Abstract
In this paper, we report on the fabrication of micron-sized dendrimer hydrogels (μDHs) using the water-in-oil (w/o) inverse microemulsion method coupled with the highly efficient aza-Michael addition. EDA core polyamidoamine (PAMAM) dendrimer G5 (10 w%) and polyethylene glycol diacrylate (PEG-DA, Mn = 575 g/mol) (the molar ratio of amine/acrylate = 1/1) were dissolved in the water phase and added to hexane in the presence of surfactants span 80/tween 80 (5/1, w/w) (volume ratio of hexane to surfactants: 70:1) to form w/o microemulsions, in which PAMAM G5 cross-links with PEG-DA via the aza-Michael addition reaction. The resulting microgels are within 3–5 μm with relatively narrow size distribution. μDHs are pH-responsive degradable. They show good cytocompatibility and do not cause acute toxicity in vivo. Furthermore, they can realize a high loading of the hydrophobic drug CPT and enter the cells in the form of particles. The CPT and CPT/dendrimer complex can be slowly released following the zero-order release kinetics. Taken together, μDHs possessing hierarchically ordered dendrimers in micron domains represent a new class of microparticles with expanded structural features for programmable drug delivery and release.
Keywords: dendrimer, microgel, inverse microemulsion, aza-Michael addition, camptothecin, degradation, fluorescent dendritic micro-hydrogel
TOC Graphic
INTRODUCTION
Polyamidoamine (PAMAM) dendrimers are best-known for their well-defined hyperbranched structures and have been intensively studied to construct nanoparticulate delivery systems.1–4 We pioneered an unconventional concept of using PAMAM dendrimers to form a cross-linked network with polyethylene glycol (PEG), i.e., dendrimer hydrogel (DH). DHs possess unique spatial structure and configuration with tunable physiochemical properties and high flexibility in delivering drugs of different types for therapeutic applications.5–9 We made the first generation of DHs using photoinitiated polymerization. We found photoinitiator residue and UV-induced free radicals during the gel formation affect the AKT signaling molecule and its phosphorylated form, a biomarker indicative of proliferation and stress of cells, raising safety issue for long-term application.10 Therefore, we sought new methods to make safer formulations. We successfully made two new types of DHs with the strain-promoted azide-alkyne cycloaddition (SPAAC) bioorthogonal chemistry (commonly known as copper-free click chemistry)8 and the aza-Michael addition (aMA) reaction.9, 11 Although both reactions proceed efficiently in water at room temperature without the use of a catalyst, the SPAAC approach requires coupling of a strained alkyne, e.g., DBCO, to the dendrimer before the click reaction. In contrast, the aMA method directly utilizes the existing functional groups (amines of dendrimers to react with acrylate groups in PEG-DA) to form a hydrogel. We can control gel solidification (from minutes to hours) and degradation kinetics by varying reactant concentration or converting dendrimer surface amines to non-reactive acetyl (Ac) groups.11 The aMa is environmentally friendly (green), efficient, and pre-functionalization of dendrimers is not needed for non-acetylated dendrimer-based formulation. Furthermore, dendrimer cryogels prepared using the aMa method shows superelasticity.12
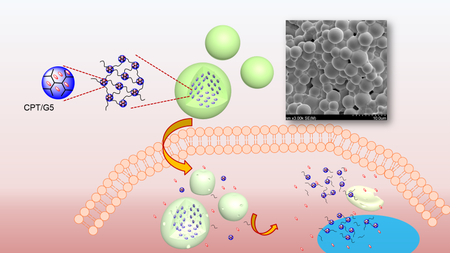
Microgels are particles with a micrometer-scale three-dimensional network.13–15 Microgels not only inherit the properties of macroscopic hydrogels including highly hydrated and tunable mechanical and chemical properties16–21 but also exhibit particle features, possessing greater structural flexibility for drug delivery.22–29 Microgels can be fabricated using various methods such as microemulsion, microfluidics, and nano-precipitation.23, 30–46 In this work, we prepared micron-sized dendrimer hydrogels (μDHs), i.e., dendrimer microgels, using the inverse microemulsion aza-Michael addition (IMaMA) method. Both PAMAM dendrimer and polyethylene glycol diacrylate (PEG-DA) are dissolved in water and which then forms micro-droplets in a continuous organic phase in the presence of surfactants. The microdroplets quickly gelate to form gel particles as a result of cross-linking of dendrimer and PEG via the highly efficient aza-Michael addition reaction. We investigated μDHs’ degradation behaviors at various pHs and cytocompatibility. We found that μDHs degrade via pH-dependent aminolysis. We also prepared μDHs loaded with the anticancer drug camptothecin (CPT) by solubilizing CPT into PAMAM dendrimer in the water phase before the IMaMA procedure. We studied the kinetics of CPT release from μDHs and found that CPT release kinetics was zero-order, the highly desirable sustained drug release profile. In addition, we found μDHs facilitated CPT cellular uptake and potentiated CPT toxicity. μDHs represent an advanced platform for programmable drug delivery and release.
MATERIALS AND METHODS
Materials.
EDA-core PAMAM dendrimer generation 5 (G5) was purchased from Dendritech (Midland, MI, USA). PEG-DA (Mn = 575 g/mol), span 80, tween 80, and cell proliferation reagent WST-1 were purchased from Sigma-Aldrich (St. Louis, MO). Camptothecin (CPT) was obtained from AK Scientific (Union City, CA). Hexane, sodium phosphate dibasic anhydrous (Na2HPO4), sodium phosphate monobasic anhydrous (NaH2PO4), potassium phosphate monobasic (KHPO4), and phosphate buffered saline (PBS, 10×) were purchased from Fisher Scientific (Pittsburgh, PA). SnakeSkin dialysis tubings (3500 and 50 000 MWCO) were obtained from Thermo Scientific (Rockford, IL). Dulbecco’s modified Eagle’s medium (DMEM), trypsin-EDTA (0.25%), and penicillin−streptomycin (10,000 U/mL) were purchased from Life Technologies (Carlsbad, CA).
Preparation of CPT-encapsulating G5 solution.
CPT in excess amounts (20 mg) was added to 1 mL water containing 100 mg G5. The mixture was vortexed briefly, sonicated for 1 h, and then left in the dark overnight. The saturated CPT/G5 solution was obtained by removing undissolved CPT via centrifugation (10 000 rpm, 3 min). CPT concentration in the CPT/G5 solution was determined at 365 nm by using an Evolution 201 UV-Visible spectrophotometer.
Preparation of μDHs.
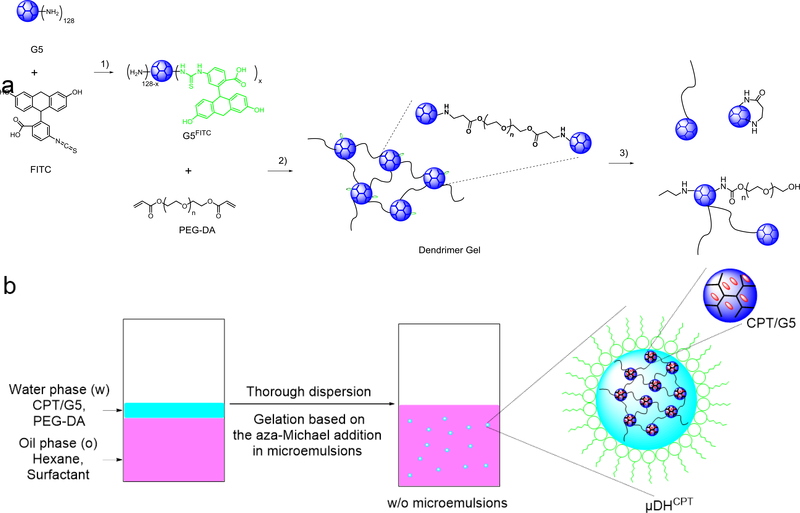
μDHs were prepared by using the inverse microemulsion method. Hexane was selected as the continuous oil phase, and a mixture of span 80/tween 80 (5/1, w/w) was used as the surfactant. The oil phase was composed of hexane and surfactant (hexane/surfactants=70:1 by volume). The aqueous solution was prepared by mixing 20 mg of G5 and 25.5 mg of PEG-DA (the molar ratio of amine/acrylate = 1/1) in 180 μL of deionized water followed by thorough vortexing for 10 s. Then the aqueous solution was immediately added to 7 mL of the oil phase and stirred at 30 000 rpm for 1 min by using an IKA disperser. The formed microemulsions were stirred at 500 rpm for 2 h, during which G5 and PEG-DA reacted to form μDHs within the stabilized emulsions. The emulsion solution was then centrifuged at 3000 rpm for 3 min, and the upper surfactant and hexane layer was discarded. The remainder was washed with deionized water three times to obtain surfactant-free μDHs. Following the same method, CPT loaded μDHs (μDHCPT) and FITC-labeled μDHs (μDHFITC) were also prepared using CPT-encapsulating G5 (CPT/G5) and FITC-labeled G5 (Scheme 1a), respectively. Fourier transform infrared spectroscopy (FTIR) spectra of PAMAM G5, PEG-DA, and μDHs were recorded on Nicolet iS5 (ThermoFisher Scientific) equipped with iD7 ATR.
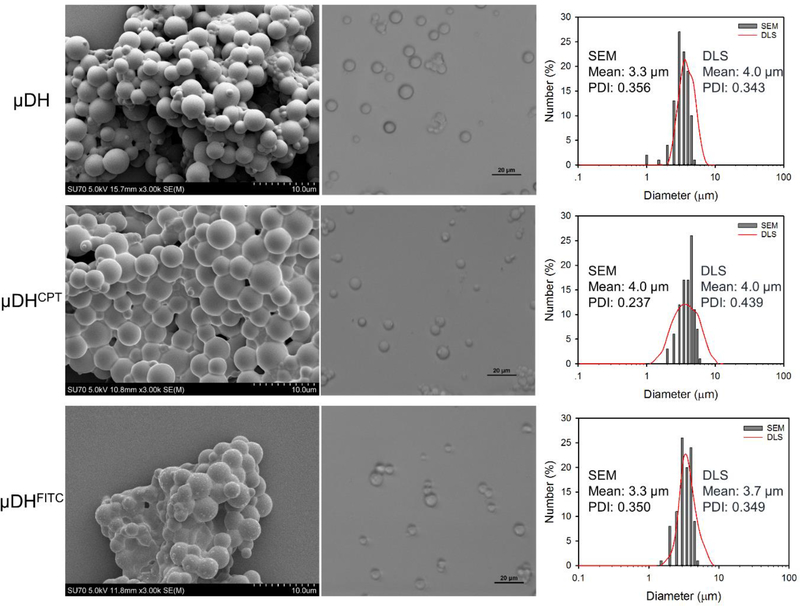
Figure 1.
SEM image (left panel), optical micrographs of gel particles in suspension (middle panel), and particle size and size distribution (right panel) of μDH, μDHCPT, and μDHFITC.
Morphology, ζ Potential, size and size distribution.
SEM images of μDH particles were taken under a scanning electron microscope (Hitachi FE-SEM Su-70, Japan). The μDH suspension in water was dropwise added onto a silicon wafer. The samples were air dried and sputter-coated with a thin layer of Pt-Au for 90 s to make the sample conductive before testing. Size distributions based on SEM results were analyzed using ImageJ2 software. The analysis was performed on a minimum of 100 particles. Morphology of μDHs in suspension was recorded on an Eclipse Ti-U inverted microscope (Nikon Instruments Inc., Melville, NY). ζ Potential, hydrodynamic size and size distribution of μDHs in suspensions (1 mg/mL) were determined by using a Malvern Zetasizer Nano ZS90 apparatus (Malvern Instruments, Worcestershire, U.K.).
Drug loading and entrapment testing.
To estimate CPT loading content in μDHCPT, pre-weighed μDHCPT particles were suspended in PBS until gel particles completely degraded. CPT in the solution was quantified based on its absorbance at 365 nm using UV-Vis spectrometry on Evolution 201 UV-Visible spectrophotometer (Thermo Scientific). The drug loading efficiency was also determined based on the percentage of CPT loaded into μDH particles.
μDH degradation studies.
Pre-weighed μDH particles (w0) were suspended in pH 5.3 or pH 7.4 PBS and incubated at 37 °C for different lengths of time up to 72 h. Each incubation solution was centrifuged at 1×104 rpm, and the supernatant was discarded. The residues were washed with DI water twice, freeze-dried, and weighed (wt). Mass remaining (%) is determined as wt/w0×100. The particles collected at different time points were imaged using SEM. The completely degraded μDH was analyzed by a Waters high performance liquid chromatography (HPLC) system equipped with a Waters 1515 isocratic HPLC pump, a Waters 2487 dual λ absorbance detector, and a Waters 717 plus autosampler. The mobile phase was the mixture of acetonitrile and water (acetonitrile/water =1/1 by volume). The UV detector monitored the eluents at 220 nm.
Drug release studies.
μDHCPT particles with known quantities were suspended in pH 5.3 or pH 7.4 PBS and loaded into a dialysis tube (MWCO 3500). The dialysis tube was immersed in 30 mL of PBS and incubated at 37 °C. At pre-determined time intervals, 1 mL of PBS outside of the dialysis tube was withdrawn for quantification of the released free CPT using UV-Vis spectrometry. One mL of fresh PBS was added to maintain a constant volume and sink condition. A control group of CPT/PBS was tested under the same condition. The release of total CPT including those in free form or complexed with G5 was studied using a dialysis tube of MWCO 50,000. All experiments were performed in triplicate.
Cytotoxicity assessment.
HN12 head and neck squamous cell carcinomas were seeded in a 96-well plate at a density of 1×104 cells/well. Following overnight culture in DMEM supplemented with 10% serum in 95% air/5% CO2 at 37 °C, the culture medium in each well was replaced with 200 μL of medium containing various amounts of μDHs (final concentrations 200, 500, and 1000 μg/mL) or μDHCPT (final concentrations 20, 100, and 200 μg/mL). After another 48 h of culture, cell viability was determined by using the WST-1 assay.
Cellular uptake studies.
HN12 cells were seeded in a 12-well plate at a density of 7.5 × 104 cells/well. After 24 h of cell attachment, the culture medium in each well was replaced with 2 mL of medium containing 25 μg/mL μDHFITC. Following incubation for 6, 24, and 48 h respectively, the cells were fixed with 4% paraformaldehyde for 30 min at room temperature, followed by permeation with 0.1% Triton X-100 for 10 min. Nuclei were counterstained with DAPI. The cells were imaged under an Eclipse Ti-U inverted microscope (Nikon Instruments Inc., Melville, NY).
In vivo safety assessment.
C57BL/6 mice were randomly divided into four groups (n=3) and injected intravenously with 50 μl of PBS solution containing different concentrations of μDH particles (0.1, 1, and 5 mg/mL). At 24 h post-administration, blood was collected from mice through cardiac puncture after euthanization. A blood sample taken from the untreated mice was included as a control. Inflammatory cytokines (IL-1β and TNF-α) in the blood were measured using BD OptEIA kits (BD Biosciences, San Jose, CA) following the manufacturer’s protocol.
RESULTS AND DISCUSSION
Characterization of dendrimer microgels.
PAMAM dendrimers and their analog hyperbranched polymers are ideal building units to prepare hydrogels because of the highly-functionalized surface and well-defined highly branched architecture. We explored the aza-Michael addition reaction (Scheme 1a) to make in-situ forming PAMAM dendrimer hydrogels with tunable properties and applications. Inverse microemulsions are water microdroplets suspended in a continuous organic phase (w/o) and stabilized with oil-soluble surfactants.47, 48 We combined the inverse microemulsion method with the highly efficient aza-Michael addition reaction to make dendrimer microgels (μDHs). As shown in Scheme 1b, PAMAM dendrimer G5 and PEG-DA were dissolved in the water phase, and hexane was chosen as the continuous oil phase and mixed with span 80/tween 80. Once the two phases were mixed, a strong dispersing force was applied to generate water-in-oil (w/o) microemulsions. The micro-sized water droplets in this micro-emulsion served as microreactors, in which the aza-Michael addition-based cross-linking between G5 and PEG-DA occurred and led to the formation of microgel particles. The disappearance of acrylate signals in FTIR spectra (Figure S1) confirms that aza-Michael addition-based cross-linking took place between G5 and PEG-DA.
Scheme 1.
a) Synthetic routes for 1) FITC labeling, 2) cross-linking reaction, and 3) the self-triggered aminolysis of the μDH degradation process. b) Preparation of dendrimer microgels using the inverse microemulsion aza-Michael addition. CPT can be encapsulated into dendrimer in the water phase and form CPT-loaded dendrimer microgels.
As shown in Figure 1, the dendrimer microgels appear as spherical microparticles with mean diameters of 3.3 ± 0.7 μm, 4.0 ± 0.9 μm, and 3.3 ± 0.7 μm for μDH, μDHCPT, and μDHFITC, respectively, as determined by SEM images. The aggregation of μDH particles shown in the SEM images is due to the volatilization of the solvent during sample preparation. As shown in Figure 1, the μDHs in suspension present excellent uniform dispersion. μDHs in suspensions are slightly larger than those dehydrated particles. The mean hydrodynamic diameters of μDH, μDHCPT, and μDHFITC are 4.0 ± 0.9 μm, 4.0 ± 1.0 μm, and 3.7 ± 0.8 μm, respectively.
pH-Dependent degradation of μDHs.
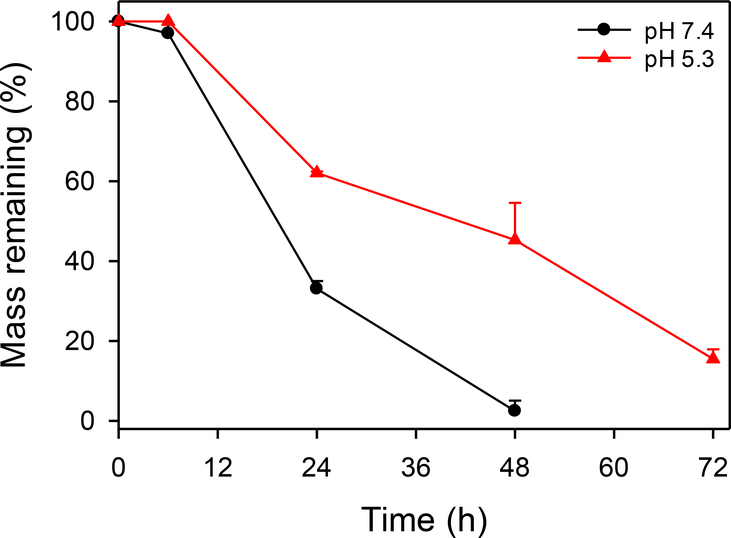
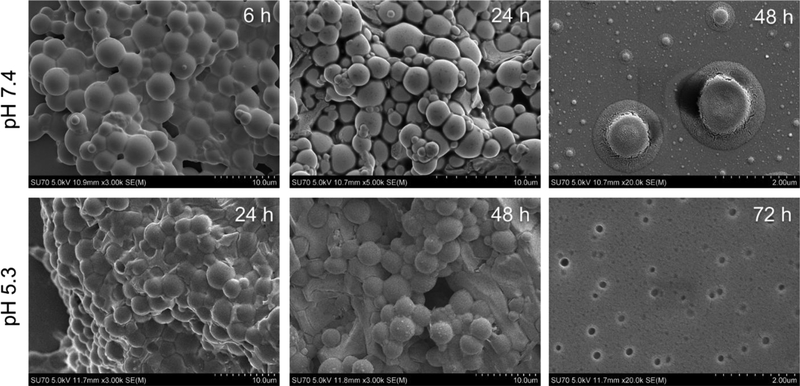
The positively charged surface of μDH was confirmed by its ζ potential value 24.1 ± 4.9 mV. It also indicates that even after cross-linking, there are still unreacted amino groups remaining. In addition, the FTIR spectrum of μDH shows the presence of unreacted amino groups (Figure S1). The unreacted amino groups in the cross-linked network structure of μDHs can self-trigger aminolysis of the ester bonds in the network (Scheme 1a). The aminolysis is highly dependent on pH, which, in turn, controls degradation kinetics of μDHs. μDHs degrade more slowly at pH5.3 than at pH7.4 (Figure 2). Based on remaining mass tracking, microgels are quite stable within 6 hours at pH 5.3, and no weight loss is observed. Even at pH 7.4, the weight loss within the first 6 hours is minimal (3%). After 24 h incubation, its 33% mass remains at pH 7.4 while its 62% mass remains at pH 5.3. μDHs degrade almost completely in 48 h at pH 7.4. In contrast, μDHs still keep 15% of the mass at pH 5.3 after 72 h incubation. The complete degradation product of the μDHs showed two peaks (1.05 min, 1.52 min) by HPLC analysis. By comparison with the spectra of raw materials (PEG-DA and G5), we believe that the peak with a 1.05 minute is G5 or G5-PEG, while the peak at 1.52 min might be extremely low cross-linked dendrimer-PEG (Figure S2).
Figure 2.
Degradation of μDH at pH 7.4 and pH 5.3.
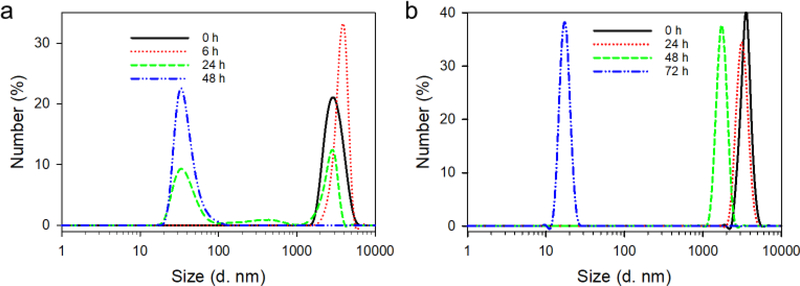
Particle size reduces along with the mass loss. At pH 7.4, particle size experiences an increase from 2.8 μm at 0 h to 3.8 μm at 6 h because of swelling. At 24 h, multiple sizes are seen in the degradation product. Approximately half of the particles remain micron-sized, and the half become nanosized (20–70 nm) (Figure 3a). After 48 h, only nano-sized particles can be detected. The size reduction process at pH 5.3 is slower than that at pH 7.4 (Figure 3b), which agrees well with the mass loss. There is almost no change in both size and size distribution after 24 h incubation at pH 5.3, indicating the stability with 24 h. At 48 h, size reduces to 1.7 μm while still being micro-sized. At 72 h, only nano-sized particles are detected, indicating the complete degradation of μDHs.
Figure 3.
Size change of μDH during incubation at pH 7.4 (a) and pH 5.3 (b).
We also imaged μDH degradation products throughout the degradation process (Figure 4, Figure S3). μDHs after 6 h-incubation at pH 7.4 do not show any apparent changes compared to their initial state. Micron-sized particles and nano-sized particles co-exist at 24 h. Among them, almost all the micro-sized particles soften and collapse. At 48 h, most particles are smaller than 100 nm and only a very small number of particles remain micro-sized, which appear as broken flat discs. In contrast, μDHs after 24 h- and 48 h-incubation at pH 5.3 do not show noticeable size reduction but only show slight interparticle coalition. At 72 h, nearly all the microparticles disappear, and only nano-sized fragments remain. The DLS, SEM, and microscopy results complementarily illustrated pH-dependent degradation of μDHs.
Figure 4.
SEM images of μDH after incubation for different lengths of time at pH 7.4 and pH 5.3.
Zero-order drug release enabled by μDHs.
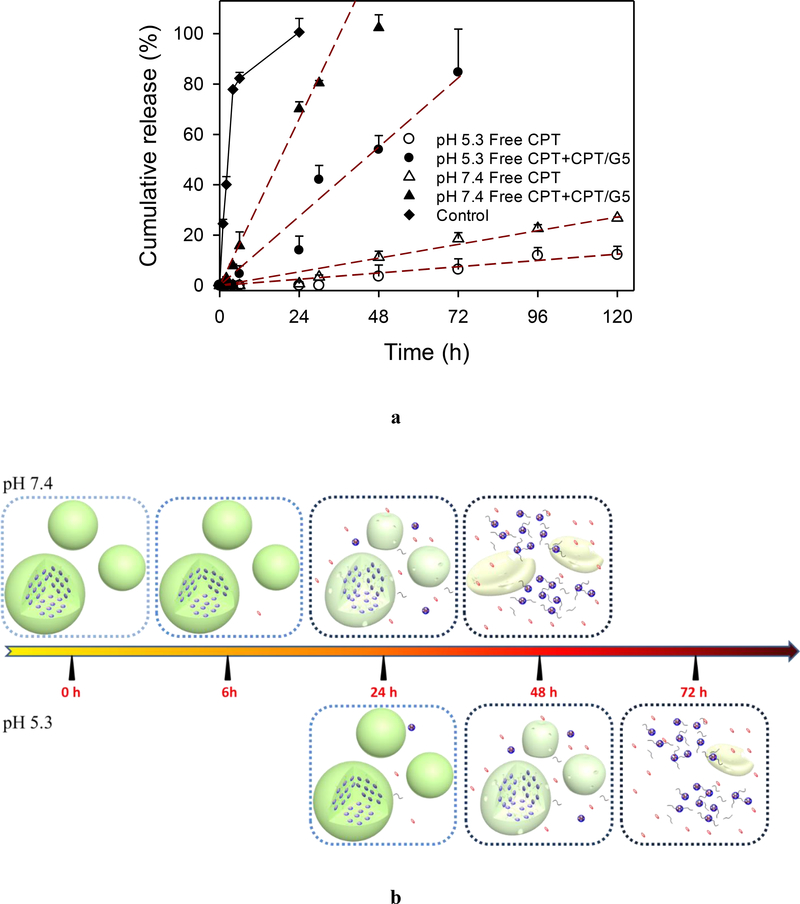
The limited solubility of CPT in aqueous medium has significantly restricted its bioavailability. Based on our measurement, the saturated solubility of CPT in pH 7.4 PBS is approximately 64 μg/mL. Herein, we prepared CPT/G5 complexes by entrapping the hydrophobic CPT molecules in the interior structure of PAMAM dendrimer G5. The results show that the saturated solubility of CPT increased significantly to 12 mg/mL in 10 wt% G5 PBS solution, suggesting approximately 10 CPT molecules are entrapped in each PAMAM G5 molecule. We used the CPT/G5 saturation solution to prepare μDHCPT particles. CPT loading capacity was determined to be 225 ± 12 μg CPT/mg μDHCPT particles, and CPT encapsulation efficiency was as high as 85 ± 5 %.
CPT release rate is also pH-dependent. CPT release is slower at pH 5.3 than at pH 7.4. Four mathematical models including the zero-order model, first-order model, Higuchi square root of time model, and Hixson and Crowell cube-root model were chosen to fit the release data.49, 50 The release of total CPT in free drug form and complexed with G5 follows zero-order release kinetics (Figure 5a, Table S2). Majority of the release CPT is found to be associated with G5. At 120 h, only 27% and 12% of free CPT was released at pH 7.4 and pH 5.3, respectively. The release of CPT/G5 is accompanied by the degradation of μDH. In the process of μDH degradation, the original solid particles gradually soften, collapse, shrink until disappear, and the building blocks, CPT/G5, is slowly released correspondingly (Figure 5b). The degradation rates at pH 7.4 and pH5.3 affect the release rate (Figure 5b). The release of free CPT is based on the diffusion of CPT from CPT/G5 complexes, which is a slow process controlled by the hydrophobic interaction between CPT (Figure 5a, b). Even though the release of free CPT is slow, we demonstrate that the CPT/G5 complexes have effective tumor cell inhibition (Figure S5b).
Figure 5.
(a) In vitro zero-order drug release of free CPT and CPT/G5 complexes from μDHCPT at pH 7.4 and pH 5.3 at 37 °C (n = 3), the control group is free CPT released from CPT/PBS at pH 7.4. (b) CPT release from μDHCPT includes burst release of free CPT and release of CPT/G5 complexes driven by pH-dependent μDH degradation.
Potentiation of anticancer drug potency by μDHs.
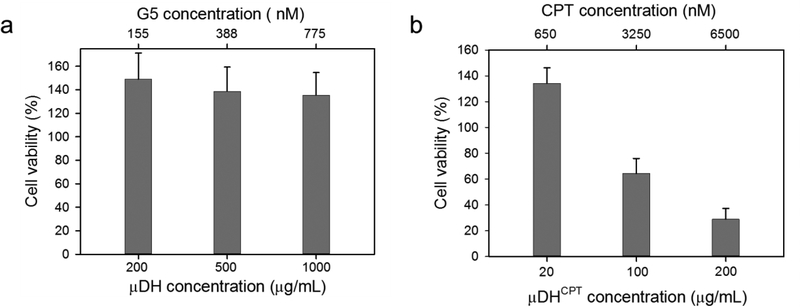
μDHs show good cytocompatibility over a broad range of concentrations. They do not cause any cytotoxicity at the concentration as high as 1000 μg/mL (Figure 6a). Instead, they promoted cell proliferation as seen in PEG and PEGylated dendrimers.51 G5 is more tolerated by cells in μDHs than in free solution form (Figure S5a), in large part due to the decreased number of amine groups by cross-linking with PEG. We further studied the in vivo safety of μDHs. μDHs of different doses were administered to mice through tail vein injection. Following injection, the mice did not show any apparent signs of discomfort or pain. At 24 h, we analyzed the plasma level of cytokines (IL-1β and TNF-α) in the treated mice. As shown in Table 1, the levels of IL-1β and TNF-α in the treated mice are comparable to those in the untreated group, indicating μDHs do not cause acute toxicity. We further tested the cytotoxicity of μDHCPT on HN12 cells (Figure 6b). The selected three doses of μDHCPT are within the safety range for both G5 (Figure S5) and μDHs. μDHCPT at 20 μg/mL does not cause cell death, but it causes 36% cell death at 100 μg/mL (corresponding to 3250 nM CPT). μDHCPT shows the highest cell-killing effect at 200 μg/mL (6500 nM CPT), which causes 71% cell death.
Figure 6.
Cytotoxicity of μDH and μDH CPT (n = 6).
Table 1.
Cytokines (IL-1β and TNF-α) in plasma at 24 h post-administration of μDH particles.
| Cytokine (ng/mL) | Untreated | μDH particles
(mg/mL) |
||
|---|---|---|---|---|
| 0.1 | 1 | 5 | ||
| IL-1β | 18.34 ± 1.39 | 17.71 ± 4.48 | 18.41 ± 5.90 | 20.51 ± 2.94 |
| TNF-α | 11.06 ± 0.69 | 16.32 ± 3.04 | 14.70 ± 0.70 | 15.63 ± 7.43 |
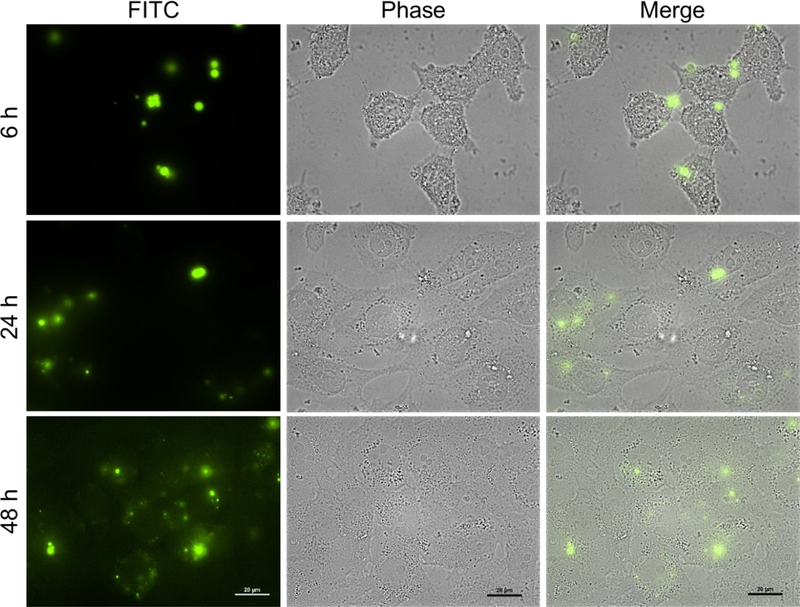
We studied the cellular uptake of μDHFITC particles (Figure 7). At 6 h, fluorescent dots are seen inside the cells. Given that μDHs do not degrade in the first 6 h of incubation, the presence of the fluorescence signals suggests that μDHs can enter the cells in the form of particles despite their relatively large size. More extended incubation with μDHFITC (24 h or 48 h) leads to accumulation of stronger fluoresce signals in the cells. This is because μDHFITC degradation products in the medium continue to be taken up by the cells. In the meantime, the internalized μDHFITC degrade into nano-sized fragments within the cells as evidenced by a relatively more uniform distribution of fluorescence signals.
Figure 7.
Cellular uptake and intracellular distribution of fluorescent μDHFITC in HN12 cells. Scale bar: 20 μm.
CONCLUSIONS
We successfully fabricated micron-sized dendrimer hydrogels (μDHs) using the water-in-oil inverse microemulsion method coupled with the highly efficient aza-Michael addition. μDHs are pH-responsive degradable. They show good cytocompatibility and do not cause acute toxicity in vivo. Furthermore, they can realize a high loading of the hydrophobic drug CPT and enter the cells in the form of particles. The CPT and CPT/dendrimer complex can be slowly released following the zero-order release kinetics. Taken together, μDHs possessing hierarchically ordered dendrimers in micron domains represent a new class of microparticles enabling programmable drug delivery and release.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported, in part, by the National Institutes of Health (R01EY024072).
Footnotes
Notes
The authors declare no competing interest.
ASSOCIATED CONTENTS
Supporting Information
REFERENCES
- 1.Yang H; Lopina ST Penicillin V-conjugated PEG-PAMAM star polymers. J Biomater Sci Polym Ed 2003, 14 (10), 1043–56. [DOI] [PubMed] [Google Scholar]
- 2.Yang H; Morris JJ; Lopina ST Polyethylene glycol-polyamidoamine dendritic micelle as solubility enhancer and the effect of the length of polyethylene glycol arms on the solubility of pyrene in water. J. Colloid Interface Sci 2004, 273 (1), 148–154. [DOI] [PubMed] [Google Scholar]
- 3.Yang H; Kao JW Synthesis and characterization of nanoscale dendritic RGD clusters for potential applications in tissue engineering and drug delivery. Int J Nanomedicine 2007, 2 (1), 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarkar K; Yang H Encapsulation and extended release of anti-cancer anastrozole by stealth nanoparticles. Drug Deliv 2008, 15 (5), 343–6. [DOI] [PubMed] [Google Scholar]
- 5.Yang H; Tyagi P; Kadam RS; Holden CA; Kompella UB Hybrid Dendrimer Hydrogel/PLGA Nanoparticle Platform Sustains Drug Delivery for One Week and Antiglaucoma Effects for Four Days Following One-Time Topical Administration. ACS Nano 2012, 6 (9), 7595–606 DOI: 10.1021/nn301873v. [DOI] [PubMed] [Google Scholar]
- 6.Holden CA; Tyagi P; Thakur A; Kadam R; Jadhav G; Kompella UB; Yang H Polyamidoamine dendrimer hydrogel for enhanced delivery of antiglaucoma drugs. Nanomedicine 2012, 8 (5), 776–83 DOI: S1549–9634(11)00355–8 [pii] 10.1016/j.nano.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 7.Yang H; Leffler CT Hybrid Dendrimer Hydrogel/Poly(Lactic-Co-Glycolic Acid) Nanoparticle Platform: An Advanced Vehicle for Topical Delivery of Antiglaucoma Drugs and a Likely Solution to Improving Compliance and Adherence in Glaucoma Management. Journal of Ocular Pharmacology and Therapeutics 2013, 29 (2), 166–172 DOI: 10.1089/jop.2012.0197. [DOI] [PubMed] [Google Scholar]
- 8.Xu L; Cooper RC; Wang J; Yeudall WA; Yang H Synthesis and Application of Injectable Bioorthogonal Dendrimer Hydrogels for Local Drug Delivery. ACS Biomater Sci Eng 2017, 3 (8), 1641–1653 DOI: 10.1021/acsbiomaterials.7b00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J; Williamson G; Lancina M; Yang H Mildly Cross-Linked Dendrimer Hydrogel Prepared via Aza-Michael Addition Reaction for Topical Brimonidine Delivery. Journal of Biomedical Nanotechnology 2017, 13 (9), 1089–1096 DOI: 10.1166/jbn.2017.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu L; Sheybani N; Yeudall WA; Yang H The effect of photoinitiators on intracellular AKT signaling pathway in tissue engineering application. Biomaterials Science 2015, 3 (2), 250–255 DOI: 10.1039/c4bm00245h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J; He H; Cooper RC; Yang H In Situ-Forming Polyamidoamine Dendrimer Hydrogels with Tunable Properties Prepared via Aza-Michael Addition Reaction. ACS Appl Mater Interfaces 2017, 9 (12), 10494–10503 DOI: 10.1021/acsami.7b00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J; Yang H Superelastic and pH-Responsive Degradable Dendrimer Cryogels Prepared by Cryo-aza-Michael Addition Reaction. Sci Rep 2018, 8 (1), 7155 DOI: 10.1038/s41598-018-25456-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh J; Drumright R; Siegwart D; Matyjaszewski K The development of microgels/nanogels for drug delivery applications. Progress in Polymer Science 2008, 33 (4), 448–477 DOI: 10.1016/j.progpolymsci.2008.01.002. [DOI] [Google Scholar]
- 14.Bonham JA; Faers MA; van Duijneveldt JS Non-aqueous microgel particles: synthesis, properties and applications. Soft Matter 2014, 10 (47), 9384–9398 DOI: 10.1039/c4sm01834f. [DOI] [PubMed] [Google Scholar]
- 15.Graham NB; Cameron A Nanogels and microgels: The new polymeric materials playground. Pure and Applied Chemistry 1998, 70 (6), 1271–1275 DOI: 10.1351/pac199870061271. [DOI] [Google Scholar]
- 16.Aguirre G; Khoukh A; Chougrani K; Alard V; Billon L Dual-responsive biocompatible microgels as high loaded cargo: understanding of encapsulation/release driving forces by NMR NOESY. Polymer Chemistry 2018, DOI: 10.1039/C7PY02111A. [DOI] [Google Scholar]
- 17.Asoh T-A; Kinoshita H; Shoji T; Kikuchi A; Tsuboi Y Rapid hydrogel repair utilizing microgel architectures. Materials Chemistry Frontiers 2017, 1 (8), 1594–1599 DOI: 10.1039/C6QM00370B. [DOI] [Google Scholar]
- 18.Di Lorenzo F; Seiffert S Macro- and Microrheology of Heterogeneous Microgel Packings. Macromolecules 2013, 46 (5), 1962–1972 DOI: 10.1021/ma302255x. [DOI] [Google Scholar]
- 19.Di Lorenzo F; Seiffert S Counter-effect of Brownian and elastic forces on the liquid-to-solid transition of microgel suspensions. Soft Matter 2015, 11 (26), 5235–5245 DOI: 10.1039/C5SM00881F. [DOI] [PubMed] [Google Scholar]
- 20.Hoare T; Pelton R Functionalized Microgel Swelling: Comparing Theory and Experiment. The Journal of Physical Chemistry B 2007, 111 (41), 11895–11906 DOI: 10.1021/jp072360f. [DOI] [PubMed] [Google Scholar]
- 21.Liu R; Milani AH; Freemont TJ; Saunders BR Doubly crosslinked pH-responsive microgels prepared by particle inter-penetration: swelling and mechanical properties. Soft Matter 2011, 7 (10), 4696–4704 DOI: 10.1039/C1SM05216K. [DOI] [Google Scholar]
- 22.Jans A; Rosencrantz RR; Mandic AD; Anwar N; Boesveld S; Trautwein C; Moeller M; Sellge G; Elling L; Kuehne AJC Glycan-Functionalized Microgels for Scavenging and Specific Binding of Lectins. Biomacromolecules 2017, 18 (5), 1460–1465 DOI: 10.1021/acs.biomac.6b01754. [DOI] [PubMed] [Google Scholar]
- 23.Rossow T; Heyman JA; Ehrlicher AJ; Langhoff A; Weitz DA; Haag R; Seiffert S Controlled Synthesis of Cell-Laden Microgels by Radical-Free Gelation in Droplet Microfluidics. Journal of the American Chemical Society 2012, 134 (10), 4983–4989 DOI: 10.1021/ja300460p. [DOI] [PubMed] [Google Scholar]
- 24.Shen J; Ye T; Chang A; Wu W; Zhou S A colloidal supra-structure of responsive microgels as a potential cell scaffold. Soft Matter 2012, 8 (48), 12034–12042 DOI: 10.1039/C2SM26885J. [DOI] [Google Scholar]
- 25.Clarke KC; Lyon LA Microgel Surface Modification with Self-Assembling Peptides. Macromolecules 2016, 49 (15), 5366–5373 DOI: 10.1021/acs.macromol.6b01497. [DOI] [Google Scholar]
- 26.Guermani E; Shaki H; Mohanty S; Mehrali M; Arpanaei A; Gaharwar AK; Dolatshahi-Pirouz A Engineering complex tissue-like microgel arrays for evaluating stem cell differentiation. Scientific reports 2016, 6, DOI: 10.1038/srep30445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill MJ; Sarkar D Polyurethane Microgel Based Microtissue: Interface-Guided Assembly and Spreading. Langmuir : the ACS journal of surfaces and colloids 2017, 33 (24), 6167–6181 DOI: 10.1021/acs.langmuir.7b01493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li YT; Meng H; Liu Y; Narkar A; Lee BP Gelatin Microgel Incorporated Poly(ethylene glycol)-Based Bioadhesive with Enhanced Adhesive Property and Bioactivity. ACS applied materials & interfaces 2016, 8 (19), 11980–11989 DOI: 10.1021/acsami.6b01364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia Y; He X; Cao M; Chen C; Xu H; Pan F; Lu JR Thermoresponsive Microgel Films for Harvesting Cells and Cell Sheets. Biomacromolecules 2013, 14 (10), 3615–3625 DOI: 10.1021/bm4009765. [DOI] [PubMed] [Google Scholar]
- 30.Guerzoni LPB; Bohl J; Jans A; Rose JC; Koehler J; Kuehne AJC; De Laporte L Microfluidic fabrication of polyethylene glycol microgel capsules with tailored properties for the delivery of biomolecules. Biomaterials Science 2017, 5 (8), 1549–1557 DOI: 10.1039/C7BM00322F. [DOI] [PubMed] [Google Scholar]
- 31.Saxena S; Hansen CE; Lyon LA Microgel Mechanics in Biomaterial Design. Accounts of Chemical Research 2014, 47 (8), 2426–2434 DOI: 10.1021/ar500131v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seiffert S; Thiele J; Abate AR; Weitz DA Smart Microgel Capsules from Macromolecular Precursors. Journal of the American Chemical Society 2010, 132 (18), 6606–6609 DOI: 10.1021/ja102156h. [DOI] [PubMed] [Google Scholar]
- 33.Yan C; Yang T; Zhu S; Wu H Synthesis and properties of poly(DEX-GMA/AAc) microgel particle as a hemostatic agent. Journal of Materials Chemistry B 2017, 5 (20), 3697–3705 DOI: 10.1039/C7TB00768J. [DOI] [PubMed] [Google Scholar]
- 34.Gulzar A; Gai S; Yang P; Li C; Ansari MB; Lin J Stimuli responsive drug delivery application of polymer and silica in biomedicine. Journal of Materials Chemistry B 2015, 3 (44), 8599–8622 DOI: 10.1039/C5TB00757G. [DOI] [PubMed] [Google Scholar]
- 35.Islam N; Ferro V Recent advances in chitosan-based nanoparticulate pulmonary drug delivery. Nanoscale 2016, 8 (30), 14341–14358 DOI: 10.1039/C6NR03256G. [DOI] [PubMed] [Google Scholar]
- 36.Jijie R; Barras A; Boukherroub R; Szunerits S Nanomaterials for transdermal drug delivery: beyond the state of the art of liposomal structures. Journal of Materials Chemistry B 2017, 5 (44), 8653–8675 DOI: 10.1039/C7TB02529G. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y; Guo Y; Wu S; Liang H; Xu H Photodegradable Coordination Polymer Particles for Light-Controlled Cargo Release. ACS Omega 2017, 2 (6), 2536–2543 DOI: 10.1021/acsomega.7b00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saunders JM; Tong T; Le Maitre CL; Freemont TJ; Saunders BR A study of pH-responsive microgel dispersions: from fluid-to-gel transitions to mechanical property restoration for load-bearing tissue. Soft Matter 2007, 3 (4), 486–494 DOI: 10.1039/B613943D. [DOI] [PubMed] [Google Scholar]
- 39.Steinhilber D; Witting M; Zhang XJ; Staegemann M; Paulus F; Friess W; Kuchler S; Haag R Surfactant free preparation of biodegradable dendritic polyglycerol nanogels by inverse nanoprecipitation for encapsulation and release of pharmaceutical biomacromolecules. Journal of Controlled Release 2013, 169 (3), 289–295 DOI: 10.1016/j.jconrel.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 40.Tang Z; Guan Y; Zhang Y Contraction-type glucose-sensitive microgel functionalized with a 2-substituted phenylboronic acid ligand. Polymer Chemistry 2014, 5 (5), 1782–1790 DOI: 10.1039/C3PY01190A. [DOI] [Google Scholar]
- 41.Tang Z; Guan Y; Zhang Y The synthesis of a contraction-type glucose-sensitive microgel working at physiological temperature guided by a new glucose-sensing mechanism. Polymer Chemistry 2018, DOI: 10.1039/C8PY00072G. [DOI] [Google Scholar]
- 42.Zhang XJ; Malhotra S; Molina M; Haag R Micro- and nanogels with labile crosslinks - from synthesis to biomedical applications. Chemical Society Reviews 2015, 44 (7), 1948–1973 DOI: 10.1039/c4cs00341a. [DOI] [PubMed] [Google Scholar]
- 43.Li YY; Yang J; Wu WB; Zhang XZ; Zhuo RX Degradable Nanogels as a Nanoreactor for Growing Silica Colloids. Langmuir : the ACS journal of surfaces and colloids 2009, 25 (4), 1923–1926 DOI: 10.1021/la803902r. [DOI] [PubMed] [Google Scholar]
- 44.Månsson R; Frenning G; Malmsten M Factors Affecting Enzymatic Degradation of Microgel-Bound Peptides. Biomacromolecules 2013, 14 (7), 2317–2325 DOI: 10.1021/bm400431f. [DOI] [PubMed] [Google Scholar]
- 45.Schimka S; Lomadze N; Rabe M; Kopyshev A; Lehmann M; von Klitzing R; Rumyantsev AM; Kramarenko EY; Santer S Photosensitive microgels containing azobenzene surfactants of different charges. Physical Chemistry Chemical Physics 2017, 19 (1), 108–117 DOI: 10.1039/C6CP04555C. [DOI] [PubMed] [Google Scholar]
- 46.Zheng X; Qian J; Tang F; Wang Z; Cao C; Zhong K Microgel-Based Thermosensitive MRI Contrast Agent. ACS Macro Letters 2015, 4 (4), 431–435 DOI: 10.1021/acsmacrolett.5b00058. [DOI] [PubMed] [Google Scholar]
- 47.Su H; Jia Q; Shan S Synthesis and characterization of Schiff base contained dextran microgels in water-in-oil inverse microemulsion. Carbohydrate polymers 2016, 152, 156–162 DOI: https://doi.org/10.1016/j.carbpol.2016.06.091. [DOI] [PubMed] [Google Scholar]
- 48.Zhang J; Yang F; Shen H; Wu D Controlled Formation of Microgels/Nanogels from a Disulfide-Linked Core/Shell Hyperbranched Polymer. ACS Macro Letters 2012, 1 (11), 1295–1299 DOI: 10.1021/mz300489n. [DOI] [PubMed] [Google Scholar]
- 49.Dredán J; Antal I; Rácz I Evaluation of mathematical models describing drug release from lipophilic matrices. International journal of pharmaceutics 1996, 145 (1), 61–64 DOI: https://doi.org/10.1016/S0378-5173(96)04725-4. [Google Scholar]
- 50.Karasulu E; Yeşim Karasulu H; Ertan G; Kirilmaz L; Güneri T Extended release lipophilic indomethacin microspheres: formulation factors and mathematical equations fitted drug release rates. European Journal of Pharmaceutical Sciences 2003, 19 (2), 99–104 DOI: 10.1016/S0928-0987(03)00048-4. [DOI] [PubMed] [Google Scholar]
- 51.Yang H; Lopina ST; DiPersio LP; Schmidt SP Stealth dendrimers for drug delivery: correlation between PEGylation, cytocompatibility, and drug payload. J. Mater. Sci.: Mater. Med 2008, 19 (5), 1991–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.