Abstract
Building on the principals that the adoptive transfer of T cells can lead to the regression of established tumors in humans, investigators are now further manipulating these cells using genetic engineering. Two decades of human gene transfer experiments have resulted in the translation of laboratory technology into robust clinical applications. The purpose of this review is to give the reader an introduction to the 2 major approaches being developed to redirect effector T-cell specificity. Primary human T cells can be engineered to express exogenous T-cell receptors or chimeric antigen receptors directed against multiple human tumor antigens. Initial clinical trial results have demonstrated that both T-cell receptor- and chimeric antigen receptor-engineered T cells can be administered to cancer patients and mediate tumor regression.
Keywords: human gene transfer, T-cell receptors (TCRs), chimeric antigen receptors (CARs), tumor regression
The first hypothesis-driven approach to harness the power of the immune system to treat human disease was described more than 200 years ago in Edward Jenner’s report to the Royal Society on inoculation. Although infectious disease research has had 2 centuries to build on approaches with biomedical foundations that would not be understood for another 150 years, the concept of cancer immunotherapy and, in particular, adoptive cell therapy (ADC) can be considered as still in its beginning stages of development as a medical science. In particular, the subject of this chapter dealing with genetic modification of T cells has its genesis in the discovery of recombinant DNA in the 1970s, followed by the development of efficient gene transfer methods in the early 1980s. These developments led to the first report on T-cell receptor (TCR) gene transfer in 19861 and the first Food and Drug Administration-approved gene transfer experiment in humans in 1989.2 In the 2 decades since these pioneering reports, great progress has been made in improving gene transfer technology and in developing methods to augment T-cell effector function. These advances have now culminated in the first successful clinical applications of T-cell engineering to mediate the regression of large established tumors in humans.
ADOPTIVE CELL THERAPY
ACT has laid the groundwork for the current interest in genetic engineering to redirect effector cell specificity. The transfer of viral antigen-specific T cells is a now a well-established procedure resulting in effective treatments of transplant associated viral infections and rare viral-related malignancies. Riddell et al3 first reported that it was possible to transfer T-cell clones to patients undergoing hematopoietic stem cell transplantation as a way of preventing cytomegalovirus (CMV) reactivation post-transplant. In these reports, allogeneic donor peripheral blood lymphocytes (PBL) were cultured with autologous fibroblasts that were infected with CMV and subsequently CD8+ anti-CMV–specific T-cell clones were isolated by limiting dilution, expanded, and returned to patients. Allogeneic hematopoietic stem cell transplantation can also be associated with the development of post-transplant lymphoproliferative disease (PTLD) secondary to reactivation of latent Epstein-Barr virus (EBV) infections. The rate of PTLD can be up to 20% in solid organ transplants. Beginning in 1994, investigators demonstrated that donor lymphocyte transfer could effectively treat EBV-associated PTLD by the transfer of ex vivo-expanded allogeneic cytotoxic T lymphocytes (CTL).4 These approaches have been expanded to target a greater variety of viral-related malignancies including nasopharyngeal carcinoma and EBV+ Hodgkin disease.5,6
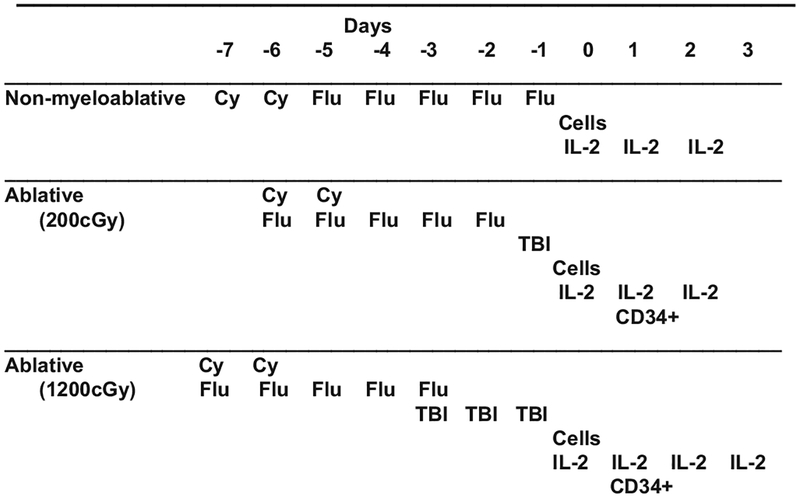
The first examples of ACT for the treatment of nonviral related malignancies were in the context of allogeneic hematopoietic stem cells for the treatment of leukemia and melanoma. The addition of donor lymphocyte infusion in the setting of nonmyeloablative hematopoietic stem cells for the treatment of chronic myelogenous leukemia was initially reported by Kolb et al7 in 1990 and further developed by several groups. Autologous tumor infiltrating lymphocytes (TIL) were first demonstrated to mediate the regression of melanoma in 1988.8 In these early studies, response rates were modest (about 1 in 3 patients responding) and responses were often not durable. A substantial increase in the effectiveness of TIL therapy came with addition of host preconditioning using nonmyleoablative chemotherapy (Fig. 1) as reported by Dudley et al.9 In this report, up to 50% of patients achieved an objective clinical response with many of these responses being quite durable, including completely responding patients rendered disease free.
FIGURE 1.
Preparative regimens for cell transfer. To facilitate engraftment and persistence of adoptively transferred T cells, patients received 3 separate conditionings treatments. Nonmyeloblative chemotherapy consisted of 2 days of cyclophosphamide (Cy, 60 mg/kg) then fludarabine (Flu, 25mg/m2) for 5 days. For additional ablation, total body irradiation (TBI) of 200 cGy and 1200 cGy was added at the days indicated. All patients received high-dose IL-2 (720,000 U/kg) every 8 hours to tolerance. Patients receiving TBI where administered autologous CD34+ mobilized peripheral blood cells (previously harvested and cryopreserved) on day 1 after cell infusion.
More recently it was shown that increasing the intensity of preconditioning regimen (Fig. 1) could increase response rates.10 Updated results from 3 sequential clinical trials performed in the Surgery Branch, National Cancer Institute, using selected tumor-reactive autologous tumor-infiltrating lymphocytes infused along with IL-2 after lymphodepleting regimens of increasing intensity in patients with metastatic melanoma are shown in Table 1. Objective response rates using Response Evaluation Criteria in Solid Tumors criteria reached 72% with maximum lymphodepletion including 32% of patients with complete tumor regressions. These responses were durable, and only 1 of 16 patients who achieved a complete response ever recurred at times ongoing from 32 to 84 months. Responses were seen at all visceral sites, and there was no relationship between the bulk of disease and the likelihood of achieving an objective response. Although the initial methodology involved in TIL generation was laborious and time consuming, recent refinements in TIL propagation have resulted in a stream-lined turnkey approach to TIL generation that can easily be adapted by any major medical center.11–13
TABLE 1.
Results for TIL Adoptive Cell Transfer Therapy at the Surgery Branch NCI*
| Treatment | Total | PR | CR | OR (%) |
|---|---|---|---|---|
| No TBI | 43 | 16 (84,36,29,28,14,13,11,8,8,7,4,3,3,2,2,2) | 5 (82+,78+,76+,75+,61+) | 21 (49%) |
| 200 TBI | 25 | 10 (57+,51+,14,9,6,6,5,4,3,3) | 3 (65+,61+,54+) | 13 (52%) |
| 1200 TBI | 25 | 10 (42+,35+,21,13,7,6,6,5,3,2) | 8 (45+,41+,41+,36+,35+,35+,34+,19) | 18 (72%) |
Data are as of May 1, 2010.
Response in months, based on RECIST. Of the 52 responding patients, 42 had prior IL-2, 21 had prior IL-2 + chemotherapy.
indicates ongoing response; CR, complete response; OR, objective response; and PR, partial response.
DEVELOPMENT OF ENGINEERED T CELLS USING T-CELL RECEPTORS
The cloning of the first bona fide tumor-associated antigen (TAA) in 1991 was made possible by the ability of the human immune system to generate T cells capable of recognizing not only mutated proteins but also nonmutated self-antigens.14 Dozens of potential tumor antigens have now been well characterized and include those normal proteins that are often overexpressed in malignancies (eg, p53 or carcinoembryonic antigen), differentiation antigens, such as melanoma antigen recognized by T cells 1 (MART-1), or members of the cancer testis antigen (CTA) family. The first description of the engineering of human T cells with a TAA TCR was by Clay et al15 using gamma retroviral vector transduction of PBL. This was followed by reports targeting the MDM2 and WT-1 TAAs using similar gene transfer methods.16,17 In the decade since these initial reports, investigators have made significant advances in the technologies associated with increasing the efficiency of TCR gene transfer.
The first step in the development of successful TCR gene transfer is the choice of gene transfer method. The targeT cells for TCR gene transfer (human T cells) have proven to be difficult to transfect using standard laboratory-based chemical methods of gene transfer. In contrast, electroporation/nucleofection has been demon strated to yield very good levels of gene transfer with RNA-based expression systems (DNA-based gene transfer results in lower efficiencies and poor cell viability postelectroporation).18 Although RNA electroporation is a valuable tool for laboratory investigations, it is more difficult to develop as a clinical-scale product for human applications. The main drawback in RNA-based TCR gene transfer is the short half-life of RNA expression post-transfer. A new system for the electroporation of DNA expression cassettes based on transposons has achieved some success in human applications and is in development as a clinical product.19
Nearly all clinical trials using TCR gene transfer are based on viral vector-based expression systems. Gamma retroviral vectors have been used in human clinical applications for more than 20 years and are a robust and well-defined clinical reagent. The only known toxicity associated with gamma retroviral vector engineering of human cells was reported in the context of the engineering of hematopoietic stem cells in immune-deficient patients. Insertional leukemogenesis was reported in 3 children in a gene therapy trial treating X-linked severe combined immunodeficiency disease in 2003.20 There have been no similar reports of vector-associated toxicities in the engineering of mature cells such as adult T lymphocytes. Examples of efficient gamma retroviral vector expression platforms include the MFG/SFG-, MP71/SF91-, and MSGV1-based vectors systems.21–23 High-level transcription mediated by the long terminal repeats of these optimized vectors is the key to successful human T-cell engineering. Alternatives to the gamma retroviral vector are systems based on lentiviral vectors. Although the lentiviral vectors have only recently been used in human applications,24 they have a fundamental biologic advantage in their ability to productively infect minimally stimulated T cells.25 Lentiviral vectors also afford the potential for transferring more complex and larger gene expression cassettes and may have a safer chromosomal integration profile than gamma retroviral vectors. In the choice of viral expression systems, there seems to be little difference in gene transfer efficiencies and expression potential between these 2 retroviral vector systems.
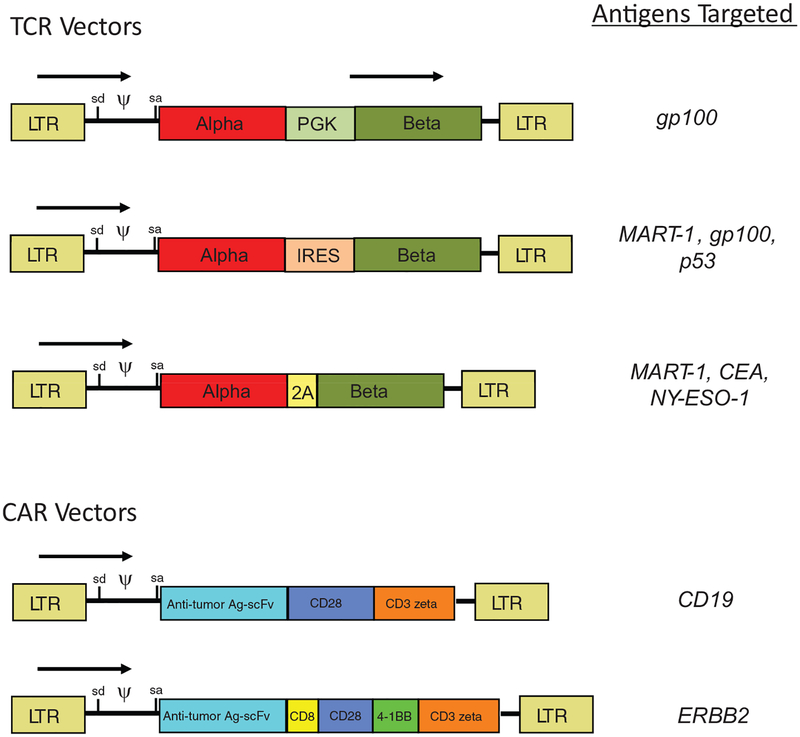
Having an efficient expression vector is important as is the design of the specific TCR expression cassette (Fig. 2). The TCR molecule is a heterodimer composed of 1 alpha and 1 beta chain that must be coexpressed at similar levels in the engineered cell. Initially the 2 TCR chains were expressed using 2 individual vectors, in 1 vector using 2 promoters, or by having the chains linked via an internal ribosomal entry site. These methods often resulted in poor expression and have now been replaced by the more efficient use of picornavirus ribosomal skip peptides to link the chains.26,27 Although the use of ribosomal skip peptides leaves several residual amino acids at the COOH terminus of the first chain, the intercellular tails of the TCR alpha and beta chains are not involved in T-cell signaling.
FIGURE 2.
Gammaretroviral vector designs. Shown are examples of gammaretroviral vector designs that have been used in the Surgery Branch, National Cancer Institute, to treat cancer patients with genetically modified T cells. TCR vectors (top) require expression of 2 proteins (the alpha and beta chains of the TCR), which can be done by the use of an internal promoter (such as PGK), an internal ribosome entry site (IRES), or a picornavirus ribosomal skip peptide (2A). CAR vectors (bottom) express an antitumor antigen single chain antibody (scFv) linked to T-cell signaling domains. Second-generation CAR vectors generally use a combinations of CD28 plus CD3zeta signaling domains, whereas third-generation CAR vectors include additional elements such as a hinge and transmembrane domain from CD8 and the second costimulatory elements, eg, derived from the 4–1BB gene. The specific proteins that have been targeted in Surgery Branch clinical trials using these vector designs is as indicated on the right of the figure. LTR indicates long terminal repeat; sd, RNA splice donor; sa, RNA splice acceptor; ψ, packaging signal; CEA, carcinoembryonic antigen; and arrows, direction of transcription.
Perhaps the most important step in development of an efficient TCR gene transfer system is the choice of the specific receptor to transfer. Clear differences can exist between TCRs that target the same antigen and include different affinities as well as poorly understood elements of the protein thermodynamics that give rise to strong or dominant TCRs.28,29 High-affinity TCRs have been generated by mutagenesis followed by selection using methods such as phage display.30 These techniques yield extremely high-affinity TCRs that can have remarkable properties as soluble regents (detecting picomolar amounts of peptide), but these ultrahigh-affinity receptors can lose specificity when transferred back into T cells.31 A more directed approach using single or dual amino acid substitutions in the complementary determining regions has demonstrated effectiveness with multiple TCRs without significant loss of specificity.32 High-avidity (the termed used to describe the sum total of T cell-TAA binding recognition) T-cell clones can be often found by a dedicated screening of multiple CTL clones, and the transfer of these TCRs can transfer the high-avidity phenotype to transduced cells.33 Finally, the use of HLA-A2 transgenic mice has been demonstrated to be an extremely useful method to isolate murine TCRs that recognize human TAAs.34,35 These TCRs are generated by immunization in an immunologic environment where T cells have not undergone central tolerance against human peptide epitopes and generally yield high-avidity CTL.
Protein engineering has proven particularly effective in optimizing the function of transferred TCRs. These modifications include the removal of glycosylation sites, the flipping of amino acids between the different chains, the addition of a second cysteine between the 2 chains, and the production of chimeric proteins containing the constant regions of the murine TCRs with the variable regions of human TCRs.36,37 The rational for making these changes goes beyond increasing TCR avidity and are designed to foster the specific pairing of the introduced TCR chains. Specific TCR chain pairing can increase the overall activity of the engineered T cell against the targeted antigen. To date, the most effective of these strategies to increase specific chain pairing has been the use of chimeric TCRs in which murine constant regions not only facilitate specific chain pairing but also associate more tightly with the CD3 proteins.38 Independent work by 2 groups has recently demonstrated that only a subset of murine amino acids needs to be substituted into human constant regions to achieve increased pairing and activity.39,40 In murine models of TCR gene transfer, self-reactive T cells can be generated by TCR mispairing,41 and this self-reactivity can be lessened by some of the techniques described above. Although self-reactivity is a theoretical concern in TCR gene transfer and has been reported in animal models,41 in our clinical experience treating more than 100 patients with T cells engineered with a second human TCR, no toxicities directly attributed to the introduced TCR have been observed.
DEVELOPMENT OF ENGINEERED T CELLS USING CHIMERIC ANTIGEN RECEPTORS
TCR-based redirection of effector cell specificity is limited by major histocompatibility complex (MHC) restriction, which has directed this technology to the development of reagents that target the common HLA haplotypes such as HLA-A0201. The pioneering works by Eshhar and coworkers42 led to the development of non-MHC restricted methods for tumor cell detection based on antibody recognition. In principle, the chimeric antigen receptor (CAR) combines any ligand-binding domain with membrane spanning and T-cell signaling proteins such that the engineered T cells can be stimulated by a cell surface antigen.43,44 This technology has primarily been applied to produce hybrid molecules derived from antibodies but cytokines have also been used. In addition to the lack of MHC restriction, CAR-engineered cells can also be redirected to recognize nonprotein determinants such as glycolipids, which significantly increase the potential antigen targets that CARs can detect. The main disadvantage of CAR-based systems is that the recognition element must be present on the cell surface and by definition CARs are hybrid proteins that may contain immunogenic determinants.
Eshhar’s “T-bodies” illustrate the paradigm of CAR design (Fig. 2). Antigen recognition is mediated by a single-chain antibody fragment (scFv) that links heavy- and light-chain variable regions of the antibody together by a flexible linker peptide. The scFv is then fused to a protein spacer element followed by a transmembrane spanning domain and intracellular signaling elements. The length of the extracellular protein spacer or stock can be important when the scFv needs to recognize determinants that are topologically recessed as illustrated by CARs directed to a particular domain of the MUC-1 antigen in which a longer spacer region based on IgD was required for the CAR to “reach” the antigenic determinant on the tumor cell surface.45 A variety of membrane spanning domains has been used for CARs, and there seems to be significant flexibility in the choice of these elements. A CAR cannot function to elicit T-cell effector functions unless it has the appropriate intracellular signaling domains. In the early investigations, a single cytoplasmic signaling element based on the CD3z or the Fc receptor gamma (FcRg) was used to transmit the signal for antigen recognition to the T cell. Presumably this occurs when multiple CARs are brought in proximity leading to phosphorylation of the immunoreceptor tyrosine-based activation motifs elements and subsequent T-cell activation.
Although much investigation of these first generation CAR designs demonstrated that they transmitted appropriate signals for the initial steps in effector function, eg, target cell lysis and cytokine release, there was little evidence that CAR-engineered T cells would undergo significant antigen-mediated cell proliferation that is critical to the normal T-cell response to antigen recognition. T cells require a second signal or costimulation to avoid antigen-dependent cell cytotoxicity, and this second signal is generally provided by the CD28 molecule. When investigators coupled the CD28 intracellular signaling domains to the CD3z molecule, these second-generation CARs were demonstrated to have significantly enhanced cell proliferation capabilities as well as retaining the ability to lyse targeT cells and release effector cytokines.46,47 Still more recently, investigators have coupled a third intracellular signaling element (eg, from 41BB or OX40) to create third-generation CAR vectors.48–50 The advantage of having multiple intracellular signaling domains is to further enhance effector function and cell survival. The potential utility of these third-generation CAR designs has recently been demonstrated using in vivo animal models in which second-generation and third-generations CARs were compared side by side.50–52 In these studies using multiple tumor models, third-generation CARs demonstrated both prolonged cell survival and enhanced tumor clearance.
CLINICAL TRIALS USING ENGINEERED T CELLS
In the United States, all clinical trials using gene transfer technology for the treatment of human disease are reviewed by the National Institutes of Health Office of Biotechnology Activities, and a list of gene therapy protocols can be found on the Office of Biotechnology Activities web site (http://oba.od.nih.gov). In addition, there is a registry of federally and privately supported clinical trials conducted in the United States and around the world at the ClinicalTrials web site (www.clinicaltrials.gov). Herein, we will discuss the results of clinical trials for which published results have been presented.
We have reported on the results from 2 clinical trials using TCR gene-engineered lymphocytes in the treatment of metastatic melanoma. In the first report, a TCR was cloned from a patient who had been administered TIL therapy, and upon long-term follow-up, a single predominant MART-1–reactive T-cell clone was demonstrated to display remarkable persistence in this responding patient. This TCR gene was cloned and inserted into a gamma retroviral vector that demonstrated the transfer of effector function to engineered T cells in vitro.23 Fifteen patients were treated with MART-1 TCR gene-engineered T cells after nonmyleoablative lymphodepletion.53 This protocol demonstrated that MART-1 TCR-specific T cells could be safely administered to patients and further demonstrated that 2 of 15 of these patients (13%) had sustained regression of large established tumors (both patients remain alive now >5 years post-treatment).
In an attempt to increase the effectiveness of this therapy, efforts were made to isolate more reactive TCRs. This was accomplished by the screening and isolation of highly active MART-1–reactive T-cell clones and by the immunization of HLA-A2 transgenic mice with peptides specific for the human gp100 melanocyte differentiation antigen. In a second reported TCR receptor gene therapy trial,54 similar conditioning and treatment protocols were followed with 6 of 20 (30%) and 3 of 16 (19%) patients demonstrating clinical responses to MART-1 and gp100 TCR-engineered T cells, respectively (Table 2). These high-avidity T-cell receptors target melanocyte differentiation antigens that are highly overex-pressed in melanoma and are also expressed in normal melanocytes. The targeting of normal melanocytes in the skin, eye, and ear was observed leading to on-target toxicity associated with inflammation and destruction of normal melanocytes (Table 2). The on-target toxicities to the eye and ear can be managed by steroid eye drops and transtympanic steroid injections, and manipulations to further improve this therapy are in progress. It should be noted that the success of both of these initial reports was in the context of lymphodepleting conditioning, which is known to be important for successful ACT based on both animal models55 and clinical trials.56
TABLE 2.
Results for TCR Gene Therapy in Patients With Metastatic Melanoma*
| Response (Number of Patients) | Toxicity (Grade 1/2/3) | ||||
|---|---|---|---|---|---|
| TCR | Total | OR | Skin | Uveitis | Auditory |
| MART-1TCR (DMF5) | 20 | 6 (30%) | 11/3/0 | 2/9/0 | 2/0/7 |
| gp100TCR (gp154) | 16 | 3 (19%) | 11/4/0 | 0/4/0 | 2/2/3 |
| Total | 36 | 9 (25%) | 22/7/0 (81%) | 2/13/0 (42%) | 4/2/3 (25%) |
Trials performed at the Surgery Branch, NCI. Response based on RECIST. Toxicity graded as shown below:
The use of first-generation CAR-engineered T cells has been reported in a variety of malignancies. In studies using transfected T-cell clones engineered to target CD171 or CD20, selected T cells were expanded long term in culture and administered to patients with neuroblastoma and lymphoma.57,58 The transfected T-cell clones demonstrated short-term persistence, and no clinical benefit was reported. Use of more efficient viral vector-mediated gene transfer methods has been reported in 3 clinical trials. Folate binding protein is overexpressed in ovarian cancer and was targeted by infusion of CAR-engineered T cells.59 In this trial, there was rapid disappearance of the engineered cells from the circulation, and no biologic affect was observed. Similarly, a first-generation CAR was administered in kidney cancer in which the target protein was carbonic anhydrase IX.60 Although no efficacy was reported, liver toxicity was observed, presumptively by the recognition of carbonic anhydrase IX on bile ducT cells. This first successful application of CAR gene therapy was reported by Pule et al61 after administration of T cells targeting the glycoprotein GD2 in neuroblastoma. In this report, 2 different T-cell populations were engineered: bulk T cells and virus-specific CTL. Interestingly, the viral-specific cells were demonstrated to have long-term persistence, suggesting that distinct T-cell subsets may have better utility in mediating effective tumor treatment. Several clinical trials using second- and third-generation CARs are currently in progress. Although no clinical responses have been reported, there have been 2 adverse event reports resulting in patient deaths, which occurred after administration of CAR-engineered T cells.62,63 In our report targeting ERBB2, the administration of a third-generation CAR resulted in immediate pulmonary toxicity and rapid decline in the patients’ clinical condition. Postmortem analysis revealed spikes in several serum cytokines similar to cytokine release syndrome. Although a definitive cause of death was not established, it is likely that low levels of ERBB2 expression on normal lung epithelial cells was sufficient to mediate the release of effector cytokines such as interferon-g that may have initiated a cytokine storm.
FUTURE DIRECTIONS
The genetic engineering of T cells to redirect effector function is in its initial clinical stage. On the basic research front, significant work is being done to enhance the activity of both TCR- and CAR-engineered T cells. T-cell receptor activity can be strengthened by manipulations that improve the specific pairing of the introduced TCR chains as well as site-directed mutagenesis of the variable region complementary-determining regions to enhance peptide/MHC recognition. In the case of CAR-engineered T cells, the use of multiple costimulatory domains in third-generation vectors is still an area of active investigation. Although it is certainly possible to improve the activity of the genetically engineered T cell to recognize tumor antigens and to initiate effector functions, it clear that the tumor microenvironment presents a hostile environment to T cells, with numerous mechanisms designed to blunt T-cell activity. Many of these inhibitory mechanisms such as TGF-β synthesis64 and PD-L1 expression65 are tumor cell-specific functions, but equally as important may be the influence of inhibitory cells such as myeloid-derived suppressor cells.66
Although there are few published reports of clinical outcomes from T-cell gene therapy trials, these initial results suggest 3 things. First, as was true for TIL therapy, preconditioning of patients may be helpful in promoting T-cell engraftment and activity. Second, it is likely that the choice of specific T-cell subsets may be associated with increased persistence if not activity. Finally, it is clear that targeting normal self-proteins with highly active T cells will result in the potential for on-target toxicity. It would therefore be prudent to attempt to target proteins that have a limited tissue distribution (such as CD19) or TAAs that are either tumor specific or not expressed in normal tissues (eg, CTAs67). The targeting of CTAs may be particularly fruitful given that their normal expression is limited to the non-MHC–expressing male germ cells, and our preliminary clinical experience targeting the NY-ESO-1 antigen has not demonstrated any on-target related toxicity.
| Grade 1 | Grade 2 | Grade 3 | |
|---|---|---|---|
| Skin | Erythema | Desquamation <50% | Desquamation >50% |
| Eye | No symptoms | Anterior | Pan uveitis |
| Ear | 15–25 dB, 2 freq. | >25 dB, 2 freq. | >25 dB, 3 freq. |
REFERENCES
- 1.Dembic Z, Haas W, Weiss S, et al. Transfer of specificity by murine alpha and beta T-cell receptor genes. Nature. 1986;320:232–238. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Aebersold P, Cornetta K, et al. Gene transfer into humans—immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N Engl J Med. 1990; 323:570–578. [DOI] [PubMed] [Google Scholar]
- 3.Riddell SR, Watanabe KS, Goodrich JM, et al. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257:238–241. [DOI] [PubMed] [Google Scholar]
- 4.Papadopoulos EB, Ladanyi M, Emanuel D, et al. Infusions of donor leukocytes to treat Epstein-Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N Engl J Med. 1994;330:1185–1191. [DOI] [PubMed] [Google Scholar]
- 5.Louis CU, Straathof K, Bollard CM, et al. Enhancing the in vivo expansion of adoptively transferred EBV-specific CTL with lymphodepleting CD45 monoclonal antibodies in NPC patients. Blood. 2009;113:2442–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bollard C, Aguilar ML, Straathof KC, et al. Cytotoxic T lymphocyte therapy for Epstein-Barr virus+ Hodgkin’s disease. J Exp Med. 2004;200:1623–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolb HJ, Mittermuller J, Clemm C, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990;76:2462–2465. [PubMed] [Google Scholar]
- 8.Rosenberg SA, Packard BS, Aebersold PM, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with meta-static melanoma. A preliminary report. N Engl J Med. 1988;319:1676–1680. [DOI] [PubMed] [Google Scholar]
- 9.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Besser MJ, Shapira-Frommer R, Treves AJ, et al. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2010;16:2646–2655. [DOI] [PubMed] [Google Scholar]
- 12.Tran KQ, Zhou J, Durflinger KH, et al. Minimally cultured tumor-infiltrating lymphocytes display optimal characteristics for adoptive cell therapy. J Immunother. 2008;31:742–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dudley ME, Gross CA, Langhan MM, et al. CD8+ enriched “young” tumor infiltrating lymphocytes can mediate regression of metastatic melanoma. Clin Cancer Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Bruggen P, Traversari C, Chomez P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–1647. [DOI] [PubMed] [Google Scholar]
- 15.Clay TM, Custer MC, Sachs J, et al. Efficient transfer of a tumor antigen-reactive TCR to human peripheral blood lymphocytes confers anti-tumor reactivity. J Immunol. 1999;163:507–513. [PubMed] [Google Scholar]
- 16.Stanislawski T, Voss RH, Lotz C, et al. Circumventing tolerance to a human MDM2-derived tumor antigen by TCR gene transfer. Nat Immunol. 2001;2: 962–970. [DOI] [PubMed] [Google Scholar]
- 17.Xue SA, Gao L, Hart D, et al. Elimination of human leukemia cells in NOD/SCID mice by WT1-TCR gene-transduced human T cells. Blood. 2005;106:3062–3067. [DOI] [PubMed] [Google Scholar]
- 18.Zhao Y, Zheng Z, Cohen CJ, et al. High-efficiency transfection of primary human and mouse T lymphocytes using RNA electroporation. Mol Ther. 2006;13:151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hackett PB, Largaespada DA, Cooper LJ. A transposon and transposase system for human application. Mol Ther. 2010;18:674–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hacein-Bey-Abina S, von Kalle C, Schmidt M, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2003;348:255–256. [DOI] [PubMed] [Google Scholar]
- 21.Riviere I, Brose K, Mulligan RC. Effects of retroviral vector design on expression of human adenosine deaminase in murine bone marrow transplant recipients engrafted with genetically modified cells. Proc Natl Acad Sci USA. 1995;92:6733–6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schambach A, Swaney WP, van der Loo JC. Design and production of retroand lentiviral vectors for gene expression in hematopoietic cells. Methods Mol Biol. 2009;506:191–205. [DOI] [PubMed] [Google Scholar]
- 23.Hughes MS, Yu YY, Dudley ME, et al. Transfer of a TCR gene derived from a patient with a marked antitumor response conveys highly active T-cell effector functions. Hum Gene Ther. 2005;16:457–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levine BL, Humeau LM, Boyer J, et al. Gene transfer in humans using a conditionally replicating lentiviral vector. Proc Natl Acad Sci USA. 2006; 103:17372–17377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cavalieri S, Cazzaniga S, Geuna M, et al. Human T lymphocytes transduced by lentiviral vectors in the absence of TCR activation maintain an intact immune competence. Blood. 2003;102:497–505. [DOI] [PubMed] [Google Scholar]
- 26.Szymczak AL, Workman CJ, Wang Y, et al. Correction of multi-gene deficiency in vivo using a single ‘self-cleaving’ 2A peptide-based retroviral vector. Nat Biotechnol. 2004;22:589–594. [DOI] [PubMed] [Google Scholar]
- 27.Wargo JA, Robbins PF, Li Y, et al. Recognition of NY-ESO-1+ tumor cells by engineered lymphocytes is enhanced by improved vector design and epigenetic modulation of tumor antigen expression. Cancer Immunol Immunother. 2009;58:383–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hart DP, Xue SA, Thomas S, et al. Retroviral transfer of a dominant TCR prevents surface expression of a large proportion of the endogenous TCR repertoire in human T cells. Gene Ther. 2008;15:625–631. [DOI] [PubMed] [Google Scholar]
- 29.Liang X, Weigand LU, Schuster IG, et al. A single TCR alpha-chain with dominant peptide recognition in the allorestricted HER2/neu-specific T cell repertoire. J Immunol. 2010;184:1617–1629. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Moysey R, Molloy PE, et al. Directed evolution of human T-cell receptors with picomolar affinities by phage display. Nat Biotechnol. 2005; 23:349–354. [DOI] [PubMed] [Google Scholar]
- 31.Zhao Y, Bennett AD, Zheng Z, et al. High-affinity TCRs generated by phage display provide CD4+ T cells with the ability to recognize and kill tumor cell lines. J Immunol. 2007;179:5845–5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robbins PF, Li YF, El-Gamil M, et al. Single and dual amino acid substitutions in TCR CDRs can enhance antigen-specific T cell functions. J Immunol. 2008;180:6116–6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson LA, Heemskerk B, Powell DJ Jr, et al. Gene transfer of tumor-reactive TCR confers both high avidity and tumor reactivity to nonreactive peripheral blood mononuclear cells and tumor-infiltrating lymphocytes. J Immunol. 2006;177:6548–6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang S, Cohen CJ, Peng PD, et al. Development of optimal bicistronic lentiviral vectors facilitates high-level TCR gene expression and robust tumor cell recognition. Gene Ther. 2008;15:1411–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Theobald M, Biggs J, Dittmer D, et al. Targeting p53 as a general tumor antigen. Proc Natl Acad Sci USA. 1995;92:11993–11997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Govers C, Sebestyen Z, Coccoris M, et al. T cell receptor gene therapy: strategies for optimizing transgenic TCR pairing. Trends Mol Med. 2010;16:77–87. [DOI] [PubMed] [Google Scholar]
- 37.Kieback E, Uckert W. Enhanced T cell receptor gene therapy for cancer. Expert Opin Biol Ther. 2010;10:749–762. [DOI] [PubMed] [Google Scholar]
- 38.Cohen CJ, Zhao Y, Zheng Z, et al. Enhanced antitumor activity of murine-human hybrid T-cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability. Cancer Res. 2006;66:8878–8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bialer G, Horovitz-Fried M, Ya’acobi S, et al. Selected murine residues endow human TCR with enhanced tumor recognition. J Immunol. 2010;184: 6232–6241. [DOI] [PubMed] [Google Scholar]
- 40.Sommermeyer D, Uckert W. Minimal amino acid exchange in human TCR constant regions fosters improved function of TCR gene-modified T cells. J Immunol. 2010;184:6223–6231. [DOI] [PubMed] [Google Scholar]
- 41.Bendle GM, Linnemann C, Hooijkaas AI, et al. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat Med. 2010;16: 565–570. [DOI] [PubMed] [Google Scholar]
- 42.Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci USA. 1989;86:10024–10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bridgeman JS, Hawkins RE, Hombach AA, et al. Building better chimeric antigen receptors for adoptive T cell therapy. Curr Gene Ther. 2010;10:77–90. [DOI] [PubMed] [Google Scholar]
- 44.Sadelain M, Brentjens R, Riviere I. The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol. 2009;21:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilkie S, Picco G, Foster J, et al. Retargeting of human T cells to tumor-associated MUC1: the evolution of a chimeric antigen receptor. J Immunol. 2008;180:4901–4909. [DOI] [PubMed] [Google Scholar]
- 46.Alvarez-Vallina L, Hawkins RE. Antigen-specific targeting of CD28-mediated T cell co-stimulation using chimeric single-chain antibody variable fragment-CD28 receptors. Eur J Immunol. 1996;26:2304–2309. [DOI] [PubMed] [Google Scholar]
- 47.Gong MC, Latouche JB, Krause A, et al. Cancer patient T cells genetically targeted to prostate-specific membrane antigen specifically lyse prostate cancer cells and release cytokines in response to prostate-specific membrane antigen. Neoplasia. 1999;1:123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pule MA, Straathof KC, Dotti G, et al. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther. 2005;12:933–941. [DOI] [PubMed] [Google Scholar]
- 49.Wang J, Jensen M, Lin Y, et al. Optimizing adoptive polyclonal T cell immunotherapy of lymphomas, using a chimeric T cell receptor possessing CD28 and CD137 costimulatory domains. Hum Gene Ther. 2007;18:712–725. [DOI] [PubMed] [Google Scholar]
- 50.Zhao Y, Wang QJ, Yang S, et al. A herceptin-based chimeric antigen receptor with modified signaling domains leads to enhanced survival of transduced T lymphocytes and antitumor activity. J Immunol. 2009;183:5563–5574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhong XS, Matsushita M, Plotkin J, et al. Chimeric antigen receptors combining 4–1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Mol Ther. 2010;18:413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Milone MC, Fish JD, Carpenito C, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17:1453–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson LA, Morgan RA, Dudley ME, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Overwijk WW, Theoret MR, Finkelstein SE, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park JR, Digiusto DL, Slovak M, et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol Ther. 2007;15:825–833. [DOI] [PubMed] [Google Scholar]
- 58.Till BG, Jensen MC, Wang J, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kershaw MH, Westwood JA, Parker LL, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12:6106–6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lamers CH, Sleijfer S, Vulto AG, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006;24:e20–e22. [DOI] [PubMed] [Google Scholar]
- 61.Pule MA, Savoldo B, Myers GD, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brentjens R, Yeh R, Bernal Y, et al. Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: case report of an unforeseen adverse event in a phase I clinical trial. Mol Ther. 2010;18:666–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morgan RA, Yang JC, Kitano M, et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bollard CM, Rossig C, Calonge MJ, et al. Adapting a transforming growth factor beta-related tumor protection strategy to enhance antitumor immunity. Blood. 2002;99:3179–3187. [DOI] [PubMed] [Google Scholar]
- 65.Dotti G Blocking PD-1 in cancer immunotherapy. Blood. 2009;114:1457–1458. [DOI] [PubMed] [Google Scholar]
- 66.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scanlan MJ, Gure AO, Jungbluth AA, et al. Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunol Rev. 2002;188:22–32. [DOI] [PubMed] [Google Scholar]