Abstract
Otolaryngologists increasingly use patient-specific 3-dimensional (3D)–printed anatomic physical models for preoperative planning. However, few reports describe concomitant use with virtual models. Herein, we aim to (1) use a 3D-printed patient-specific physical model with lateral skull base navigation for preoperative planning, (2) review anatomy virtually via augmented reality (AR), and (3) compare physical and virtual models to intraoperative findings in a challenging case of a symptomatic petrous apex cyst. Computed tomography (CT) imaging was manually segmented to generate 3D models. AR facilitated virtual surgical planning. Navigation was then coupled to 3D-printed anatomy to simulate surgery using an endoscopic approach. Intraoperative findings were comparable to simulation. Virtual and physical models adequately addressed details of endoscopic surgery, including avoidance of critical structures. Complex lateral skull base cases may be optimized by surgical planning via 3D-printed simulation with navigation. Future studies will address whether simulation can improve patient outcomes.
Keywords: 3D printing, simulation, navigation, skull base, transcanal, endoscopic, residency education, augmented reality
Three-dimensional (3D) printing is a useful tool for surgical planning.1Surgeons use patient-specific 3D-printed physical models for preoperative planning,2,3such as custom plates or surgical guides.4,5Recent 3D-printed simulators render complex anatomy accurately.1,5-8Augmented reality (AR) projects virtual objects into real-life environments. Although AR applications originated over a decade ago,9,10feasibility was limited by ergonomics and accurate patient/image registration.11Current technology facilitates conversion of medical imaging to 3D models in AR for mobile devices.9
Combining AR with surgical navigation enables personalized preoperative planning. In otologic surgery, virtual cues could identify structures, such as vessels and cranial nerves along the skull base. To date, isolated reports describe personalized preoperative planning.12,13Furthermore, no reports discuss planning via virtual and physical surgical simulations with concurrent navigation for a complete visuospatial and tactile experience. Herein, we describe a challenging case of a petrous apex cyst accessible through a subcochlear surgical corridor. Virtual rendering, 3D printing, and navigation enabled a safe transcanal endoscopic approach.
Methods
Imaging
A 48-year-old pilot presenting with left-sided otalgia, tinnitus, and vertex headache exacerbated by elevation changes mid-flight was found to have an ipsilateral petrous apex cyst. No cranial nerve deficits were present. Computed tomography (CT) images were obtained on a SOMATOM Definition AS (Siemens, Munich, Germany) with 0.6-mm slice thickness, 120 kVp, and 1000-ms exposure time. Imaging revealed a cystic mass in the left petrous apex measuring 1.8 × 1.1 × 1.2cm with medial and anterior bony dehiscence abutting the horizontal portion of the carotid artery ( Figure 1A ). The study was deemed exempt by the Massachusetts Eye and Ear Institutional Review Board.
Figure 1.
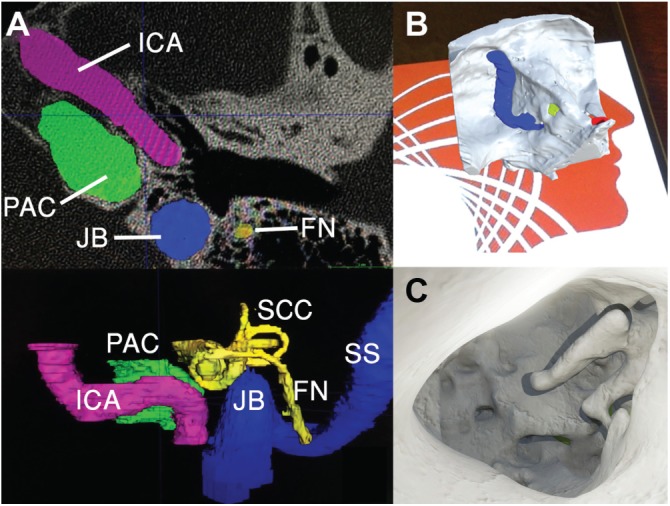
Left ear 3-dimensional (3D) reconstruction. (A) Manual segmentation from computed tomography images into 3D meshes using ITK-SNAP. (B, C) Augmented reality mobile phone application visualized anatomy preoperatively, registered with target image. FN, facial nerve; ICA, internal carotid artery; JB, jugular bulb; PAC, petrous apex cyst; SCC, semicircular canal; SS, sigmoid sinus.
3D Modeling
Manual segmentation, the process by which the digital image was partitioned into individual components to isolate anatomic structures, was performed to create 3D models (meshes) from highlighted voxels in DICOM images in ITK-SNAP.14Bone, vessel, and nerve were segmented into separate meshes, exported as stereolithography (STL) files, and postprocessed with smoothing filters.
Augmented Reality
Meshes were imported into Unity v5.6 (San Francisco, California) with Vuforia (Needham, Massachusetts). Custom-scripted user controls enabled zoom, rotation, and opacity changes to surgically oriented models.
3D Printing and Navigation Preparation
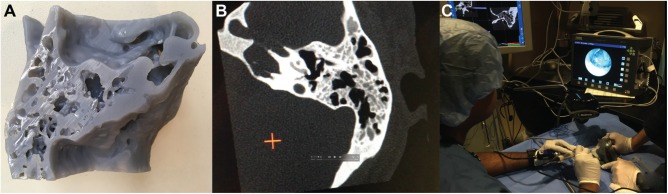
The temporal bone STL file was 3D-printed directly using a Form2 with 25-µm resolution (FormLabs, Somerville, Massachusetts) ( Figure 2A ). Six titanium screws were instrumented as fiducial markers, and another axial CT scan was performed.
Figure 2.
(A) A 3-dimensional (3D) print of temporal bone used for preoperative simulation. (B) Computed tomography scan of a 3D print was a 1:1 match with the original and able to be registered for navigation. (C) Transcanal approach to petrous apex was simulated on 3D print with navigation.
Navigation
Original and 3D-printed CT scans were imported onto a Stealth3D workstation (Medtronic, Minneapolis, Minnesota). Image registration compared structural differences between patient and 3D-printed anatomy. Calibration used fiducial markers. For visualization, rigid otoendoscopes were coupled to high-definition video (Karl Storz, Tuttlingen, Germany).
Preoperative Simulation
The navigation workstation and 3D-printed model were brought to the surgical training laboratory with a full operating theater setup. The 3D-printed model provided a tactile representation of surgical anatomic landmarks. Following registration, navigation used the original CT data. The procedure was performed as would be done in surgery.
Results
3D Modeling and Augmented Reality
Anatomy was segmented with structures comprising temporal bone, petrous apex cyst, internal carotid artery, jugular bulb, sigmoid sinus, cranial nerves VII/VIII, cochlea, and vestibular apparatus ( Figure 1A ). Physically based rendering materials were applied, and an AR app built for Android OS allowed the interrelationship of critical landmarks to be evaluated from a virtual transcanal view ( Figure 1B , C ).
Preoperative Simulation
Surgical simulation using navigation was performed on the 3D-printed model preoperatively ( Figure 2 ). During fiducial registration, the 3D-printed model was a 1:1 match with original CT imaging, with a 0.7-mm margin of error in Stealth3D ( Figure 2B ). Petrous apex cyst access and drainage was performed via a transcanal, endoscopic-assisted (3-mm diameter, 14-cm rigid endoscope, 0, 30-degree) infracochlear approach coupled with navigation ( Figure 2B , C ). A limited inferior canalplasty was performed for hypotympanum access. Navigation remained accurate without physical model landmarks deviating from observed coordinates. Of note, during simulation, the carotid canal was encountered anteriorly by the 1-mm burr. This was confirmed using navigation probes placed at the defect.
Live Surgery
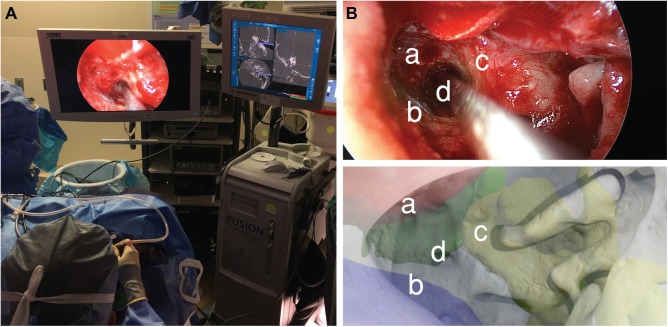
Anatomic constraints observed during simulation provided insight for live surgery. An optimized transcanal endoscopic approach achieved successful cyst drainage while avoiding the carotid artery anteriorly, jugular bulb inferiorly, and basal turn of the cochlea superiorly within a 1.5-mm surgical corridor ( Figure 3B ). There were no perioperative complications and the patient experienced symptomatic relief 1 year postoperatively.
Figure 3.
(A) Intraoperative photo of the live surgery performed using a transcanal endoscopic approach. (B) Comparison of intraoperative (upper panel) with virtual, preoperative otoendoscopic views (lower panel) demonstrated that the virtual render predicted the trajectory of the real surgical approach based on structures at risk: (a) internal carotid artery, (b) jugular bulb, (c) basal turn of the cochlea, and (d) access to petrous apex cyst.
Discussion
A personalized 3D-printed temporal bone was successfully fabricated and coupled with surgical navigation. The 3D-printed model was a high-fidelity replica of patient-specific anatomy, evidenced by registration with original CT imaging and comparisons between simulation and intraoperative findings. Although data loss can occur during segmentation, postprocessing, and 3D printing, the margin of error along landmarks was 0.7 mm, close to 0.6-mm cuts. For delicate lateral skull base microanatomy, results exceeded expectations. Moreover, small open structures, including mastoid air cells and foramina, remained patent.
That the carotid canal was encountered during simulation underscores the value of preoperative surgical planning. Subjectively, 3D-printed bone had good haptic feedback during drilling, and simulation strongly aided in preparation. However, further cases are required to demonstrate improved safety using preoperative planning.
Several studies investigated preoperative planning in otology; however, no studies used multiple formats, including AR.2,12,13,15Spine surgery studies involving cadaveric and 3D-printed models demonstrated decreased error with instrumentation.16,17Within otolaryngology, surgical planning using combined physical/virtual models may ultimately optimize patient outcomes.
Conclusion
Complex lateral skull base cases may be optimized by surgical planning using AR and 3D-printed simulation with concurrent navigation. High-fidelity patient-specific models are fabricated using consumer technology. Future studies will address whether simulation can improve outcomes, including patient safety.
Author Contributions
Samuel R. Barber, study design and conduct, collection, analysis, interpretation, and writing; Kevin Wong, study design and conduct, collection, analysis, interpretation, and writing; Vivek Kanumuri, study design and conduct, collection, analysis, interpretation, and writing; Ruwan Kiringoda, study design and conduct, collection, analysis, interpretation, and writing; Judith Kempfle, study design and conduct, collection, analysis, interpretation, and writing; Aaron K. Remenschneider, study design and conduct, collection, analysis, interpretation, and writing; Elliott D. Kozin, study design and conduct, collection, analysis, interpretation, and writing; Daniel J. Lee, study design and conduct, collection, analysis, interpretation, and writing.
Disclosures
Competing interests: None.
Sponsorships: None.
Funding source: None.
Footnotes
No sponsorships or competing interests have been disclosed for this article.
References
- 1. Hoang D, Perrault D, Stevanovic M, et al. Surgical applications of three-dimensional printing: a review of the current literature & how to get started. Ann Transl Med. 2016;4:456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Crafts TD, Ellsperman SE, Wannemuehler TJ, et al. Three-dimensional printing and its applications in otorhinolaryngology–head and neck surgery. Otolaryngol Head Neck Surg. 2017;156:999-1010. [DOI] [PubMed] [Google Scholar]
- 3. Hsieh TY, Dedhia R, Cervenka B, et al. 3D Printing: current use in facial plastic and reconstructive surgery. Curr Opin Otolaryngol Head Neck Surg. 2017;25:291-299. [DOI] [PubMed] [Google Scholar]
- 4. Mendez BM, Chiodo MV, Patel PA. Customized “in-office” three-dimensional printing for virtual surgical planning in craniofacial surgery. J Craniofac Surg. 2015;26:1584-1586. [DOI] [PubMed] [Google Scholar]
- 5. Kaye R, Goldstein T, Zeltsman D, et al. Three dimensional printing: a review on the utility within medicine and otolaryngology. Int J Pediatr Otorhinolaryngol. 2016;89:145-148. [DOI] [PubMed] [Google Scholar]
- 6. Barber SR, Kozin ED, Dedmon M, et al. 3D-printed pediatric endoscopic ear surgery simulator for surgical training. Int J Pediatr Otorhinolaryngol. 2016;90:113-118. [DOI] [PubMed] [Google Scholar]
- 7. Dedmon MM, O’Connell BP, Kozin ED, et al. Development and validation of a modular endoscopic ear surgery skills trainer. Otol Neurotol. 2017;38:1193-1197. [DOI] [PubMed] [Google Scholar]
- 8. Mowry SE, Jammal H, Myer CT, et al. A novel temporal bone simulation model using 3D printing techniques. Otol Neurotol. 2015;36:1562-1565. [DOI] [PubMed] [Google Scholar]
- 9. Zhu E, Hadadgar A, Masiello I, et al. Augmented reality in healthcare education: an integrative review. PeerJ. 2014;2:e469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Berryman DR. Augmented reality: a review. Med Ref Serv Q. 2012;31:212-218. [DOI] [PubMed] [Google Scholar]
- 11. Shuhaiber JH. Augmented reality in surgery. Arch Surg. 2004;139:170-174. [DOI] [PubMed] [Google Scholar]
- 12. Rose AS, Webster CE, Harrysson OL, et al. Pre-operative simulation of pediatric mastoid surgery with 3D-printed temporal bone models. Int J Pediatr Otorhinolaryngol. 2015;79:740-744. [DOI] [PubMed] [Google Scholar]
- 13. Ritacco LE, Di Lella F, Mancino A, et al. 3D printed models and navigation for skull base surgery: case report and virtual validation. Stud Health Technol Inform. 2015;216:1025. [PubMed] [Google Scholar]
- 14. Yushkevich PA, Piven J, Hazlett HC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. NeuroImage. 2006;31:1116-1128. [DOI] [PubMed] [Google Scholar]
- 15. Wanibuchi M, Noshiro S, Sugino T, et al. Training for skull base surgery with a colored temporal bone model created by three-dimensional printing technology. World Neurosurg. 2016;91:66-72. [DOI] [PubMed] [Google Scholar]
- 16. Guo F, Dai J, Zhang J, et al. Individualized 3D printing navigation template for pedicle screw fixation in upper cervical spine. PLoS One. 2017;12(2):e0171509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bohm PE, Arnold PM. Simulation and resident education in spinal neurosurgery. Surg Neurol Int. 2015;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]