Abstract
Transforming growth factor β (TGF-β) is a cytokine with complex biological functions that may involve tumor promotion or tumor suppression. It has been reported that multiple types of tumors secrete TGF-β, which can inhibit tumor-specific cellular immunity and may represent a major obstacle to the success of tumor immunotherapy. In this study, we sought to enhance tumor immunotherapy using genetically modified antigen-specific T cells by interfering with TGF-β signaling. We constructed three γ-retroviral vectors, one that expressed TGF-β-dominant-negative receptor II (DNRII) or two that secreted soluble TGF-β receptors: soluble TGF-β receptor II (sRII) and the sRII fused with mouse IgG Fc domain (sRIIFc). We demonstrated that T cells genetically modified with these viral vectors were resistant to exogenous TGF-β-induced smad-2 phosphorylation in vitro. The functionality of antigen-specific T cells engineered to resist TGF-β signaling was further evaluated in vivo using the B16 melanoma tumor model. Antigen-specific CD8+ T cells (pmel-1) or CD4+ T cells (tyrosinase-related protein-1) expressing DNRII dramatically improved tumor treatment efficacy. There was no enhancement in the B16 tumor treatment using cells secreting soluble receptors. Our data support the potential application of the blockade of TGF-β signaling in tumor-specific T cells for cancer immunotherapy.
Keywords: adoptive T-cell therapy, antigen-specific T cell, TGF-β
INTRODUCTION
T cells specific for tumor antigens have been observed both within tumors and in the peripheral blood. Encouraging results have been reported using adoptive transfer of the tumor infiltrating lymphocytes resulting in tumor regression in patients with metastatic melanoma.1,2 For many patients though, even the administration of large numbers of tumor-reactive cells does not mediate clinical response. One of the explanations for this treatment failure is that the tumors may have acquired immune evasion mechanisms. Secretion of transforming growth factor β (TGF-β) by tumor cells is one of the widely observed strategies for tumor evasion.3,4 The role of TGF-β in cancer biology is complex and involves tumor suppression as well as tumor promotion, depending on when or where the cytokine is secreted. As an immune suppressor factor, the biological actions of TGF-β include the inhibition of proliferation and effector functions of T cells and regulation of differentiation of functionally distinct subsets of T cells.5,6 In addition, tumor cells may avoid the differentiation and apoptotic effects of TGF-β by expressing a nonfunctional TGF-β receptor.7,8
The signaling pathway of TGF-β is mediated by its receptors including TGF-β receptor I (TGF-β-RI), TGF-β receptor II (TGF-β-RII) and TGF-β receptor III (TGF-β-RIII).9,10 The interaction between the receptor complex and ligand causes phosphorylation of transcription factors smad2 and smad3, resulting in their translocation to the nucleus and regulation of gene expression.11 Inhibitors targeting the TGF-β signaling pathway are being evaluated in preclinical models and early clinical trials, including oligonucleotide AP12009, TGF-β antibody GC1008 and TGF-β2 antisense vaccine et al.3 Though systemic blockade of TGF-β using anti-TGF-β antibody was well tolerated in preclinical studies,12 given the pleiotropic effect of this cytokine, one potential concern of this systemic therapy is the development of autoimmune toxicities in human. Other potential toxicities related to systemic blockade might result from the cytokine’s homeostatic function in other tissues outside of the immune system, including angiogenesis and development of musculoskeletal tissues. To control the toxicity related to systemic inhibition of the TGF-β pathway, we evaluated three strategies to generate the antigen-specific T cell resistant to TGF-β by expressing a dominant-negative TGF-β receptor type II (DNRII) or two types of decoy-soluble TGF-β receptor II.
RESULTS AND DISCUSSION
Tumor cells or immature myeloid cells secrete TGF-β to evade immune surveillance through inhibition of effector T-cell proliferation, cytokine release and cytolytic activity. Those effects might affect the treatment efficacy of adoptively transferred tumor-specific cytotoxic T lymphocytes (CTL) in tumor immunotherapy. Owing to the highly pleiotropic properties of TGF-β and the presence of TGF-β receptors on most cell types, neutralization efforts using monoclonal antibodies targeting TGF-β or its receptors may have unpredictable consequences in vivo. It was demonstrated using TGF-β-dominant-negative receptor transgenic mouse model that specific blockade of TGF-β on T cells leads to the enhancement of antitumor immunity.13,14 More recently, Bollard et al. had reported that human Epstein-Barr virus-CTLs transduced with a retrovirus vector expressing a DNRII were resistant to the antiproliferative and anticytotoxic effects of exogenous TGF-β.15,16 The TGF-β-resistant CTL had a functional advantage over unmodified CTL in the presence of TGF-β-secreting Epstein-Barr virus-positive lymphoma, and had enhanced antitumor activity in vivo.15,16
An alternative strategy to specifically neutralize TGF-β at the tumor site and shield the immune cells from negative effects of the cytokine is the use of soluble TGF-β receptors, which abrogate TGF-β signaling by competitive binding of the ligand to its receptor.17 Furthermore, it had been reported that systemic administration of an oncolytic adenovirus expressing a soluble form of TGF-β receptor II fused with human Fc IgG1 (sRIIFc) resulted in significant inhibition of tumor growth of established bone metastases in a human xenograft mouse model.18–20
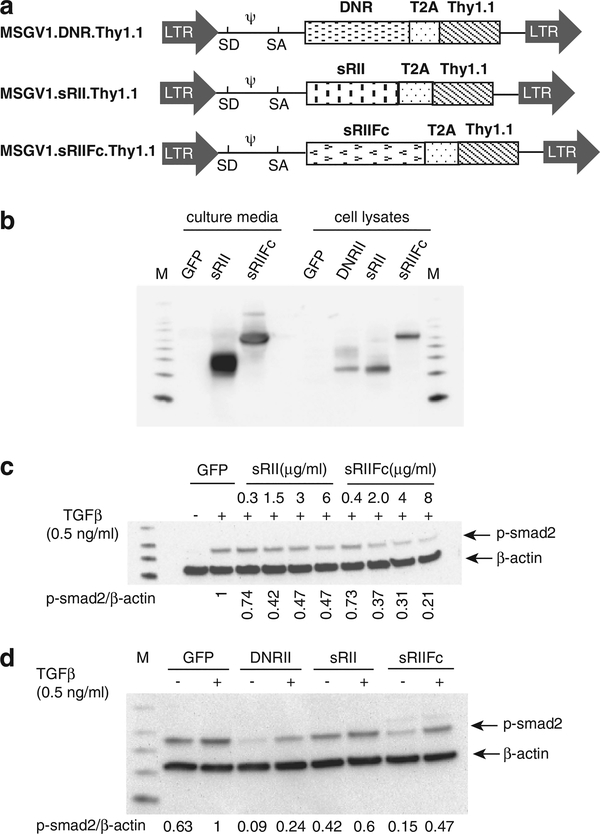
In this study, we evaluated the strategies to deliver modified TGF-β receptors to the tumor environment by antigen-specific T cells. We constructed three γ-retroviral vectors, one that expressed mouse TGF-β-dominant-negative receptor II (MSGV1.DNRII), a second that secreted a soluble TGF-β-RII containing the extracellular domain of TGF-β-RII (MSGV1.sRII) and third a soluble TGF-β-RIIFc, in which the extracellular domain was fused to the mouse immunoglobulin (IgG2a) Fc fragment (MSGV1.sRIIFc)(Figure 1a). In order to track the transduced cells in vitro and in vivo, the Thy1.1 gene was inserted downstream of the receptor genes and separated by a picornavirus T2A linker (Figure 1a). The vector-expressing green fluorescent protein (GFP) (MSGV1.GFP) was used as an experimental control. To evaluate the expression and functionality of these receptors, mouse splenocytes were transduced with three vectors expressing DNRII, sRII and sRIIFc, respectively. Using western blot analysis, we readily detected the expression of DNRII, sRII and sRIIFc in transduced lymphocytes. As expected, both soluble sRII and sRIIFc were detected in the cell culture media as well as in total cell lysates (Figure 1b).
Figure 1.
DNRII-, sRII-, sRIIFc-transduced T cells were resistant to TGF-β-mediated smad2 phosphorylation. (a) Schematic representation of retroviral vectors: MSGV1.DNRII, MSGV1.sRII and MSGV1.sRIIFc. LTR, long terminal repeat; SD, splice donor; SA, splice acceptor; T2A, ribosomal skip peptide. (b) Mouse splenocytes were transduced with the MSGV1.GFP, MSGV1.DNRII, MSGV1.sRII and MSGV1.sRIIFc. The cells and culture supernatant were harvested 48 h later. The DNRII, sRII and sRIIFc expression were measured by immunoblotting with anti-TGF-β-RII antibody. (c) Different amount of partially concentrated conditioned media was added to T cells treated with exogenous TGF-β1 (0.5 ng ml−1) for 1 h. Phosphorylation smad2 (p-smad2) was measured by western blot. The relative level of p-smad2 was normalized by β-actin. The p-smad2 level in the cells treated with TGF-β1 and the supernatant from GFP-transduced cells was set as 1. (d) The T cells were transduced with GFP, DNRII, sRII or sRIIFc individually and treated without or with exogenous TGF-β1 (0.5 ng ml−1, 1 h). The smad2 phosphorylation was measured by western blot. The relative level of p-smad2 was normalized by β-actin. The relative p-smad2 level in the GFP-transduced cells treated with TGF-β1 and was set as 1.
To determine the biological activity of the soluble decoy receptors, culture medium from transduced cells was collected and applied to mouse T cells. The decoy receptors prevented exogenous TGF-β1-induced smad-2 phosphorylation in a dosage-dependent manner (Figure 1c). It was also demonstrated that the cells transduced with soluble receptors were resistant to phosphorylation of smad-2 induced by exogenous TGF-β1 (Figure 1d); however, the TGF-β blockade was less than that observed in cells transduced with DNRII. These results indicated that both DNRII and decoy vectors could successfully transduce mouse T cells and block TGF-β signaling pathways in vitro.
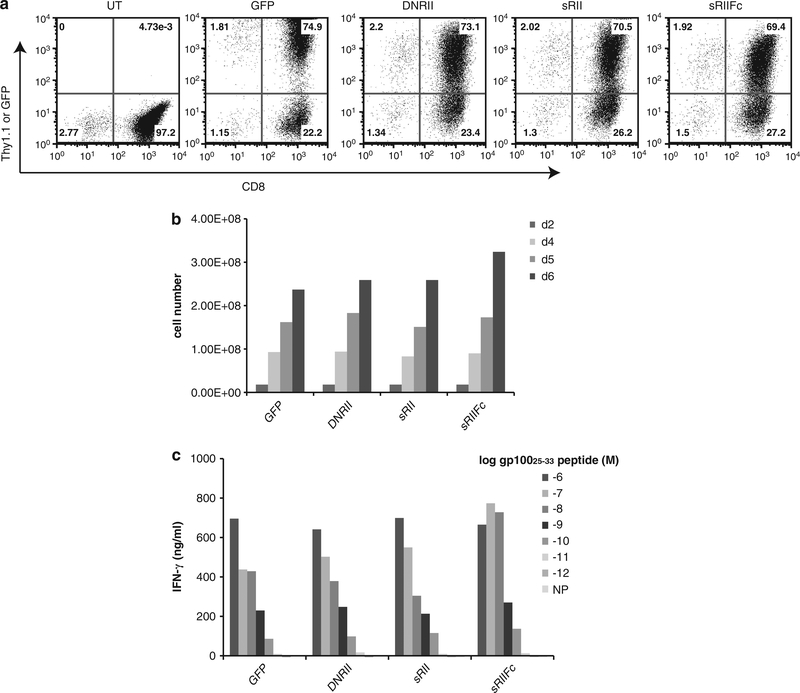
B16 melanoma, derived from C57BL/6 mice, is a ‘poorly immunogenic’ tumor. Penafuerte et al. had found that B16 tumor secreted biologically active TGF-β, which in turn inhibited cytokine-induced immune cell proliferation and downregulated interleukin-2Rβ expression and interferon-γ secretion by natural killer cells.4 Using real-time PCR and enzyme-linked immunosorbent assay, the B16-F10 melanoma line cultured in the Surgery Branch NCI was confirmed to express TGF-β (Supplementary 1). We have previously reported that large established B16 tumors can be specifically treated using adoptive transfer of antigen-specific T cells (Pmel-1 cells).21 Pmel-1 cells were stimulated and transduced with viral vectors expressing DNRII, sRII, sRIIFc or GFP. The transduction efficiency of each vector was around 70% measured by flow cytometry analysis using Thy1.1-FITC antibody (Figure 2a). There was no effect on cell proliferation in cells transduced by different vectors (Figure 2b). The genetically modified cells also retained similar antigen recognition as measured by interferon-γ secretion following antigen-specific peptide stimulation (Figure 2c).
Figure 2.
Pmel-1 T cells expressing DNRII, sRII or sRIIFc did not affect cell proliferation or antigen recognition. (a) The pmel-1 cells were transduced with GFP, DNRII, sRII or sRIIFc, and analyzed by fluorescence-activated cell sorting using Thy1.1-FITC and CD8-PE antibody. (b). The transduced cells were enumerated every 2 days by trypan blue exclusion. (c) The cells transduced with DNRII, sRII or sRIIFc vector were co-cultured with various concentration (from 10−6 M to 10−12 M) of hgp10025–33 peptide-pulsed cells for 16 h. The interferon-γ level in the culture was measured by enzyme-linked immunosorbent assay (shown are the mean values of duplicate determinations). NP, negative control peptide.
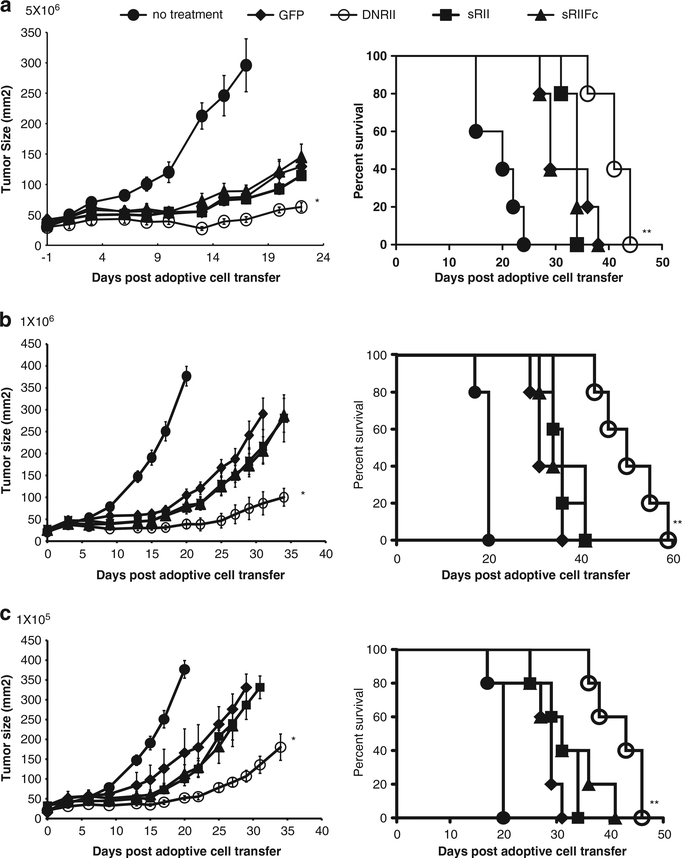
To determine the in vivo efficacy of these cells, different doses of genetically modified cells (5 × 106, 1 × 106 or 1 × 105) were infused into B16 tumor-bearing mice (n = 5) along with administration of rVVhgp100 and interleukin-2. As previously reported, compared with animals receiving no treatment, animals receiving Pmel-1 cell (GFP control) showed delayed tumor growth and prolonged survival (Figure 3). We observed that tumor-bearing mice receiving T cells transduced with DNRII vector displayed an augmented tumor treatment compared with the mice giving cells modified by GFP (P = 0.009) and this was observed at all dose levels (Figure 3). In addition, the tumor-bearing mice treated by DNRII-genetically modified pmel-1 cells had significantly prolonged survival compared with the control group (P<0.01, Figure 3). However, cells expressing the soluble receptors did not enhance treatment compared with GFP-engineered control Pmel-1 cells in these experiments (Figure 3).
Figure 3.
DNRII expressing pmel-1 cells had enhanced antitumor activity against B16 melanoma tumor. Pmel-1 cells were transduced with vector-expressing GFP, DNRII, sRII or sRIIFc. B16 tumor-bearing mice (n = 5) were adoptively transferred with 5 × 106 (a), 1 × 106 (b) or 1 × 105 (c) cells genetically modified by pmel-1 cells as described in Materials and methods. Tumor sizes were assessed with serial measurements. Error bars represent s.e.m. (*P = 0.009, DNRII compared with GFP). The survival of tumor-bearing mice that received 5 × 106 (a), 1 × 106 (b) or 1 × 105 (c) of genetic-modified cell transfer were determined as shown (**P<0.05, DNRII compared with GFP).
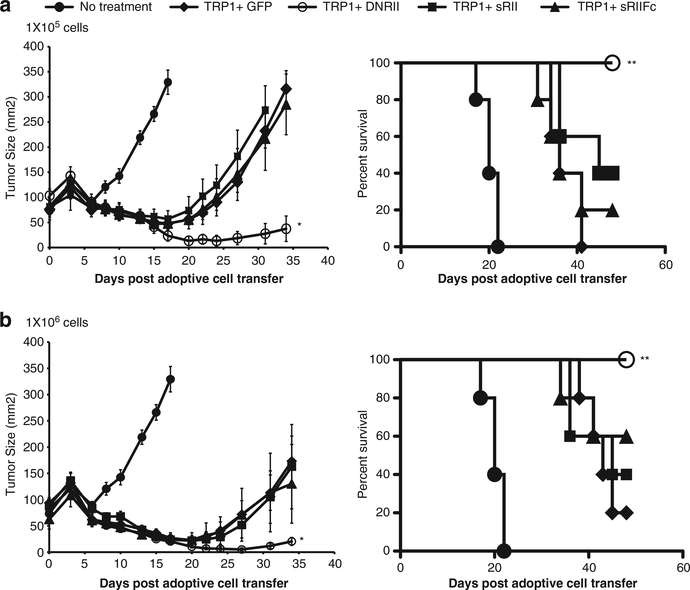
While CD8+ Pmel-1 T cells are an example of a classic effector T cell, CD4+ T cells can display a variety of phenotypes, including both suppressor and effector T-cell functions. For example, we and others had reported that CD4 T cells transduced with a major histocompatibility complex class II-restricted T-cell receptor (TCR) specific for tyrosinase-related protein-1 (TRP1) could eradicate established B16 tumor.22–24 The differentiation status of CD4+ T cells can be influenced by several cytokines, including TGF-β. It was reported that TGF-β promoted differentiation of naïve CD4 T cells into regulatory T cells (iTreg) and Th17 cells via a paracrine mechanism.25–27,28 Based on these observations, we next investigated the effect of blockade TGF-β signaling on the in vivo function of CD4 anti-TRP1 T cells. CD4 T cells were stimulated and cotransduced with viral vectors expressing the TRP1-TCR and sRII, sRIIFc, DNRII or GFP vectors. B16 tumor-bearing mice were given 1 × 106 or 1 × 105 double-engineered cells along with vaccine rVVTRP1 and interleukin-2 administration. Consistent with reported results, CD4 T cells genetically modified by TCR targeting TRP1 resulted in B16 tumor regression with as few as 100 000 cells and prolonged the survival of tumor-bearing mice (P<0.01, Figure 4a). The CD4 cells double engineered with TRP1 and DNRII significantly augmented this tumor treatment efficacy and displayed longer survival compared with cells modified by TRP1 and GFP (P<0.05) (Figures 4a and b). Again, there was no treatment difference among the mice receiving cells cotransduced by TRP1 and sRII, sRIIFc versus GFP (Figures 4a and b).
Figure 4.
TRP1 CD4 cells co-expressing DNRII dramatically augmented the tumor treatment in B16 melanoma tumor model. CD4 T cells were isolated from normal mouse splenocytes and stimulated with anti-CD3 and anti-CD28 in vitro. The cells were than cotransduced with TRP1-TCR and GFP, DNRII, sRII or sRIIFc. B16 tumor-bearing mice (n = 5) were adoptively transferred with 1 × 105 (a) or 1 × 106 (b) double-engineered CD4 T cells. Tumor sizes were assessed with serial measurements. Error bars represent s.e.m. (*P<0.05, TRP1 + DNRII compared with TRP1 + GFP). The survival of tumor-bearing mice that received 1 × 105 (a) or 1 × 106 (b) of genetically modified cell transfer were determined as shown (**P<0.05, TRP1 + DNRII compared with TRP1 + GFP).
In its function as a tumor suppressive cytokine, TGF-β has been reported to enhance tumor migration and invasion as well as inhibit antitumor immune responses.29–31 The effect of neutralizing TGF-β on antitumor activity has been evaluated by expressing of soluble TGF-β receptors in a variety of cell lines and animal tumor models for pancreatic, prostate or breast cancer.32–35 In these tumor models, the soluble receptor was delivered either by engineered tumor cells or intraperitoneal injection. Systemic delivery of the oncolytic adenovirus Ad.sTβRFc, which expressing soluble TGF-β receptor sRIIFc, was reported to inhibit the progression of established bone metastases and conferred a survival advantage to mice in a breast cancer model.20 The success of this treatment relied on the combination of sTGF-β-RIIFc production and tumor destruction by adenovirus.20
In this study, we aimed to improve adoptive T-cell therapy by abrogating TGF-β in the tumor microenvironment. Our in vitro experimental data indicated that two types of soluble receptors secreted by the T cells were effective in inhibiting smad-2 phosphorylation mediated by exogenous TGF-β1, and the engineered antigen-specific T cells maintained their antigen recognition property. However, the in vitro blocking activity in the cells expressing sRII and sRIIFc were weaker than that in the cells expressing DNRII, possibly owing to an insufficient amount of soluble proteins required to neutralize the added TGF-β1. In vivo, there was no toxicity observed upon transferring the cells constitutively secreting soluble receptor, but also no treatment benefit. The loss of efficacy could be owing to inadequate local concentration of the receptor antagonists, or possibly owing to the TGF-β being presented to the T cells in a form that is inaccessible to the soluble receptors, which may occur via direct presentation of cell surface-bound TGF-β on Tregs or myeloid cells.36,37
It was reported that Epstein-Barr virus-CTL genetically modified by TGF-β-dominant-negative receptor had greater antitumor activity in an immunodeficient mouse model.16 Our data demonstrated that blockade of TGF-β signaling in tumor antigen-specific CD8 T cells (anti-gp100) and CD4 T cells (antiTRP1) dramatically improved the adoptive T-cell treatment in an immunocompetent mouse melanoma tumor treatment model. This augmentation appeared to be more significant in CD4 (TRP1) T cells than the CD8 (Pmel-1) T cells. This observation was not associated with significant differences in blockade of smad-2 phosphorylation. As TGF-β is known to be involved in the differentiation of CD4 T cells into Treg, blockade TGF-β on CD4 cell would possibly generate fewer Treg cells, which could favor the antitumor activity of immune effector CD4 T cells. The use of antigen-specific T cells engineered with a TGF-β DNRII may be a useful strategy to potentially improve the outcome of adoptive T-cell therapy targeting cancers that express TGF-β.
MATERIALS AND METHODS
Mice and cell lines
Splenocytes from C57BL/6 mice were used to generate CD8+ and CD4+ murine T cells. Murine CD4+ T cells were purified from splenocytes using mouse CD4+ T-cell enrichment kit (Stem cell Tech, Vancouver, BC, Canada). The cells were then stimulated with 1μgml−1 of anti-CD3 and anti-CD28 antibody (BD Biosciences, San Jose, CA, USA) for 36h and cultured in 60IUml−1 of interleukin-2 (Chiron, Emeryville, CA, USA). Platinum-E retroviral package cell line (Plat-E, Cell Biolab, San Diego, CA, USA) was used to produce retrovirus and cultured in Dulbecco’s Modified Eagle media (Invitrogen, Carlsbad, CA, USA) with 10% fetal bovine serum (Biofluid Inc., Gaithersburg, MD, USA), 100Uml−1 penicillin and 100μgml−1 streptomycin, 2mM l-glutamine and 25mM HEPES buffer solution (Invitrogen).
Vector design
Murine TGF-β-DNRII and soluble TGF-β receptor fusion with IgG Fc fragment (sRIIFc) were synthesized as codon-optimization sequences (Invitrogen). TGF-β-soluble receptor (sRII) was amplified from sRIIFc using the primer pair: 5′-TTTCCATGGGTCGGGGGCTGCTCVAGGGGCCT-3′ and 5′-TT TGAATTCTCGTCAGGATTGCTGGTGTTATA-3′ by PCR. The genes were cut by NcoI/EcoRI and ligated to 2A.Thy1.1 fragment with EcoRI/BamH1 restriction sites and inserted into MSGV1 vector38 at NcoI/BamH1 enzyme sites. All vectors have been confirmed by enzyme digestion and DNA sequencing.
Western blot
The C57BL/6 mice splenocytes were stimulated by anti-CD3/anti-CD28 and transduced by DNRII, sRII and sRIIFc vectors viral supernatant. The cells were lysed by using RIPA buffer (Thermo Scientific, Rockford, IL, USA). Total cell protein was separated at 12% SDS–polyacrylamide gel eletrophoresis (SDS-PAGE) (Invitrogen) and transferred to nitrocellulose membrane (Invitrogen). The membrane was then probed with antibodies against TGF-β RII (R&D, Minneapolis, MN, USA), p-smad2 (Cell Signaling, Danvers, MA, USA) and β-actin (Santa Cruz, Santa Cruz, CA, USA).
Retroviral vector preparation and transduction
To generate retrovirus, 293 GP cells, which stably express GAG and POL proteins, were transfected as previously described.39 In brief, 9 μg of vector DNA and 4 μg of RD114 envelope plasmid DNA were mixed with lipofectamine 2000 (Invitrogen) in serum-free medium and incubated at room temperature for 20 min. The mixture was applied to 293 GP cells that had been plated the prior day on a 100-mm2 polylysine-coated plate (Becton Dickinson, Franklin Lakes, NJ, USA). After 6 h of incubation, the medium was replaced with Dulbecco’s Modified Eagle Medium (Invitrogen) with 10% fetal bovine serum and the viral supernatants were harvested 48 h later. Platinum-E cell, a retroviral package cell line, was infected by 293 GP produced by the retroviral vector and cultured in Dulbecco’s Modified Eagle Medium. Retrovirus harvested from the platinum-E cells was used for splenocyte transduction as described before.40 Briefly, the stimulated murine T cells were transduced with retroviral vectors in 24-well plates with 1 μgml−1 protamine sulfate, centrifuged at 1000 g, 1.5 h.
Adoptive cell transfer
C57BL/6 mice were housed at the National Institutes of Health (NIH). B16 (H-2b), a poorly immunogenic gp100+ murine melanoma cell line, was maintained in RPMI-1640 (Invitrogen) with 10% fetal bovine serum.
C57BL/6 mice at 6–12 weeks of age were injected with 2 × 105 to 5 × 105 B16 melanoma cells. Ten days later, groups of tumor-bearing mice (n = 5) were treated with 5 Gy lymphodepleting irradiation and given retroviral vectors-engineered CD4+ or CD8+ T cells, respectively, by tail vein injection. The perpendicular diameters of the tumors were measured with a caliper by an independent investigator in a blinded manner. The tumor curve data were shown as mean±s.e.m. The NCI Animal Care and Use Committee of the NIH approved all animal experiments.
Statistic analysis
Tumor growth slopes were compared using Wilcoxon rank sum test. Survival curves at different treatment groups were compared using Mantel–Cox test. P<0.05 was considered significant.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr Lalage Wakefield for kindly providing TGF-β DNRII vector and help in explaining data. FACS laboratory and the TIL laboratory in the Surgery Branch, National Cancer Institute provide technical support and maintenance of tumor cells from patients. This work is supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, and Center for Cancer Research.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on Gene Therapy website (http://www.nature.com/gt)
REFERENCES
- 1.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 2002; 298: 850–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res 2011; 17: 4550–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yingling JM, Blanchard KL, Sawyer JS. Development of TGF-beta signalling inhibitors for cancer therapy. Nat Rev Drug Discov 2004; 3: 1011–1022. [DOI] [PubMed] [Google Scholar]
- 4.Penafuerte C, Galipeau J. TGF beta secreted by B16 melanoma antagonizes cancer gene immunotherapy bystander effect. Cancer Immunol Immunother 2008; 57: 1197–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorelik L, Flavell RA. Transforming growth factor-beta in T-cell biology. Nat Rev Immunol 2002; 2: 46–53. [DOI] [PubMed] [Google Scholar]
- 6.Gorelik L, Constant S, Flavell RA. Mechanism of transforming growth factor beta-induced inhibition of T helper type 1 differentiation. J Exp Med 2002; 195: 1499–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park K, Kim SJ, Bang YJ, Park JG, Kim NK, Roberts AB et al. Genetic changes in the transforming growth factor beta (TGF-beta) type II receptor gene in human gastric cancer cells: correlation with sensitivity to growth inhibition by TGF-beta. Proc Natl Acad Sci USA 1994; 91: 8772–8776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knaus PI, Lindemann D, DeCoteau JF, Perlman R, Yankelev H, Hille M et al. A dominant inhibitory mutant of the type II transforming growth factor beta receptor in the malignant progression of a cutaneous T-cell lymphoma. Mol Cell Biol 1996; 16: 3480–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebner R, Chen RH, Shum L, Lawler S, Zioncheck TF, Lee A et al. Cloning of a type I TGF-beta receptor and its effect on TGF-beta binding to the type II receptor. Science 1993; 260: 1344–1348. [DOI] [PubMed] [Google Scholar]
- 10.Attisano L, Carcamo J, Ventura F, Weis FM, Massague J, Wrana JL. Identification of human activin and TGF beta type I receptors that form heteromeric kinase complexes with type II receptors. Cell 1993; 75: 671–680. [DOI] [PubMed] [Google Scholar]
- 11.Ikushima H, Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer 2010; 10: 415–424. [DOI] [PubMed] [Google Scholar]
- 12.Terabe M, Ambrosino E, Takaku S, O’Konek JJ, Venzon D, Lonning S et al. Synergistic enhancement of CD8 + T cell-mediated tumor vaccine efficacy by an anti-transforming growth factor-beta monoclonal antibody. Clin Cancer Res 2009; 15: 6560–6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity 2000; 12: 171–181. [DOI] [PubMed] [Google Scholar]
- 14.Gorelik L, Flavell RA. Immune-mediated eradication of tumors through the blockade of transforming growth factor-beta signaling in T cells. Nat Med 2001; 7: 1118–1122. [DOI] [PubMed] [Google Scholar]
- 15.Bollard CM, Rossig C, Calonge MJ, Huls MH, Wagner HJ, Massague J et al. Adapting a transforming growth factor beta-related tumor protection strategy to enhance antitumor immunity. Blood 2002; 99: 3179–3187. [DOI] [PubMed] [Google Scholar]
- 16.Foster AE, Dotti G, Lu A, Khalil M, Brenner MK, Heslop HE et al. Antitumor activity of EBV-specific T lymphocytes transduced with a dominant negative TGF-beta receptor. J Immunother 2008; 31: 500–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russo LM, Brown D, Lin HY. The soluble transforming growth factor-beta receptor: advantages and applications. Int J Biochem Cell Biol 2009; 41: 472–476. [DOI] [PubMed] [Google Scholar]
- 18.Seth P, Wang ZG, Pister A, Zafar MB, Kim S, Guise T et al. Development of oncolytic adenovirus armed with a fusion of soluble transforming growth factorbeta receptor II and human immunoglobulin Fc for breast cancer therapy. Hum Gene Ther 2006; 17: 1152–1160. [DOI] [PubMed] [Google Scholar]
- 19.Hu Z, Zhang Z, Guise T, Seth P. Systemic delivery of an oncolytic adenovirus expressing soluble transforming growth factor-beta receptor II-Fc fusion protein can inhibit breast cancer bone metastasis in a mouse model. Hum Gene Ther 2010; 21: 1623–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu Z, Gerseny H, Zhang Z, Chen YJ, Berg A, Stock S et al. Oncolytic adenovirus expressing soluble TGFbeta receptor II-Fc-mediated inhibition of established bone metastases: a safe and effective systemic therapeutic approach for breast cancer. Mol Ther 2011; 19: 1609–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med 2003; 198: 569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerkar SP, Sanchez-Perez L, Yang S, Borman ZA, Muranski P, Ji Y et al. Genetic engineering of murine CD8 + and CD4 + T cells for preclinical adoptive immunotherapy studies. J Immunother 2011; 34: 343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie Y, Akpinarli A, Maris C, Hipkiss EL, Lane M, Kwon EK et al. Naive tumor-specific CD4( + ) T cells differentiated in vivo eradicate established melanoma. J Exp Med 2010; 207: 651–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood 2008; 112: 362–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huter EN, Stummvoll GH, DiPaolo RJ, Glass DD, Shevach EM. Cutting edge: antigen-specific TGF beta-induced regulatory T cells suppress Th17-mediated autoimmune disease. J Immunol 2008; 181: 8209–8213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cejas PJ, Walsh MC, Pearce EL, Han D, Harms GM, Artis D et al. TRAF6 inhibits Th17 differentiation and TGF-beta-mediated suppression of IL-2. Blood 2010; 115: 4750–4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu L, Wang J, Zhang F, Chai Y, Brand D, Wang X et al. Role of SMAD and non-SMAD signals in the development of Th17 and regulatory T cells. J Immunol 2010; 184: 4295–4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muranski P, Restifo NP. Adoptive immunotherapy of cancer using CD4( + ) T cells. Curr Opin Immunol 2009; 21: 200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muraoka RS, Dumont N, Ritter CA, Dugger TC, Brantley DM, Chen J et al. Blockade of TGF-beta inhibits mammary tumor cell viability, migration, and metastases. J Clin Invest 2002; 109: 1551–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruffini PA, Rivoltini L, Silvani A, Boiardi A, Parmiani G. Factors, including transforming growth factor beta, released in the glioblastoma residual cavity, impair activity of adherent lymphokine-activated killer cells. Cancer Immunol Immunother 1993; 36: 409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kobie JJ, Wu RS, Kurt RA, Lou S, Adelman MK, Whitesell LJ et al. Transforming growth factor beta inhibits the antigen-presenting functions and antitumor activity of dendritic cell vaccines. Cancer Res 2003; 63: 1860–1864. [PubMed] [Google Scholar]
- 32.Rowland-Goldsmith MA, Maruyama H, Matsuda K, Idezawa T, Ralli M, Ralli S et al. Soluble type II transforming growth factor-beta receptor attenuates expression of metastasis-associated genes and suppresses pancreatic cancer cell metastasis. Mol Cancer Ther 2002; 1: 161–167. [PubMed] [Google Scholar]
- 33.Suzuki E, Kapoor V, Cheung HK, Ling LE, DeLong PA, Kaiser LR et al. Soluble type II transforming growth factor-beta receptor inhibits established murine malignant mesothelioma tumor growth by augmenting host antitumor immunity. Clin Cancer Res 2004; 10: 5907–5918. [DOI] [PubMed] [Google Scholar]
- 34.Bandyopadhyay A, Lopez-Casillas F, Malik SN, Montiel JL, Mendoza V, Yang J et al. Antitumor activity of a recombinant soluble betaglycan in human breast cancer xenograft. Cancer Res 2002; 62: 4690–4695. [PubMed] [Google Scholar]
- 35.Bandyopadhyay A, Wang L, Lopez-Casillas F, Mendoza V, Yeh IT, Sun L. Systemic administration of a soluble betaglycan suppresses tumor growth, angiogenesis, and matrix metalloproteinase-9 expression in a human xenograft model of prostate cancer. Prostate 2005; 63: 81–90. [DOI] [PubMed] [Google Scholar]
- 36.Ostroukhova M, Seguin-Devaux C, Oriss TB, Dixon-McCarthy B, Yang L, Ameredes BT et al. Tolerance induced by inhaled antigen involves CD4( þ ) T cells expressing membrane-bound TGF-beta and FOXP3. J Clin Invest 2004; 114: 28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J Immunol 2009; 182: 240–249. [DOI] [PubMed] [Google Scholar]
- 38.Hughes MS, Yu YY, Dudley ME, Zheng Z, Robbins PF, Li Y et al. Transfer of a TCR gene derived from a patient with a marked antitumor response conveys highly active T-cell effector functions. Hum Gene Ther 2005; 16: 457–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wargo JA, Robbins PF, Li Y, Zhao Y, El-Gamil M, Caragacianu D et al. Recognition of NY-ESO-1 þ tumor cells by engineered lymphocytes is enhanced by improved vector design and epigenetic modulation of tumor antigen expression. Cancer Immunol Immunother 2009; 58: 383–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kerkar SP, Muranski P, Kaiser A, Boni A, Sanchez-Perez L, Yu Z et al. Tumor-specific CD8 + T cells expressing interleukin-12 eradicate established cancers in lymphodepleted hosts. Cancer Res 2010; 70: 6725–6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.