the day of parturition (P0) as their expression levels in the spleen were not significantly different from non-pregnant females treated with LPS (IL-1β: p = 0.114, IFNγ: p = 0.441). Additionally, we found main effects of both pregnancy condition and LPS treatment for the expression of IL-4 in the spleen where non-pregnant controls are significantly different than E11 and E22 time-points (Pregnancy: F5,81 = 4.465; p = 0.001, Treatment: F1,81 = 4.293; p = 0.041; Fig. 1D). We also found a significant interaction of pregnancy condition and LPS treatment for the expression of TLR4 (Treatment × Condition: TLR4: F5,84 = 3.15; p = 0.012; Fig. 1E) such that LPS-treated females at E11, E22, P0, and P2 had a significantly attenuated response compared to the LPS-treated non-pregnant females (p < 0.05). These data confirm that pregnancy significantly attenuates the expression of TLR4 and the expression of specific inflammatory molecules, in this case IL-1β, IL-6, IFNγ, and IL-4 in the periphery, even in the presence of an immune challenge, but that these effects of pregnancy are largely reversed immediately after parturition.
Fig. 1.
Examination of cytokine expression in maternal spleen at various time-points throughout pregnancy and the postpartum period. There is a significant interaction of pregnancy condition and LPS treatment for the expression of IL-1β, IL-6, and IFNγ in the spleen (Treatment × Condition: IL-1β: F5,84 = 3.22; p = 0.010, IL-6: F5,84 = 2.34; p = 0.048, IFNγ: F5,84 = 2.32; p = 0.050; ABC). The expression of both IL-β and IL-6 were significantly attenuated in LPS-treated females at E11 compared to the expression of these same genes in LPS-treated non-pregnant females (IL-1β: p < 0.001; IL-6: p < 0.001; AB). This attenuation was also apparent at E22 for IL-β, IL-6, and IFNγ (IL-1β: p < 0.001; IL-6: p < 0.001, IFNγ: p < 0.001; ABC). The expression of IL-1β and IFNγ immediately returned to pre-pregnancy levels on the day of parturition (P0) as the expression levels of these cytokines in the spleen were not significantly different from non-pregnant females treated with LPS (IL-1β: p = 0.114, IFNγ: p = 0.441; AC). There was a main effect of pregnancy condition and LPS treatment for the expression of IL-4 in the spleen where non-pregnant controls are significantly different than E11 and E22 time-points (Condition: F5,81 = 4.465; p = 0.001, Treatment: F1,81 = 4.293; p = 0.041; D). There was also a significant interaction of pregnancy condition and LPS treatment for the expression of TLR4 (Treatment × Condition: TLR4: F5,84 = 3.15; p = 0.012; Fig. 1E), such that LPS-treated females at E11, E22, P0, and P2 had a significantly attenuated response compared to the LPS-treated non-pregnant females. n = 7–8 rats per group. Condition × Treatment interaction: groups that do not share any capital letters are considered significantly significant (p < 0.05). Main effect of Condition: groups that do not share any lowercase letters are considered statistically significant (p < 0.05). Main effect of Treatment: groups with an asterisk are considered statistically significant (p < 0.05).
3.2. Maternal brain
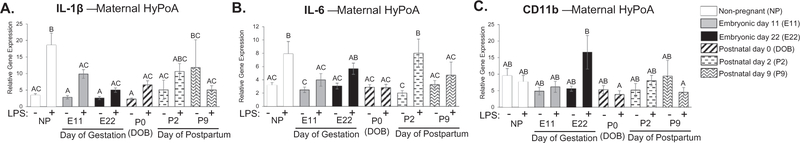
We analyzed the hypothalamus/preoptic area (HyPoA), hippocampus, and medial prefrontal cortex (mPFC) from females across all points of gestation and the postpartum period to determine whether neuroimmune function was significantly attenuated in a manner similar to the effects seen in the spleen (Fig. 1). The hypothalamus and preoptic area are brain regions important for the generation of febrile responses, induction of sickness behaviors, and has been shown to be altered during pregnancy and the postpartum period for the development of appropriate postpartum maternal behaviors (Elmquist et al., 1997; Insel and Harbaugh, 1989; Numan et al., 1977). In the HyPoA, there was a significant treatment by condition interaction for the expression of IL-1β, IL-6, and CD11b (IL-1β: F5,82 = 2.614, p = 0.030, Fig. 2A; IL-6: F5,82 = 2.822; p = 0.021, Fig. 2B; CD11b: F5,82 = 2.368; p = 0.047, Fig. 2C). Specifically, IL-1β gene expression following the LPS challenge in the HyPoA was significantly attenuated during pregnancy at E11, E22, P0, and P9 compared to non-pregnant controls (E11: p = 0.038; E22: p = 0.001; P0: p = 0.005; P9: p = 0.002, Fig. 2A). IL-6 gene expression was attenuated following LPS treatment at E11, P0, and P9 compared to non-pregnant controls (E11: p = 0.008, P0: p = 0.001, P9: p = 0.001, Fig. 2B). Post hoc analysis of CD11b expression revealed very few significant differences, only that CD11b expression following LPS treatment on P0 and P9 were significantly different than CD11b expression following LPS treatment on E22 (P0: p = 0.023, P9: 0.038; Fig. 2C). There were no significant differences in TLR4 expression between groups (data not shown).
Fig. 2.
Examination of cytokine expression in maternal hypothalamus/preoptic area at various time-points throughout pregnancy and the postpartum period. There was a significant treatment by condition interaction in the expression of IL-1β, IL-6, and CD11b (IL-1β: F5,82 = 2.614, p = 0.030; IL-6: F5,82 = 2.822; p = 0.021; CD11b: F5,82 = 2.368; p = 0.047; ABC). Specifically, IL-1β gene expression following the LPS challenge in the HyPoA is significantly attenuated during pregnancy at both E11, E22, P0, and P9 compared to non-pregnant controls (E11: p = 0.038; E22: p = 0.001; P0: p = 0.005; P9: p = 0.002; A). IL-6 gene expression is attenuated following LPS treatment at E11, P2, and P9 compared to non-pregnant controls (E11: p = 0.008, P0: p = 0.001, P9: p = 0.001; B). There was a significant attenuation in CD11b gene expression following LPS during postpartum days P0 and P9 compared to LPS treated animals on day E22 (P0: p = 0.023, P9: 0.038; C). n = 7–8 rats per group. Condition × Treatment interaction: groups that do not share any capital letters are considered significantly significant (p < 0.05).
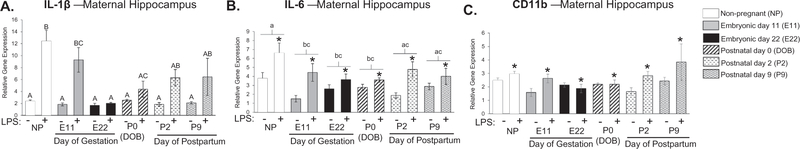
We analyzed cytokine expression within the hippocampus because of its importance in cognitive function as well as its implications in the etiology of depression, two components of behavior that are also altered during pregnancy and the postpartum period (Buckwalter et al., 1999; Kronfol, 2000; Maes, 1995; Raison and Miller, 2013). In the hippocampus, there was a significant treatment by condition interaction, such that IL-1β expression on E22 and P0 were significantly suppressed following LPS treatment relative to LPS treated non-pregnant controls (IL-1β: F5,81 = 3.685; p = 0.005; Fig. 3A). There was a main effect of both pregnancy condition and treatment for IL-6 expression (Condition: F5,82 = 2.972; p = 0.016; Treatment: F1,82 = 20.736; p < 0.001; Fig. 3B) where IL-6 expression at E11, E22, and P0 were significantly decreased within the hippocampus compared to non-pregnant controls (E11: p = 0.023; E22: p = 0.040; P0: p = 0.030; Fig. 3B). Notably, however, IL-6 expression was still induced following LPS treatment at all points during gestation and the postpartum period despite differences in baseline expression. We also found a main effect of treatment for CD11b expression, where CD11b expression is increased following LPS treatment (F1,83 = 9.661; p = 0.021; Fig. 3C). We did not find significant differences in TLR4 expression between groups (data not shown).
Fig. 3.
Examination of cytokine expression in maternal hippocampus at various time-points throughout pregnancy and the postpartum period. There was a significant treatment by condition interaction for IL-1β gene expression following LPS exposure where time-points E22 and P0 were significantly different than non-pregnant controls (IL-1β: F5,81 = 3.685; p = 0.005; A). There was a main effect of both condition and treatment for IL-6 expression where time-points E11, E22, and P0 were significantly different than non-pregnant controls (E11: p = 0.023; E22: p = 0.040; P0: p = 0.030; B). There was also a main effect of treatment for CD11b expression, where CD11b expression is generally increased following LPS treatment (F1,83 = 9.661; p = 0.021; C). n = 7–8 rats per group. Condition × Treatment interaction: groups that do not share any capital letters are considered significantly significant (p < 0.05). Main effect of Condition: groups that do not share any lowercase letters are considered statistically significant (p < 0.05). Main effect of Treatment: groups with an asterisk are considered statistically significant (p < 0.05).
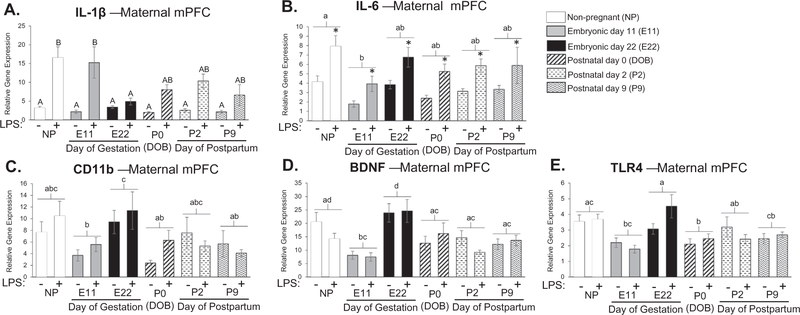
We also examined cytokine expression in the mPFC during pregnancy and the postpartum period because of its well-known role in cognition and the regulation of mood and emotion, which is also known to be altered during pregnancy and the postpartum period (Buckwalter et al., 1999; Damasio et al., 1990; Morgan et al., 1993; Raison and Miller, 2013; Teuber, 1964). In the mPFC, there was a treatment by condition interaction for IL-1β gene expression (F5,80 = 2.668; p = 0.028, Fig. 4A). Specifically, LPS-induced IL-1β expression was significantly attenuated at E22 compared to non-pregnant controls (E22: p = 0.002; Fig. 4A). Additionally, we found a main effect of condition and treatment for IL-6 gene expression where the E11 pregnancy time-point was significantly different than non-pregnant controls (Condition: F5,78 = 2.550; p = 0.034, Treatment: F1,78 = 26.209; p < 0.001, Fig. 4B). There was also a main effect of condition for CD11b, where E22 was significantly different than E11, P0, and P9 (F5,79 = 3.144; p = 0.012, Fig. 4C). We also examined the expression of the neurotrophic factor, BDNF, because we had previously seen that it is significantly modulated in its expression within the mPFC around birth (Posillico and Schwarz, 2016). We found a main effect of condition for BDNF expression and post hoc tests revealed that BDNF in the mPFC at E11 is significantly decreased compared to the non-pregnant group and that E22 is significantly increased compared to all other time-points with the exception of the non-pregnant group (F5,79 = 8.451; p = < 0.001, Fig. 4D). We analyzed the expression of BDNF in other brain regions (hippocampus and HyPoA), but found no significant effects (data not shown). Additionally, we analyzed TLR4 expression and found a significant main effect of pregnancy. Post hoc analyses revealed that TLR4 expression at P0 was significantly suppressed compared to the non-pregnant group, and TLR4 gene expression at E22 was significantly higher than E11, P0, and P9 (F5,80 = 5.608; p = < 0.001, Fig. 4E). Taken together, these data suggest that pregnancy can significantly influence the neuroimmune response or cytokine production in the maternal brain. It is important to note, however, that cytokine expression is altered differentially in the brain relative to the periphery, and slightly different across various brain regions, which we will discuss in further detail, below.
Fig. 4.
Examination of cytokine expression in maternal medial prefrontal cortex at various time-points throughout pregnancy and the postpartum period. There was a treatment by condition interaction for IL-1β gene expression (F5,80 = 2.668; p = 0.028) where LPS-induced IL-1β expression was significantly attenuated at E22 compared to non-pregnant controls (E22: p = 0.002; A). There was a main effect of condition and treatment for IL-6 gene expression where E11 pregnancy time-point was significantly different than non-pregnant controls (Condition: F5,78 = 2.550; p = 0.034, Treatment: F1,78 = 26.209; p < 0.001; B). There was also a main effect of condition for CD11b where E11, P0, and P9 were significantly different than non-pregnant controls (F5,79 = 3.144; p = 0.012; C). We found a main effect of condition for BDNF where E11 expression is significantly decreased compared to the non-pregnant group and that E22 is significantly increased compared to all other time-points with the exception of the non-pregnant group (F5,79 = 8.451; p = < 0.001; D). There was a main effect in TLR4 expression where P0 was significantly suppressed compared to the non-pregnant group, and E22 gene expression was significantly higher in E11, P0, and P9 (F5,80 = 5.608; p = < 0.001; E). n = 7–8 rats per group. Condition × Treatment interaction: groups that do not share any capital letters are considered significantly significant (p < 0.05). Main effect of Condition: groups that do not share any lowercase letters are considered statistically significant (p < 0.05). Main effect of Treatment: groups with an asterisk are considered statistically significant (p < 0.05).
3.3. How does maternal immune activation alter inflammatory gene expression in the fetus and placenta?
3.3.1. Fetal brain
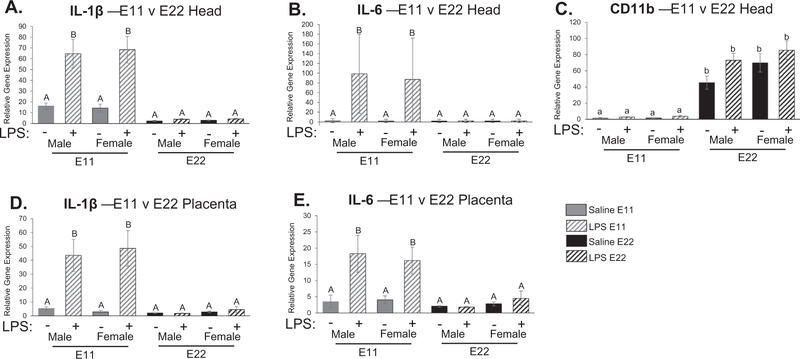
Fetal brain tissue was collected at E11 and E22 following LPS treatment of the dam, this allowed us the opportunity to analyze the fetal neuroimmune response to maternal immune challenge. We found a significant (p < 0.001) treatment by condition (fetal age) interaction for IL-1β expression (F1,46 = 36.067; p < 0.001, Fig. 5A) as well as IL-6 expression (F1,45 = 18.225; p < 0.001, Fig. 5B), where gene expression was significantly higher in E11 pups following maternal LPS administration compared to saline-treated controls (p < 0.05), but also compared to all E22 pups (p < 0.05). Additionally, we found a main effect of condition (fetal age) on CD11b gene expression. Specifically, E22 pups had significantly higher CD11b expression compared to E11 pups (F1,46 = 131.846 p < 0.001, Fig. 5C). This effect could be representative of microglial infiltration and subsequent proliferation which only begins just prior to embryonic day 10 (Chan et al., 2007; Ginhoux et al., 2010; Male and Rezaie, 2001), thus explaining why there would be lower CD11b expression on E11 compared to E22. Notably, there was no effect of maternal LPS treatment at either age on the expression of CD11b in the fetal brain. There were also no significant differences between the sexes at these fetal ages.
Fig. 5.
Examination of cytokine expression in fetal head and placenta at embryonic day 11 (E11) and embryonic day 22 (E22). There was a significant treatment by condition interaction for IL-1β expression (F1,46 = 36.067; p < 0.001; A) as well as IL-6 expression (F1,45 = 18.225; p < 0.001; B) where gene expression was significantly higher in E11 pups compared to saline treated controls but also compared to E22 pups following LPS administration (p < 0.05). There was a main effect of condition in CD11b gene expression where E22 pups showed significantly higher gene expression compared to E11 pups (F1,46 = 131.846 p < 0.001; C). Similar to fetal brain, there was a significant treatment by condition interaction for IL-1β gene expression (F1,46 = 25.705; p < 0.001; D) and IL-6 expression (F1,46 = 13.472; p = 0.001; E), where inflammatory gene expression was significantly higher in placentas collected at E11 compared to placentas taken at E22 following maternal LPS administration. There were no significant differences between the sexes in the immune response seen in the placenta. *p < 0.05. n = 5–8 rats per group. Condition × Treatment interaction: groups that do not share any capital letters are considered significantly significant (p < 0.05). Main effect of Condition: groups that do not share any lowercase letters are considered statistically significant (p < 0.05).
3.3.2. Placenta
Placental tissue was also collected at E11 and E22. Similar to fetal brain, there was a significant treatment by condition (fetal age) interaction for IL-1β gene expression (F1,46 = 25.705; p < 0.001, Fig. 5D) and IL-6 expression (F1,46 = 13.472; p = 0.001, Fig. 5E), where inflammatory gene expression was significantly higher in placentas collected at E11 compared to placentas taken at E22 following maternal LPS administration. There were no significant differences in TLR4 expression in the placenta. There were no significant differences between the sexes in the immune response seen in the placenta.
4. Discussion
Our results demonstrate that peripheral IL-1β, IFNγ, and IL-4 cytokine expression in the spleen is significantly suppressed during pregnancy and returns to baseline function immediately after parturition. We see a similar suppression of the cytokine response during pregnancy in the maternal brain, particularly during late gestation; however, in contrast to our findings in the maternal spleen, this immune suppression extends moderately into the postpartum period. We found that the fetal immune response in the brain and placenta is also suppressed, particularly during late pregnancy, suggesting that cytokine production in the fetus and placenta mirrors the peripheral cytokine response of the mother. Taken together, our results suggest that the peripheral and central immune systems respond similarly to an immune challenge during pregnancy and the immediate postpartum period, however, the time course of the immune suppression associated with pregnancy is slightly different between the periphery and the brain. In addition, the immune response measured in the fetal brain in response to maternal immune activation may largely be driven by the placenta and/or by the peripheral immune response in the mother.
There are two main factors that can affect peripheral and central immune activation during pregnancy: 1) cellular and molecular signals that originate from the decidua and placenta, and 2) hormonal modulation of immune function. There are several immune cells localized at the point of contact between the mother (via the decidua) and the fetus (via the placenta). The decidua contains cells originated from the uterine lining that contain several adaptive immune cells such as macrophages, T cells, and dendritic cells (DCs). Trophoblasts, however, are immunocompetent cells derived from the fetus and are located within the placenta (Sanguansermsri and Pongcharoen, 2008). These maternal and fetal cells release cellular and molecular signals that thereby determine the immune environment associated with a successful pregnancy. It is generally understood that following both peripheral and central immune activation, immune cells can express a variety of immune profiles, ranging from the classical pro-inflammatory phenotype (Th1) to the alternative anti-inflammatory phenotype (Th2) (Benakis et al., 2014; David and Kroner, 2011; Hanisch and Kettenmann, 2007; Ransohoff et al., 2012). Researchers have revealed thymic stromal lymphoprotein (TSLP) as an important cytokine necessary to modulate maternal peripheral immune function into the alternative Th2 response during pregnancy. TSLP activates dendritic cells within the maternal decidua via its cognate receptor to induce a local Th2 cytokine response (Ito et al., 2005). In addition, trophoblasts from the placenta directly reduce the production of Th1 cytokines from T cells and simultaneously increase two Th2 transcription factors, GATA-3 and STAT 6, to further decrease the Th1/Th2 cytokine ratio (Liu et al., 2011). Together, these local interactions between the maternal immune cells and the placental trophoblasts may explain the significant immune suppression we see in the maternal and fetal immune systems, and further, may explain how the detachment of the placenta on the day of birth may result in the rapid return of a more “classical” inflammatory immune response in the mother’s peripheral immune system shortly after parturition. Additionally, our data show that the immune response of the fetus reflects the immune response of the placental and maternal tissue, which could potentially put the fetus at risk in the face of maternal infection or immune challenge. Taken together, these data demonstrate how much the maternal and placental immune environment can impact the immune environment of the fetus.
The hormones of pregnancy are also known to have an important immunomodulatory effect on the peripheral immune system during pregnancy and the postpartum period. Estrogen, progesterone, and lactogenic hormones are the primary hormones that influence the physiological changes accompanying pregnancy and parturition. In rodents, progesterone increases throughout pregnancy, then undergoes a precipitous drop in blood plasma concentrations right before parturition, while estrogen and prolactin undergo a steep incline during late gestation and immediately postpartum (Krasnegor and Bridges, 1990). In general, the activity of hormonal signaling through their receptors can suppress the transcriptional regulation of inflammatory cytokines (Robinson and Klein, 2012). For example, progesterone has been found to be anti-inflammatory in the face of an ischemic challenge, nerve damage, or even infection (Brotfain et al., 2016; Kipp et al., 2016; Labombarda et al., 2015; Lammerding et al., 2016; Paris et al., 2016; Perez-Alvarez and Wandosell, 2016). Additionally, during lactation, progesterone contributes to an increase in astrocytes in the rodent cingulate cortex (Salmaso et al., 2009) and the dentate gyrus of the hippocampus (Cabrera et al., 2013). Therefore it is very likely that high levels of progesterone associated with pregnancy can impact the function of glial cells, including microglia, thereby altering the expression of cytokines throughout pregnancy and the postpartum period (Posillico and Schwarz, 2016). Additionally, estrogen has been found to increase the number of CD4+ and CD25 + Treg cells (Polanczyk et al., 2004), enhance the function of NK cells (important for altering the decidual tissue to support implantation via the production of IFNγ) (Hao et al., 2007), and stimulate Th2 cytokine production from peripheral lymphocytes (Al-Shammri et al., 2004). Further, fluctuations in lactogenic hormones can also alter immune function. Prolactin has been found to enhance the inflammatory response and proliferation of glia following a traumatic brain injury (Möderscheim et al., 2007), and oxytocin administration has been found to alleviate tissue damage in various animal models of injury (Akdemir et al., 2014; Houshmand et al., 2009; Karelina et al., 2011). The protective mechanisms by which prolactin and oxytocin act may be related to a decrease in pro-inflammatory cytokines from microglia, macrophages, and endothelial cells (Akman et al., 2015; Szeto et al., 2008; Tug˘tepe et al., 2013; Yuan et al., 2016).
The increase in pregnancy-related hormone levels throughout gestation correlates with our results in that the greatest amount of suppression in central and peripheral immune function occurs during late gestation. In the periphery, the suppression of cytokine expression is immediately reversed, while in the brain, this effect persists to some extent postpartum. This might suggest that as the hormone levels decrease over the next few days after parturition, that hormonal modulation of immune function in the brain may similarly take longer to return to its non-pregnant state than the periphery, however this remains to be determined. We hypothesize that the function of the immune system is not solely modulated by either hormones or immune signals from the placenta alone, but rather it is a combination of both that results in the dramatic changes we see in maternal peripheral and central immune function during the peripartum period.
In addition to the general decrease in cytokine production that we observe following an LPS challenge in the maternal brain during pregnancy, we found some other interesting patterns in the data that are worth noting. There was a significant increase in BDNF gene expression in the maternal mPFC on the day before parturition (E22). We have previously reported this effect in a separate study from our lab, where BDNF levels were significantly increased in the maternal mPFC on the day of birth (Posillico and Schwarz, 2016). We hypothesize that the increase in BDNF expression just before or during parturition may prime the new mother’s brain for the expression of appropriate maternal behaviors after birth (Leuner and Sabihi, 2016).
We also see a significant suppression of TLR4 expression in the maternal spleen during pregnancy. With reduced receptor expression, LPS might have a reduced effect in stimulating the immune system to produce a subsequent increase in cytokines. We also saw a main effect of condition in TLR4 expression in the mPFC, however, the post hoc did not reveal a robust pattern in immune regulation like we see in the spleen, and moreover, the pattern does not reflect the robust changes seen in cytokine production in the maternal brain. Therefore, the reduction in receptor expression in peripheral immune cells and organs, such as that seen in the spleen, could be a possible mechanism by which the peripheral immune system is regulated, thereby indirectly influencing central immune function and cytokine production during pregnancy.
In the mPFC and hippocampus, we see an interesting and distinct pattern in the expression of IL-1β and IL-6. Specifically, baseline levels of IL-1β gene expression (in the absence of the LPS challenge) were quite low, almost undetectable, and did not differ across various points of gestation. Following LPS treatment, however, these levels increased differentially dependent on the stage of pregnancy. In contrast, our data indicate that IL-6 expression in the mPFC and hippocampus changes throughout the various stages of pregnancy, independent of LPS treatment. After LPS treatment, however, the relative fold-increase in IL-6 expression did not differ throughout the various stages of pregnancy or the postpartum period. More simply stated, we hypothesize these effects on neuroimmune function are driven by the inability of the neuroimmune system to produce IL-1β within these brain regions during pregnancy in response to an immune challenge. In contrast, the relative difference in IL-6 gene expression across pregnancy and the postpartum period appears to be driven by a decrease in IL-6 expression in the brain during pregnancy, even though the brain is still capable of inducing the expression of IL-6 in response to the immune challenge. This pattern of inflammatory gene expression could inform us about the molecular or cellular mechanisms underlying the changes in neuroimmune function that we see during pregnancy and the postpartum period. For example, it is possible that pregnancy inhibits the mechanisms or the cellular machinery by which immune cells in the brain such as microglia induce the expression of IL-1β. In contrast, our data suggest that pregnancy may alter the baseline expression of IL-6 or the number of microglial cells that produce IL-6 (Posillico and Schwarz, 2016) within the maternal mPFC and hippocampus, for reasons still unknown. In this case, LPS treatment and Toll-like receptor 4 activation can still recruit the machinery necessary to induce IL-6 expression in response to an immune challenge.
One possible caveat of this study is that we utilized LPS to evoke an immune response, and LPS treatment only activates the TLR4 pathway and does not represent a widespread infection like that of a bacterial or viral pathogen. Thus, it is possible that if this study were repeated using a more robust bacterial or viral pathogen, one that stimulates a different pattern recognition receptor than TLR4, we may see a larger maternal and fetal immune response, despite pregnancy. Given previous reports of peripheral immunosuppression during pregnancy, we feel confident that these data accurately present the systematic immunosuppression that also occurs within the brain during pregnancy, regardless of the type of challenge used in the study. Additionally, we used the spleen as a proxy for the peripheral immune system, however the findings seen here may not apply to all cells and tissues of the peripheral immune system.
Notably, these data were all collected from first-time mothers, and we wonder whether the immune response may be different if we were to have used rats that had previously been pregnant and borne offspring. In support of this idea, previous research has shown that fetal progenitor cells, which have been found to differentiate into leukocytes and endothelial cells, enter the maternal circulation during pregnancy and can be found there for a long time afterwards (Khosrotehrani et al., 2005; Nassar et al., 2012). This phenomenon is referred to as fetal cell microchimerism (Khosrotehrani et al., 2005); there is evidence that these persisting fetal cells affect the maternal immune system. They have been found to aid the mother in wound healing and have also been implicated in autoimmune disease in mothers (Boddy et al., 2015). As such, it would not be surprising to find that that these circulating fetal progenitor cells would also be able to affect the mother’s central immune system. Also in support of this idea, it is well-known that first-time pregnancy results in significant plasticity within the female brain, both in rodents (Leuner and Sabihi, 2016) and in humans (Hoekzema et al., 2016). In rodents, this plasticity is necessary to induce appropriate postpartum maternal behaviors, and once a dam has given birth for the first time, she is maternal towards any pups she encounters thereafter, even if she is not their biological mother or actively lactating (Bridges, 2015; Fleming et al., 1999). Additionally, it has been shown that rats exposed to environmental enrichment, which is also known to increase plasticity, have a significantly blunted proinflammatory response to LPS in the hippocampus (Williamson et al., 2012). Therefore, it is possible, that there may be a unique period of increased plasticity that persists within the postpartum brain of first-time mothers that may explain the lingering changes in immunosuppression we report here. Thus in subsequent pregnancies, the duration or magnitude of this change in neuroimmune function that we measured here may be different.
Various studies have shown that the peripheral immune system is suppressed during pregnancy (Mellor et al., 2001; Raghupathy, 1997; Trowsdale and Betz, 2006); however, this is the first study to systematically examine maternal neuroimmune function by challenging the immune system in the pregnant and postpartum brain. Therefore, this study provides the foundation to further explore and understand the connection between neuroimmune modulation during pregnancy and various neurological disorders – perhaps even informing the connections to neurodevelopmental disorders in offspring, autoimmune disorders that target the nervous system in women, and, importantly, mood and anxiety related disorders that are common during pregnancy and the postpartum period.
Acknowledgments
We would like to acknowledge and thank Julie Gomez and Pragyan Khanal for collecting data presented in this manuscript. This research was supported by a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation and NIH grants R21MH104280 and R01MH106553 to JMS.
References
- Akdemir A, Erbas O, Gode F, Ergenoglu M, Yeniel O, Oltulu F, Ellipsis Taskiran D, 2014. Protective effect of oxytocin on ovarian ischemia-reperfusion injury in rats. Peptides 55, 126–130. 10.1016/j.peptides.2014.02.015. [DOI] [PubMed] [Google Scholar]
- Akman T, Akman L, Erbas O, Terek MC, Taskiran D, Ozsaran A, 2015. The preventive effect of oxytocin to cisplatin-induced neurotoxicity: an experimental rat model. BioMed Res. Int 2015, 13–17. 10.1155/2015/167235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shammri S, Rawoot P, Azizieh F, AbuQoora A, Hanna M, Saminathan TR, Raghupathy R, 2004. Th1/Th2 cytokine patterns and clinical profiles during and after pregnancy in women with multiple sclerosis. J. Neurol. Sci 222 (1–2), 21–27. 10.1016/j.jns.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Benakis C, Garcia-Bonilla L, Iadecola C, Anrather J, 2014. The role of microglia and myeloid immune cells in acute cerebral ischemia. Front. Cell. Neurosci 8 (January), 461 10.3389/fncel.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi S, Bertollini MGR, Orzi F, Fieschi C, 1991. The influence of pregnancy on relapses in multiple sclerosis: a cohort study. Acta Neurol. Scand 84 (5), 403–406. 10.1111/j.1600-0404.1991.tb04977.x. [DOI] [PubMed] [Google Scholar]
- Boddy AM, Fortunato A, Wilson Sayres M, Aktipis A, 2015. Fetal microchimerism and maternal health: a review and evolutionary analysis of cooperation and conflict beyond the womb. BioEssays 37 (10), 1106–1118. 10.1002/bies.201500059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges RS, 2015. Neuroendocrine regulation of maternal behavior. Front. Neuroendocrinol 36, 178–196. 10.1016/j.yfrne.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotfain E, Gruenbaum SE, Boyko M, Kutz R, Zlotnik A, Klein M, 2016. Neuroprotection by estrogen and progesterone in traumatic brain injury and spinal cord. Injury, 641–653. [DOI] [PMC free article] [PubMed]
- Buckwalter GJ, Stanczyk FZ, McCleary CA, Bluestein BW, Buckwalter DK, Rankin KP, Goodwin MT, 1999. Pregnancy, the postpartum, and steroid hormones: effects on cognition and mood. Psychoneuroendocrinology 24, 4530. [DOI] [PubMed] [Google Scholar]
- Cabrera V, Ramos E, González-Arenas A, Cerbón M, Camacho-Arroyo I, Morales T, 2013. Lactation reduces glial activation induced by excitotoxicity in the rat hippocampus. J. Neuroendocrinol 25 (6), 519–527. 10.1111/jne.12028. [DOI] [PubMed] [Google Scholar]
- Chan WY, Kohsaka S, Rezaie P, 2007. The origin and cell lineage of microglia-New concepts. Brain Res. Rev 53 (2), 344–354. 10.1016/j.brainresrev.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T, 1998. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N. Engl. J. Med 339 (5), 285–291. 10.1056/NEJM199807303390501. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio H, 1990. Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behav. Brain Res 41 (2), 81–94. 10.1016/0166-4328(90)90144-4. [DOI] [PubMed] [Google Scholar]
- David S, Kroner A, 2011. Repertoire of microglial and macrophage responses after spinal cord injury. Nat. Rev. Neurosci 12 (7), 388–399. 10.1038/nrn3053. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Scammell TE, Saper CB, 1997. Mechanisms of CNS response to systemic immune challenge: the febrile response. Trends Neurosci 20 (12), 565–570. 10.1016/S0166-2236(97)01138-7. [DOI] [PubMed] [Google Scholar]
- Fleming AS, O’Day DH, Kraemer GW, 1999. Neurobiology of mother-infant interactions: experience and central nervous system plasticity across development and generations. Neurosci. Biobehav. Rev 23 (5), 673–685. 10.1016/S0149-7634(99)00011-1. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Mehler MF, Ellipsis Merad M, 2010. NIH public access. Science 330 (6005), 841–845. 10.1126/science.1194637.Fate. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haim A, Julian D, Albin-Brooks C, Brothers HM, Lenz KM, Leuner B, 2016. A survey of neuroimmune changes in pregnant and postpartum female rats. Brain Behav. Immun 59, 67–78. 10.1016/j.bbi.2016.09.026. [DOI] [PubMed] [Google Scholar]
- Hanisch U-KK, Kettenmann H, 2007. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci 10 (11), 1387–1394. 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Hao S, Zhao J, Zhou J, Zhao S, Hu Y, Hou Y, 2007. Modulation of 17??-estradiol on the number and cytotoxicity of NK cells in vivo related to MCM and activating receptors. Int. Immunopharmacol 7 (13), 1765–1775. 10.1016/j.intimp.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Hazes JMW, Coulie PG, Geenen V, Vermeire S, Carbonnel F, Louis E, De Keyser F, 2011. Rheumatoid arthritis and pregnancy: evolution of disease activity and pathophysiological considerations for drug use. Rheumatology (Oxford, England) 50 (11), 1955–1968. 10.1093/rheumatology/ker302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hench PS, 1938. Effect of spontaneous jaundice on rheumatoid (atrophic) arthritis. Br. Med. J 394–398. 10.1136/bmj.2.4050.394. [DOI] [PMC free article] [PubMed]
- Hoekzema E, Barba-Müller E, Pozzobon C, Picado M, Lucco F, García-García D, Ellipsis Vilarroya O, 2016. Pregnancy leads to long-lasting changes in human brain structure. Nat. Neurosci 20 (2). 10.1038/nn.4458. [DOI] [PubMed] [Google Scholar]
- Houshmand F, Faghihi M, Zahediasl S, 2009. Biphasic protective effect of oxytocin on cardiac ischemia/reperfusion injury in anaesthetized rats. Peptides 30 (12), 2301–2308. 10.1016/j.peptides.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Insel TR, Harbaugh CR, 1989. Lesions of the hypothalamic paraventricular nucleus disrupt the initiation of maternal behavior. Physiol. Behav 45, 1033–1041. [DOI] [PubMed] [Google Scholar]
- Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, Ellipsis Liu YJ, 2005. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J. Exp. Med 202 (9), 1213–1223. 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karelina K, Stuller KA, Jarrett B, Zhang N, Wells J, Norman GJ, Courtney Devries A, 2011. Oxytocin mediates social neuroprotection after cerebral ischemia. Stroke 42 (12), 3606–3611. 10.1161/STROKEAHA.111.628008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosrotehrani K, Johnson KL, Guégan S, Stroh H, Bianchi DW, 2005. Natural history of fetal cell microchimerism during and following murine pregnancy. J. Reprod. Immunol 66 (1), 1–12. 10.1016/j.jri.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Kipp M, Hochstrasser T, Schmitz C, Beyer C, 2016. Female sex steroids and glia cells: impact on multiple sclerosis lesion formation and fine tuning of the local neurodegenerative cellular network. Neurosci. Biobehav. Rev 67, 125–136. 10.1016/j.neubiorev.2015.11.016. [DOI] [PubMed] [Google Scholar]
- Krasnegor NA, Bridges RS, 1990. Mammalian Parenting: Biochemical, Neurobiological, and Behavioral Determinants Oxford University Press, New York. [Google Scholar]
- Kronfol Z, 2000. Cytokines and the brain: implications for clinical psychiatry. Am. J. Psychiatry 157 (5), 683–694. 10.1176/appi.ajp.157.5.683. [DOI] [PubMed] [Google Scholar]
- Labombarda F, Jure I, Gonzalez S, Lima A, Roig P, Guennoun R, Ellipsis De Nicola AF, 2015. A functional progesterone receptor is required for immunomodulation, reduction of reactive gliosis and survival of oligodendrocyte precursors in the injured spinal cord. J. Steroid Biochem. Mol. Biol 154, 274–284. 10.1016/j.jsbmb.2015.09.011. [DOI] [PubMed] [Google Scholar]
- Lammerding L, Slowik A, Johann S, Beyer C, Zendedel A, 2016. Poststroke inflammasome expression and regulation in the peri-infarct area by gonadal steroids after transient focal ischemia in the rat brain. Neuroendocrinology 103 (5), 460–475. 10.1159/000439435. [DOI] [PubMed] [Google Scholar]
- Leuner B, Sabihi S, 2016. The birth of new neurons in the maternal brain: hormonal regulation and functional implications. Front. Neuroendocrinol 41, 99–113. 10.1016/j.yfrne.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Tang Y, Feng J, 2011. Cross talk between activation of microglia and astrocytes in pathological conditions in the central nervous system. Life Sci 89 (5–6), 141–146. 10.1016/j.lfs.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Maes M, 1995. Evidence for an immune response in major depression: a review and hypothesis. Progr. Neuropsychopharmacol. Biol. Psychiatry 19 (1), 11–38. 10.1016/0278-5846(94)00101-M. [DOI] [PubMed] [Google Scholar]
- Male D, Rezaie P, 2001. Colonisation of the human central nervous system by microglia: the roles of chemokines and vascular adhesion molecules. Prog. Brain Res 132 (4), 81–93. 10.1016/S0079-6123(01)32067-8. [DOI] [PubMed] [Google Scholar]
- Mellor AL, Munn DH, 2001. Extinguishing maternal immune responses during pregnancy: implications for immunosuppression. Seminars Immunol 13 (4), 213–218. 10.1006/smim.2000.0317. [DOI] [PubMed] [Google Scholar]
- Möderscheim TAE, Gorba T, Pathipati P, Kokay IC, Grattan DR, Williams CE, Scheepens A, 2007. Prolactin is involved in glial responses following a focal injury to the juvenile rat brain. Neuroscience 145 (3), 963–973. 10.1016/j.neuroscience.2006.12.053. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Romanski LM, LeDoux JE, 1993. Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci. Lett 163 (1), 109–113. 10.1016/0304-3940(93)90241-C. [DOI] [PubMed] [Google Scholar]
- Nassar D, Droitcourt C, Mathieu-d’Argent E, Kim MJ, Khosrotehrani K, Aractingi S, 2012. Fetal progenitor cells naturally transferred through pregnancy participate in inflammation and angiogenesis during wound healing. FASEB J 26 (1), 149–157. 10.1096/fj.11-180695. [DOI] [PubMed] [Google Scholar]
- Nelson JL, Ostensen M, 1997. Pregnancy and rheumatoid arthritis. Rheum. Dis. Clin. North Am 840, 45–50. [DOI] [PubMed] [Google Scholar]
- Numan M, Rosenblatt J, Komisaruk B, 1977. Medial preoptic area and onset of maternal behavior in the rat. J. Compar. Physiol. Psychol 91 (1), 146–164. [DOI] [PubMed] [Google Scholar]
- Osborne LM, Monk C, 2013. Perinatal depression-the fourth inflammatory morbidity of pregnancy? theory and literature review. Psychoneuroendocrinology 38 (10), 1929–1952. 10.1016/j.psyneuen.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris JJ, Zou SP, Hahn YK, Knapp PE, Hauser KF, 2016. 5??-reduced progestogens ameliorate mood-related behavioral pathology, neurotoxicity, and microgliosis associated with exposure to HIV-1 Tat. Brain Behav. Immun 55, 202–214. 10.1016/j.bbi.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Alvarez M, Wandosell F, 2016. Stroke and neuroinflammation: role of sexual hormones. Curr. Pharm. Des 22 (10), 1334–1349. [DOI] [PubMed] [Google Scholar]
- Polanczyk MJ, Carson BD, Subramanian S, Afentoulis M, Vandenbark AA, Ziegler SF, Offner H, 2004. Cutting edge: estrogen drives expansion of the CD4 + CD25 + regulatory T cell compartment. J. Immunol 173 (4), 2227–2230. 10.4049/jimmunol.173.4.2227. [DOI] [PubMed] [Google Scholar]
- Posillico CK, Schwarz JM, 2016. An investigation into the effects of antenatal stressors on the postpartum neuroimmune profile and depressive-like behaviors. Behav. Brain Res 298, 218–228. 10.1016/j.bbr.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghupathy R, 1997. Th1-type immunity is incompatible with successful pregnancy. Immunol. Today 18 (10), 478–482. 10.1016/S0167-5699(97)01127-4. [DOI] [PubMed] [Google Scholar]
- Raison CL, Miller AH, 2013. Role of inflammation in depression: implications for phenomenology, pathophysiology and treatment. Modern Trends Pharmacopsychiat [DOI] [PubMed]
- Raison C, Miller A, 2017. Pathogen-host defense in the evolution of depression: insights into epidemiology, genetics, bioregional differences and female preponderance. Neuropsychopharmacology 42 (1), 5–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Brown Ma., 2012. Review series Innate immunity in the central nervous system. J. Clin. Investig 122 (4), 1164–1171. 10.1172/JCI58644.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remus JL, Dantzer R, 2016. Inflammation models of depression in rodents: relevance to psychotropic drug discovery. Int. J. Neuropsychopharmacol 19 (9), 1–13. 10.1093/ijnp/pyw028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DP, Klein SL, 2012. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm. Behav 62 (3), 263–271. 10.1016/j.yhbeh.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmaso N, Nadeau J, Woodside B, 2009. Steroid hormones and maternal experience interact to induce glial plasticity in the cingulate cortex. Eur. J. Neurosci 29 (4), 786–794. 10.1111/j.1460-9568.2009.06627.x. [DOI] [PubMed] [Google Scholar]
- Sanguansermsri D, Pongcharoen S, 2008. Pregnancy immunology: decidual immune cells. Asian Pac. J. Allergy Immunol 26, 171–181. [PubMed] [Google Scholar]
- Szeto A, Nation DA, Mendez AJ, Dominguez-Bendala J, Brooks LG, Schneiderman N, McCabe PM, 2008. Oxytocin attenuates NADPH-dependent superoxide activity and IL-6 secretion in macrophages and vascular cells. Am. J. Physiol. Endocrinol. Metab 295 (6), E1495–E1501. 10.1152/ajpendo.90718.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuber H-L, 1964. The riddle of frontal lobe function in man. Neuropsychol. Rev 19 (1), 25–46. 10.1007/s11065-009-9088-z. [DOI] [PubMed] [Google Scholar]
- Trowsdale J, Betz AG, 2006. Mother’s little helpers: mechanisms of maternal-fetal tolerance. Nat. Immunol 7 (3), 241–246. 10.1038/ni1317. [DOI] [PubMed] [Google Scholar]
- Tug˘tepe H, S,ener G, Bıyıklı NK, Yüksel M, Çetinel S,, Gedik N, Yeg˘en BÇ, 2013. The protective effect of oxytocin on ischemia/reperfusion injury in rat urinary bladder. Peptides 40, 82–88. 10.1016/j.peptides.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Williamson LL, Chao A, Bilbo SD, 2012. Environmental enrichment alters glial antigen expression and neuroimmune function in the adult rat hippocampus. Brain Behav. Immun 26 (3), 500–510. 10.1016/j.bbi.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Liu S, Bai X, Gao Y, Liu G, Wang X, Ellipsis Welch M, 2016. Oxytocin inhibits lipopolysaccharide-induced inflammation in microglial cells and attenuates microglial activation in lipopolysaccharide-treated mice. J. Neuroinflamm 13 (1), 77 10.1186/s12974-016-0541-7. [DOI] [PMC free article] [PubMed] [Google Scholar]