Abstract
Liver cancer is one of the most common malignant tumors and prognosis remains poor. It has been increasingly recognized that liver cancer stem cells (LCSCs) are responsible for the carcinogenesis, recurrence, metastasis and chemoresistance of hepatocellular carcinoma (HCC). Targeting LCSCs is promising to be a new direction for the treatment of HCC. Herein, we summarize the potentially therapeutic targets in LCSCs at the level of genes, molecules and cells, such as knockout of oncogenes or oncoproteins, restoring the silent tumor suppressor genes, inhibition of the transcription factors and regulation of noncoding RNAs (including microRNAs and long noncoding RNAs) in LCSCs at the genetic level; inhibition of markers and blockade of the key signaling pathways of LCSCs at the molecular level; and inhibiting autophagy and application of oncolytic adenoviruses in LCSCs at the cellular level. Moreover, we analyze the potential targets in LCSCs to eliminate chemoresistance of HCC. Thereinto, the suppression of autophagy and Nanog by chloroquine and shRNA respectively may be the most promising targeting approaches. These targets may provide novel therapeutic strategies for the treatment of HCC by targeting LCSCs.
Keywords: drug resistance, HCC treatment, hepatocellular carcinoma, liver cancer stem cells, targeting LCSCs
Introduction
Liver cancer is one of the most common human malignancies in the world, it takes the second place of mortality, only next to lung cancer. Liver cancer includes several subtypes, hepatocellular carcinoma (HCC) is by far the most common worldwide, accounting for 78%, other subtypes such as bile-duct cancer (15%), hepatoblastoma and various liver sarcomas and carcinomas (7%).1 The main risk factors for HCC are definite, including hepatitis B or C virus infection, alcohol abuse, intake of the fungal metabolite aflatoxin B1 and an emerging cause that named nonalcoholic fatty liver disease or nonalcoholic steatohepatitis (NASH).2 In the past few decades, cancer stem cells (CSCs) have similar characteristics to normal stem cells, such as self-renewal and pluripotent activity, which have been found to be associated with the major malignant phenotypes of cancer, including recurrence, metastasis and chemoresistance.3 Liver cancer stem cells (LCSCs) which are identified by certain surface markers, are also known as hepatic cancer stem cells or liver tumor-initiating cells (T-ICs). Recently, the malignant behaviors of LCSCs have been increasingly recognized. They are identified to be responsible for the initiation, relapse, metastasis and chemoresistance of HCC.3–6 Thus, targeting LCSCs may be a new strategy for the treatment of HCC. This paper reviews the potentially therapeutic targets against LCSCs at the level of genes, molecules and cells, including targets of drug resistance.
Targeting LCSCs at the level of genes
Targeting LCSCs at the genetic level includes knockout of oncogenes or oncoproteins, and restoration of the silent tumor suppressor genes (Table 1).
Table 1.
Targets at the level of genes.
| Targets | Effect* | Mechanism | Species | References |
|---|---|---|---|---|
| Oncogenes or oncoproteins | ||||
| BC047440 | + | BC047440 increased the activation of NF-κB signaling. | Mouse | You and colleagues7 |
| 14-3-3ζ | + | Knockout of 14-3-3ζ improved IR-induced apoptosis by upregulating the expression of pro-apoptotic proteins. | Human | Lee and colleagues8 |
| HSP90 | + | Inhibition of HSP90 induced LCSC apoptosis by downregulating the HSP90 effector proteins. | Mouse | Yang and colleagues9 |
| ANXA3 | + | ANXA3 overexpression activated JNK pathway, leading to oncogenic events. | Human | Pan and colleagues10; Tong and colleagues11 |
| Hepatitis B virus PreS 1 | + | PreS1 upregulated CSCs-related genes including Klf4, Nanog, Sox2, Oct4, and c-Myc. | Human | Liu and colleagues12 |
| SIRT1 | + | SIRT1 regulated the transcriptional activity of Sox2, and was maintained by MEK1 signaling. | Human | Liu and colleagues13; Cheng and colleagues14 |
| ADAM17 | + | ADAM17 was a key point to activate Notch signaling pathway, silencing ADAM17 reduced the activity of Notch signaling pathway. | Human | Hong and colleagues15 |
| Tumor suppressor genes | ||||
| Shp2 and Pten | – | Shp2 and Pten worked together to promoted tumorigenesis of LCSCs by the upregulation of the proto-oncogene c-jun. | Human | Luo and colleagues16 |
| C8orf4 | – | C8orf4 impaired the self-renewal of LCSCs through inhibition of NOTCH2 signaling. | Human | Zhu and colleagues17 |
| p53 | – | Autophagy suppressed p53 which otherwise could be activated by PINK1 to downregulate the expression of Nanog. | Human | Liu and colleagues18 |
| Numb | – | The phosphorylation of Numb destabilized p53 and promoted self-renewal of LCSCs in a Nanog-dependent manner. | Human | Siddique and colleagues19 |
+, promoting the stemness of LCSCs;–,suppressing the stemness of LCSCs.
ADAM17, a disintegrin and metalloproteinase-17; ANXA3, annexin A3; LCSC, liver cancer stem cell; PINK1, Pten-induced putative kinase 1; Pten, phosphatase and tensin homologue; Shp2, src-homology 2 domain-containing phosphatase 2; SIRT1, histone deacetylatase sirtuin 1.
Knockout or inactivation of the oncogenes or oncoproteins in LCSCs
In recent years, increasing genes are reported to have relations with the biological behaviors of LCSCs. The activation of oncogenes or overexpression of oncoproteins increase the proliferation and tumorigenicity of LCSCs. The functions of these oncogenes and oncoproteins which may be taken as targets against LCSCs are as followed.
BC047440
BC047440 (GeneBank accession number: BC047440) is a novel HCC-related gene, the full length of BC047440 cDNA was cloned from human HCC tissues, the activation of BC047440 contributed to development of HCC.20 You and colleagues7 showed that BC047440 played an important role in maintaining stemness properties of LCSCs in the nude mouse model. The oncogenic effect of BC047440 depended on the increasing activation of nuclear factor kappa B (NF-κB), which was a critical nuclear transcription factor (TF) in tumor development. On the other hand, knockout of BC047440 resulted in tumorigenicity inhibition and hepatocyte-differentiation induction of LCSCs through enhancing the expression of hepatocyte nuclear factor 4α (HNF4α), which was important to regulate hepatocyte differentiation.7 Removal of the BC047440 gene may counteract the malignant behaviors of LCSCs.
14-3-3ζ
14-3-3ζ belongs to the 14-3-3 family, which is a group of evolutionarily highly conserved acidic proteins.21The abnormal expression of 14-3-3ζ has been detected in multiple cellular pathways in cancers. Lee and colleagues8 reported that 14-3-3ζ was upregulated in LCSCs after γ-irradiation (IR), it contributed to radiation resistance and survival of LCSCs. Knockout of 14-3-3ζ reduced the stemness properties of LCSCs and improved IR-induced apoptosis by upregulating the expression of pro-apoptotic proteins. The combination of radiotherapy and 14-3-3ζ silencing may be potential strategy for HCC therapy by targeting LCSCs.
HSP90
Heat-shock protein 90 (HSP90) can induce the heat-shock response as a chaperone protein.22 It facilitates cellular proteins folding and degradation, keeps protein stability under oxidative and heat stress. It also plays a critical role in the stabilization and activity of various oncogenic proteins.22,23 Yang and colleagues9 found that HSP90 in CD90+ LCSCs was upregulated under hyperthermic condition in nude mice, the HSP90 inhibitor could sensitize CD90+LCSCs to hyperthermia and induce CD90+LCSCs apoptosis by downregulating the HSP90 effector proteins, which regulated cell survival and apoptosis. Thus, blockage of HSP90 may improve the thermotherapy sensitivity and reduce thermoresistance of LCSCs. HSP90 may become a promising target against LCSCs, especially in thermotherapy of HCC.
Annexin A3 (ANXA3)
ANXA3 is a group of Ca2+-dependent phospholipid-binding secretory proteins.24 It contributed to the propagation and self-renewal of LCSCs.10 Upregulation of ANXA3 promoted the tumorigenicity of LCSCs through aberrant regulated c-Jun NH2-terminal kinase (JNK) pathway, the JNK inhibitor could suppress the oncogenic events caused by ANXA3 overexpression.11 Thus, ANXA3 silencing and blockage of JNK pathway may suppress the malignant behaviors of LCSCs.
Hepatitis B virus PreS 1
Hepatitis B virus (HBV) infection is involved in HCC development. It is the initial step of HBV infection for the HBV envelope protein PreS1 to bind to the specific cellular surface receptor of hepatocytes.25 Liu and colleagues12 found that PreS1 acted as an oncoprotein in HCC development, it drove the expression of CSCs-related genes including Klf4, Nanog, Sox2, octamer 4 (Oct4), and c-Myc. PreS1 also upregulated CSCs-related markers, such as CD133, CD90 and CD117. Knockout of PreS1 counteracted the stemness properties of LCSCs, indicating that the suppression of Pres1 may be potential method for the therapy of HBV-related HCC through targeting LCSCs.
SIRT1
Histone deacetylatase sirtuin 1 (SIRT1) is class III histone deacetylase, taking a role in the regulation of cellular stress responses.26 SIRT1 participates in the process of carcinogenesis by regulating lipid metabolism. In HCC, Liu and colleagues13 reported that SIRT1 promoted self-renewal and tumorigenicity of LCSCs by regulating the transcriptional activity of Sox2, which was essential TF for LCSCs. Knockdown of SIRT1 impaired the stemness capacities of LCSCs. On the other hand, the stabilization of SIRT1 was maintained by MEK1 signaling which belongs to the mitogen-activated protein kinase (MAPK) pathway.14 So, both SIRT1 and MEK1 signaling may be novel potential targets against LCSCs.
ADAM17
A disintegrin and metalloproteinase-17 (ADAM17), also known as tumor necrosis factor (TNF)-α converting enzyme (TACE), is important in processing single-spanning membrane proteins.15 Many proteins processed by ADAM17 including cytokines, receptors and growth factors are involved in cancer development. In HCC, it was revealed that ADAM17 contributed to radio resistance and was responsible for invasion and metastasis of CD133+ LCSCs. The mechanism was that ADAM17 was a key point to activate Notch signaling pathway, silencing ADAM17 reduced the radio resistance of LCSCs by suppressing Notch signaling.15 So, silencing ADAM17 may become novel method to improve the efficacy of radiotherapies and reduce the metastasis of HCC.
Restoring the silent tumor suppressor genes in LCSCs
In recent years, it has been recognized that the silencing of tumor suppressor genes caused by mutations, deletions, promoter inactivation or other epigenetic changes are relevant to malignant change in normal cells. Accordingly, it may become a potential strategy for HCC therapy to restore the expression of silent tumor suppressor genes in LCSCs.
Ptpn11/shp2 and Pten
Src-homology 2 domain-containing phosphatase 2 (Shp2) is an oncogenic tyrosine phosphatase, encoded by Ptpn11.27 Shp2 mutation was detected in several types of leukemia.28 The role of Shp2 on HCC development is controversial, it was demonstrated that Shp2 played a role as tumor suppressor in HCC on one hand,29 but on the other hand it promoted HCC development,30 and enhanced the invasion of LCSCs by activating β-catenin signaling.31
Phosphatase and tensin homologue (Pten), deleted from chromosome 10, is a classical tumor suppressor which could block the activation of PI3K/Akt signaling pathway.32 Pten and Shp2 work together to exert the inhibitory effect on LCSCs. It was identified that both deficiencies of Shp2 and Pten promoted tumorigenesis in LCSCs, Pten deficiency promoted Akt over-activation and Shp2 loss induced JNK activation, resulting in the upregulation of the proto-oncogene c-jun.16 Both silencing of Shp2 and Pten were related to poor prognosis in patients with HCC. So, Shp2 and Pten may become novel potential targets against LCSCs, but further research is needed.
C8orf4
C8orf4, also named thyroid cancer 1 (TC1), was cloned from thyroid cancer.33 Overexpression of C8orf4 was reported being involved in tumorigenesis in several types of human cancers. It could promote clonogenicity of human lung cancer cells and also acts as an oncogene in breast cancer.17,34,35 But, Zhu and colleagues17 found an inhibitory effect of C8orf4 in HCC, it impaired the self-renewal of LCSCs through inhibition of Notch2 signaling. It counteracted the nuclear translocation of Notch2 intracellular domain. By contrast, C8orf4 silencing drove the activation of Notch2 signaling, then improved self-renewal properties of LCSCs. Anyhow, C8orf4 was found to act oncogenic role in several other types of cancer, it needs further studies about the role of C8orf4 gene in targeted therapy of HCC based on LCSCs.
p53
As a classical tumor suppressor gene, p53 could restrain stem cells expansion by restricting self-renewal, inhibiting symmetric division and blocking the somatic/progenitor cells reprogramming into stem cells.36,37 Liu and colleagues18 found that autophagy, a catabolic process of cells removing damaged organelles and protein aggregates, could positively regulate LCSCs by suppressing p53 which otherwise could be phosphorylated on mitochondria by Pten-induced putative kinase 1 (PINK1), a kinase associated with mitophagy, to downregulate the expression of CSC-related gene Nanog. So, both the restoration of p53 and blockade of autophagy may be promising methods to control LCSCs.
Numb
Numb is originally discovered in drosophila embryos as a cell fate determinant during sensory organ formation.38 Accumulating evidences support that Numb acts as tumor suppressor in several types of cancer by inhibiting Notch signaling and epithelial-mesenchymal transition (EMT).39 In pancreatic cancer, Numb was downregulated by Musashi2, a RNA-binding protein which was required for tumorigenesis as a translational repressor.40,41 In HCC, Siddique and colleagues19 reported that Numb could conjunct with p53 forming the Numb-p53 complex to prevent the degradation of p53, the phosphorylation of Numb destabilized p53 and promoted self-renewal of LCSCs in a Nanog-dependent manner. Nanog phosphorylated Numb by enhancing the kinase activities of both Aurora A kinase (AURKA) and atypical protein kinase C zeta (aPKCζ) through the Nanog-AURKA-aPKCζ pathway. Thus, the Nanog-Numb-p53 signaling axis is important in the self-renewal and tumorigenesis of LCSCs, it may provide novel strategies for the treatment of HCC.
Targeting LCSCs at the level of molecules
Targeting LCSCs at the molecular level includes inhibition of the TFs, regulation of noncoding RNA (including microRNA and long noncoding RNA), decrease of markers and interruption of the essential signaling pathways of LCSCs (Table 2).
Table 2.
Targets at the level of molecules.
| Targets | Effect* | Mechanism | Species | References |
|---|---|---|---|---|
| TFs | ||||
| Twist | + | Twist is associated with EMT and self-renewal of LCSCs by regulating the CSCs marker CD24. | Human | Liu and colleagues42; He and colleagues43;
Ren and colleagues44 |
| HIF-1 and ELK3 | + | ELK3 promoted the migration and invasion of LCSCs by targeting HIF-1α. | Human | Lee and colleagues45 |
| KLF8 | + | KLF8 facilitated the activation of the Wnt/β-catenin signaling pathway. | Human | Shen and colleagues46 |
| Noncoding RNAs | ||||
| MicroRNAs (miRNAs) | ||||
| MiR-122 | – | MiR-122 suppressed Wnt/β-catenin signaling pathway and inhibited glycolysis by targeting glycolytic genes PDK4. | Human | Song and colleagues47 |
| MiR-152 | – | MiR-152 directly binding to 3′ untranslated region and downregulating the expression of KIT which is a proto-oncogene. | Human | Huang and colleagues48 |
| MiR-21 | – | MiR-21 positively regulated the expression of LCSC markers CD13, EpCAM, CD90 and Oct4. | Human | Jiang and colleagues49 |
| MiR-155 | + | MiR-155 mediated TP53INP1 to regulate CSC phenotype, and enhanced the LCSC markers CD90, CD133 and Oct4. | Human | Liu and colleagues50 |
| MiR-25 | + | Knockdown of miR-25 increased apoptosis of LCSCs induced by TRAIL, via Pten/PI3K/Akt/Bad signaling pathway. | Human | Feng and colleagues51 |
| MiR-200 family | +/– | MiR-429 decreased RBBP4 expression and resulted in the activation of Oct4. But, miR-200a suppressed the EMT phenotype of LCSCs. | Human | Li and colleagues52; Wang and colleagues53 |
| MicroRNA let-7 | – | Let-7a negatively regulating EMT and Wnt signaling pathway. Let-7c targeted PBX3 and suppressed the transcriptional activity of CSCs-related genes including CACNA2D1, EpCAM, Sox2 and Notch3. | Human | Jin and colleagues54; Han and colleagues55 |
| MiR-1246 | + | MiR-1246 activated the Wnt/β-catenin pathway through inhibiting the expression of Axin2 and GSK3β. | Human | Chai and colleagues56 |
| Long noncoding RNAs (lncRNAs) | ||||
| HULC and MALAT1 | + | They cooperated to regulate the TRF2. | Human | Wu and colleagues57 |
| LncDILC | – | LncDILC inhibited the autocrine IL-6/STAT3 signaling, and mediated the crosstalk between TNF-α/NF-κB signaling and IL-6/JAK2/STAT3 cascade. | Human | Wang and colleagues58 |
| CUDR and H19 | + | Pten depletion promoted the binding of CUDR to the oncogene CyclinD1, the CUDR-cyclinD1 complex then enhanced the H19 expression. | Human | Pu and colleagues59 |
| HOTAIR | + | HOTAIR accelerated LCSC malignant proliferation through downregulating SETD2. | Human | Li and colleagues60 |
| LncTCF7 | + | LncTCF7 recruited the SWI/SNF complex to activation of Wnt signaling. | Human | Wang and colleagues61 |
| LncSox4 | + | LncSox4 recruited the TF Stat3 to the Sox4 promoter to trigger the expression of Sox4. | Human | Chen and colleagues62 |
| LncBRM | + | lncBRM associated with BRM to trigger the BRG1/BRM switch and BAF, leading to activation of the transcriptional cofactors YAP1. | Human | Zhu and colleagues63 |
| Lncβ-Catm | + | Lncβ-Catm associated with β-catenin and the methyltransferase EZH2, promoting the methylation and stability of β-catenin. | Human | Zhu and colleagues64 |
| LncCAMTA1 | + | LncCAMTA1 associated with CAMTA1 promoter to inhibit its transcription. | Human | Ding and colleagues65 |
| LCSC biomarkers | ||||
| CD133 | + | The downregulation of CD133 decreased the level of NF-κB. | Human | Liu and colleagues66 |
| ICAM-1 | + | ICAM-1 is upregulated by Nanog, promoting the stemness of LCSCs. | Human | Liu and colleagues67 |
| Signaling pathways | ||||
| Wnt/β-catenin pathway | + | The Wnt/β-catenin pathway promoted the self-renewal and unlimited cell proliferation of CSCs. | Human | Chen and colleagues68; Kim and colleagues69; Seto and colleagues70 |
| PI3K/Akt/mTOR pathway | + | HBV X protein facilitates AFP expression, which activates PI3K/Akt signal pathways. | Human | Zhu and colleagues71 |
| Akt/GSK-3β/β-catenin pathway | + | Inhibition of the protein kinaseAkt reduced the self-renewal of LCSCs. | Human | Xu and colleagues72; Zhai and colleagues73; Kim and colleagues69 |
| STAT3 signaling pathway | + | TAMs produced IL-6, activating STAT3 and elevating the cellular glucose uptake. TLR4 cooperated with STAT3 via Nanog to activate Twist1. | Human | Zhang and colleagues74; Wan and colleagues75; Uthaya and colleagues76 |
| RAS/RAF/ERK pathway | + | Depleting MEK or reducing ERK1/2 phosphorylation suppressed the proliferation, invasion and migration of LCSCs. MEK maintained the stabilization of SIRT1 protein. | Human | Galuppo and colleagues77; Sun and colleagues78; Cheng and colleagues14 |
| JNK signaling pathway | + | ANXA3 could enhance the activity of JNK pathway in CD133+LCSCs by upregulating the expression of c-MYC. | Human | Tong and colleagues11 |
| Notch signaling pathway | + | The Notch signaling cascade associated with Wnt, MAPK and NF-κB signaling. | Human | Luo and colleagues79; Wang and colleagues80 |
+, promoting the stemness of LCSCs; –, suppressing the stemness of LCSCs.
BAF, BRG1-associated factor; BRM, Brahma; CAMTA1, the calmodulin binding transcription activator 1; CUDR, cancer upregulated drug resistant; DILC, downregulated in LCSCs; ELK3, Net/SAP-2/Erp; GSK3β, glycogen synthase kinase 3β; HIF-1, hypoxia-inducible factor 1; HOTAIR, HOX transcript antisense RNA; HULC, highly upregulated in liver cancer; ICAM-1, intercellular adhesion molecule 1; KLF8, Krüppel-like factor 8; LCSC, liver cancer stem cell; MALAT1, nuclear-enriched transcript 2 (NEAT2); NF-κB, nuclear factor-κB; RBBP4, Rb binding protein 4; STAT3, signal transducer and activator of transcription 3; TAM, tumor-associated macrophage; TF, Transcription factor; TLR4, Toll-like receptor 4; TP53INP1, tumor protein 55-induced nuclear protein 1; TRAIL, tumor necrosis factor-related apoptosis inducing ligand; TRF2, telomere repeat binding factor 2.
Inhibition of the key TFs in LCSCs
Twist
The Twist proteins belong to the highly conserved basic heli-loop-helix TF family; the Twist genes include Twist1 and Twist2.81 It is reported that Twist is associated with EMT and self-renewal of LCSCs by regulating the CSCs marker CD24, promoting the development of HCC.42,82 Some phytochemicals show inhibitory effect on Twist signaling in LCSCs, such as casticin and 8-bromo-7-methoxychrysin (BrMC). Casticin is derived from Fructus Viticis (Chinese name, Manjingzi).43 BrMC is a novel synthetic analogue of chrysin (5,7-dihydroxyflavone).44 Both casticin and BrMC could inhibit EMT and the stemness of LCSCs by negatively regulating Twist.43,44 Thus, Twist inhibitors might be therapeutic agents by targeting LCSCs.
HIF-1α and ELK3
Hypoxia-inducible factor 1 (HIF-1) is a basic-helix-loop-helix-PAS heterodimeric TF which mediates transcriptional responses in hypoxic cells.83 HIF-1 is composed of two subunits, the HIF-1α and HIF-1β. HIF-1α is identified an important role in tumor development, including the regulation of oncogenes expression, cellular metabolism and proliferation, tumor metastasis and invasion.45 Zou and colleagues84 showed that the downregulation of HIF-1α inhibited the biological characteristics of LCSCs, such as self-renewal, migration, invasion.
The TF ELK3, also named Net/SAP-2/Erp, is part of the large family of ETS-domain TFs, belonging to the ternary complex factor subfamily.85 ELK3 is associated with wound healing, angiogenesis, and tumor growth as a downstream of the RAS/ERK signaling pathway.86 Lee and colleagues45 showed that ELK3 promoted the migration and invasion of LCSCs by targeting HIF-1α. So, both HIF-1α and ELK3 might act as the therapeutic targets for HCC treatment.
KLF8
Krüppel-like factor 8 (KLF8) belongs to the KLF family of TFs as DNA-binding transcriptional regulators in many cellular processes.87 KLF8 was highly expressed not only in LCSCs, but also in HCC tumors and distant migrated tissues.46 Overexpression of KLF8 promoted the maintenance and chemoresistance of LCSCs, accordingly, played a role in HCC tumorigenesis. Mechanistically, KLF8 facilitated the activation of the Wnt/β-catenin signaling pathway.46 The knockout of KLF8 gene showed markedly apoptosis of LCSCs. So, KLF8 could be taken as a novel target to suppress LCSCs.
Targeting noncoding RNAs in LCSCs
Noncoding RNAs include microRNAs (miRNAs) and long noncoding RNAs (lncRNAs). MiRNAs are a class of small noncoding RNAs which can post-transcriptionally regulate the expression of target genes and play roles as tumor suppressors or oncogenes in different tumorigenic progression.88 Long noncoding RNAs (lncRNAs) are >200 bases long and have low or no protein-coding potential.89 They are known to regulate splicing, recruit TFs, and regulate mRNA stability. Increasing studies demonstrate that both miRNAs and lncRNAs play important roles in LCSCs, they may be taken as therapeutic targets for the treatment of HCC.
Regulation of microRNAs in LCSCs
a. MicroRNA 122
MicroRNA 122 (miR-122) is a liver-specific miRNA, accounting for 70% of the total miRNAs in human liver.90 MiR-122 was identified as a tumor suppressor in HCC.47 Upregulation of miR-122 inhibited EMT, proliferation and invasion of hepatoma cells by suppressing the Wnt/β-catenin signaling pathway.91 Moreover, miR-122 inhibited glycolysis by targeting glycolytic genes PDK4, accordingly, suppressed the stemness properties and growth of CD133+ LCSCs.47
b. MicroRNA 152
MicroRNA-152 (miR-152) is involved in diverse biological functions and induces apoptosis in HCC as a tumor suppressor.48,92,93 In HBV-related HCC, the decrease of miR-152 contributed to the epigenetic inactivation of RIZ1, a candidate HCC suppressor gene which could be repressed by HBV X protein.93 In LCSCs, miR-152 inhibited clonogenicity and growth of CD133+LCSCs by directly binding to 3’ untranslated region and downregulating the expression of KIT which is a proto-oncogene.48
c. MicroRNA 21
MicroRNA 21 (miR-21) was identified as a pro-metastatic miRNA, which regulated the expression of metastasis-related proteins in HCC.94 MiR-21 was upregulated in LCSCs.95 Overexpression of miR-21 promoted the tumorigenesis, invasion and migration of LCSCs and positively regulated the expression of Oct4 and LCSC markers CD13, EpCAM and CD90.49 MiR-21 played an oncogenic role in LCSCs which might be taken as a target.
d. MicroRNA 155
MicroRNA 155 (miR-155) could either be an oncogene or a tumor suppressor in several type of cancers.96 In HCC, miR-155 was demonstrated to contribute to tumorigenesis.97 MiR-155 facilitated the formation and self-renewal of LCSCs by mediating TP53INP1(tumor protein 55-induced nuclear protein 1) to regulate CSC phenotype.50 Moreover, the overexpression of miR-155 enhanced the levels of Oct4 and LCSC markers CD90 and CD133, knockdown of miR-155 led to a decrease of LCSCs populations.50
e. MicroRNA 25
Abnormal expression of MicroRNA 25 (miR-25) mediates tumorigenesis in many human malignant tumors.98 In HCC, miR-25 played a role in promoting proliferation, invasion and migration of hepatoma cells.99 It was overexpressed in LCSCs compared with non-LCSCs, knockdown of it increased apoptosis of LCSCs induced by TNF-related apoptosis inducing ligand (TRAIL), via the Pten/PI3K/Akt/Bad signaling pathway51. It indicates that both miR-25 and TRAIL may become novel anticancer agents by targeting LCSCs.
f. MicroRNA 200 family
MicroRNA 200 family (miR-200 family) consist of five evolutionarily conserved members in the human genome with miR-200b, miR-200a and miR-429 located in 1p36 cluster, and miR-200c, miR-141 located in 12p13 cluster.100 MiR-429 was demonstrated as oncogene in HCC, its upregulation promoted the self-renewal, tumorigenicity and chemoresistance of EpCAM+LCSCs, it led to the activated transcription of Oct4 by E2F transcription factor 1 (E2F1) via reducing Rb binding protein 4 (RBBP4) expression, which otherwise could inhibit E2F1 transcriptional activity.52 Whereas, miR-200a acted as tumor suppressor, its upregulation suppressed the EMT phenotype of LCSCs by increasing the epithelial marker E-cadherin and decreasing the mesenchymal markers, fibronectin, vimentin, and N-cadherin.53 Moreover, miR-200b/miR-200c/miR-429 subfamily was found to suppress HCC metastasis by inhibiting Rho/ROCK signaling pathway.101 However, the miR-200 family play indispensable roles in maintenance of LCSCs which still need further studies.
g. MicroRNA let-7
The let-7 family consists of 12 members: let-7a-1, -2, -3; let-7b; let-7c; let-7d; let-7e; let-7f-1, -2; let-7g; let-7i; MIR98, most of them are found to be downregulated in several cancers, restoration of normal expression prevents tumorigenesis.102 In HCC, let-7a and let-7c were identified as tumor suppressors by targeting LCSCs.54,55 Let-7a suppressed the self-renewal of LCSCs by negatively regulating EMT and Wnt signaling pathway.54 Let-7c targeted PBX3 which was essential for the maintenance of LCSCs and suppressed the transcriptional activity of CSCs-related genes including CACNA2D1, EpCAM, Sox2 and Notch3.55
h. MicroRNA 1246
The upregulation of microRNA 1246 (miR-1246) was observed in several types of cancer, which was identified to play an oncogenic role.56,103 In HCC, miR-1246 was demonstrated to promote migration and invasion of hepatoma cells.103 Circulating miR-1246 has been shown to be a predictor of survival and tumor recurrence in HCC patients after liver transplantation.104 MiR-1246 promoted stemness of LCSCs by activating the Wnt/β-catenin pathway through inhibiting the expression of Axin2 and glycogen synthase kinase 3β (GSK3β), which could induce the degradation of β-catenin.56 Moreover, Oct4 acted as the direct upstream regulator of miR-1246 by direct promoter binding which drove β-catenin activation in LCSCs.
Regulation of lncRNAs in LCSCs
a. LncRNA highly upregulated in liver cancer and lncRNA MALAT1
Highly upregulated in liver cancer (HULC) is an lncRNA which was reported to act an oncogenic role in HCC progression.57,105,106 MALAT1, also known as nuclear-enriched transcript 2 (NEAT2), is a highly conserved lncRNA involving in cell cycle control.57,107 It was reported that the overexpression of HULC and MALAT1 promoted the proliferation of LCSCs, they cooperated to exert the oncogenic effect in LCSCs through regulating telomere repeat binding factor 2 (TRF2).57
b. LncRNA downregulated in LCSCs
The absence of lncRNA downregulated in LCSCs (DILC) was identified in patients of HCC with poor prognosis, blockade of lncDILC significantly enhanced the expansion of LCSCs and then promoted the progression of HCC.58 The mechanism was that lncDILC inhibited the autocrine interleukin (IL)-6/STAT3 signaling, and mediated the crosstalk between TNF-α/NF-κB signaling and IL-6/JAK2/STAT3 cascade, overexpression of lncDILC bound IL-6 promoter and blocked the IL-6 transcription, while lncDILC depletion enhanced IL-6 expression which induced by NF-κB in LCSCs.58 So, restoration of lncDILC may be a novel method against LCSCs.
c. LncRNA cancer upregulated drug resistant and LncRNA H19
Cancer upregulated drug resistant (CUDR) is a novel lncRNA which was found overexpressed in doxorubicin-resistant sublines of human squamous carcinoma cells.108 CUDR was also found overexpressed in many other tumors and promoted tumorigenesis.109 In human HCC, CUDR cooperated with SET1A, component of histone methyltransferase complex, to promote tumor growth.110 In addition, CUDR could induce upregulation of HULC and β-catenin, and control human liver stem cells malignant differentiation, which was identified the possible origination of LCSCs.109,59,111 CUDR and lncRNA H19 were found to have a combined action on LCSCs. H19 is encoded by a highly conserved imprinted gene and is essential for human tumor growth, its high expression was associated with poor clinical outcomes of patients with solid tumors.112,113 In human HCC, H19 was reported to play a role in tumorigenesis of HCC.113 It stimulated angiogenesis and promoted the adhesion of CD90+ hepatoma cells to endothelial cell monolayer.114 It was demonstrated that Pten depletion promoted the binding of CUDR to the oncogene CyclinD1, the CUDR-CyclinD1 complex then enhanced the H19 expression by loading onto the promoter region of H19, which improved the cell telomerase activity and extended the telomere length in LCSCs, finally promoting LCSCs growth.59
d. LncRNA HOX transcript antisense RNA
LncRNA HOX transcript antisense RNA (HOTAIR) acts as a transcriptional modulator in various fundamental biological activities, its overexpression is also related with poor prognosis of patients with HCC.115 Excessive HOTAIR promoted the malignant transformation of normal liver stem cells to LCSCs via inducing EMT.111 HOTAIR also governed the oncogenic action of inflammatory related gene IKKα, IKKβ, and IKKγ in LCSCs.116 Most of all, it accelerated human LCSCs malignant proliferation through downregulating SETD2, which is an essential enzyme in DNA double-strand breaks.60
e. Lnc RNA TCF7
LncTCF7 is a novel lncRNA which is highly expressed both in HCC and LCSCs, the protein-coding gene TCF7 acts as an upstream trigger to activate the Wnt signaling cascade.61 LncTCF7 recruited the SWI/SNF complex, a group of evolutionally conserved multi-subunits, to the promoter of TCF7 to initiate its expression, leading to activation of Wnt signaling and promoting self-renewal and tumorigenic capacity of LCSCs.61
f. LncRNA Sox4
LncSox4 is also a novel lncRNA which is recently discovered to be highly expressed in HCC and LCSCs.62 LncSox4 facilitated the self-renewal of LCSCs, mechanistically by targeting STAT3-Sox4 pathway, it recruited the TF STAT3 to the Sox4 promoter to trigger the expression of Sox4, which was required for the self-renewal of LCSCs.62
g. LncRNA Brahma
LncBRM (gene symbol LINCR-0003) is recently identified to be highly expressed in LCSCs and is required for the oncogenicity and self-renewal of LCSCs.63 The mechanism was that lncBRM associated with Brahma (BRM) to trigger the BRG1/BRM switch and the BRG1-associated factor (BAF) complex, leading to activation of the transcriptional cofactors YAP1 which was required for the self-renewal of LCSCs.63
h. LncRNA β-Catm
Lncβ-Catm (gene symbol LINC00184) is a novel lncRNA which is recently found to be highly expressed both in LCSCs and HCC.64 Lncβ-Catm contributed to LCSCs self-renewal by activating Wnt/β-catenin signaling. Mechanistically, it associated with β-catenin and the methyltransferase EZH2, promoting the methylation and stability of β-catenin, which resulted in the activation of Wnt/β-catenin signaling.64
i. LncRNA CAMTA1
LncCAMTA1 (gene symbol RP11-312B8.1) is a novel lncRNA which is identified to be highly expressed both in LCSCs and HCC, promoting the stemness and tumorigenesis of LCSCs.65 Mechanistically, lncCAMTA1 associated with the calmodulin binding transcription activator 1 (CAMTA1) promoter to inhibit the transcription of CAMTA1 which served as a tumor suppressor in HCC.65
Decreasing LCSCs biomarkers
CD133
CD133 is an essential LCSCs biomarker which participates in the regulation of EMT, tumorigenicity and invasion of LCSCs. The downregulation of CD133 decreased the level of nuclear factor-κB (NF-κB), leading to inhibition of EMT and the stemness of LCSCs.66 The expression of CD133 in HCC could be negatively regulated by Ikaros, a member of the Krüppel family, which interacted with the transcription repressor CtBP to form a complex directly bounding to the CD133 promoter.117
ICAM-1
Intercellular adhesion molecule 1 (ICAM-1) is a transmembrane molecule which is involved in many important cellular processes and also in the development of various human cancers, such as HCC, breast cancer and renal cancer.118 ICAM-1 is considered as a stem cell marker which is upregulated by Nanog, it is also high expressed in LCSCs, promoting the tumorigenicity and stemness of LCSCs.67
Interruption of the essential signaling pathways in LCSCs
Several signaling pathways are essential in the development of LCSCs and HCC, which provide some promising targets against LCSCs for the treatment of HCC.
Wnt/β-catenin pathway
The Wnt/β-catenin pathway is responsible for the self-renewal and unlimited cell proliferation of CSCs. It has also been widely confirmed in maintenance of LCSCs. Chen and colleagues68 revealed that constitutive expression of Wnt/β-catenin was detected in LCSCs, knockdown of it suppressed the cell phenotype of LCSCs. The Wnt/β-catenin inhibitor could impair the viability of LCSCs.69,70,119 Moreover, some phytochemicals, such as casticin, triptolide and BrMC, also have been demonstrated to restrain the self-renewal and proliferation of LCSCs by suppressing Wnt/β-catenin signaling.120,121
PI3K/Akt/mTOR pathway
The PI3K/Akt signal pathway plays a key role in tumorigenesis.122 In HCC, The PI3K/Akt/mTOR pathway promoted formation of LCSCs, leading to hepatocarcinogenesis.123,124 The inhibitor of PI3K or mTOR could suppress the proliferation of LCSCs. Moreover, HBV contributes to the activation of the pathways, HBV X protein facilitates alpha fetoprotein (AFP) expression, which promotes the proliferation of LCSCs, by activating PI3K/Akt signal pathways.71
Akt/GSK-3β/β-catenin pathway
In HCC, the Akt/GSK-3β/β-catenin pathway promotes the proliferation and invasion of LCSCs.72,73 Inhibition of Akt which is a protein kinase in multiple cellular processes, reduced the self-renewal and propagation of LCSCs.73,122 However, increasing evidence shows that adverse effects including liver injury and inflammation, hyperglycemia and hyperinsulinemia accompany the complete deletion of Akt.122 Thus, the definite mechanisms of systemic inhibition of Akt or the pathways need further study.
STAT3 signaling pathway
Signal transducer and activator of transcription 3 (STAT3) plays an oncogenic role in cancer progression involving in the regulation of EMT and CSCs, it could be activated by Toll-like receptors, microRNA or cytokine receptors such as the IL-6 family of cytokines.125 It was identified that IL-6 activated STAT3 and elevated the cellular glucose uptake, finally promoting the stemness of LCSCs.74,75 On the other hand, TLR4 could also trigger the expression of Nanog, the latter directly interacted with STAT3 which could also phosphorylated by the leptin receptor (OB-R) pathways to promote the generation and invasion of LCSCs by upregulating Twist1.76 Moreover, STAT3 meditated Sox4 expression to favor the activity of LnxSox4 as mentioned above.62
RAS/RAF/MEK/ERK signaling pathway
Aberrant regulation of the RAS/RAF/MEK/ERK pathway has been detected in several malignancies including HCC.126,127 Experimental research demonstrated that blockade of the RAS/RAF/ERK pathway suppressed the proliferation, invasion and migration of LCSCs by depleting MEK or reducing ERK1/2 phosphorylation.71,77,78 Moreover, MEK signaling was identified to promote the self-renewal of LCSCs by maintaining the stabilization of SIRT1 protein which was involved in carcinogenesis.14
JNK signaling pathway
The JNK signaling pathway is involved in cancer development by regulating the tumor-initiating capacity of CSCs.128 In HCC, increased JNK activity is associated with tumor proliferation.126 The CSCs-related gene ANXA3 could enhance the activity of the JNK pathway in CD133+LCSCs by upregulating the expression of the proto-oncogene c-MYC, finally improving the self-renewal of LCSCs.11
Notch signaling pathway
The Notch receptors consist of four members (Notch1-4) in mammals, which can combine with various ligands and be activated.17 The Notch signaling cascade participates in the progress of various cancers associating with Wnt, MAPK and NF-κB signaling.79 In HCC, the activated Notch pathway was detected in LCSCs promoting the expression of CSC-related genes and maintaining the stemness of LCSCs.79 Notch1 in LCSCs was identified to be a downstream of Wnt/β-catenin.80 Notch2 which was activated in LCSCs could be suppressed by C8orf4.17
Targeting LCSCs at the level of cells
Targeting LCSCs at the cellular level includes inhibiting autophagy and application of oncolytic adenoviruses in LCSCs.
Blocking the process of autophagy in LCSCs
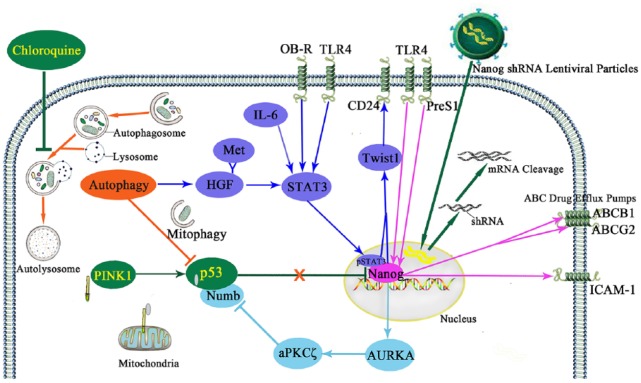
Autophagy is a conserved lysosomal degradation process during which the cytoplasmic components including macromolecules and organelles are degraded by the lysosome, accordingly maintaining the cellular homeostasis including anti-stress, immunity and antiaging.129,130 The effect of autophagy varies in different phases of tumorigenesis, it suppresses malignant transformation in healthy cells, but reduces intracellular and extracellular stress of cancer cells to promote tumor development.130 In healthy human liver, basal rates of autophagy maintain hepatocyte homeostasis by degrading misfolded proteins, protein aggregates and damaged mitochondria.131 Autophagy increased during the development of cirrhosis.132 Increased autophagy triggered the expression of hepatocyte growth factor (HGF) which bound with its receptor Met, leading to activation of JNK and STAT3 signaling to induce the formation of the Axin2+CD90+ LCSCs in liver cirrhosis.133 In another way, mitophagy which selectively removes the mitochondria by autophagy, positively regulated LCSCs by suppressing the tumor suppressor p53 which otherwise could be activated by PINK1 on mitochondria to downregulate the expression of Nanog (Figure 1).18 Moreover, autophagy contributed to the initiation and invasion of HCC. In the tumor microenvironment, autophagy copes with hypoxia and nutritional deprivation, favoring the survival of LCSCs and the chemoresistance of hepatoma cells.134 Chloroquine, the inhibitor of autophagy, showed inhibitory effect on the stemness of LCSCs.133,134 It inhibited autophagic flux by decreasing autophagosome-lysosome fusion which formed autolysosomes.135 Chloroquine may inhibit the formation of LCSCs by suppressing pSTAT3 and Nanog, and keep the p53 activity from being suppressed by autophagy. Thus, the application of chloroquine or other autophagy inhibitors in liver cirrhosis and HCC may be potential targeting approaches against LCSCs.
Figure 1.
Targeting LCSCs by suppression of autophagy and Nanog.
Autophagy positively regulated LCSCs by suppressing p53 and favoring the activity of Nanog. Mitophagy suppressed p53 which otherwise was activated by PINK1 on mitochondria to downregulate Nanog. Autophagy mediated HGF binding with its receptor Met to activate STAT3 which was also phosphorylated by IL-6, OB-R and TLR4. The pSTAT3 and Nanog directly interacted to trigger Twist1-CD24 axis. Nanog, the downstream of TLR4 and PreS1, suppressed p53 by phosphorylation of Numb and destabilization of Numbp53 complex via the Nanog-AURKA-aPKCζ pathway. Moreover, Nanog upregulated the level of ICAM-1, ABCB1 and ABCG2 which favored the self-renewal and chemoresistance of LCSCs. The autophagy inhibitor chloroquine could block the self-renewal of LCSCs. It inhibited autophagy by decreasing autophagosome–lysosome fusion which formed autolysosomes to degrade the cytoplasmic components. Chloroquine may block pSTAT3 and Nanog and keep the p53 activity from being suppressed by autophagy in LCSCs. On the other hand, the Nanog-targeting shRNA via lentiviral particles could efficiently block the expression of Nanog at both mRNA and protein levels by cleavage of Nanog mRNA. Nanog silence by shRNA which showed a long-term depletion of the targeted gene impaired both the self-renewal and the chemoresistance of LCSCs. Chloroquine and Nanog shRNA may provide potential LCSC-targeting approaches by the suppression of autophagy and Nanog respectively.
ABCG2, ATP-binding cassette G 2; ABCB1, ATP-binding cassette subfamily B member 1; AURKA, Aurora A kinase; aPKCζ, atypical protein kinase C zeta; HGF, hepatocyte growth factor; ICAM-1, intercellular adhesion molecule 1; LCSC, liver cancer stem cell; OB-R, the leptin receptor; PINK1, Pten-induced putative kinase 1; STAT3, signal transducer and activator of transcription 3; shRNA, short hairpin RNA; TLR4, Toll-like receptor 4.
Application of oncolytic adenovirus in LCSCs
In recent years, the antitumor use of viruses has been studied in depth. Oncolytic adenoviruses which are genetically modified, could selectively enter and spread inside tumors, show their cytotoxicity and suppression of tumors.136 In HCC, it was reported that a new oncolytic adenovirus GD55 demonstrated a stronger killing effect on human LCSCs.137 Moreover, Zhang and colleagues designed a novel oncolytic adenovirus which carried the tumor suppressor gene TSLC1 and targeted Wnt signaling pathway, the adenovirus inhibited growth and metastasis of LCSCs in vivo by mediating TSLC1 and suppressing Wnt signaling.138 Therefore, oncolytic adenovirus may serve as a potentially therapeutic application by targeting LCSCs.
Eliminating the chemoresistance of LCSCs
Targeting LCSCs by depleting or inactivating the drug resistance genes or proteins and blocking the drug resistance-associated signaling pathways in LCSCs may be effective to eliminate its chemoresistance (Table 3).
Table 3.
Targets to eliminate the chemoresistance of LCSCs.
| Targets | Mechanism | Species | References |
|---|---|---|---|
| Oncogenes or oncoproteins | |||
| ABCG 2 | ABCG 2 is involved in drug efflux pumps, which is mediated by Oct4 and Nanog. | Human | Jia and colleagues139, Zhou and colleagues140 |
| ZIC-2, PML and Oct4 | ZIC-2 and PML act as upstream of Oct4 which has a positive association between ABCG2. | Human | Zhu and colleagues141; Tang al.142; Jia and colleagues139 |
| Nanog | Nanog decreased the expression of ABCB1 and ABCG2 in LCSCs. | Human | Zhou and colleagues140 |
| Laminin-332 | Laminin-332 acted as part of the human LCSC niches, upregulated K19 expression, and downregulated phospho-histone H3 expression and induced phosphorylation of mTOR. | Human | Govaere and colleagues143 |
| CHD4 | CHD4 contributed to the repair of DNA damage in a PARP-dependent manner in EpCAM+LCSCs as a chromatin remodeling enzyme. | Human | Nio and colleagues144 |
| MSI2 | MSI2 upregulated Lin28A, which is critical CSC-related RNA-binding proteins. | Human | Fang and colleagues145 |
| GRAMD1A | GRAMD1A regulated the transcriptional activity of STAT5. | Human | Fu and colleagues146 |
| Dysadherin | Dysadherin might upregulated drug efflux pumps in LCSCs. | Human | Jiang and colleagues147 |
| Signaling pathway | |||
| JNK signaling pathway | The number of LCSCs and phosphorylation of SAPK/JNK increased upon anticancer treatment. | Human | Kim and colleagues148 |
| Akt signaling pathway | The inhibition of Akt signaling enhanced the sensitivity of LCSCs to sorafenib by upregulating ERK signaling, which was a primary target of sorafenib. | Human | Xu and colleagues72 |
ABCB1, ATP-binding cassette subfamily B member 1; ABCG2, ATP-binding cassette G 2; CHDs, chromodomain-helicase-DNA-binding proteins; GRAMD1A, GRAM domain-containing protein 1A; K 19, keratin 19; LCSC, liver cancer stem cell; MSI2, Musashi 2; Oct4, octamer-binding transcription factor 4; PARP, poly (ADP-ribose) polymerase; PML, promyelocytic leukemia; SAPK, phospho-stress-activated protein kinase; STAT5, signal transducer and activator of transcription 5.
Depletion or inactivating the drug resistance genes or proteins in LCSCs
ABCG2
The ATP-binding cassette (ABC)G2 is a member of the superfamily of ABC transporters drug efflux pumps, which lead to multidrug resistance in liver cancer.139 ABCG2 is mediated by Oct4 for the chemotherapeutic resistance in hepatoma cells via a potential Oct4-Akt-ABCG2 pathway.149 Both Oct4 and Nanog, another upstream of ABCG2, have a positive association with ABCG2, cooperating to favor the chemoresistance of LCSCs.139,140 Thus, ABCG2 may become one of the most important targets for drug resistance of LCSCs.
ZIC-2–Octamer-binding transcription factor 4 (Oct4) axis and promyelocytic leukemia–Oct4 axis
Octamer-binding transcription factor 4 (Oct4) which is encoded by the Pou5f1 gene belongs to the POU (Pit, Oct and Unc) family of DNA-binding proteins.150 As mentioned above, the overexpression of Oct4 favored the stemness of LCSCs by upregulating miR-1246 to drive β-catenin activation.56 Its expression in LCSCs can be upregulated by PreS1, miR-21, miR-155 and miR-429/RBBP4/E2F1/Oct4 pathway.12,49,50,52 It was also found that the zinc finger TF ZIC2 which was highly expressed in LCSCs acted upstream of Oct4, ZIC2 recruited the nuclear remodeling factor (NURF) complex binding to the promoter of Oct4, thereby initiating Oct4 activation.141Another protein upstream of Oct4 is the promyelocytic leukemia (PML) protein which was originally identified in acute promyelocytic leukemia.151,142 PML took a role as tumor suppressor in multiple pathways, but it was found to preserve the activity of Oct4 gene in stem cells.151,152 In LCSCs, suppression of PML decreased the level of Oct4, indicating that the PML favored the stemness of LCSCs via the PML/Oct4 axis.142 However, Oct4 overexpression is also involved in the acquisition of the drug-resistant phenotype of cancer cells.153 It has a positive association between ABCG2, both of them contributed to drug resistance of CD90+CD133+LCSCs.139 Oct4 is one of the most promising therapeutic targets for HCC treatment by targeting LCSCs.
Nanog
Nanog is a homeodomain-containing TF which plays a key role in the regulation of stemness acquirement and cancer development.154 As mentioned above, Nanog is critical to the stemness of LCSCs, and can be activated by PreS1 and TLR4.12,76 Its abnormal expression upregulated the level of ICAM-1.67 The activated Nanog directly interacted with pSTAT3 to drive the expression of Twist1 which activated CD24.42,76 Autophagy is associated with Nanog. It favors the activity of Nanog not only by activating the HGF/Met/STAT3 pathway, but also by suppressing p53, which otherwise downregulates the expression of Nanog.18,133 On the other hand, Nanog suppresses p53 by phosphorylation of Numb and destabilization of the Numb-p53 complex via the Nanog-AURKA-aPKCζ pathway.19 Moreover, Nanog was found to be involved in chemoresistance of LCSCs; knockdown of it led to increased chemosensitivity to antitumor agents by decreasing the expression of ABCB1 (ABC subfamily B member 1) and ABCG2 in LCSCs which belong to the ABC drug efflux pumps (Figure 1).140 It was found that Nanog silence by short hairpin RNA (shRNA) or small interfering RNA (siRNA) impaired both the self-renewal and the chemoresistance of LCSCs.19,67,140 The Nanog shRNA via lentiviral particles could efficiently block the expression of Nanog at both mRNA and protein levels by the cleavage of Nanog mRNA. It showed a long-term depletion of the targeted gene. Thus, the suppression of Nanog via shRNA lentiviral particles or other gene therapy vectors may be potential LCSCs-targeting approaches.
Laminin-332
Laminin-332 belongs to the laminin family which is a kind of extracellular matrix proteins.143 Laminin acts as part of hepatic progenitor cell (HPC) niches to maintain the phenotype of HPCs during human liver damage.155 Govaere and colleagues143 demonstrated that laminin-332 also acted as part of the human LCSCs niches, it supported stemness and chemoresistance of LCSCs under doxorubicin and sorafenib treatment by upregulating keratin(K)19 expression, downregulating phospho-histone H3 expression and inducing phosphorylation of mTOR. However, the effect of laminin-332 was decreased upon the inhibition of mTORC1 and mTORC2, and enhanced when mTORC1 was inhibited alone,143 which need further studies.
CHD4
The chromodomain-helicase-DNA-binding proteins (CHDs) is part of the histone deacetylation (NuRD) complex protein, which was essential in transcriptional events of oncogenesis.156 CHD4 was found to contribute to the repair of DNA damage in a poly (ADP-ribose) polymerase (PARP)-dependent manner in EpCAM+LCSCs as a chromatin remodeling enzyme.144 The overexpression of CHD4 enhanced the chemoresistance of LCSCs to anticancer drugs, while inhibition of CHD4 by doubly suppressing PARP and histone deacetylase (HDAC) suppressed LCSC proliferation.144 Thus, knockout of CHD4 may enhance the chemosensitivity of LCSCs which deserves further investigation.
Musashi 2
The Musashi family is a kind of evolutionarily conserved RNA-binding protein which comprises Musashi 1 and Musashi 2 (MSI2).40 MSI2 is required in the tumorigenesis of several human cancers, and participates in inhibiting the tumor suppressor Numb and wildtype p53.19,40 Fang and colleagues145 found that MSI2 also contributed to the chemoresistance of LCSCs by upregulation of Lin28A, which is critical CSC-related RNA-binding proteins. Knockdown of MSI2 or Lin28A impaired the chemoresistance of LCSCs, moreover, MSI2 knockdown also reduce the level of Nanog, Oct4 and Sox2.145 Thus, MSI2 may become new target to improve the chemosensitivity of LCSCs.
GRAM domain-containing protein 1A
GRAM domain-containing protein 1A (GRAMD1A) is a novel protein, the function of which has not been explored.146 It is highly expressed in HCC and sustains the self-renewal and chemotherapy resistance of LCSCs by regulating the transcriptional activity of STAT5 (signal transducer and activator of transcription 5). However, other evidence showed that STAT5 could prevent the formation of aggressive HCC.157 So, both GRAMD1A and STAT5 need more research.
Dysadherin
Dysadherin is a cell membrane glycoprotein which upregulates the production of chemokine and downregulates cell adhesion mediated by E-cadherin, accordingly, creating survival conditions for many types of human cancer.158 In HCC, high expression of dysadherin sustained high resistance to chemotherapeutic drugs in LCSCs which might depend on upregulation of drug efflux pumps.147 Knockdown of dysadherin showed increased sensitivity to chemotherapy agents and apoptotic cell death, as well as remarkable decreasing expression of stemness-related proteins.147
Blockade of the drug resistance-associated signaling pathways in LCSCs
JNK signaling pathway
As mentioned above, the JNK signaling pathway is associated with the self-renewal and tumorigenicity of LCSCs.11 JNK signaling was also involved in mutidrug resistance of HCC. It was found that the number of side population (SP) of cells and phosphorylation of phospho-stress-activated protein kinase (SAPK)/JNK increased upon anticancer treatment, the increase of SP cells which was considered as LCSCs could be blocked by JNK signaling inhibition, indicating that the activation of JNK may be responsible for drug resistance in HCC.148
Akt signaling pathway
As mentioned above, the Akt signaling pathway is involved in the proliferation of LCSCs. Also, Akt signaling contributed to the chemotherapeutic resistance of LCSCs, the inhibition of Akt signaling enhanced the sensitivity of LCSCs to sorafenib by upregulating ERK signaling, which was a primary target of sorafenib.72
Conclusion
In recent years, the important role of LCSCs in the initiation, relapse, metastasis and drug resistance of HCC has been identified. Targeting LCSCs is expected to be a promising approach for the treatment of HCC. In this review, we summarize potentially therapeutic targets which are key points of LCSCs at the genetic, molecular and cellular level. Moreover, we analyze the promising approaches to eliminate chemoresistance of LCSCs. These targets provide new opportunities for HCC treatment. However, it has been recognized that these key elements in the differentiation and self-renewal of LCSCs interacted with each other through a complex crosstalk. Thus, combined therapies by targeting LCSCs may enhance the therapeutic efficacy of HCC treatment. Moreover, although many of the targets mentioned above are highly expressed in LCSCs compared with normal liver cells or normal liver stem cells, but the systemic inhibition of these targets may result in injury of normal cells, how to save normal cells from damage in the clinical application is certainly a concern, which needs further research. In summary, targeting LCSCs could be a promising therapeutic strategy for the treatment of HCC.
Acknowledgments
Ying Zhu conceived and designed the work and approved the final version. Na Li acquired data and prepared the manuscript.
Footnotes
Funding: This work was supported by the National Natural Science Foundation of China (no. 81673728).
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Ying Zhu  https://orcid.org/0000-0003-4198-5515
https://orcid.org/0000-0003-4198-5515
Contributor Information
Na Li, The First Affiliated Hospital of Dalian Medical University, Dalian, China.
Ying Zhu, Department of Infectious Disease, The First Affiliated Hospital of Dalian Medical University, Dalian, Liaoning 116011, China.
References
- 1. Laursen L. A preventable cancer. Nature 2014; 516: S2–S3. [DOI] [PubMed] [Google Scholar]
- 2. Llovet JM, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2016; 2: 16018. [DOI] [PubMed] [Google Scholar]
- 3. Nio K, Yamashita T, Kaneko S. The evolving concept of liver cancer stem cells. Mol Cancer 2017; 16: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheng Z, Li X, Ding J. Characteristics of liver cancer stem cells and clinical correlations. Cancer Lett 2015; 379: 230. [DOI] [PubMed] [Google Scholar]
- 5. Lee TKW, Cheung VCH, Ng IOL. Liver tumor-initiating cells as a therapeutic target for hepatocellular carcinoma. Cancer Lett 2013; 338: 101–109. [DOI] [PubMed] [Google Scholar]
- 6. Chan LH, Luk ST, Ma S. Turning hepatic cancer stem cells inside out–a deeper understanding through multiple perspectives. Mol Cells 2015; 38: 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. You N, Zheng L, Liu W, et al. Proliferation inhibition and differentiation induction of hepatic cancer stem cells by knockdown of BC047440: a potential therapeutic target of stem cell treatment for hepatocellular carcinoma. Oncol Rep 2014; 31: 1911–1920. [DOI] [PubMed] [Google Scholar]
- 8. Lee YK, Hur W, Lee SW, et al. Knockdown of 14-3-3ζ enhances radio sensitivity and radio-induced apoptosis in CD133+ liver cancer stem cells. Exp Mol Med 2014; 46: e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang R, Tang Q, Miao F, et al. Inhibition of heat-shock protein 90 sensitizes liver cancer stem-like cells to magnetic hyperthermia and enhances anti-tumor effect on hepatocellular carcinoma-burdened nude mice. Int J Nanomedicine 2015; 10: 7345–7358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pan Q, Pan K, Wang Q, et al. Annexin A3 as a potential target for immunotherapy of liver cancer stem-like cells. Stem Cells 2015; 33: 354–366. [DOI] [PubMed] [Google Scholar]
- 11. Tong M, Fung TM, Luk ST, et al. ANXA3/JNK signaling promotes self-renewal and tumor growth, and its blockade provides a therapeutic target for hepatocellular carcinoma. Stem Cell Reports 2015; 5: 45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu Z, Dai X, Wang T, et al. Hepatitis B virus PreS1 facilitates hepatocellular carcinoma development by promoting appearance and self-renewal of liver cancer stem cells. Cancer Lett 2017; 400: 149–160. [DOI] [PubMed] [Google Scholar]
- 13. Liu L, Liu C, Zhang Q, et al. SIRT1-mediated transcriptional regulation of SOX2 Is important for self-renewal of liver cancer stem cells. Hepatology 2016; 64: 814–827. [DOI] [PubMed] [Google Scholar]
- 14. Cheng J, Liu C, Liu L, et al. MEK1 signaling promotes self-renewal and tumorigenicity of liver cancer stem cells via maintaining SIRT1 protein stabilization. Oncotarget 2016; 7: 20597–20611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hong SW, Hur W, Choi JE, et al. Role of ADAM17 in invasion and migration of CD133-expressing liver cancer stem cells after irradiation. Oncotarget 2016; 7: 23482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Luo X, Liao R, Hanley KL, et al. Dual Shp2 and Pten deficiencies promote non-alcoholic steatohepatitis and genesis of liver tumor-initiating cells. Cell Rep 2016; 17: 2979–2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhu P, Wang Y, Du Y, et al. C8orf4 negatively regulates self-renewal of liver cancer stem cells via suppression of NOTCH2 signalling. Nat Commun 2015; 6: 7122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu K, Lee J, Kim JY, et al. Mitophagy controls the activities of tumor suppressor p53 to regulate hepatic cancer stem cells. Mol Cell 2017; 68: 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Siddique HR, Feldman DE, Chen CL, et al. Numb phosphorylation destabilizes p53 and promotes self-renewal of tumor-initiating cells by Nanog-dependent mechanism in liver cancer. Hepatology 2015; 62: 1466–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zheng L, Liang P, Li J, et al. Expression of BC047440 protein in hepatocellular carcinoma and its relationship to prognosis. Chin J Cancer 2010; 29: 931–936. [DOI] [PubMed] [Google Scholar]
- 21. Neal CL, Yu D. 14-3-3ζ as a prognostic marker and therapeutic target for cancer. Expert Opin Ther Targets 2010; 14: 1343–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haque A, Alam Q, Alam MZ, et al. Current understanding of HSP90 as a novel therapeutic target: an emerging approach for the treatment of cancer. Curr Pharm Des 2016; 22. [DOI] [PubMed] [Google Scholar]
- 23. Esfahani K, Cohen V. HSP90 as a novel molecular target in non-small-cell lung cancer. Lung Cancer(Auckl) 2016; 7: 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Raynal P, Pollard HB. Annexins: the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim Biophys Acta 1994; 1197: 63. [DOI] [PubMed] [Google Scholar]
- 25. Tang KH, Yusoff K, Tan WS. Display of hepatitis B virus PreS1 peptide on bacteriophage T7 and its potential in gene delivery into HepG2 cells. J Virol Methods 2009; 159: 194–199. [DOI] [PubMed] [Google Scholar]
- 26. Simmons GE, Pruitt WM, Pruitt K. Diverse roles of SIRT1 in cancer biology and lipid metabolism. Int J Mol Sci 2015; 16: 950–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Feng G, Hui C, Pawson T. SH2-containing phosphotyrosine phosphatase as a target of protein-tyrosine kinases. Science 1993; 259: 1607–1611. [DOI] [PubMed] [Google Scholar]
- 28. Chan RJ, Feng GS. PTPN11 is the first identified proto-oncogene that encodes a tyrosine phosphatase. Blood 2007; 109: 862–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bard-Chapeau EA, Li S, Ding J, et al. Ptpn11/Shp2 acts as a tumor suppressor in hepatocellular carcinogenesis. Cancer Cell 2011; 19: 629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Han T, Xiang DM, Sun W, et al. PTPN11/Shp2 overexpression enhances liver cancer development and predicts poor prognosis of patients. J Hepatol 2015; 63: 651–660. [DOI] [PubMed] [Google Scholar]
- 31. Xiang D, Cheng Z, Liu H, et al. Shp2 promotes liver cancer stem cell expansion by augmenting β-catenin signaling and predicts chemotherapeutic response of patients. Hepatology 2017; 65: 1566. [DOI] [PubMed] [Google Scholar]
- 32. Cantley LC. The phosphoinositide 3-kinase pathway. Science 2002; 296: 1655–1657. [DOI] [PubMed] [Google Scholar]
- 33. Chua EL, Young L, Wu WM, et al. Cloning of TC-1 (C8orf4), a novel gene found to be overexpressed in thyroid cancer. Genomics 2000; 69: 342–347. [DOI] [PubMed] [Google Scholar]
- 34. Lei J, Li W, Yang Y, et al. TC-1 overexpression promotes cell proliferation in human nonsmall cell lung cancer that can be inhibited by PD173074. PLoS One 2014; 9: e100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang ZQ, Streicher KL, Ray ME, et al. Multiple interacting oncogenes on the 8p11-p12 amplicon in human breast cancer. Cancer Res 2006; 11632–11643. [DOI] [PubMed] [Google Scholar]
- 36. Bonizzi G, Cicalese A, Insinga A, et al. The emerging role of p53 in stem cells. Trends Mol Med 2012; 18: 6–12. [DOI] [PubMed] [Google Scholar]
- 37. Lin T, Chao C, Saito S, et al. p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nat Cell Biol 2005; 7: 165–171. [DOI] [PubMed] [Google Scholar]
- 38. Uemura T, Shepherd S, Ackerman L, et al. Numb, a gene required in determination of cell fate during sensory organ formation in Drosophila embryos. Cell 1989; 58: 349–360. [DOI] [PubMed] [Google Scholar]
- 39. Bocci F, Jolly MK, Tripathi SC, et al. Numb prevents a complete epithelial-mesenchymal transition by modulating Notch signalling. J R Soc Interface 2017; 14 20170512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sheng W, Dong M, Chen C, et al. Musashi2 promotes the development and progression of pancreatic cancer by down-regulating Numb protein. Oncotarget 2016; 8: 14359–14373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sheng W, Dong M, Chen C, et al. Cooperation of Musashi-2, Numb, MDM2, and P53 in drug resistance and malignant biology of pancreatic cancer. FASEB J 2017; 31: 2429. [DOI] [PubMed] [Google Scholar]
- 42. Liu AY, Cai Y, Mao Y, et al. Twist2 promotes self-renewal of liver cancer stem-like cells by regulating CD24. Carcinogenesis 2014; 35: 537. [DOI] [PubMed] [Google Scholar]
- 43. He M, Cao XC, He GC, et al. Casticin inhibits epithelial-mesenchymal transition of liver cancer stem cells of the SMMC-7721 cell line through downregulating Twist. Oncol Lett 2014; 7: 1625–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ren KQ, Cao XZ, Liu ZH, et al. 8-bromo-5-hydroxy-7-methoxychrysin targeting for inhibition of the properties of liver cancer stem cells by modulation of Twist signaling. Int J Oncol 2013; 43: 1719–1729. [DOI] [PubMed] [Google Scholar]
- 45. Lee J H, Hur W, Hong S W, et al. ELK3 promotes the migration and invasion of liver cancer stem cells by targeting HIF-1α. Oncol Rep 2017; 37: 813. [DOI] [PubMed] [Google Scholar]
- 46. Shen YN, He HG, Shi Y, et al. Krüppel-like factor 8 promotes cancer stem cell-like traits in hepatocellular carcinoma through Wnt/β-catenin signaling. Mol Carcinog 2016; 56. [DOI] [PubMed] [Google Scholar]
- 47. Song K, Kwon H, Chang H, et al. Active glycolytic metabolism in CD133+ hepatocellular cancer stem cells: regulation by MIR-122. Oncotarget 2015; 6: 40822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Huang H, Hu M, Li P, et al. Mir-152 inhibits cell proliferation and colony formation of CD133+ liver cancer stem cells by targeting KIT. Tumour Biol 2015; 36: 921–928. [DOI] [PubMed] [Google Scholar]
- 49. Jiang J, Yang P, Guo Z, et al. Overexpression of microRNA-21 strengthens stem cell-like characteristics in a hepatocellular carcinoma cell line. World J Surg Oncol 2016; 14: 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liu F, Kong X, Lv L, et al. MiR-155 targets TP53INP1 to regulate liver cancer stem cell acquisition and self-renewal. FEBS Lett 2015; 589: 500–506. [DOI] [PubMed] [Google Scholar]
- 51. Feng X, Jiang J, Shi S, et al. Knockdown of miR-25 increases the sensitivity of liver cancer stem cells to TRAIL-induced apoptosis via Pten/PI3K/Akt/Bad signaling pathway. Int J Oncol 2016; 49: 2600–2610. [DOI] [PubMed] [Google Scholar]
- 52. Li L, Tang J, Zhang B, et al. Epigenetic modification of MiR-429 promotes liver tumour-initiating cell properties by targeting Rb binding protein 4. Gut 2014; 64: 156. [DOI] [PubMed] [Google Scholar]
- 53. Wang J, Yang X, Bai R, et al. Overexpression of miR-200a suppresses epithelial-mesenchymal transition of liver cancer stem cells. Tumor Biol 2015; 36: 2447–2456. [DOI] [PubMed] [Google Scholar]
- 54. Jin B, Wang W, Meng X, et al. Let-7 inhibits self-renewal of hepatocellular cancer stem-like cells through regulating the epithelial-mesenchymal transition and the Wnt signaling pathway. BMC Cancer 2016; 16: 863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Han H, Du Y, Zhao W, et al. PBX3 is targeted by multiple miRNAs and is essential for liver tumour-initiating cells. Nat Commun 2015; 6: 8271. [DOI] [PubMed] [Google Scholar]
- 56. Chai S, Ng KY, Tong M, et al. Octamer-4/microRNA-1246 signaling axis drives Wnt/β-catenin activation in liver cancer stem cells. Hepatology 2017; 64: 2062–2076. [DOI] [PubMed] [Google Scholar]
- 57. Wu M, Lin Z, Li X, et al. HULC cooperates with MALAT1 to aggravate liver cancer stem cells growth through telomere repeat-binding factor 2. Sci Rep 2016; 6: 36045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang X, Sun W, Shen W, et al. Long non-coding RNA DILC regulates liver cancer stem cells via IL-6/STAT3 axis. J Hepatol 2016; 64: 1283. [DOI] [PubMed] [Google Scholar]
- 59. Pu H, Zheng Q, Li H, et al. CUDR promotes liver cancer stem cell growth through upregulating TERT and C-Myc. Oncotarget 2015; 6: 40775–40798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Li H, An J, Wu M, et al. LncRNA HOTAIR promotes human liver cancer stem cell malignant growth through downregulation of SETD2. Oncotarget 2015; 6: 27847–27864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang Y, He L, Du Y, et al. The long noncoding RNA lncTCF7 promotes self-renewal of human liver cancer stem cells through activation of Wnt signaling. Cell Stem Cell 2015; 16: 413–425. [DOI] [PubMed] [Google Scholar]
- 62. Chen Z, Huang L, Wu Y, et al. LncSox4 promotes the self-renewal of liver tumour-initiating cells through Stat3-mediated Sox4 expression. Nat Commun 2016; 7: 12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhu P, Wang Y, Wu J, et al. LncBRM initiates YAP1 signalling activation to drive self-renewal of liver cancer stem cells. Nat Commun 2016; 7: 13608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhu P, Wang Y, Huang G, et al. lnc-β-Catm elicits EZH2-dependent β-catenin stabilization and sustains liver CSC self-renewal. Nat Struct Mol Biol 2016; 23: 631–639. [DOI] [PubMed] [Google Scholar]
- 65. Ding LJ, Li Y, Wang SD, et al. Long noncoding RNA lncCAMTA1 promotes proliferation and cancer stem cell-like properties of liver cancer by inhibiting CAMTA1. Int J Mol Sci 2016, 17: 1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Liu Y M, Li XF, Liu H, et al. Ultrasound-targeted microbubble destruction-mediated downregulation of CD133 inhibits epithelial-mesenchymal transition, stemness and migratory ability of liver cancer stem cells. Oncol Rep 2015; 34: 2977. [DOI] [PubMed] [Google Scholar]
- 67. Liu S, Li N, Yu X, et al. Expression of intercellular adhesion molecule 1 by hepatocellular carcinoma stem cells and circulating tumor cells. Gastroenterology 2013; 144: 1031–1041. [DOI] [PubMed] [Google Scholar]
- 68. Chen W, Zhang YW, Li Y, et al. Constitutive expression of Wnt/β-catenin target genes promotes proliferation and invasion of liver cancer stem cells. Mol Med Rep 2016; 13: 3466–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kim JY, Lee HY, et al. CWP232228 targets liver cancer stem cells through Wnt/β-catenin signaling: a novel therapeutic approach for liver cancer treatment. Oncotarget 2016; 7: 20395–20409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Seto K, Sakabe T, et al. A novel small-molecule Wnt inhibitor, IC-2, has the potential to suppress liver cancer stem cells. Anticancer Res 2017; 37: 3569. [DOI] [PubMed] [Google Scholar]
- 71. Zhu M, Li W, Lu Y, et al. HBx drives alpha fetoprotein expression to promote initiation of liver cancer stem cells through activating PI3K/AKT signal pathway. Int J Cancer 2017; 140: 1346. [DOI] [PubMed] [Google Scholar]
- 72. Xu Q, Xu HX, Li JP, et al. Growth differentiation factor 15 induces growth and metastasis of human liver cancer stem-like cells via AKT/GSK-3β/β-catenin signaling. Oncotarget 2017; 8: 16972–16987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhai B, Zhang X, et al. MK2206 overcomes the resistance of human liver cancer stem cells to sorafenib by inhibition of pAkt and upregulation of pERK. Tumour Biol 2015; 37: 8047–8055. [DOI] [PubMed] [Google Scholar]
- 74. Zhang HL, Wang MD, Zhou X, et al. Blocking preferential glucose uptake sensitizes liver tumor-initiating cells to glucose restriction and sorafenib treatment. Cancer Lett 2017; 388: 1–11. [DOI] [PubMed] [Google Scholar]
- 75. Wan S, Zhao E, Kryczek I, et al. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology 2014; 147: 1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Uthaya Kumar DB, Chen CL, Liu JC, et al. TLR4 Signaling via Nanog cooperates with STAT3 to activate twist1 and promote formation of tumor-initiating stem-like cells in livers of mice. Gastroenterology 2016; 150: 707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Galuppo R, Maynard E, Shah M, et al. Synergistic inhibition of HCC and liver cancer stem cell proliferation by targeting RAS/RAF/MAPK and Wnt/β-catenin pathways. Anticancer Res 2014; 34: 1709–1713. [PMC free article] [PubMed] [Google Scholar]
- 78. Sun J, Luo Q, Liu L, et al. Salinomycin attenuates liver cancer stem cell motility by enhancing cell stiffness and increasing F-actin formation via the FAK-ERK1/2 signalling pathway. Toxicology 2017; 384: 1–10. [DOI] [PubMed] [Google Scholar]
- 79. Luo J, Wang P, Wang R, et al. The Notch pathway promotes the cancer stem cell characteristics of CD90+cells in hepatocellular carcinoma. Oncotarget 2016; 7: 9525–9537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang R, Sun Q, Wang P, et al. Notch and Wnt/β-catenin signaling pathway play important roles in activating liver cancer stem cells. Oncotarget 2016; 7: 5754–5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ansieau S, Morel AP, Hinkal G, et al. TWISTing an embryonic transcription factor into an oncoprotein. Oncogene 2010; 29: 3173–3184. [DOI] [PubMed] [Google Scholar]
- 82. Zou H, Feng X, Cao JG. Twist in hepatocellular carcinoma: pathophysiology and therapeutics. Hepatol Int 2015; 9: 399–405. [DOI] [PubMed] [Google Scholar]
- 83. Wang GL, Jiang BH, Rue EA, et al. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA 1995; 92: 5510–5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zou H, Cao X, Xiao Q, et al. Synergistic inhibition of characteristics of liver cancer stem-like cells with a combination of sorafenib and 8-bromo-7-methoxychrysin in SMMC-7721 cell line. Oncol Rep 2016; 36: 1731. [DOI] [PubMed] [Google Scholar]
- 85. Kasza A. Signal-dependent Elk-1 target genes involved in transcript processing and cell migration. Biochim Biophys Acta 2013; 1829: 1026–1033. [DOI] [PubMed] [Google Scholar]
- 86. Wasylyk C, Zheng H, Castell C, et al. Inhibition of the Ras-net (Elk-3) pathway by a novel pyrazole that affects microtubules. Cancer Res 2008; 68: 1275–1283. [DOI] [PubMed] [Google Scholar]
- 87. Yan Q, Zhang W, Yao W, et al. KLF8 promotes tumorigenesis, invasion and metastasis of colorectal cancer cells by transcriptional activation of FHL2. Oncotarget 2015; 6: 25402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Iorio MV, Croce CM. MicroRNA involvement in human cancer. Carcinogenesis 2012; 33: 1126–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Dey BK, Mueller AC, Dutta A. Long non-coding RNAs as emerging regulators of differentiation, development, and disease. Transcription 2014, 5: e944014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Jopling C. Liver-specific microRNA-122: Biogenesis and function. RNA Biol 2012; 9: 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Jin Y, Wang J, Han J, et al. MiR-122 inhibits epithelial-mesenchymal transition in hepatocellular carcinoma by targeting Snail1 and Snail2 and suppressing Wnt/β-cadherin signaling pathway. Exp Cell Res 2017; 360: 210–217. [DOI] [PubMed] [Google Scholar]
- 92. Dang YW, Zeng J, He RQ, et al. Effects of miR-152 on cell growth inhibition, motility suppression and apoptosis induction in hepatocellular carcinoma cells. Asian Pac J Cancer Prev 2014; 15: 4969–4976. [DOI] [PubMed] [Google Scholar]
- 93. Zhao Z, Hu Y, Shen X, et al. HBx represses RIZ1 expression by DNA methyltransferase 1 involvement in decreased miR-152 in hepatocellular carcinoma. Oncol Rep 2017; 37: 2811. [DOI] [PubMed] [Google Scholar]
- 94. Zhou L, Yang ZX, Song WJ, et al. MicroRNA-21 regulates the migration and invasion of a stem-like population in hepatocellular carcinoma. Int J Oncol 2013; 43: 661–669. [DOI] [PubMed] [Google Scholar]
- 95. Li R, Qian N, Tao K, et al. MicroRNAs involved in neoplastic transformation of liver cancer stem cells. J Exp Clin Cancer Res 2010; 29: 169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Chen Z, Ma T, Huang C, et al. The pivotal role of microRNA-155 in the control of cancer. J Cell Physiol 2014; 229: 545–550. [DOI] [PubMed] [Google Scholar]
- 97. Han ZB, Chen HY, Fan JW, et al. Up-regulation of microRNA-155 promotes cancer cell invasion and predicts poor survival of hepatocellular carcinoma following liver transplantation. J Cancer Res Clin Oncol 2012; 138: 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Caiazza C, Mallardo M1. The roles of miR-25 and its targeted genes in development of human cancer. Microrna 2016; 5: 113–119. [DOI] [PubMed] [Google Scholar]
- 99. Wang C, Wang X, Su Z, et al. miR-25 promotes hepatocellular carcinoma cell growth, migration and invasion by inhibiting RhoGDI1. Oncotarget 2015; 6: 36231–36244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wong CM, Wei L, Au SL, et al. MiR-200b/200c/429 subfamily negatively regulates Rho/ROCK signaling pathway to suppress hepatocellular carcinoma metastasis. Oncotarget 2015; 6: 13658–13670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Tsai SC, Lin CC, Shih TC, et al. The miR-200b-ZEB1 circuit regulates diverse stemness of human hepatocellular carcinoma. Mol Carcinog 2017; 56: 2035–2047. [DOI] [PubMed] [Google Scholar]
- 102. Barh D, Malhotra R, Ravi B, et al. MicroRNA let-7: an emerging next-generation cancer therapeutic. Curr Oncol 2010; 17: 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sun Z, Meng C, Wang S, et al. microRNA-1246 enhances migration and invasion through CADM1 in hepatocellular carcinoma. BMC Cancer 2014; 14: 616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ng KT, Lo CM, Wong N, et al. Earlyphase circulating miRNAs predict tumor recurrence and survival in hepatocellular carcinoma patients after liver transplantation. Oncotarget 2016; 7: 19824–19839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Cui M, Xiao Z, Wang Y, et al. Long noncoding RNA HULC modulates abnormal lipid metabolism in hepatoma cells through an miR-9-mediated RXRA signaling pathway. Cancer Res 2015; 75: 846–857. [DOI] [PubMed] [Google Scholar]
- 106. Cui M, Zheng M, Sun B, et al. A long noncoding RNA perturbs the circadian rhythm of hepatoma cells to facilitate hepatocarcinogenesis. Neoplasia 2015; 17: 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Tripathi V, Shen Z, Chakraborty A, et al. Long noncoding RNA MALAT1 controls cell cycle progression by regulating the expression of oncogenic transcription factor B-MYB. PLoS Genet 2013; 9: e1003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Tsang WP, Wong TW, Cheung AH, et al. Induction of drug resistance and transformation in human cancer cells by the noncoding RNA CUDR. RNA 2007; 13: 890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Gui X, Li H, Li T, et al. Long noncoding RNA CUDR regulates HULC and β-Catenin to govern human liver stem cell malignant differentiation. Mol Ther 2015; 23: 1843–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Li T, Zheng Q, An J, et al. SET1A cooperates with CUDR to promote liver cancer growth and hepatocyte-like stem cell malignant transformation epigenetically. Mol Ther 2016; 24: 261. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 111. Ye P, Wang T, Liu W H, et al. Enhancing HOTAIR/MiR-10b drive liver normal stem cells to arise malignant transformation tendency through inducing epithelial-mesenchymal transitions. Rejuvenation Res 2015; 18. [DOI] [PubMed] [Google Scholar]
- 112. Liu F, Hua P, Xia G, et al. Prognostic and clinicopathological significance of long noncoding RNA H19 overexpression in human solid tumors: evidence from a meta-analysis. Oncotarget 2016; 7: 83177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Matouk IJ, DeGroot N, Mezan S, et al. The H19 non-coding RNA is essential for human tumor growth. PLoS One 2007; 2: e845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Conigliaro A, Costa V, Dico A L, et al. CD90+ liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Mol Cancer 2015; 14: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wu L, Zhang L, Zheng S. Role of the long non-coding RNA HOTAIR in hepatocellular carcinoma. Oncol Lett 2017; 14: 1233–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. An J, Wu M, Xin X, et al. Inflammatory related gene IKKα, IKKβ, IKKγ cooperates to determine liver cancer stem cells progression by altering telomere via heterochromatin protein 1-HOTAIR axis. Oncotarget 2016; 7: 50131–50149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Zhang L, Li H, Ge C, et al. Inhibitory effects of transcription factor Ikaros on the expression of liver cancer stem cell marker CD133 in hepatocellular carcinoma. Oncotarget 2014; 5: 10621–10635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Kang JH, Choi MY, Cui YH, et al. Regulation of FBXO4-mediated ICAM-1 protein stability in metastatic breast cancer. Oncotarget 2017; 8: 83100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Gedaly R, Galuppo R, Daily MF, et al. Targeting the Wnt/β-catenin signaling pathway in liver cancer stem cells and hepatocellular carcinoma cell lines with FH535. PLoS One 2014; 9: e99272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. He G, Cao X, He M, et al. Casticin inhibits self-renewal of liver cancer stem cells from the MHCC97 cell line. Oncol Lett 2014; 7: 2023–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Quan MF, Xiao LH, Liu ZH, et al. 8-bromo-7-methoxychrysin inhibits properties of liver cancer stem cells via downregulation of β-catenin. World J Gastroenterol 2013; 19: 7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Wang Q, Chen X, Hay N. Akt as a target for cancer therapy: more is not always better (lessons from studies in mice). Br J Cancer 2017; 117: 159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Peng YC, Lu SD, Zhong JH, et al. Combination of 5-fluorouracil and 2-morphilino-8-phenyl-4H-chromen-4-one may inhibit liver cancer stem cell activity. Tumor Biol 2016; 37: 10943. [DOI] [PubMed] [Google Scholar]
- 124. Gedaly R1, Galuppo R, Musgrave Y, et al. PKI-587 and sorafenib alone and in combination on inhibition of liver cancer stem cell proliferation. J Surg Res 2013; 185: 225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Yuan J, Zhang F, Niu R. Multiple regulation pathways and pivotal biological functions of STAT3 in cancer. Sci Rep 2015; 5: 17663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Low HB, Zhang Y. Regulatory roles of MAPK phosphatases in cancer. Immune Netw 2016; 16: 85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Masliah-Planchon J, Garinet S, Pasmant E. RAS-MAPK pathway epigenetic activation in cancer: miRNAs in action. Oncotarget 2016; 7: 38892–38907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kitanaka C, Sato A, Okada M. JNK signaling in the control of the tumor-initiating capacity associated with cancer stem cells. Genes Cancer 2013; 4: 388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Levine B. Autophagy in mammalian development and differentiation. Nat Cell Biol 2010; 12: 823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Galluzzi L, Pietrocola F, BravoSan Pedro JM, et al. Autophagy in malignant transformation and cancer progression. Embo J 2015; 34: 856–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Puri P, Chandra A. Autophagy modulation as a potential therapeutic target for liver diseases. J Clin Exp Hepatol 2014; 4: 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Hung TM, Yuan RH, Huang WP, et al. Increased autophagy markers are associated with ductular reaction during the development of cirrhosis. Am J Pathol 2015; 185: 2454–2467. [DOI] [PubMed] [Google Scholar]
- 133. Li J, Hu SB, Wang LY, et al. Autophagy-dependent generation of Axin2+ cancer stem-like cells promote hepatocarcinogenesis in liver cirrhosis. Oncogene 2017; 36: 6725–6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Song YJ, Zhang SS, Guo XL, et al. Autophagy contributes to the survival of CD133+ liver cancer stem cells in the hypoxic and nutrient-deprived tumor microenvironment. Cancer Lett 2013; 339: 70–81. [DOI] [PubMed] [Google Scholar]
- 135. Mauthe M, Orhon I, Rocchi C, et al. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018; 14: 1435–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Alemany R. Oncolytic adenoviruses in cancer treatment. Biomedicines 2014; 2: 36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Zhang X, Meng S, Rong Z, et al. GP73-regulated oncolytic adenoviruses possess potent killing effect on human liver cancer stem-like cells. Oncotarget 2016; 7: 29346–29358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Zhang J, Lai W, Li Q, et al. A novel oncolytic adenovirus targeting Wnt signaling effectively inhibits cancer-stem like cell growth via metastasis, apoptosis and autophagy in HCC models. Biochem Biophys Res Commun 2017; 491: 469–477. [DOI] [PubMed] [Google Scholar]
- 139. Jia Q, Zhang X, Deng T, et al. Positive correlation of Oct4 and ABCG2 to chemotherapeutic resistance in CD90+CD133+ liver cancer stem cells. Cell Reprogram 2013; 15: 143. [DOI] [PubMed] [Google Scholar]
- 140. Zhou J, Chen R, Deng X. Knockdown of Nanog reverses the chemoresistance to doxorubicin in liver cancer stem-like cells by reducing ABCB1 and ABCG2 expression. J Hepatol 2015; 62: S417–S418. [Google Scholar]
- 141. Zhu P, Wang Y, He L, et al. ZIC2-dependent OCT4 activation drives self-renewal of human liver cancer stem cells. J Clin Invest 2015; 125: 3795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Tang H, Jin Y, Jin S, et al. Arsenite inhibits the function of CD133+ CD13+ liver cancer stem cells by reducing PML and Oct4 protein expression. Tumour Biol 2016; 37: 14103–14115. [DOI] [PubMed] [Google Scholar]
- 143. Govaere O, Wouters J, Petz M, et al. Laminin-332 sustains chemoresistance and quiescence as part of the human hepatic cancer stem cell niche. J Hepatol 2016; 64: 609. [DOI] [PubMed] [Google Scholar]
- 144. Nio K, Yamashita T, Okada H, et al. Defeating EpCAM+ liver cancer stem cells by targeting chromatin remodeling enzyme CHD4 in human hepatocellular carcinoma. J Hepatol 2015; 63: 1164–1172. [DOI] [PubMed] [Google Scholar]
- 145. Fang T, Lv H, Wu F, et al. Musashi 2 contributes to the stemness and chemoresistance of liver cancer stem cells via LIN28A activation. Cancer Lett 2016; 384: 50–59. [DOI] [PubMed] [Google Scholar]
- 146. Fu B, Wei M, Zhao H, et al. GRAM domain-containing protein 1A (GRAMD1A) promotes the expansion of hepatocellular carcinoma stem cell and hepatocellular carcinoma growth through STAT5. Sci Rep 2016; 6: 31963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Jiang N, Chen W, Zhang JW, et al. Aberrantly regulated dysadherin and B-cell lymphoma 2/B-cell lymphoma 2-associated X enhances tumorigenesis and DNA targeting drug resistance of liver cancer stem cells. Mol Med Rep 2015; 12: 7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Kim JB, Park SY, Kim HR, et al. JNK signaling in hepatocarcinoma cells is associated with the side population upon treatment with anticancer drugs. Mol Med Rep 2015; 11: 263–268. [DOI] [PubMed] [Google Scholar]
- 149. Wang XQ, Ongkeko WM, Chen L, et al. Octamer 4 (Oct4) mediates chemotherapeutic drug resistance in liver cancer cells through a potential Oct4-AKT-ATP-binding cassette G2 pathway. Hepatology 2010; 52: 528–539. [DOI] [PubMed] [Google Scholar]
- 150. Zeineddine D, Hammoud AA, Mortada M, et al. The Oct4 protein: more than a magic stemness marker. Am J Stem Cells 2014; 3: 74–82. [PMC free article] [PubMed] [Google Scholar]
- 151. Guan D, Kao HY. The function, regulation and therapeutic implications of the tumor suppressor protein, PML. Cell Biosci 2015; 5: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Chuang YS, Huang WH, Park SW, et al. Promyelocytic leukemia protein in retinoic acid-induced chromatin remodeling of Oct4 gene promoter. Stem Cells 2011; 29: 660–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Linn DE, Yang X, Sun F, et al. A Role for OCT4 in tumor initiation of drug-resistant prostate cancer cells. Genes Cancer 2010; 1: 908–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Wang ML, Chiou SH, Wu CW. Targeting cancer stem cells: emerging role of Nanog transcription factor. Onco Targets Ther 2013; 6: 1207–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Lorenzini S, Bird TG, Boulter L, et al. Characterisation of a stereotypical cellular and extracellular adult liver progenitor cell niche in rodents and diseased human liver. Gut 2010; 59: 645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Lai AY, Wade PA. Cancer biology and NuRD: a multifaceted chromatin remodelling complex. Nat Rev Cancer 2011; 11: 588–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Friedbichler K, Themanns M, Mueller KM, et al. Growth-hormone-induced signal transducer and activator of transcription 5 signaling causes gigantism, inflammation, and premature death but protects mice from aggressive liver cancer. Hepatology 2012; 55: 941–952. [DOI] [PubMed] [Google Scholar]
- 158. Nam JS, Hirohashi S, Wakefield LM. Dysadherin: a new player in cancer progression. Cancer Lett 2007; 255: 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]