Abstract
No previous meta-analysis has evaluated the efficacy and safety of pulmonary vasodilators in Fontan physiology. Recent relative trials have obtained conflicting results regarding improvements in peak oxygen consumption; the relatively small number of patients in each study may be a limiting factor. We aimed to evaluate the efficacy and safety of pulmonary vasodilators in Fontan patients. Relevant studies were identified by searching the PubMed, Embase, and Cochrane Library databases. Pooled outcomes were determined to assess the efficacy and safety of pulmonary vasodilators in Fontan patients. Nine randomized controlled studies involving 381 patients with Fontan circulation were included. Pulmonary vasodilator therapy led to significant improvement (mean difference = −0.39, 95% CI: [−0.72, −0.05]) in the New York Heart Association (NYHA) functional class. The 6-minute walking distance (6MWD) was significantly increased by 134 m (95% CI: [86.07, 181.94]), and the peak VO2 was also significantly improved (mean difference = 1.42 ml·(kg·min)-1, 95% CI: [0.21, 2.63]). Additionally, the mean pulmonary artery pressure (mPAP) was significantly reduced (mean difference = −2.25 mmHg, 95% CI: [−3.00, −1.50]). No significant change was found in mortality or in brain natriuretic peptide (BNP) or N-terminal pronatriuretic peptide (NT-proBNP). Four studies reported no side effects and good drug tolerance, and two studies reported mild adverse effects. The present meta-analysis indicated that pulmonary vasodilators (primarily the PDE-5 inhibitor and endothelin-1 receptor antagonist) significantly improved the hemodynamics of Fontan patients, reduced the NYHA functional class and increased the 6MWD. The peak oxygen consumption was also improved. No significant change was observed in mortality or in the BNP or NT-proBNP level. Overall, the pulmonary vasodilators were well tolerated. This finding needs to be confirmed in future studies.
Keywords: Fontan procedure, PDE-5 inhibitor, endothelin-1 receptor antagonist, outcomes
Introduction
Fontan circulation is a palliative procedure performed in patients born with single-ventricular circulation who are unable to undergo biventricular repair for a range of congenital heart diseases.1 The Fontan surgical procedure passes the venous return directly into the pulmonary arteries without a pumping chamber in between. The 10-year post-surgery survival rate for the first Fontan patients was only 50%, but this rate has increased to 93% at present.2–5 Therefore, the Fontan procedure has become the typical treatment for complex congenital heart disease.
In the short and medium term, this procedure shows favorable results, with a stable clinical condition permitting neural development and growth in children, and the procedure is therefore an effective and safe surgery. Pulmonary blood flow (PBF) increases after the Glenn or total cavopulmonary connection procedure, which conflicts with the limitation of pulmonary small vessels and results in pulmonary interstitial edema. Previous studies have demonstrated that the absence of a pulsatile pulmonary flow induces endothelial dysfunction with impaired pulmonary vasodilation,6 which may play a role in increasing pulmonary vascular resistance (PVR). This factor may play an important role in the failing Fontan circulation with preserved systolic function. Furthermore, long-term pulmonary vasculature remodeling after Fontan surgery has been shown to be responsible for the increase in pulmonary resistance in patients who die during follow-up.7 In Fontan circulation, there is no pump to propel blood into the pulmonary arteries and overcome a possible increased afterload, since the systemic veins are directly connected to the pulmonary arteries. The remaining postcapillary energy is harnessed to drive blood through the lungs. Therefore, keeping the PVR low is mandatory both before and after Fontan completion, suggesting a potential role for pulmonary vasodilators in reinforcing PBF in these patients. Otherwise, increasing complications have been observed with prolonged post-surgical follow-up, including reduced exercise capacity, arrhythmia, thrombosis, cyanosis, protein-losing enteropathy, and plastic bronchitis.8 In addition, patients’ quality of life is severely affected. Standard heart failure treatment with afterload reduction fails in most Fontan patients.9 These cases have highlighted the significance of the early detection and management of these complications.
Physiological studies have depicted a reduced preload of the single ventricle.10,11 Six observational studies have estimated the importance of pulmonary vasodilators, because reducing PVR consequently benefits a patient’s cardiac function and exercise capacity.12–17 However, these one-arm trials did not include corresponding control groups and only compared the pre- and post-treatment effects. Recently, some randomized controlled trials (RCTs)18–22 have been conducted to investigate the efficacy of pulmonary vasodilators. Some disagreement exists concerning the improvement in peak oxygen consumption. In addition, the relatively small number of patients enrolled in each study may be a limiting factor for evaluations of the efficacy and safety of the procedure. In response to this recent accumulation of evidence, we sought to evaluate the effect and safety of pulmonary vasodilators in Fontan patients by conducting a meta-analysis of RCTs.
Methods
Search strategy
We performed a review of the literature and a meta-analysis of studies that reported the effects and safety of pulmonary vasodilators on the exercise capacity, 6-minute walking distance (6MWD) and functional class in Fontan patients. Relevant studies were identified by searching the PubMed and Embase databases and the Cochrane Central Register of Controlled Trials using the following search terms: (“Fontan procedure” OR “Fontan”) AND (“drug therapy” OR “drug” OR “pharmac agent” OR “medication” OR “administration” OR “phosphodiesterase type 5 inhibitor” OR “sildenafil” OR “tadalafil” OR “vardenafil” OR “Viagra” OR “endothelial receptor antagonist” OR “bosentan” OR “ambrisentan” OR “prostacyclin” OR “Epoprostenol” OR “Treprostinil” OR “Remodulin” OR “iloprost” OR “nitric oxide” OR “NO” OR “calcium channel blockers” OR “nifedipine” OR “diltiazem” OR “amlodipine”). In addition, the reference lists of the studies were retrieved from the databases, and conference reviews of Fontan circulation and medical management of Fontan patients were evaluated. This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.23 All studies included in this meta-analysis were published between 2008 and 2016.
Selection criteria
Based on screening of the “titles/abstracts and full texts,” studies were included if they met all of the following criteria: (i) the subjects enrolled in the studies had Fontan circulation; (ii) the patients were administered pulmonary vasodilators, and the efficacy or safety of the medication was evaluated; (iii) control groups, including non-treatment and placebo groups, were presented in the studies; (iv) the primary exposures investigated included mortality, hemodynamics, New York Heart Association (NYHA) functional assessments, 6MWD and exercise capacity, and adverse effects were also measured; and (v) the study design was a RCT. There were no restrictions concerning the publication language or type of Fontan surgery. We excluded self-controlled design studies, case reports, and conference papers. Only one randomized controlled study24 examined the echocardiographic indices of myocardial performance and thus had to be excluded.
Data extraction
The following information was independently extracted by two authors (W.W. and X.H.): the first author; publication year; study design; drug; number of patients; age; outcomes and conclusions.
Assessment of study quality
The risk of bias within each study was assessed using the Cochrane risk of bias tool, which contained the following seven aspects: random sequence generation (selection bias); allocation concealment (selection bias); blinding of participants and personnel (performance bias); blinding of outcome assessment (detection bias); incomplete outcome data (attrition bias); selective reporting (reporting bias); and other bias.25 If more than two types of high-risk bias were found among all types of measured bias, then the article was excluded. The quality assessment was independently conducted by two authors (W.W and X.H.); their results were compared, and a third author (W.L.) intervened if a consensus could not be reached.
Statistical analyses
The pooled treatment effects, point estimate of the mortality rate, mean pulmonary arterial pressure (mPAP), NYHA functional class, 6MWD, and peak oxygen uptake (peak VO2) were calculated using the STATA software (version 12). A P-value less than 0.05 for any test or model was considered significant unless otherwise indicated. For continuous data, the inverse variance statistical method was used to measure the effect of the mean difference in each outcome. For dichotomous data, the risk ratio was measured.
We used Cochran’s χ2-based Q test and the I-squared test to assess inter-study heterogeneity.26 If no significant heterogeneity (defined as P > 0.10 or I2 < 50%) was detected, the pooled outcome was determined with the fixed effects model (Mantel–Haenszel); conversely, the random effects model (DerSimonian and Laird) was used when significant heterogeneity was found.27 In addition, a sensitivity analysis was conducted to determine the influence of individual trials on the overall pooled results.
Possible publication bias was considered using Begg’s rank correlation test28 and Egger’s linear regression test.29 In addition, funnel plots were employed to assess potential publication bias.
Results
Literature search
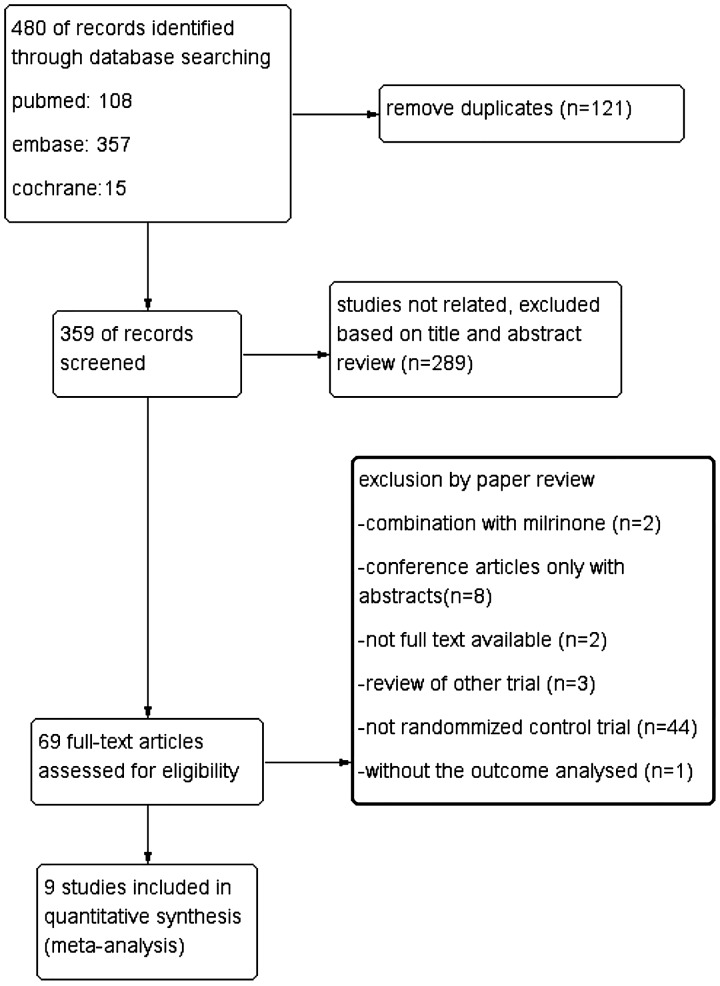
Initially, 480 citations were identified, and 121 duplicates were removed, leaving 359 studies for the screening step. After reviewing the titles and abstracts, 289 publications were excluded, primarily because they did not focus on the effects and safety of pulmonary vasodilation. Full-text reviews were conducted for 60 studies, of which two30,31 were excluded because they combined the drug of focus with milrinone, and eight articles were excluded because they were conference articles that only provided abstracts. In addition, two studies without the full available text and three32–34 repetitive reviews of other trials were all excluded. Moreover, 44 studies that were not RCTs were excluded due to their study designs. Another RCT24 was also excluded because no outcomes were reported. After reviewing the remaining studies, nine studies met the inclusion criteria and were added to the pooled analysis18–22, 35–38 (Fig. 1).
Figure 1.
A flow diagram of the study selection. The flow diagram shows the literature search for the relevant studies of the effect on and safety of pulmonary vasodilators for Fontan patients.
Eligible studies
The characteristics of the nine included studies are summarized in Table 1. The most recent study was published in 2016. A total of 381 patients were allocated to the pulmonary vasodilator and control groups. The age range of the patients was 11–28 years. For these two-arm clinical trials, the data were extracted to compare the efficacy of the pulmonary vasodilators. Among the studies, four evaluated mortality,35–38 two focused on mPAP,36,37 three focused on the NYHA functional class,19,20,36 two focused on the 6MWD,35,36 and five focused on the peak VO2.18–22
Table 1.
Characteristics of the trials included in the meta-analysis.
| Studies | Study design | Drug | Sample size | Patient age | Outcomes | Conclusions |
|---|---|---|---|---|---|---|
| Giardini A, 2008 | RCT | Sildenafil (0.7 mg/kg, range 25–50 mg) | 55 | 22.8 ± 4.9 (16–32) | Peak VO2 | Sildenafil improves exercise capacity and increases PBF and CI |
| Goldberg DJ, 2011 | RCT | Sildenafil (20 mg 3 × daily) | 55 | 14.9 ± 5.1 | Peak VO2 | Sildenafil did not cause an improvement in VO2 max; but there was an increase in ventilatory efficiency and a suggestion of improved oxygen consumption in two subgroups |
| Giordano R, 2015 | Retrospective RCT | Sildenafil (0.35 mg/kg × 4 h) | 30 | >18 years | Mortality, mPAP | Sildenafil may be used in postoperative Fontan operations, with positive effectiveness |
| Mendoza A, 2015 | Prospective RCT | Sildenafil and nitric oxide (4.6 ± 1.6 mg/kg/day) | 48 | 5.4 ± 1.7(man) 5.7 ± 2.7 (woman) | Mortality | Postoperative administration of sildenafil and nitric oxide had no influence on early postoperative outcomes after the modified Fontan procedure, in terms of duration of pleural effusions, mechanical ventilation time, length of stay in the ICU, and length of hospital stay |
| Mark JS, 2013 | RCT | Bosentan (125 mg 2 × daily) | 48 | 28 (18–55) | NYHA class, peak VO2, BNP | An increased NT-proBNP level was present in the majority of Fontan patients. Six months of bosentan treatment was not beneficial |
| Shang SK, 2013 | RCT | Bosentan (≤10 kg: 7.8125 mg 2× daily; 10–20 kg: 15.625 mg 2× daily; 20–30 kg: 31.25 mg 2× daily; >30 kg: 62.5 mg 2× daily) | 39 | 2.5–18 | Mortality, NYHA class. 6MWD, BNP, | Bosentan reduces the incidence of pulmonary arteriovenous fistulae and protein-losing enteropathy and improve heart function |
| Hebert A, 2014 | RCT | Bosentan (62.5 mg 2× daily for 2 weeks; 125 mg 2× daily for 12 weeks) | 69 | 20 ± 7.4 | NYHA class, Peak VO2, BNP | Bosentan improves exercise capacity and time, as well as NYHA functional class, without serious adverse effects |
| Cedars AM, 2016 | RCT | Ambrisentan (5 mg × daily) | 28 | >18 years | Peak VO2 | Ambrisentan improves exercise capacity in adult Fontan patients |
| Shang SK, 2016 | RCT | Bosentan (≤10 kg: 7.8125 mg, ×2 daily; 10–20 kg: 15.625 mg × 2 daily; 20–30 kg: 31.25 mg × 2 daily; >30 kg: 62.5 mg × 2 daily) | 9 | 11.13 ± 4.13 (6.5–16) | Mortality, mPAP, NYHA class, 6MWD | Bosentan improves NYHA functional status and improves the results of the 6-min walking test (6MWT) in Fontan patients post-surgery, and no other benefits were observed |
BNP: brain natriuretic peptide; mPAP: mean pulmonary artery pressure; NYHA: New York Heart Association; peak VO2: peak oxygen uptake; RCT: randomized controlled trials; 6MWD: six-minute walking distance.
Methodological quality assessment
All of the included studies were RCTs and clearly described the randomization methods. Two studies did not provide the randomization and blinding methods.37,38 Three studies did not adequately describe the methods used for concealed treatment allocation.18,35,36 Two studies did not adequately describe blinding of the participants and personnel.22,35 No risk of attrition bias, reporting bias, or other bias concerning the company sponsors was found among the studies.
Overall analysis of efficacy endpoints
Mortality
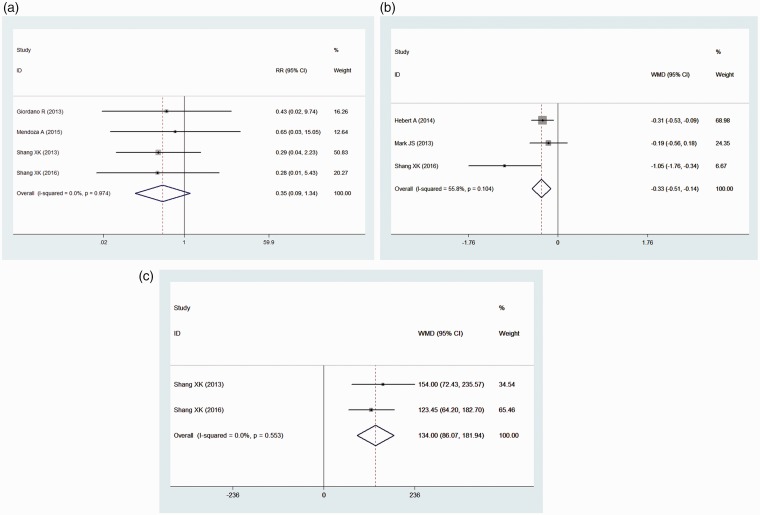
Mortality was reported in four studies. Compared with those in the control group, the patients undergoing pulmonary vasodilator therapy did not show significant differences in mortality (RR = 0.35, 95% CI: [0.09, 1.34], P = 0.126) under the fixed effects model (heterogeneity: I2 = 0.0%) (Fig. 2a).
Figure 2.
A forest plot of mortality and cardiac function. (a) Forest plot of mortality. (b) Forest plot of the NYHA class. (c) Forest plot of the 6MWD. The squares indicate the risk ratio (RR), and the horizontal lines indicate the 95% confidence interval (CI) for each included trial. The squares indicated the mean difference (MD), and the horizontal lines indicate the 95% confidence interval (CI) for each included trial. The statistical weight of a trial in the meta-analysis was proportional to the size of each square. The diamonds indicate the pooled risk ratio and 95% confidence interval, with the center indicating the point estimate, and the left and right ends indicating the 95% CIs.
Functional capacity
Three of the included studies reported the NYHA functional status. A significant improvement (mean difference = −0.39, 95% CI: [−0.72, −0.05], P = 0.000) was observed in patients with pulmonary vasodilators under the randomized model. Moderate heterogeneity was observed among these studies, with I2 = 55.8% (Fig. 2b).
The 6MWD was reported in only two studies. The 6MWD was significantly improved by 134 m (95% CI: [86.07, 181.94], P = 0.000) under the fixed effects model (heterogeneity: I2 = 0.0%) compared with the 6MWD in the control group (Fig. 2c).
In addition, we evaluated the brain natriuretic peptide (BNP) or N-terminal pronatriuretic peptide (NT-proBNP) levels before and after administration and the safety of the drugs. Two of the studies19,20,35 demonstrated a significant reduction in both the pro-BNP and BNP levels, which might indicate mild improvement in atrial distension. However, one study20 showed increased NT-proBNP levels in the majority of Fontan patients. The results showed that the pro-BNP and BNP levels did not differ between the two groups with and without vasodilator treatment.
Exercise capacity
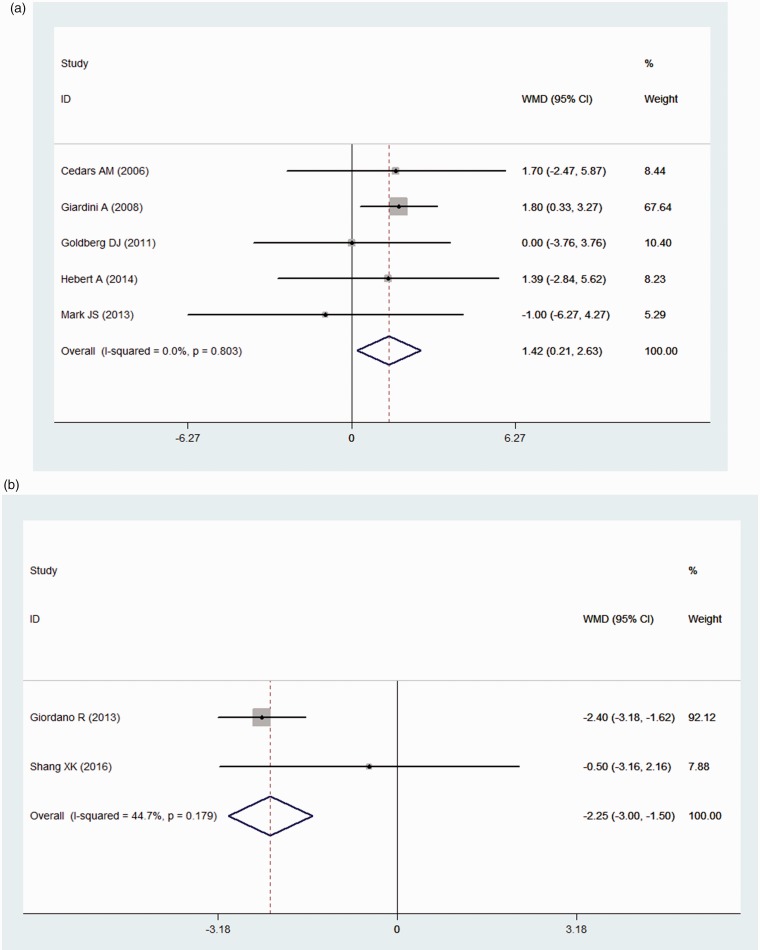
The peak VO2 was reported in five studies. The peak VO2 was also significantly improved (mean difference = 1.42 ml·(kg·min)-1, 95% CI: [0.21, 2.63], P = 0.021) under the fixed effects model (heterogeneity: I2 = 0.0%) compared with that in the control group (Fig. 3a).
Figure 3.
A forest plot of the exercise capacity and hemodynamic parameters. (a) Forest plot of the peak VO2. (b) Forest plot of the mPAP. The squares indicate the mean difference (MD), and the horizontal lines indicate the 95% confidence interval (CI) for each included trial. The statistical weight of a trial in the meta-analysis is proportional to the size of each square. The diamonds indicate the pooled risk ratio and 95% confidence interval, with the center indicating the point estimate, and the left and right ends indicating the 95% CIs.
Hemodynamic parameters
The mPAP was reported in only two studies. Concerning the hemodynamic parameters during right cardiac catheterization, the mPAP was significantly reduced in the experimental group (mean difference = −2.25 mmHg, 95% CI: [−3.00, −1.50], P = 0.000) with mild heterogeneity (I2 = 44.7%) (Fig. 3b).
Safety and side effects
Four of the studies18,22,35,36 reported no side effects with good drug tolerance, and two19–21 studies reported mild adverse effects, primarily flushing, back pain, itching, gastroenteritis, dyspepsia, resultant acidosis, elevated aminotransferases, and fatigue. Overall, the pulmonary vasodilators were well tolerated.
Sensitivity analysis and publication bias
We performed sensitivity analyses to identify the potential sources of heterogeneity in the efficacy of pulmonary vasodilators in Fontan patients. For the NYHA functional class with moderate heterogeneity, when a single study was omitted in turn, the pooled improvement ranged from a mean difference of −0.60 (95% CI: [−0.31, 0.11]) to −0.39 (95% CI: [−0.72, −0.05]). Moreover, when we removed one study,36 the heterogeneity changed from I2 = 55.8% to I2 = 0.0%, accompanied by the resulting change from (mean difference = −0.39, 95% CI: [−0.72, −0.05], P = 0.000) to (mean difference = −0.28, 95% CI: [−0.47, −0.09], P = 0.004). Although it contributed to the heterogeneity, this study was retained in the analysis. Thus, we chose a random model to estimate this pooled outcome.
Although the statistical tests suggested that there was no evidence of publication bias (Begg’s test (P = 0.089 for mortality, P = 1.000 for NYHA class and P = 0.221 for peak VO2) and Egger’s test (P = 0.338 for mortality, P = 0.490 for NYHA class and P = 0.156 for peak VO2)), ruling out the existence of publication bias through visual inspection of the funnel plot is difficult.
Discussion
The present meta-analysis, which included nine randomized controlled studies, examined the efficacy and safety of pulmonary vasodilators in patients with complex congenital heart disease and Fontan physiology. Our results demonstrated that pulmonary vasodilators (primarily the phosphodiesterase type 5 (PDE-5) inhibitor and endothelin-1 receptor antagonist) significantly improved the hemodynamics based on a reduced mPAP, NYHA functional class, 6MWD, and peak VO2 exercise capacity. However, no significant change in mortality was observed between the pulmonary vasodilator and control groups.
An ongoing large RCT, Phase III study (RUBATO) of Macitentan, will assess the efficacy and safety of Macitentan in stable Fontan-palliated adolescent and adult subjects. The study was started in December 2017, and no results have been posted to date. Recent studies have reported that high PVR is a predictive factor for early death following the Fontan procedure.39 Fontan circulation requires systemic venous pressure to maintain pulmonary circulation. Chronic high venous pressures in excess of 18–20 mmHg are poorly tolerated in patients with Fontan circulation and result in symptoms such as congestion, edema, ascites, lymphatic failure, and progressive veno-venous collaterals with cyanosis.40 In addition, because the pulmonary circuit has no pump, hypertension in the systemic veins acts to overcome PVR. As a result, the overall cardiac output is diminished, and the circulation remains in a chronic state of systemic venous hypertension. Maintaining low PVR and systemic blood pressure is essential to guarantee the long-term durability of the Fontan circuit,2 and thus pulmonary vasodilators play an important role. On the other hand, headache and flushing are common in pulmonary vasodilators due to the effect of dilating systematic artery. The exact effect of the pulmonary vasodilator on the systemic circulation in Fontan patients is unknown. Decreased pulmonary artery pressure versus no significant change of systemic artery pressure may indicate the vasodilation effects dominantly through pulmonary circulation. Another potential effect of increasing ventricular volumes by pulmonary vasodilators is increased collateral PBF. The ventricle is exposed to very different and extreme loading conditions that can cause structural remodeling and cardiac dysfunction throughout the Fontan strategy, which may lead to ventricular overgrowth and eccentric hypertrophy. Collateral flow to the lungs may mitigate these changes. Thus, the ventricle evolves from volume overload and overstretch to overgrowth and (severe) deprivation.
Many previous studies have assessed the efficacy of pulmonary vasodilators, although the majority of these studies are one-arm trials with self-control comparisons.12–17 Their results have shown improvements in the cardiac functional class and exercise capacity as a consequence of the reduction of PVR. For the mPAP, NYHA functional class, and 6MWD, the improvements were significant. However, there is no consensus regarding whether pulmonary vasodilators enhance the peak VO2 during exercise. From the forest plot, we found that two studies18,20 demonstrated opposite results compared with the others. In our meta-analysis, peak oxygen consumption was significantly improved. We propose the following reasons for the discrepancy. First, many factors may influence the results, including the age and region of the patients, treatment duration, follow-up period, and drug dose. Second, not all Fontan patients are affected by this subtle pulmonary vascular disease. Third, the improvement in exercise capacity after drug administration may be related to a poorer baseline condition, which may mean that patients with poor health profit more from pulmonary vasodilators than patients with optimal health.41 In terms of mortality, we considered the following reasons for the negative results. The follow-up time required to determine mortality may be relatively short, and the sample sizes of these studies are limited. In addition, in the range of slightly increased values, BNP and NT-proBNP are nonspecific for circulatory failure due to these old-fashioned types of Fontan modifications.42 However, several studies have shown that some patients with BNP levels above 100 pg/ml present the most prominent drug effects.18,24 The pulmonary vasodilators were well tolerated, and only mild side effects were reported, primarily including flushing, back pain, itching, gastroenteritis, dyspepsia, resultant acidosis, elevated aminotransferases, and fatigue.13,19,20,43
Due to the heterogeneity observed among the included studies, pooled estimates were calculated using different effects models. For the assessments of mortality, 6MWD, and peak VO2, no heterogeneity was found (I2 = 0.0%), indicating the reliability of the results. For mPAP, mild heterogeneity was found (I2 = 44.7%). We used a fixed model to assess the outcomes. However, the NYHA functional class showed moderate heterogeneity with I2 = 55.8%, which was largely attributed to one study after the sensitivity analysis;36 thus, the randomized model was chosen, assuming that underlying true effects differed between studies. Formal statistical tests suggested that there was no evidence of publication bias based on Begg’s and Egger’s tests.
This study has several limitations that should be noted. First, the results were limited by the small number of studies and the moderate degree of heterogeneity observed. Thus, the analysis results should be interpreted with caution. Second, very few studies have subjected Fontan patients to cardiac catheterization because the findings are difficult to understand, especially the effect of collateral vessels. As a result of this, evidence of evaluating mPAP became weaker. Although mPAP was evaluated in two studies with poor evidence, PVR was not calculated due to insufficient data and information. Third, although the potential impact of pulmonary vasodilators on Fontan physiology has been evaluated, no study to date has addressed the essential question of therapy for Fontan patients: will treatment with pulmonary vasodilators alter the long-term outcomes of patients with this imperfect physiology?44 In addition, whether chronic daily use of pulmonary vasodilators will reduce or eliminate the risk of late consequences after Fontan surgery is a question that must be answered.45 Therefore, larger sample sizes and RCTs with long-term follow-up are needed to evaluate the efficacy and safety of pulmonary vasodilator therapy in post-Fontan patients.
Conclusion
Pulmonary vasodilators (primarily the PDE-5 inhibitor and endothelin-1 receptor antagonist) significantly improved the hemodynamics of Fontan patients, reduced the NYHA functional class, and increased the 6MWD. Peak VO2 was also significantly improved in Fontan patients with poor exercise capacity. No significant change was observed in patient mortality, BNP, or NT-proBNP. Overall, the pulmonary vasodilators were well tolerated. This conclusion should be considered carefully and confirmed with large RCTs.
Conflict of interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers 81170188 and 30971212), the Natural Science Foundation of Chongqing (grant number CSCT 2009BB5069), and the Chongqing Municipal Health and Family Planning Commission (grant numbers 2016HBRC001 and 2016XMSB 0003767).
References
- 1.Kreutzer G, Galíndez E, Bono H, et al. An operation for the correction of tricuspid atresia. J Thorac Cardiovasc Surg 1973; 66: 613–621. [PubMed] [Google Scholar]
- 2.Hosein RB, Clarke AJ, McGuirk SP, et al. Factors influencing early and late outcome following the Fontan procedure in the current era. The ‘Two Commandments’? Eur J Cardiothorac Surg 2007; 31: 344–352; discussion 53. [DOI] [PubMed] [Google Scholar]
- 3.McGuirk SP, Winlaw DS, Langley SM, et al. The impact of ventricular morphology on midterm outcome following completion total cavopulmonary connection. Eur J Cardiothorac Surg 2003; 24: 37–46. [DOI] [PubMed] [Google Scholar]
- 4.Ono M, Boethig D, Goerler H, et al. Clinical outcome of patients 20 years after Fontan operation – effect of fenestration on late morbidity. Eur J Cardiothorac 2006; 30: 923–929. [DOI] [PubMed] [Google Scholar]
- 5.Diller GP, Giardini A, Dimopoulos K, et al. Predictors of morbidity and mortality in contemporary Fontan patients: Results from a multicenter study including cardiopulmonary exercise testing in 321 patients. Eur Heart J 2010; 31: 3073–3083. [DOI] [PubMed] [Google Scholar]
- 6.Henaine R, Vergnat M, Bacha EA, et al. Effects of lack of pulsatility on pulmonary endothelial function in the Fontan circulation. J Thorac Cardiovasc Surg 2013; 146: 522–529. [DOI] [PubMed] [Google Scholar]
- 7.Ridderbos FJ, Wolff D, Timmer A, et al. Adverse pulmonary vascular remodeling in the Fontan circulation. J Heart Lung Transplant 2015; 34: 404–413. [DOI] [PubMed] [Google Scholar]
- 8.Deal BJ, Jacobs ML. Management of the failing Fontan circulation. Heart 2012; 98: 1098–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francois K, Bove T, De Groote K, et al. Pleural effusions, water balance mediators and the influence of lisinopril after completion Fontan procedures. Eur J Cardiothorac Surg 2009; 36: 57–62. [DOI] [PubMed] [Google Scholar]
- 10.Eicken A, Petzuch K, Marek J, et al. Characteristics of Doppler myocardial echocardiography in patients with tricuspid atresia after total cavopulmonary connection with preserved systolic ventricular function. Int J Cardiol 2007; 116: 212–218. [DOI] [PubMed] [Google Scholar]
- 11.Hager A, Fratz S, Schwaiger M, et al. Pulmonary blood flow patterns in patients with Fontan circulation. Ann Thorac Surg 2008; 85: 186–191. [DOI] [PubMed] [Google Scholar]
- 12.Mori H, Park IS, Yamagishi H, et al. Sildenafil reduces pulmonary vascular resistance in single ventricular physiology. Int J Cardiol 2016; 221: 122–127. [DOI] [PubMed] [Google Scholar]
- 13.Ovaert C, Thijs D, Dewolf D, et al. The effect of bosentan in patients with a failing Fontan circulation. Cardiol Young 2009; 19: 331–339. [DOI] [PubMed] [Google Scholar]
- 14.Rhodes J, Ubeda-Tikkanen A, Clair M, et al. Effect of inhaled iloprost on the exercise function of Fontan patients: A demonstration of concept. Int J Cardiol 2013; 168: 2435–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabri MR, Zolfi-Gol A, Ahmadi A, et al. Effect of tadalafil on myocardial and endothelial function and exercise performance after modified Fontan operation. Pediatr Cardiol 2016; 37: 55–61. [DOI] [PubMed] [Google Scholar]
- 16.Uhm JY, Jhang WK, Park JJ, et al. Postoperative use of oral sildenafil in pediatric patients with congenital heart disease. Pediatr Cardiol 2010; 31: 515–520. [DOI] [PubMed] [Google Scholar]
- 17.Van De Bruaene A, La Gerche A, Claessen G, et al. Sildenafil improves exercise hemodynamics in Fontan patients. Circ Cardiovasc Imaging 2014; 7: 265–273. [DOI] [PubMed] [Google Scholar]
- 18.Goldberg DJ, French B, McBride MG, et al. Impact of oral sildenafil on exercise performance in children and young adults after the fontan operation: A randomized, double-blind, placebo-controlled, crossover trial. Circulation 2011; 123: 1185–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hebert A, Mikkelsen UR, Thilen U, et al. Bosentan improves exercise capacity in adolescents and adults after Fontan operation: The TEMPO (Treatment With Endothelin Receptor Antagonist in Fontan Patients, a Randomized, Placebo-Controlled, Double-Blind Study Measuring Peak Oxygen Consumption) study. Circulation 2014; 130: 2021–2030. [DOI] [PubMed] [Google Scholar]
- 20.Schuuring MJ, Vis JC, van Dijk AP, et al. Impact of bosentan on exercise capacity in adults after the Fontan procedure: A randomized controlled trial. Eur J Heart Fail 2013; 15: 690–698. [DOI] [PubMed] [Google Scholar]
- 21.Cedars AM, Saef J, Peterson LR, et al. Effect of ambrisentan on exercise capacity in adult patients after the Fontan procedure. Am J Cardiol 2016; 117: 1524–1532. [DOI] [PubMed] [Google Scholar]
- 22.Giardini A, Balducci A, Specchia S, et al. Effect of sildenafil on haemodynamic response to exercise and exercise capacity in Fontan patients. Eur Heart J 2008; 29: 1681–1687. [DOI] [PubMed] [Google Scholar]
- 23.Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg 2011; 39: 91–92. [DOI] [PubMed] [Google Scholar]
- 24.Goldberg DJ, French B, Szwast AL, et al. Impact of sildenafil on echocardiographic indices of myocardial performance after the Fontan operation. Pediatr Cardiol 2012; 33: 689–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins J and Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. 2014.
- 26.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 27.Overton RC. A comparison of fixed-effects and mixed (random-effects) models for meta-analysis tests of moderator variable effects. Psychol Methods 1998; 3: 354–379. [Google Scholar]
- 28.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1995; 50: 1088–1101. [PubMed] [Google Scholar]
- 29.Stuck AE, Rubenstein LZ, Wieland D. Bias in meta-analysis detected by a simple, graphical test. BMJ 1998; 316: 469–470. [PMC free article] [PubMed] [Google Scholar]
- 30.Cai J, Su Z, Shi Z, et al. Nitric oxide and milrinone: combined effect on pulmonary circulation after Fontan-type procedure: a prospective, randomized study. Ann Thorac Surg 2008; 86: 882–888. discussion 888. [DOI] [PubMed] [Google Scholar]
- 31.Cai J, Su Z, Shi Z, et al. Nitric oxide in conjunction with milrinone better stabilized pulmonary hemodynamics after Fontan procedure. Artif Organs 2008; 32: 864–869. [DOI] [PubMed] [Google Scholar]
- 32.Ciliberti P, Alessandro G. Siliberti P2011-Impact of oral chronic administration of sildenafil in children and young adults after the Fontan operation. Future Cardiol 2011; 7: 609–612. [DOI] [PubMed] [Google Scholar]
- 33.Hebert AH, Mikkelsen UR, Thilen U, et al. Effects of bosentan on peak oxygen consumption, hemodynamics and functional class in Fontan Patients: A randomized, placebo controlled, double blind study. J Am Coll Cardiol 2014; 63: A478. [DOI] [PubMed] [Google Scholar]
- 34.Aboulhosn JA, Derk G, Houser L, et al. Efficacy of endothelin blockade in adults with Fontan physiology. J Am Coll Cardiol 2013; 61: E1268. [DOI] [PubMed] [Google Scholar]
- 35.Shang XK, Li YP, Liu M, et al. Efficacy of endothelin receptor antagonist bosentan on the long-term prognosis in patients after Fontan operation. Chin J Cardiol 2013; 41: 1025–1028. [PubMed] [Google Scholar]
- 36.Shang XK, Lu R, Zhang X, et al. Efficacy of bosentan in patients after Fontan procedures: A double-blind, randomized controlled trial. J Huazhong Univ Sci Technolog Med Sci 2016; 36: 534–540. [DOI] [PubMed] [Google Scholar]
- 37.Giordano R, Palma G, Poli V, et al. First experience with sildenafil after Fontan operation: Short-term outcomes. J Cardiovasc Med (Hagerstown) 2015; 16: 552–555. [DOI] [PubMed] [Google Scholar]
- 38.Mendoza A, Albert L, Belda S, et al. Pulmonary vasodilator therapy and early postoperative outcome after modified Fontan operation. Cardiol Young 2015; 25: 1136–1140. [DOI] [PubMed] [Google Scholar]
- 39.Lewis G, Thorne S, Clift P, et al. Cross-sectional imaging of the Fontan circuit in adult congenital heart disease. Clin Radiol 2015; 70: 667–675. [DOI] [PubMed] [Google Scholar]
- 40.Gewillig M, Brown SC. The Fontan circulation after 45 years: Update in physiology. Heart 2016; 102: 1081–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hager A, Weber R, Muller J, et al. Predictors of sildenafil effects on exercise capacity in adolescents and adults with Fontan circulation. Clin Res Cardiol 2014; 103: 641–646. [DOI] [PubMed] [Google Scholar]
- 42.Heck PB, Muller J, Weber R, et al. Value of N-terminal pro brain natriuretic peptide levels in different types of Fontan circulation. Eur J Heart Fail 2013; 15: 644–649. [DOI] [PubMed] [Google Scholar]
- 43.Shabanian R, Shahbaznejad L, Razaghian A, et al. Sildenafil and ventriculo-arterial coupling in Fontan-palliated patients: A noninvasive echocardiographic assessment. Pediatr Cardiol 2013; 34: 129–134. [DOI] [PubMed] [Google Scholar]
- 44.Goldberg DJ, Paridon SM. Fontan circulation: The search for targeted therapy. Circulation 2014; 130: 1999–2001. [DOI] [PubMed] [Google Scholar]
- 45.Rychik J, Goldberg DJ. Late consequences of the fontan operation. Circulation 2014; 130: 1525–1528. [DOI] [PubMed] [Google Scholar]