Abstract
Purpose
To present the case of a patient with a hypertensive choroidopathy and her follow-up using multimodal imaging; and to assess how wide-field swept-source (SS) Optical Coherence Tomography Angiography (OCTA) contributes to detecting the areas of hypoperfusion.
Observations
A 25-year-old white woman with terminal renal insufficiency, myopericarditis, and cerebrospinal fluid pressure of 37 mmHg indicating intracranial hypertension, presented with a painless loss of vision in both eyes. Her blood pressure was 190/135 mmHg. A thorough diagnosis work-up failed to reveal the etiology. The fundus examination showed arterial narrowing and moderate venous dilation in both eyes. Deep yellow spots were found bilaterally, associated with slight pigment epithelium detachments and exudative retinal detachments. Multimodal imaging showed characteristic features of choroidal involvement in hypertension. Wide-field 12 × 12 mm PlexElite map montage at the choriocapillaris slab identified areas of non-perfusion of the choriocapillaris. These areas mostly correlate with late indocyanine green angiography (ICGA)-presumed choroidal ischemia. During the follow-up, the patient's blood pressure normalized and the choriocapillaris flow improved.
Conclusions and importance
In this case of malignant hypertensive retinopathy with exudative retinal detachment of the posterior pole, SS-OCTA showed multiple and widespread flow voids on the choriocapillaris slabs, corresponding to the areas of hypofluorecence on ICGA, demonstrating an associated hypertensive choroidopathy. It would appear that SS-OCTA used alone is capable to show choroidal vascularization impairment in cases of hypertensive retinopathy.
Keywords: Hypertensive choroidopathy, Multimodal imaging, Swept-source optical coherence tomography angiography
1. Introduction
Malignant high blood pressure can lead to severe multiple organ damage,1 and can affect the eyes in several ways. Patients with malignant high blood pressure may develop retinopathy, optic neuropathy and choroidopathy.1 While hypertensive retinopathy is the most frequently identified manifestation in the eyes, choroidopathy is far less common.2 The latter is characterized as a widespread of hypofluorescent zones observed using Indocyanine Green-Angiography (ICGA), which are presumed to indirectly show damage to the choriocapillaris.
Recently, optical coherence tomography angiography (OCTA) has allowed for the non-invasive assessment of retinal and choroidal microvasculature. However, choriocapillaris flow is harder to analyze due to the masking effect of the retinal pigment epithelium (RPE). The advantage of swept-source (SS) devices, which use longer wavelengths, is that they better penetrate the retinal and choroidal layers and show the choroidal tissue more accurately. SS-OCTA has already been used to show and confirm choriocapillaris damage in several diseases (Acute Posterior Multifocal Placoid Pigment Epitheliopathy, Central Serous Chorioretinopathy etc.).3,4
We report here the findings of wide-field SS-OCTA in hypertensive choroidopathy showing large flow signal voids at the choriocapillaris layer, which match the hypofluorescent areas on the ICGA.
2. Case report
A 25-year-old white woman with terminal renal insufficiency, myopericarditis and cerebrospinal fluid pressure of 37 mmHg indicating intracranial hypertension presented with a painless loss of vision in both eyes. Her blood pressure was 190/135 mmHg. A thorough diagnosis work-up failed to reveal the etiology of the hypertension and the renal failure.
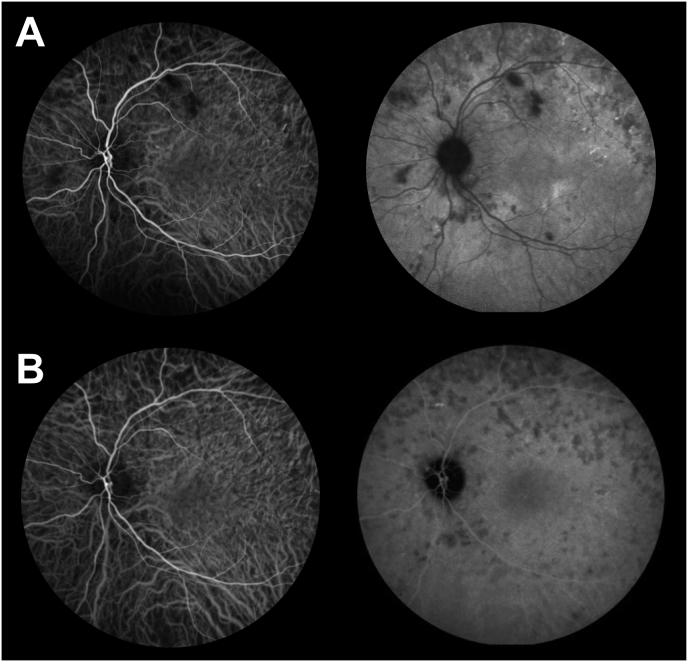
Corrected visual acuity (VA) was 0.7 in the right eye (RE) and 0.4 in the left eye (LE). The ocular motility exam was normal. Furthermore, no relative afferent pupillary defects were identified. Intraocular pressure and the anterior chamber slit lamp examination were unremarkable for both eyes. The fundus examination revealed arterial narrowing and moderate venous dilation in both eyes. Deep yellow spots were found bilaterally, associated with exudative retinal detachments. Multimodal imaging showed characteristic features of choroidal involvement in hypertension. Fundus autofluorescence of both eyes revealed areas of hypofluorescence due to retinal hemorrhages, cotton-wool spots and retinal pigment epithelium (RPE) alterations, and areas of hyperautofluorescence corresponding to deep retinal yellow spots which highlight acute ischemic damage to the RPE. Fundus Fluorescein Angiography (FA) showed areas of hypofluorescence in the early phase which become hyperfluorescent in the late phase (Fig. 1). Spectral Domain (SD) OCT B-scan through the fovea demonstrated exudative retinal detachment involving the fovea. The enhance depth imaging (EDI) revealed choroidal thickness of 347 μm in the RE, and 387 μm in the LE. The SD-OCT B-scan also showed minor pigment epithelial detachments (Fig. 2). The initial ICGA montage showed choroidal ischemia. The ischemic hypofluorescent areas were predominantly found in the superior part of the fundus and remained hypofluorescent throughout the ICGA exam. The choriocapillaris slab on the SS-OCTA showed flow signal voids mostly in the superior part of the eye. These areas correlate with late ICGA choroidal non-perfusion, but seem to be larger on SS-OCTA (Fig. 3).
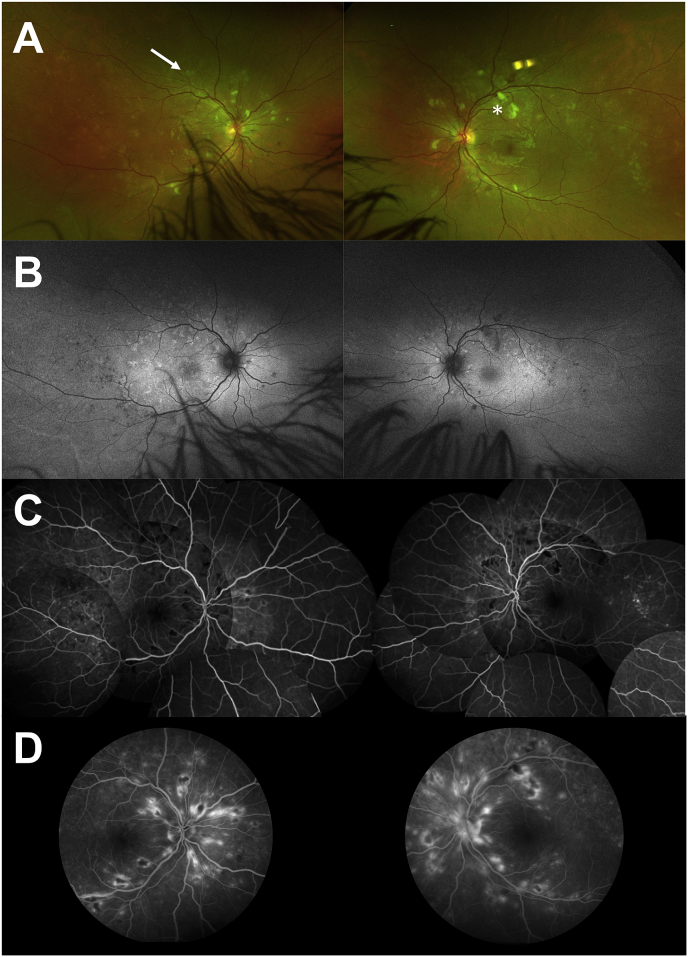
Fig. 1.
Multimodal imaging at baseline. A Ultra-wide field retinophotography of both eyes showing cotton-wool spots (asterix), hemorrhages and deep yellow spots (arrow).B Fundus autofluorescence of both eyes revealing areas of hypo-autofluorescence due to retinal hemorrhages and cotton-wool spots, and areas of hyperautofluorescence corresponding to deep retinal yellow spots. In the temporal areas of both eyes, there are hypo-autofluorescent dots that are due y to the RPE scars already present.C Early phase of Fluorescein Angiography (FA) montage demonstrating hypofluorescent areas. D Late phase of FA showing hyperfluorescent spots. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
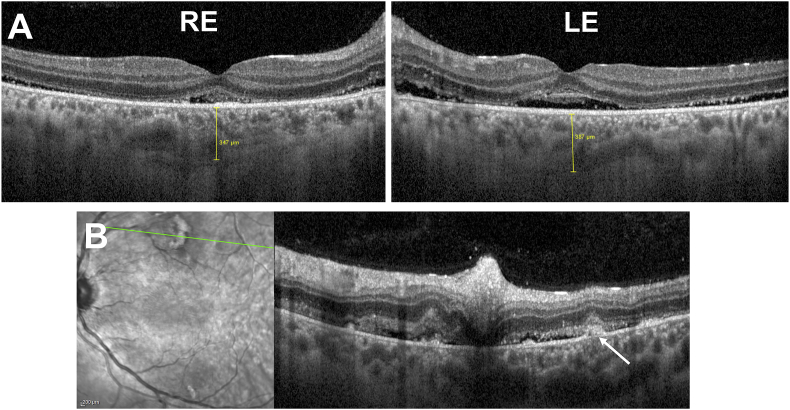
Fig. 2.
Horizontal Spectral Domain optical coherence tomography (SD-OCT) B-scan of both eyes. A Horizontal SD-OCT B-scan through the fovea demonstrating shallow exudative retinal detachment involving the fovea. Retrofoveal choroidal thickness was 347 μm and 387μm in the right eye (RE) and left eye (LE), respectively.B Horizontal SD-OCT B-scan of the left eye corresponding to the green line on the infrared imaging showing small pigment epithelial detachments (arrow), choroidal thickening, subretinal fluid, and cotton-wool spots in the inner retina. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
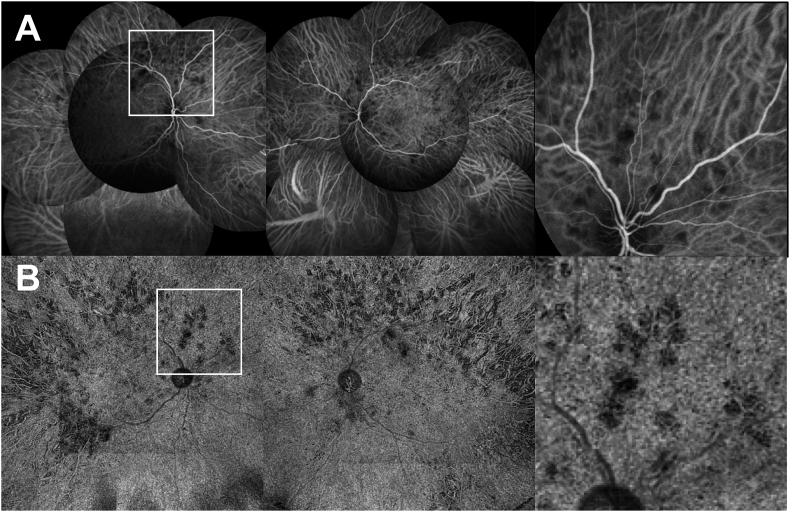
Fig. 3.
Initial indocyanine green angiography (ICGA) and swept source Optical Coherence Tomography-Angiography (SS-OCTA) montage. A Initial ICGA showing hypofluorescent areas preferentially located within the posterior pole.B Widefield 12X12 montage SS-OCTA showing flow signal voids in the choriocapillaris slab in both eyes. Inset: close-up view showing the matching between ICGA and SS-OCTA.
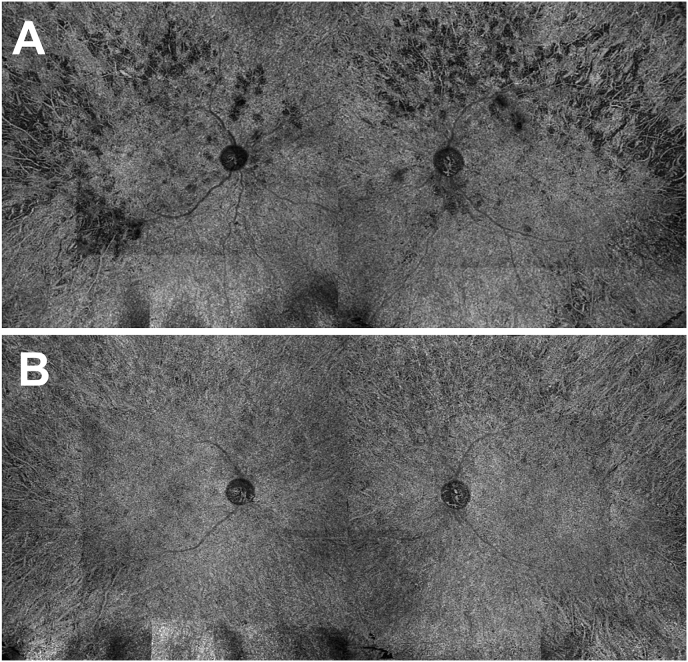
One month later, blood pressure was well controlled. VA had increased to 1.0 RE and 1.0 LE. There were less deep yellow spots and less hyperautofluorescent areas on the fundus autofluorescence. The exudative retinal detachment had resolved. Although some minor attenuations of the ellipsoid zone (EZ) signal were present on the nasal edge of the macula in both eyes, EZ in the macular area remained normal. Choroidal thickness had decreased in both eyes, which suggests initial choroidal thickening. Multimodal imaging showed the improvement in fundus damage (Fig. 4). ICGA seems normal in the early phase and showed hypofluorescence areas in the late phase. Moreover, ICGA also showed a decrease in choroidal hyperpermeability and in non-perfusion areas (Fig. 5). The choriocapillaris slab on the SS-OCTA showed improvement in choroidal circulation in both eyes with resolution of flow voids (Fig. 6).
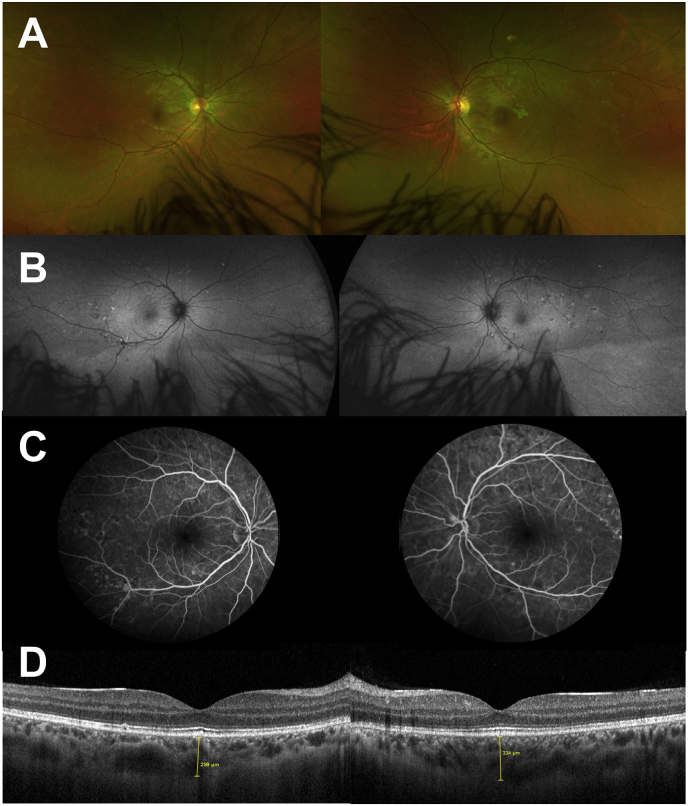
Fig. 4.
Multimodal Imaging at one month. A Ultra-wide field retinophotography of both eyes showing regression of cotton-wool spots, disappearance of hemorrhages and reduction, but persistence of deep yellow spots.B Fundus autofluorescence of both eyes showing a decrease in the number of hypofluorescent areas. C Fundus Fluorescein Angiography showing a reduction in the hyperfluorescent areas. D Horizontal Spectral Domain Optical coherence tomography B-scan through the fovea of both eyes showing a regression of the exudative retinal detachment and a decrease in the retrofoveolar choroidal thickness in both eyes. (299μm and 339μm in the RE and LE, respectively). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 5.
Early and late phases of Indocyanine green angiography (ICGA) at baseline and one-month visit. A Initial ICGA showing hypofluorescence spots in both the early and late phases. In this case, the hypofluorescent pattern is explained by the choriocapillaris non-perfusion. B ICGA at one-month follow-up. The hypofluorescent spots appeared only in the late phase, demonstrating the alteration of the retinal pigment epithelium.
Fig. 6.
Swept source Optical Coherence Tomography-Angiography (SS-OCTA) montage at baseline and one-month visit. A Montage of 12 × 12mm SS-OCTA in the choriocapillaris slab of both eyes, showing flow voids. B Montage of 12 × 12mm SS-OCTA in the choriocapillaris slab of both eyes, showing the significant reduction in the number and surface area of flow voids.
3. Discussion
Malignant hypertension, the most severe form of hypertension, is characterized by high blood pressure with a diastolic pressure over 130 mmHg, associated with bilateral retinal hemorrhages and/or exudates, with or without papilledema.5 The prevalence of malignant hypertension is low in the general population. Over five decades of observation around 1–2 new cases per 100,000 individuals per year have been recorded in a large cohort in Europe.6 Prognosis is very poor if the disease is not treated. Malignant hypertension still constitutes a clinically relevant and challenging form of hypertension and it should be always considered when assessing patients with poorly-controlled hypertension.5 SS-OCTA provides a unique means of non-invasively studying the vasculature of retinal and choroidal lesions in vivo. The choroid is dependent on the vegetative nervous system. In malignant hypertension, the system's capacities are exceeded. This results in fibrinoid necrosis of the arterioles, and non-perfusion of the choriocapillaris due to the contraction of the flexible vessels.7 This requires contractile and flexible vessels which explains why younger patients are more likely to develop this pathology.8
Choriocapillaris non-perfusion alters the RPE and can weaken its pump function which causes retinal serous detachment and then outer retinal alterations.9,10 Ischemic choriocapillaris produces an early and a late hypofluorescence signal on ICGA,11 as found on the initial examination of our patient. However, at the one-month-visit, there was no hypofluorescence in the early phase of ICGA, whereas the late phase did show hypofluorescent areas. These ICGA features suggest areas of RPE alteration, as these cells normally uptake the ICG molecules throughout the exam. This would explain why hypofluorescence was only found in the late phase in these areas.12
This case illustrates that the acute phase of the disease is characterized by patchy multifocal choroidal ischemia leading to acute RPE alterations. The healing phase is characterized by the restoration of choriocapillaris perfusion. However, RPE scars may persist despite this restoration of choriocapillaris perfusion. The OCTA features showing initial flow signal voids in the choriocapillaris slab which were no longer present at the follow-up visit, confirm this hypothesis.
SS-OCT instruments use a wavelength tunable swept laser, currently with a central wavelength of between 1040 nm and 1060 nm, with a super-luminescent diode (SLD) of 750 nm. Although SD-OCT is capable of showing the choriocapillaris and choroid with modern noise reduction strategies, SS-OCTA has a faster scanning speed than SD-OCTA, which allows for denser scan patterns and larger scan areas. SS-OCT technology has a longer wavelength and reduced sensitivity roll-off. Light penetration through the RPE is thereby enhanced, thus the detection of signals from the deeper layers is better. The higher power combined with the reduced sensitivity roll-off improves the likelihood of detecting the inherently weaker signals from deeper layers.13 The SS-OCT system can overcome the RPE barrier, resulting in improved observation of the choroid and the choroid vasculature, including the choriocapillaris slab.
OCTA at the choriocapillaris slab identifies the areas of non-perfusion of the choriocapillaris. Unlike ICGA, OCTA provides an isolated choriocapillaris slab analysis. These areas of non-perfusion are represented by no-flow zones (flow signal voids) which means that the decorrelation signal is no longer detected. Throughout follow-up we observed a reduction in the number and areas of choriocapillaris flow voids, along with the normalization of the patient's blood pressure. In our case RPE scars were observed at the one-month visit.
OCTA findings can help clinicians non-invasively diagnose and determine the extent of the impact of malignant hypertension on the body's microcirculation.
In malignant hypertensive retinopathy with exudative retinal detachment of the posterior pole, SS-OCTA was able to show, on choriocapillaris slabs, multiple and widespread flow voids corresponding to the area of choroidal non-perfusion seen on ICGA, demonstrating an associated hypertensive choroidopathy. It would appear that SS-OCTA used alone is able to show the choroidal vascularization impairment in cases of hypertensive retinopathy.
Further studies using SS-OCTA are needed to determine the precise quantitative characteristics of hypertensive choroidopathy and the correlation between choroidal and choriocapillary alterations and other microcirculation disorders that can be life threatening.
4. Conclusion
Wide-field SS-OCTA allows the non-invasive visualization and follow-up of areas of choriocapillaris non-perfusion. Various features in the retina, such as vessel caliber and density, flow density, microvascular changes and vascular fractal dimensions, should be investigated in further studies in order to evaluate their correlation with the systemic health of the cardiovascular system.
Patient Consent to publication
Consent to publish the case report was not obtained. This report does not contain any personal information that could lead to identification of the patient.
Conflicts of interest
The following authors have no financial disclosures: AR, TM, AA, PD, LK.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgements
This case was presented to the “Société Française de Rétine” on January 20th, 2018.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajoc.2019.01.001.
Funding
No funding or grant support.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ugarte M., Horgan S., Rassam S., Leong T., Kon C.H. Hypertensive choroidopathy: recognizing clinically significant end-organ damage. Acta Ophthalmol. 2008;86(2):227–228. doi: 10.1111/j.1600-0420.2007.01030.x. [DOI] [PubMed] [Google Scholar]
- 2.Schubert H.D. Ocular manifestations of systemic hypertension. Curr Opin Ophthalmol. 1998;9(6):69–72. doi: 10.1097/00055735-199812000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Heiferman M.J., Rahmani S., Jampol L.M. Acute posterior multifocal placoid pigment epitheluipathy on optical coherence tomography angiography. Retina. 2017;37(11):2084–2094. doi: 10.1097/IAE.0000000000001487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicolò M., Rosa R., Musetti D., Musolino M., Saccheggiani M., Traverso C.E. Choroidal vascular flow area in central serous Chorioretinopathy using swept-source optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2017;58(4):2002–2010. doi: 10.1167/iovs.17-21417. [DOI] [PubMed] [Google Scholar]
- 5.Shantsila A., Lip G.Y.H. Malignant hypertension revisited-does this still exist? Am J Hypertens. 2017;30(6):543–549. doi: 10.1093/ajh/hpx008. [DOI] [PubMed] [Google Scholar]
- 6.van den Born B.-J.H., Koopmans R.P., Groeneveld J.O., van Montfrans G.A. Ethnic disparities in the incidence, presentation and complications of malignant hypertension. J Hypertens. 2006;24(11):2299–2304. doi: 10.1097/01.hjh.0000249710.21146.38. [DOI] [PubMed] [Google Scholar]
- 7.Kishi S., Tso M.O.M., Hayreh S.S. Fundus lesions in malignant hypertension: I. A pathologic study of experimental hypertensive choroidopathy. Arch Ophthalmol. 1985;103(8):1189–1197. doi: 10.1001/archopht.1985.01050080101029. [DOI] [PubMed] [Google Scholar]
- 8.Tso M.O.M., Jampol L.M. Pathophysiology of hypertensive retinopathy. Ophthalmology. 1982;89(10):1132–1145. doi: 10.1016/s0161-6420(82)34663-1. [DOI] [PubMed] [Google Scholar]
- 9.Stern W.H., Ernest J.T. Microsphere occlusion of the choriocapillaris in rhesus monkeys. Am J Ophthalmol. 1974;78(3):438–448. doi: 10.1016/0002-9394(74)90231-1. [DOI] [PubMed] [Google Scholar]
- 10.Gaudric A., Sterkers M., Coscas G. Retinal detachment after choroidal ischemia. Am J Ophthalmol. 1987;104(4):364–372. doi: 10.1016/0002-9394(87)90226-1. [DOI] [PubMed] [Google Scholar]
- 11.Nemiroff J., Phasukkijwatana N., Vaclavik V., Nagiel A., Holz E.R., Sarraf D. The spectrum of Almaric triangular choroidal infarction. Retin Cases Brief Rep. 2017;11(Suppl 1):S113–S120. doi: 10.1097/ICB.0000000000000442. [DOI] [PubMed] [Google Scholar]
- 12.Chang A.A., Morse L.S., Handa J.T. Histologic localization of indocyanine green dye in aging primate and human ocular tissues with clinical angiographic correlation. Ophthalmology. 1998;105(6):1060–1068. doi: 10.1016/S0161-6420(98)96008-0. [DOI] [PubMed] [Google Scholar]
- 13.Miller A.R., Roisman L., Zhang Q. Comparison between spectral-domain and swept-source optical coherence tomography angiographic imaging of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2017;58(3):1499–1505. doi: 10.1167/iovs.16-20969. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.