Abstract
Background:
The intestinal vitamin D receptor (VDR) remains poorly characterized in patients with inflammatory bowel disease (IBD).
Methods:
Colonoscopic biopsies and intestinal resection specimens from the terminal ileum, ascending and sigmoid colon, from patients with and without IBD, were analyzed for VDR mRNA quantification by polymerase chain reaction, and protein localization and semi-quantification by immunohistochemistry. The relationship between VDR and intestinal inflammation, serum 25(OH)D and oral vitamin D intake was elicited.
Results:
A total of 725 biopsies from 20 patients with Crohn’s disease (CD), 15 with ulcerative colitis (UC) and 14 non-IBD controls who underwent colonoscopy were studied. VDR gene expression and protein staining intensity was similar across all three groups, and across the intestinal segments. Sigmoid colon VDR mRNA expression inversely correlated with faecal calprotectin (r = −0.64, p = 0.026) and histological score (r = −0.67, p = 0.006) in UC, and histological score (r = −0.58, p = 0.019) in patients with CD. VDR staining intensity was higher in quiescent than diseased segments. No relationship with serum 25(OH)D or oral vitamin D intake was noted. Immunohistochemical staining of 28 intestinal resection specimens from 15 patients (5 each with CD, UC and non-IBD controls) showed diffuse VDR staining in the mucosa, submucosa and circular muscle.
Conclusions:
VDR transcript expression and protein staining intensity are inversely related to inflammation in IBD, but unrelated to serum 25(OH)D, and similar to non-IBD controls. Strategies to upregulate intestinal VDR, potentially translating to modulation of disease activity, require investigation.
Keywords: Crohn’s disease, inflammatory bowel disease, ulcerative colitis, vitamin D, vitamin D receptor
Introduction
There are now extensive in vitro and animal study data outlining various potential mechanisms by which vitamin D may exert an immunomodulatory effect in patients with inflammatory bowel disease (IBD).1–15 Additionally, serum 25(OH)D status has been shown to be inversely proportional to intestinal inflammation as assessed by faecal calprotectin, clinical disease activity indices, endoscopic and histologic activity.16–19 However, some evidence suggests no clear protective effect of vitamin D in IBD,20,21 and with a few exceptions, clinical trials to date using vitamin D supplementation in patients with IBD have been largely negative or underpowered to assess response with respect to improvement in objective markers of disease activity.16,22–27
Most of the actions of the vitamin D axis involve gene regulation following the binding of the vitamin D receptor (VDR) ligand [mainly 1,25 dihydroxy vitamin D (1,25(OH)2D)] to retinoid-X receptor (RXR; VDR–ligand–RXR complex), which then binds vitamin D-responsive elements located predominantly in the promoter regions of target genes.28 In addition, some rapid actions of the VDR–ligand complex involve nongenomic cellular responses such as regulation of voltage-gated calcium and chloride channels in osteoblasts, calcium entry, contractility and myogenesis in skeletal muscle cells, and calcium uptake by intestinal epithelial cells.29,30 Transgenic human VDR expression in mouse intestinal epithelial cells, protected mice from colitis by reducing intestinal epithelial cell apoptosis.3 The VDR TaqI tt genotype was over-represented in patients with Crohn’s disease (CD),31 and was recently associated with lower levels of VDR protein in peripheral blood mononuclear cells and a higher risk of penetrating disease.32
Recent investigation has suggested that the distribution of VDR at the gene and protein level in intestinal tissue in patients with IBD has a relationship with mucosal inflammation. VDR gene expression assessed by cDNA microarrays in 10 biopsies from patients with ulcerative colitis (UC) were reduced compared with biopsies from healthy controls.3 VDR protein levels as measured by western blotting were reduced in four biopsies from areas of quiescent disease in patients with CD and four biopsies from patients with UC, compared with biopsies from healthy controls.3 In a separate study, patients with UC having biopsies 20 cm from the anal verge with serum 25(OH)D levels >25 ng/ml (>62.5 nmol/l) were noted to have less histological inflammation, VDR mRNA expression and semi-quantitative staining intensity for VDR protein than patients with serum 25(OH)D levels <20 ng/ml (<50 nmol/l).17 In a smaller study of 10 patients each with and without IBD, the intensity of immunohistochemical staining of VDR was not significantly different between colonic mucosal segments from non-IBD controls and from visually inflamed or noninflamed segments from patients with IBD. No correlation between serum 25(OH)D level and colonic mucosal VDR staining was noted across all patients in this study.33
Despite the abovementioned studies, several questions regarding VDR in the intestinal wall remain. First, no clear data exist on the relative distribution of the VDR in the terminal ileum and different regions of the colon, in healthy controls and patients with IBD, or across the depth of the intestinal wall in these segments. The relationship between VDR and intestinal inflammation remains unclear. Also, it is important to decipher whether serum 25-hydroxy vitamin D (25(OH)D) status is an accurate reflection of mucosal VDR status, and whether oral vitamin D intake is associated with altered expression of VDR at the intestinal tissue level. The mouse VDR gene locus contains intronic and upstream enhancers that bind 1,25(OH) 2D,34 but it is unknown whether serum 25(OH)D status may influence intestinal mucosal VDR expression in vivo, especially in patients with IBD.
The aims of this study, hence, were to comprehensively characterize, in patients with IBD and non-IBD controls, VDR gene expression, protein localization and semi-quantification in the terminal ileum and colon, and across the mucosal, submucosal and circular muscle layers. Furthermore, it was aimed to elicit the association of these components with inflammation, circulating vitamin D status and oral vitamin D intake. It was hypothesized that VDR mRNA expression and protein levels were lower in patients with IBD, with an inverse correlation with inflammation, and positive association with circulating 25(OH)D and oral vitamin D intake.
Materials and methods
Patients
Patients with CD and UC planned for colonoscopy for assessment of disease activity or dysplasia surveillance were invited to participate, as were non-IBD controls, patients undergoing colonoscopy to screen for bowel cancer or for evaluation of anal outlet bleeding, but not altered bowel habit. Patients with and without IBD undergoing ileal or colonic resection were additionally recruited.
Protocol
Patients with IBD and controls identified for the study were interviewed within the week prior to planned colonoscopy or intestinal resection. Historical information was elicited during the interview and via the patient records. Patients were asked to estimate sunlight exposure as described previously.35 Briefly, self-estimated average hours per week of sunlight exposure with equivalent of face and forearms exposed, during high ultraviolet B (UVB) light exposure months (September to April) and low exposure months (May to August) in Melbourne, Australia (latitude 37.8o South),36 was recorded using arbitrary ranges of <1,1–2, 2–5, 5–10 or >10 h. Dietary intake of vitamin D was assessed by food frequency questionnaire of selected foods highest in vitamin D content according to nutritional tables (NUTTAB) 2010 produced by Food Standards Australia New Zealand as listed in Supplementary Table 1.37 Total daily oral vitamin D intake was then calculated for each participant by the sum of amount of daily vitamin D supplementation and estimated dietary intake based on this questionnaire. Peripheral blood samples and a sample of faeces were obtained. At colonoscopy or intestinal resection, samples of intestinal wall or mucosa were obtained as follows.
Colonoscopy was performed following split-dose polyethylene glycol-based bowel preparation. Intubation to at least 5–10 cm proximal to the ileocaecal valve was attempted in all patients. Macroscopic descriptions of all regions of the ileum and colon visualized were noted. Biopsies using standard forceps were obtained from the ileum (5 cm proximal to the ileocaecal valve), ascending colon (5 cm distal to the ileocaecal valve) and sigmoid colon (25 cm from the anal verge), as well as two other inflamed regions. Immediately after being taken, two biopsies from each region were fixed in 10% neutral buffered formalin for routine histopathological analysis, one fixed in 4% paraformaldehyde in phosphate-buffered saline (Thermofisher Scientific, Scoresby, Australia) stored overnight at 4oC, and two placed in RNAlater solution (Life Technologies, Melbourne, Australia) and stored at −80oC until analysis.
Patients undergoing surgical resection had routine preoperative preparation. At the time of intestinal resection, a 2 × 1 cm full-thickness segment was removed with a sterile scalpel blade under aseptic conditions from the terminal ileum, ascending colon or sigmoid colon where appropriate. For patients having resection for bowel cancer, the site of the incision was at least 8 cm away from the visible and palpable margin of the tumour. The excised segment was washed twice with normal saline, then further divided in full-thickness segments, with four parts placed in 4% paraformaldehyde solution, and four parts placed in RNAlater solution at room temperature for 2 h followed by storage at −80oC until analysis.
Written informed consent was obtained from all participants. The protocol for this study was approved by the Eastern Health Office of Research and Ethics.
Clinical and laboratory measures
Information regarding disease characteristics, gastrointestinal symptoms, comorbid illnesses, medications and lifestyle factors was collected. Patients with IBD were characterized according to the Montreal Classification.38 Blood samples were analyzed for laboratory indices including serum electrolytes, renal and liver function, markers of systemic inflammation (C-reactive protein, white cell count, platelet count and albumin) via routine laboratory techniques, and 25(OH)D via the Elecsys electro-chemiluminescence assay using the Cobas modular analyzer platform (Roche Diagnostics, Castle Hill, Australia) as per manufacturer’s instructions. Intestinal inflammation was assessed by faecal calprotectin using Quantum Blue Calprotectin quantitative lateral flow assay (Bühlmann Laboratories, Schonenbüch, Switzerland) within 1 week of collection, as per manufacturer’s instructions, with values expressed in µg/g faeces. Values were quantified within the range of 30–300 µg/g, with values below this range expressed as <30 µg/g. Specimens with faecal calprotectin >300 µg/g were quantified using Bühlmann Quantum Blue High Range Calprotectin assay following dilution to 1:300.
Quantitative analysis of tissue RNA content
RNA extraction from tissue samples was performed using the RNeasy Plus Universal Mini Kit (Qiagen, Melbourne, Australia) following tissue disruption and homogenization. Briefly, tissue stored in RNAlater solution was thawed, weighed, then disrupted and homogenized using a TissueRuptor rotor-stator homogenizer. Further RNA extraction was performed using the RNeasy Universal Plus Mini Kit according to manufacturer’s instructions. Quantification of RNA in samples was performed using the Quantifluor® RNA System (Promega, Alexandria, Australia) and the Synergy HT Plate Reader (Biotek, Burleigh, Australia) using 485 nm excitation and 528 nm emission wavelengths. All samples were then assessed for RNA quality by gel electrophoresis, imaged using Bio-rad VersaDoc Imaging System with Quantity One version 4.6.7 software for purity and presence of RNA degradation products.
Reverse transcription of all RNA samples was performed using the SuperScript III First-Strand Synthesis Supermix for polymerase chain reaction (quantitative real time-PCR; Invitrogen, Mount Waverley, Australia). qRT-PCR for the VDR gene was carried out using multiplexing where both the target gene and endogenous reference gene were amplified in a single well. SYBR Green primers were designed using the Primer Express version 3 software program (Applied Biosystems, Foster City, CA, USA; Supplementary Table 1). Melt curve analysis and agarose gel electrophoresis of the amplicon indicated that a single product was amplified. Taqman predeveloped eukaryotic 18S ribosomal RNA (18S rRNA)-VIC (Probe and Primer Ltd.) was used as the endogenous reference gene (Applied Biosystems). Single reactions were carried for VDR1 using 1× SYBR buffer, 1 µl cDNA (containing 3–25 ng/µl of cDNA) and 250 nmol/l of forward and reverse primer and in separate reaction wells, 1 µl cDNA (containing 3–25 ng/µl of cDNA), predeveloped endogenous 18S rRNA-VIC mix and Platinum QPCR supermix-UDG with ROX as the passive reference dye (Invitrogen). All thermal cycling reactions were run on an Applied Biosystems 7500 Real Time PCR machine using SDS version 2.0.6 software, with at 95°C for 20 s followed by 50 cycles at 95°C for 3 s (denaturing) and 60°C for 30 s (annealing and extension). Each sample was run and analyzed in duplicate.
Quantification was performed using the threshold cycle (Ct) for the gene of interest and housekeeping gene in each multiplex reaction. The delta (Δ) Ct was calculated by subtracting the Ct of 18S rRNA-VIC from the gene of interest paired with the dye FAM. The median of the terminal ileal non-IBD control tissue Ct was then determined and subtracted from all values to obtain the delta-delta Ct. The expression of the target genes relative to healthy control terminal ileal tissue was evaluated using fold induction calculated as 2−(DDCt).
Immunohistochemistry
Colonoscopic biopsies and resection specimens were stored in 4% paraformaldehyde overnight at 4oC and were processed using Leica PELORIS Rapid Tissue Processor (Leica Biosystems, North Ryde, Australia), then paraffin embedded. Sections 3 µm thick were cut and placed on poly-L-lysine-coated slides (Thermofisher Scientific). For immunohistochemical analysis, slides were heated, dewaxed and endogenous peroxidase blocked with 3% hydrogen peroxide. Antigen retrieval was performed by microwave heating for 5 min in citrate buffer (pH 6.0). Nonspecific binding was blocked using Dako Antibody Diluent (Agilent Technologies, Mulgrave, Australia) and primary VDR mouse monoclonal antibody diluted to 1/500 [Santa Cruz VDR antibody (SC-13133)] added. After overnight incubation, secondary antibody incubation for 60 min was performed, followed by diaminobenzidine (DAB+) chromogenic substrate (Dako). Counterstaining in Mayer’s haematoxylin was performed prior to mounting.
For semi-quantitative immunohistochemical analysis, slides were photographed using Aperio Scanscope AT Turbo (Leica Biosystems, Mount Waverley, Australia). Magnified images were analyzed using ImageJ version 1.47 microscope image processing software (National Institutes of Health, Bethesda, MD, USA). Up to three images from each colonoscopic biopsy specimen were captured, then colour deconvolution was performed to identify DAB staining. The mucosal area was manually selected and quantification of particle density as a percentage of the total area highlighted performed. The mean percentage readings from each biopsy image were compared across specimens. For intestinal resection specimens, three readings from each of mucosa, submucosa and circular muscle were obtained, and analyzed in the same manner specific to location.
Qualitative analysis of the anatomical location and patterns of antigenic expression within different tissues was also performed. The assessment of histological activity in the tissue sections was independently graded by two experienced gastrointestinal histopathologists (PH, SM), with scores averaged in cases of disagreement. Individual colonic biopsies from patients with Crohn’s disease were scored using an index adapted from D’Haens and colleagues39 (Supplementary Table 2), and biopsies from patients with UC were scored using the Geboes Score40 (Supplementary Table 3).
Statistical considerations
Statistical analyses were performed using SPSS version 23 (IBM Corporation, 2015) and GraphPad Prism version 7.02 (GraphPad software, 2016). Analysis of variance and unpaired Student’s t-tests (two-sided) were used for comparison of means between groups, and independent samples z-tests for comparison of proportions between groups. The Kruskal–Wallis test was used to compare nonparametric variables between groups. The relationship between variables was assessed by bivariate and partial correlation using Pearson’s coefficient for parametric and Spearman’s coefficient for nonparametric variables as appropriate. Values for faecal calprotectin were normalized by log transformation. A p value of 0.05 or less was considered statistically significant.
Ethical considerations
The protocol for this study was approved by the Office of Research and Ethics at Eastern Health (E03-1112) and was performed in accordance with Australian regulations and the principles of the Declaration of Helsinki 1954 and its later amendments. Informed consent was obtained from all individual participants included in this study.
Results
Colonoscopic biopsy tissue
Patients
A total of 20 patients with CD, 15 with UC and 16 non-IBD controls planned for colonoscopy were recruited. Overall, two non-IBD controls were excluded from all analyses due to the presence of terminal ileal mucosal inflammation in one and a markedly elevated faecal calprotectin (2218 µg/g) in the other. Therefore, 14 non-IBD controls with normal ileocolonoscopy, apart from haemorrhoids in some, with normal biopsies on histology, were selected. As shown in Table 1, non-IBD controls were slightly older than patients with IBD. Significantly more patients with IBD were taking vitamin D supplementation (p = 0.003), and subsequently tended to have higher total daily oral vitamin D intake (p = 0.061). None of the patients were on anticonvulsant medications that may influence serum vitamin D levels. No difference in self-estimated sunlight exposure in the high ultraviolet (UV) index at latitude 37.8 South (September to April) or low UV dose (May to August) were noted across the groups (data not shown).
Table 1.
Baseline characteristics of patients undergoing colonoscopy. (Fitzpatrick skin types: I, pale white skin, blue/hazel eyes, blond/red hair; II, fair skin, blue eyes; III, darker white skin; IV, light brown skin; V, brown skin; VI, dark brown or black skin).
| Crohn’s disease (n = 20) | Ulcerative colitis (n = 15) | Non-IBD controls (n = 14) | p value | |
|---|---|---|---|---|
| Age, mean (range) years | 42 (22–69) | 40 (23–68) | 50 (23–70) | 0.092a |
| Female:male | 8:12 | 5:10 | 7:7 | 0.308a |
| Comorbid illnesses, n | ||||
| Hypertension | 4 | 1 | 1 | |
| Hyperlipidaemia | 1 | 2 | 1 | |
| Type 2 diabetes mellitus | 0 | 2 | 1 | |
| Chronic kidney disease | 1 | 0 | 0 | |
| Viral hepatitis | 1 | 0 | 0 | |
| Ethnicity, n (%) | ||||
| Australian and New Zealander | 13 (65) | 9 (60) | 8 (57) | |
| Northern and Western European | 2 (10) | 1 (7) | 1 (7) | |
| Southern European | 3 (15) | 2 (13) | 1 (7) | |
| Eastern European | 0 (0) | 1 (7) | 2 (14) | |
| Eastern and South-East Asian | 0 (0) | 0 (0) | 1 (7) | |
| Indian Subcontinental | 1 (5) | 1 (7) | 0 (0) | |
| Arab and Middle Eastern | 1 (5) | 1 (7) | 0 (0) | |
| Jewish | 0 (0) | 0 (0) | 1 (7) | |
| South and Central American | 0 (0) | 0 (0) | 1 (7) | |
| Fitzpatrick skin type, n (%) | ||||
| I | 4 (7) | 1 (2) | 3 (8) | |
| II | 35 (63) | 29 (64) | 22 (56) | |
| III | 15 (27) | 11 (24) | 12 (31) | |
| IV | 2 (4) | 3 (7) | 0 (0) | |
| V | 0 (0) | 1 (2) | 2 (5) | |
| VI | 0 (0) | 0 (0) | 0 (0) | |
| Smoking status, n (%) | ||||
| Never smoked | 4 (20) | 10 (67) | 5 (36) | |
| Ex-smokers | 10 (50) | 3 (20) | 7 (50) | |
| Current smokers | 6 (30) | 2 (13) | 2 (14) | |
| Body mass index, mean (95% CI), kg/m2 | 27.4 (24.4–30.4) | 29.0 (25.0–32.9) | 27.8 (24.6–31.0) | 0.769a |
| Waist circumference, mean (95% CI), cm | 92.2 (85.5–99.0) | 98.4 (89.0–107.8) | 97.1 (88.6–105.7) | 0.456a |
| Systolic BP, mean (95% CI), mmHg | 116 (111–122) | 119 (112–126) | 123 (116–131) | 0.277a |
| Heart rate, mean (95% CI), beats/min | 76 (71–81) | 73 (67–80) | 69 (62–76) | 0.237a |
| Vitamin D supplementation, n (%) | 14 (70) | 11 (73) | 3 (21) | 0.003 c |
| Estimated total dietary vitamin D intake, mean (range), IU/day | 1342 (31–5413) | 1005 (24–3229) | 349 (14–1091) | 0.061a |
Analysis of variance.
Kruskal–Wallis test.
Chi-squared, IBD versus healthy controls.
BP, blood pressure; CI, confidence interval; IBD, inflammatory bowel disease.
Disease characteristics of the patients with IBD are as outlined in Table 2. For CD, most patients had colonic or ileocolonic disease and just under half had nonstricturing, nonpenetrating disease, 12 of 20 patients had active disease, and 7 previously had resectional surgery. For UC, all patients had left-sided or extensive colitis. Routine laboratory indices are presented in Table 3. There was no difference in serum 25(OH)D level across the groups.
Table 2.
Characteristics of patients with IBD undergoing colonoscopy.
| Crohn’s disease (n = 20) | Ulcerative colitis (n = 15) | |||
|---|---|---|---|---|
| Montreal classification, n (%) | Age at diagnosis, years | Disease extent | ||
| <17 | 2 (10) | Proctitis | 0 (0) | |
| 17–40 | 15 (75) | Left-sided colitis | 9 (60) | |
| >40 | 3 (15) | Extensive colitis | 6 (40) | |
| Location | Disease severity | |||
| Ileal | 2 (10) | Clinical remission | 6 (40) | |
| Colonic | 9 (45) | Mild | 4 (27) | |
| Ileocolonic | 9 (45) | Moderate | 5 (33) | |
| Upper gastrointestinal | 0 (0) | Severe | 0 (0) | |
| Behaviour | ||||
| Nonstricturing, nonpenetrating | 9 (45) | |||
| Stricturing | 3 (15) | |||
| Penetrating/fistulizing | 8 (40) | |||
| Perianal | 8 (40) | |||
| Clinical disease activity |
Harvey Bradshaw index
median (range) |
5 (0–18) |
Simple clinical colitis activity index
median (range) |
3 (0–8) |
| Medical therapy, n (%) | Nil | 1 (5) | 1 (7) | |
| 5-ASA only | 2 (10) | 6 (40) | ||
| Steroids ± 5-ASA | 0 (0) | 1 (7) | ||
| Azathioprine/6-MP ± 5-ASA / steroids | 7 (35) | 6 (40) | ||
| Methotrexate ± 5-ASA/ steroids | 2 (10) | 1 (7) | ||
| Infliximab/adalimumab ± 5-ASA/steroids | 1 (5) | 0 (0) | ||
| Infliximab/adalimumab ± immunomodulators | 7 (35) | 0 (0) | ||
| Previous intestinal surgery, n (%) | Single ileocolonic resection | 5 (25) | ||
| Multiple ileocolonic resections | 2 (10) | |||
5-ASA, 5-aminosalicylates; 6-MP, 6-mercaptopurine; IBD, inflammatory bowel disease.
Table 3.
Routine laboratory indices in patient with IBD and non-IBD controls.
| Crohn’s disease (n = 20) | Ulcerative colitis (n = 15) | Non-IBD controls (n = 14) | p value | |
|---|---|---|---|---|
| Haemoglobin, mean (range), g/l | 131 (110–159) | 138 (115–169) | 142 (122–162) | 0.095a |
| White cell count, mean (range), × 109/l | 7.6 (3.5–12.7) | 6.2 (3.0–9.4) | 6.6 (4.5–9.6) | 0.192a |
| Platelet count, mean (range), × 109/l | 269 (151–424) | 255 (140–360) | 223 (129–301) | 0.141a |
| Serum albumin, mean (range), g/l | 39 (28–43) | 41 (36–47) | 40 (35–44) | 0.075a |
| Serum C-reactive protein, median (range), mg/l | 2.0 (<2.0–38) | 2.0 (<2.0–12) | <2.0 (<2.0–7) | 0.264b |
| Faecal calprotectin, median (range), µg/g | 104 (<30–5412) | 83 (<30–96389) | 40 (<30–278)d | 0.112b |
| Log faecal calprotectin, mean (range) | 2.18 (<1.48–3.88) | 2.17 (<1.48–3.92) | 1.74 (<1.48–2.21) | 0.165a |
| 25(OH) vitamin D, mean (range), nmol/l | 67 (26–127) | 65 (14–130) | 65 (24–102) | 0.936a |
Analysis of variance.
Kruskal–Wallis test.
VDR mRNA expression.
IBD, inflammatory bowel disease; VDR, vitamin D receptor.
Expression of mRNA for VDR was similar across the terminal ileum, ascending and sigmoid colon (Figure 1). No significant difference in such expression across the groups of participants with CD, UC or non-IBD controls was noted. No correlation between age and expression of VDR mRNA was seen, and no differences between males and females across the whole cohort, or subgroups of patients with IBD or non-IBD, were noted (independent samples t-test, data not shown).
Figure 1.
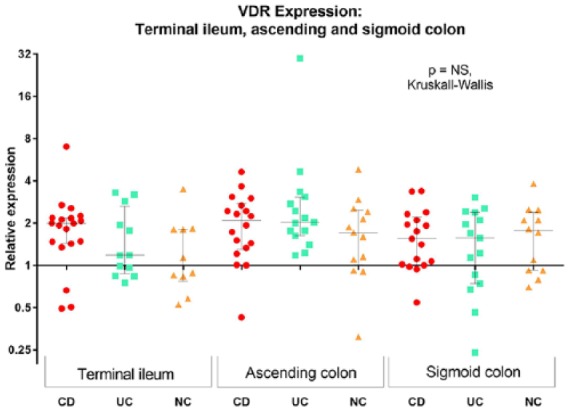
Colonoscopic biopsy VDR gene expression among patients with IBD and non-IBD controls in the terminal ileum, ascending colon and sigmoid colon. Expression is relative to the median of the non-IBD control terminal ileal specimens. Circles represent patients with CD, squares patients with UC and triangles non-IBD controls (NC).
CD, Crohn’s disease; IBD, inflammatory bowel disease; NC, non-IBD controls; UC, ulcerative colitis; VDR, vitamin D receptor.
There was no significant relationship between serum 25(OH)D and VDR gene expression across all participants (Figure 2), or in subgroups with CD, UC or in non-IBD controls (data not shown). There was no correlation between mucosal VDR mRNA expression and total oral vitamin D intake (Supplementary Figure 1) or sun exposure (data not shown).
Figure 2.
Correlation of intestinal VDR mRNA expression with serum 25(OH)D level across all participants. Expression is relative to the median of the non-IBD control terminal ileal specimens.
IBD, inflammatory bowel disease; VDR, vitamin D receptor.
A significant inverse correlation between VDR mRNA expression in the sigmoid colon and faecal calprotectin was noted in patients with UC (Figure 3). A similar trend was noted among patients with CD in the terminal ileum and sigmoid colon. VDR mRNA expression also significantly correlated with the histological scores of inflammation both in patients with CD and UC [Figure 3(c) and (d)]. No such correlation was observed in the ascending colon, but there were fewer inflamed biopsies from this segment (n = 4 for CD, n = 1 for UC, data not shown).
Figure 3.
Relationship between intestinal VDR mRNA expression and intestinal inflammation. Relationship between VDR expression and faecal calprotectin in patients with (a) CD and (b) UC; and relationship between VDR mRNA expression and degree of histological remission in patients with (c) CD and (d) UC. Expression is relative to the median of the non-IBD control terminal ileal specimens.
CD, Crohn’s disease; IBD, inflammatory bowel disease; UC, ulcerative colitis; VDR, vitamin D receptor.
VDR protein expression
Semi-quantitative immunohistochemical staining intensity for VDR protein was similar across patients with CD, UC and non-IBD controls (Supplementary Figure 2). Analogous to the findings for gene expression by PCR, there were no relationships between serum 25(OH)D or total oral vitamin D intake and semi-quantitative VDR staining intensity (data not shown). VDR staining intensity in noninflamed colonic segments from patients with IBD were significantly higher than in inflamed segments, and higher than in specimens from non-IBD controls (Figure 4). Representative immunohistochemical images are shown in Supplementary Figure 3.
Figure 4.

VDR immunohistochemical staining of mucosa of colonoscopic biopsies in inflamed and noninflamed colon. Staining is expressed as the percentage of DAB+ particle density identified at an arbitrary detection threshold using ImageJ image processing software.
DAB, diaminobenzidine; VDR, vitamin D receptor.
Surgically-resected intestinal tissue
Patients
Resection specimens from five patients each with CD, UC and non-IBD controls were analyzed for transmural VDR mRNA expression and immunohistochemical staining. The characteristics of the patients are outlined in Supplementary Tables 4 and 5. A total of two of the non-IBD controls had right hemicolectomies for right sided adenocarcinoma, one patient had an anterior resection for sigmoid adenocarcinoma, one patient had an anterior resection for a high recto-vaginal diverticular fistula, and one patient had an anterior resection for sigmoid cancer and elected to undergo a right hemicolectomy for an advanced ascending colon polyp concurrently. Terminal ileal specimens were collected from five patients with CD, four with UC and three non-IBD controls; ascending colon specimens from four patients with CD, four with UC and three non-IBD controls; and sigmoid colon specimens from three with CD, five with UC and three non-IBD controls. Ascending and sigmoid colon specimens from one patient with CD did not include mucosal, submucosal and circular muscle layers due sampling error, and were therefore omitted from further analyses.
VDR mRNA expression
There was no significant variation in overall expression of VDR mRNA across terminal ileal, ascending and sigmoid colonic segments, nor were there any significant differences between patients with CD, UC and non-IBD controls, with limited numbers in these analyses (Supplementary Figure 4).
VDR protein expression
Representative images are shown in Figure 5. Diffuse nuclear staining of VDR throughout all layers of the intestinal wall in the terminal ileum and colon was seen. Within the mucosa, this was strongest in the epithelial cells, both enterocytes and goblet cells. Qualitatively, staining in the terminal ileal epithelium from patients with CD was noticeably less intense than among that from patients with UC and non-IBD controls. A similar pattern was noted in the colon, where epithelial staining from patients with CD and UC was less intense than that from non-IBD controls. In the colonic epithelium, there was a gradient of higher staining in mature apical epithelial cells compared with basal crypt epithelium, which appeared exaggerated in inflamed epithelium from patients with IBD.
Figure 5.
Representative immunohistochemical images of VDR in the terminal ileum, ascending colon and sigmoid colon among patients with CD, UC and non-IBD controls (a) terminal ileum; (b) ascending colon; and (c) sigmoid colon. Images represent samples with the median of the semi-quantification.
CD, Crohn’s disease; IBD, inflammatory bowel disease; UC, ulcerative colitis; VDR, vitamin D receptor.
The nuclei of stromal cells, as well as the vascular endothelium of the lamina propria, submucosa and myocytes, also stained for VDR, but less intensely than epithelial cells. Colonic myocytes from some patients stained strongly for VDR throughout the muscle fibres, especially nuclei [non-IBD controls, Figure 5(b) and (c)].
Staining for VDR by immunohistochemistry was most dense in the mucosa as compared with the submucosa and circular muscle, though this was highly variable across different participants (Supplementary Figure 5).
Discussion
One key to defining the role of vitamin D in immunoregulation in the gastrointestinal tract is understanding the expression and localization of its putative receptor, the VDR, in the intestine. Previous studies have been fragmentary in scope and have involved very small cohorts. The current study comprehensively studied VDR in the intestine of larger, prospectively-collected cohorts of patients without intestinal inflammation and of a well-characterized cohort of patients with IBD. While the results overall showed no clear differences in gene and protein expression of VDR or in the tissue and cellular localization between patients with IBD and non-IBD controls, study within a more homogeneous group of patients with IBD indicated that a greater level of inflammation was associated with lower expression of VDR gene and protein that was especially evident within the sigmoid colon.
It is worth noting that most patients who underwent colonoscopy with biopsies had no or mild inflammation on histological analysis, with most colonic inflamed specimens arising from the sigmoid colon. The differences between inflamed and noninflamed mucosa in patients with IBD and non-IBD controls were, therefore, more clearly elicited in biopsies from this region of the colon. A significant inverse relationship between VDR mRNA expression and faecal calprotectin, as well as histological grading of inflammation, in colonic biopsy specimens, was demonstrated. Notably, accumulating evidence suggests that the epithelial VDR may protect against inflammation via regulation of permeability,2,23 protection against apoptosis,3,4,41 and stimulation of Paneth cell secretion of antimicrobial peptides.5–7 Intestinal epithelial cell deletion of VDR in mice was associated with an abnormal Paneth cell phenotype, reduced lysozyme secretion, reduced ATG16L1 mRNA and protein levels, and increased susceptibility to dextran sodium sulfate colitis.42 Paneth cell specific α- and β-defensin secretion in high-fat-diet fed mice were reduced in the presence of vitamin D deficiency.7 Therefore, a reduction in mucosal VDR demonstrated in this study may predispose to perpetuating inflammation in patients with IBD. Data from mice suggest that VDR deletion may also influence the intestinal microbiome, with increased abundance of Helicobacter hepaticus, and reduced abundance of Akkermansia muciniphila7, and increased Escherichia coli and Bacteroides with reduction in the butyrate-producing bacteria Butyrivibrio previously reported.42 However, whether this translates into an effect in humans is uncertain. High dose oral vitamin D supplementation in patients with UC was shown to be associated with a significant increase in Enterobacteriaceae abundance, but no change in overall diversity or other specific bacteria were found, despite a significant reduction in faecal calprotectin in patients with active UC.22
Higher VDR mRNA expression and immunohistochemical staining in noninflamed areas in patients with IBD compared with inflamed areas and biopsies from non-IBD controls is a paradox that required further exploration. It may be postulated that an upregulation of VDR in noninflamed tissue occurs as a compensatory mechanism to counter-regulate inflammation. The subsequent significant reduction in VDR in inflamed tissue may reflect a deficiency of this ability, or downregulation through other mechanisms in susceptible patients. This is supported by an inverse correlation between VDR mRNA expression and faecal calprotectin as well as histological inflammation. A third possibility is an artefactual reduction noted due to epithelial denudation in areas of ulceration, but this is not supported by the immunohistochemical analysis of the inflamed specimens, which, despite inflammation, had an intact epithelium.
The finding of nuclear VDR diffusely expressed in cells throughout the depth of the intestinal wall in both terminal ileum and colon in the resection specimens from all patients adds weight to the likely multiple roles of this protein in cellular homeostasis. Paneth cells are expressed in the small intestine, so VDR may exert other effects in the colon. Among the roles ascribed to VDR in various studies include submucosal stromal and immune cells function, and regulation of calcium-mediated myocyte contraction.5,14,29,43–45
A further interesting finding from this study was the lack of relationship between intestinal VDR gene expression and serum 25(OH)D status, the main marker of vitamin D stores in the human body. 1,25(OH)2D, the product of 25(OH)D, has been demonstrated to upregulate VDR expression in glomerular epithelial cells,46 and, therefore, it may be hypothesized that VDR gene expression and protein levels in various tissues around the body may be influenced by total body 25(OH)D stores. This was not found to be the case for terminal ileal and colonic VDR in this study. There are potentially many confounders to this finding, but it raises the possibility of regulation of intestinal VDR through vitamin D-independent mechanisms. Delving into this further, there was no significant relationship between 25(OH)D and faecal calprotectin (r = −0.05, p = 0.776) in the cohort of 35 patients with IBD analyzed in this study. This contrasts with previous studies,17,18,47 where an inverse relationship between serum 25(OH)D and intestinal inflammation was noted and is thus difficult to reconcile. A longitudinal study to assess change in intestinal VDR gene expression following vitamin D supplementation in patients with IBD would be useful, as would investigation of other ways to stimulate VDR expression, if indeed this is required for immunomodulatory effect. Though targeting a specific serum 25(OH)D level of 100–125 nmol/l did not result in a reduction of objective markers of disease activity in a pilot study of patients with IBD,16 giving high dose weekly oral vitamin D was associated with a reduction in faecal calprotectin in another study,22 raising the possibility of a dose effect on immunomodulation. On the other hand, local delivery of VDR agonists may be a more suitable approach, bypassing the need for upregulation of endogenous VDR. Measurement of intestinal mucosal or submucosal levels of 25(OH)D, or the main physiologically active 1,25(OH)2D, has not previously been reported, and may provide another interesting avenue for study.
The strengths of this study lie first in the size of the cohorts examined. The two previous studies reporting on expression of VDR mRNA and/or protein at the tissue level, both suggesting reduced expression in patients with IBD, have been performed in small cohorts where in many of the analyses the number of biopsies assessed were stated but not the number of patients.3,33 The variability noted in the key indices measured in the present study underlie the hazards in making conclusions from a small number of observations in heterogeneous samples. A second strength lies in the detail and breadth of the characterization of the current prospectively-assessed cohort covering potential confounders. For instance, serum vitamin D levels, inflammatory markers, dietary intake and other clinical characteristics were concurrently documented using a methodical and consistent approach. However, there are multiple methodological issues that require consideration. Variation in the proportions of mucosa, submucosa and circular muscle in full-thickness resection specimens, and variability in depth of colonoscopic biopsies may confound results, especially given the highest expression was in the epithelial layer. Most significant findings were noted in colonic, especially sigmoid colonic, specimens. Very few specimens from the ascending colon were inflamed in patients undergoing colonoscopy, and similarly numbers were limited from terminal ileal inflamed segments to ascertain significant differences. This limits the ability to decipher whether any immunomodulatory functions of vitamin D vary across intestinal segments.
The staining intensity of VDR was considerably lower in colonoscopic biopsies than for resection specimens, with the same dilution of primary antibody of 1/500 used for all specimens. Satisfactory staining was still noted, and qualitative comparison across different specimens and between groups was still valid in this study. Western blotting for VDR protein quantification is a superior technique than the semi-quantification provided by immunohistochemical staining intensity used in this study and is an avenue for further investigation. Analysis of proinflammatory cytokines associated with inflammation, and ultrastructural localization of the VDR in Paneth cells as well as other immune cells would be useful for further analysis.
In conclusion, VDR gene expression and protein localization is similar in patients with CD, UC and non-IBD controls, but levels are inversely related to inflammation in patients with IBD and higher in quiescent compared with active diseased segments. VDR may be upregulated as a compensatory mechanism, and a deficiency to upregulate may predispose to intestinal inflammation. Serum 25(OH)D status is unrelated to intestinal VDR gene expression or protein level as assessed by immunohistochemistry, but whether vitamin D supplementation increases intestinal VDR expression, and therefore influences disease activity in patients with IBD, or novel VDR agonists are a more effective approach, require further investigation.
Supplemental Material
Supplemental material, Supplementary_Material for The intestinal vitamin D receptor in inflammatory bowel disease: inverse correlation with inflammation but no relationship with circulating vitamin D status by Mayur Garg, Simon G. Royce, Chris Tikellis, Claire Shallue, Pavel Sluka, Hady Wardan, Patrick Hosking, Shaun Monagle, Merlin Thomas, John S. Lubel and Peter R. Gibson in Therapeutic Advances in Gastroenterology
Footnotes
Funding: This work was supported by the Gastroenterological Society of Australia Scholarship awarded to Dr Mayur Garg, and Eastern Health Clinical School.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Statement of data availability: Most of the data supporting the findings of this study are available within the paper and its supplementary information file. All other data are available from the corresponding author upon reasonable request.
Supplemental material: Supplemental material for this article is available online.
ORCID iD: Mayur Garg  https://orcid.org/0000-0002-9149-3589
https://orcid.org/0000-0002-9149-3589
Contributor Information
Mayur Garg, Department of Gastroenterology, Eastern Health Clinical School, Monash University, Level 3W, Building B, 8 Arnold St, Box Hill, Victoria, 3128, Australia.
Simon G. Royce, Department of Medicine, Central Clinical School, Monash University, Victoria, Australia
Chris Tikellis, Department of Diabetes, Central Clinical School, Monash University, Victoria, Australia.
Claire Shallue, Eastern Health Clinical School, Monash University, Victoria, Australia.
Pavel Sluka, Eastern Health Clinical School, Monash University, Victoria, Australia.
Hady Wardan, Eastern Health Clinical School, Monash University, Victoria, Australia.
Patrick Hosking, Department of Pathology, Eastern Health, Victoria, Australia.
Shaun Monagle, Department of Pathology, Eastern Health, Victoria, Australia.
Merlin Thomas, Department of Diabetes, Central Clinical School, Monash University, Victoria, Australia.
John S. Lubel, Department of Gastroenterology, Eastern Health, Victoria, Australia; Eastern Health Clinical School, Monash University, Victoria, Australia
Peter R. Gibson, Department of Gastroenterology, Alfred Hospital and Monash University, Victoria, Australia
References
- 1. Garg M, Lubel JS, Sparrow MP, et al. Review article: vitamin D and inflammatory bowel disease-established concepts and future directions. Aliment Pharmacol Ther 2012; 36: 324–344. [DOI] [PubMed] [Google Scholar]
- 2. Kong J, Zhang Z, Musch MW, et al. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol 2008; 294: G208–G216. [DOI] [PubMed] [Google Scholar]
- 3. Liu W, Chen Y, Golan MA, et al. Intestinal epithelial vitamin D receptor signaling inhibits experimental colitis. J Clin Invest 2013; 123: 3983–3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu S, Liao AP, Xia Y, et al. Vitamin D receptor negatively regulates bacterial-stimulated NF-kappaB activity in intestine. Am J Pathol 2010; 177: 686–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Flanagan P. Vitamin D enhances macrophage function and improves killing of Crohn’s associated E. coli. J Crohns Colitis 2013; 7: S20. [Google Scholar]
- 6. Yuk JM, Shin DM, Lee HM, et al. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe 2009; 6: 231–243. [DOI] [PubMed] [Google Scholar]
- 7. Su D, Nie Y, Zhu A, et al. Vitamin D signaling through induction of Paneth cell defensins maintains gut microbiota and improves metabolic disorders and hepatic steatosis in animal models. Front Physiol 2016; 7: 498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brennan A, Katz DR, Nunn JD, et al. Dendritic cells from human tissues express receptors for the immunoregulatory vitamin D3 metabolite, dihydroxycholecalciferol. Immunology 1987; 61: 457–461. [PMC free article] [PubMed] [Google Scholar]
- 9. Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol 2000; 164: 2405–2411. [DOI] [PubMed] [Google Scholar]
- 10. Szeles L, Keresztes G, Torocsik D, et al. 1,25-dihydroxyvitamin D3 is an autonomous regulator of the transcriptional changes leading to a tolerogenic dendritic cell phenotype. J Immunol 2009; 182: 2074–2083. [DOI] [PubMed] [Google Scholar]
- 11. Jeffery LE, Burke F, Mura M, et al. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol 2009; 183: 5458–5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Daniel C, Sartory NA, Zahn N, et al. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther 2008; 324: 23–33. [DOI] [PubMed] [Google Scholar]
- 13. Zhang Y, Leung DY, Richers BN, et al. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol 2012; 188: 2127–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dionne S, Duchatelier CF, Seidman EG. The influence of vitamin D on M1 and M2 macrophages in patients with Crohn’s disease. Innate Immun 2017; 23: 557–565. [DOI] [PubMed] [Google Scholar]
- 15. Zai K, Hirota M, Yamada T, et al. Therapeutic effect of vitamin D3-containing nanostructured lipid carriers on inflammatory bowel disease. J Control Release 2018; 286: 94–102. [DOI] [PubMed] [Google Scholar]
- 16. Garg M, Rosella O, Rosella G, et al. Evaluation of a 12-week targeted vitamin D supplementation regimen in patients with active inflammatory bowel disease. Clin Nutr 2017; 37. [DOI] [PubMed] [Google Scholar]
- 17. Meckel K, Li YC, Lim J, et al. Serum 25-hydroxyvitamin D concentration is inversely associated with mucosal inflammation in patients with ulcerative colitis. Am J Clin Nutr 2016; 104: 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gubatan J, Mitsuhashi S, Zenlea T, et al. Low serum vitamin D during remission increases risk of clinical relapse in patients with ulcerative colitis. Clin Gastroenterol Hepatol 2017; 15: 240–246 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Raftery T, Merrick M, Healy M, et al. Vitamin D status is associated with intestinal inflammation as measured by fecal calprotectin in Crohn’s disease in clinical remission. Dig Dis Sci 2015; 60: 2427–2435. [DOI] [PubMed] [Google Scholar]
- 20. Ghaly S, Kaakoush NO, Lloyd F, et al. High Dose Vitamin D supplementation alters faecal microbiome and predisposes mice to more severe colitis. Sci Rep 2018; 8: 11511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lund-Nielsen J, Vedel-Krogh S, Kobylecki CJ, et al. Vitamin D and inflammatory bowel disease: mendelian randomization analyses in Copenhagen studies and UK biobank. J Clin Endocrinol Metab 2018; 103: 3267–3277. [DOI] [PubMed] [Google Scholar]
- 22. Garg M, Hendy P, Ding JN, et al. The effect of vitamin D on intestinal inflammation and faecal microbiota in patients with ulcerative colitis. J Crohn Colitis 2018; 12: 963–972. [DOI] [PubMed] [Google Scholar]
- 23. Raftery T, Martineau AR, Greiller CL, et al. Effects of vitamin D supplementation on intestinal permeability, cathelicidin and disease markers in Crohn’s disease: results from a randomised double-blind placebo-controlled study. United Eur Gastroenterol J 2015; 3: 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang L, Weaver V, Smith JP, et al. Therapeutic effect of vitamin D supplementation in a pilot study of Crohn’s patients. Clin Transl Gastroenterol 2013; 4: e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jorgensen SP, Agnholt J, Glerup H, et al. Clinical trial: vitamin D3 treatment in Crohn’s disease - a randomized double-blind placebo-controlled study. Aliment Pharmacol Ther 2010; 32: 377–383. [DOI] [PubMed] [Google Scholar]
- 26. Miheller P, Muzes G, Hritz I, et al. Comparison of the effects of 1,25 dihydroxyvitamin D and 25 hydroxyvitamin D on bone pathology and disease activity in Crohn’s disease patients. Inflamm Bowel Dis 2009; 15: 1656–1662. [DOI] [PubMed] [Google Scholar]
- 27. Narula N, Cooray M, Anglin R, et al. Impact of high-dose vitamin D3 supplementation in patients with Crohn’s disease in remission: a pilot randomized double-blind controlled study. Dig Dis Sci 2017; 62: 448–455. [DOI] [PubMed] [Google Scholar]
- 28. Plum LA, DeLuca HF. Vitamin D, disease and therapeutic opportunities. Nat Rev Drug Discov 2010; 9: 941–955. [DOI] [PubMed] [Google Scholar]
- 29. Boland RL. VDR activation of intracellular signaling pathways in skeletal muscle. Mol Cell Endocrinol 2011; 347: 11–16. [DOI] [PubMed] [Google Scholar]
- 30. Norman AW. Minireview: vitamin D receptor: new assignments for an already busy receptor. Endocrinology 2006; 147: 5542–5548. [DOI] [PubMed] [Google Scholar]
- 31. Simmons JD, Mullighan C, Welsh KI, et al. Vitamin D receptor gene polymorphism: association with Crohn’s disease susceptibility. Gut 2000; 47: 211–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gisbert-Ferrandiz L, Salvador P, Ortiz-Masia D, et al. A single nucleotide polymorphism in the vitamin d receptor gene is associated with decreased levels of the protein and a penetrating pattern in Crohn’s disease. Inflamm Bowel Dis 2018; 24: 1462–1470. [DOI] [PubMed] [Google Scholar]
- 33. Abreu-Delgado Y, Isidro RA, Torres EA, et al. Serum vitamin D and colonic vitamin D receptor in inflammatory bowel disease. World J Gastroenterol 2016; 22: 3581–3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pike JW, Meyer MB. The vitamin D receptor: new paradigms for the regulation of gene expression by 1,25-dihydroxyvitamin D(3). Endocrinol Metab Clin North Am 2010; 39: 255–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Garg M. Association of circulating vitamin D concentrations with intestinal but not systemic inflammation in inflammatory bowel disease. Inflamm Bowel Dis 2013; 19: 2634–2643. [DOI] [PubMed] [Google Scholar]
- 36. Low vitamin D in Victoria: key health messages for doctors, nurses and allied health. Melbourne, 2012. [Google Scholar]
- 37. FSANZ. NUTTAB, 2010. http://www.foodstandards.gov.au/science/monitoringnutrients/nutrientables/nuttab/pages/default.aspx (accessed September 2011).
- 38. Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006; 55: 749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. D’Haens GR, Geboes K, Peeters M, et al. Early lesions of recurrent Crohn’s disease caused by infusion of intestinal contents in excluded ileum. Gastroenterology 1998; 114: 262–267. [DOI] [PubMed] [Google Scholar]
- 40. Geboes K, Riddell R, Ost A, et al. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut 2000; 47: 404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. He L, Liu T, Shi Y, et al. Gut epithelial vitamin D receptor regulates microbiota-dependent mucosal inflammation by suppressing intestinal epithelial cell apoptosis. Endocrinology 2017; 159: 967–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wu S, Zhang YG, Lu R, et al. Intestinal epithelial vitamin D receptor deletion leads to defective autophagy in colitis. Gut 2015; 64: 1082–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Giardina C, Nakanishi M, Khan A, et al. Regulation of VDR expression in Apc-mutant mice, human colon cancers and adenomas. Cancer Prev Res 2015; 8: 387–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ceglia L. Vitamin D and skeletal muscle tissue and function. Mol Aspects Med 2008; 29: 407–414. [DOI] [PubMed] [Google Scholar]
- 45. Wu-Wong JR, Nakane M, Ma J, et al. VDR-mediated gene expression patterns in resting human coronary artery smooth muscle cells. J Cell Biochem 2007; 100: 1395–1405. [DOI] [PubMed] [Google Scholar]
- 46. Verouti SN, Tsilibary EC, Fragopoulou E, et al. Vitamin D receptor activators upregulate and rescue podocalyxin expression in high glucose-treated human podocytes. Nephron Exp Nephrol 2012; 122: 36–50. [DOI] [PubMed] [Google Scholar]
- 47. Garg M, Rosella O, Lubel JS, et al. Association of circulating vitamin D concentrations with intestinal but not systemic inflammation in inflammatory bowel disease. Inflamm Bowel Dis 2013; 19: 2634–2643. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Material for The intestinal vitamin D receptor in inflammatory bowel disease: inverse correlation with inflammation but no relationship with circulating vitamin D status by Mayur Garg, Simon G. Royce, Chris Tikellis, Claire Shallue, Pavel Sluka, Hady Wardan, Patrick Hosking, Shaun Monagle, Merlin Thomas, John S. Lubel and Peter R. Gibson in Therapeutic Advances in Gastroenterology





