Abstract
Male lower urinary tract symptoms (LUTS) is an increasingly important problem for the majority of late middle aged and elderly men. Fexapotide triflutate (FT) is a first in-class compound given by local injection via the transrectal intraprostatic route under ultrasound guidance. Data from >1700 FT and control injections in prospective randomized blinded controlled multicenter trials are reviewed and discussed in relation to current developments in the field of treatments for LUTS associated with benign prostatic hyperplasia (BPH). Long-term studies of FT in the United States have shown statistically significant improvement in BPH symptoms and objective outcomes including significant reduction in both spontaneous acute urinary retention as well as the subsequent incidence of BPH surgery. FT has been shown to be well tolerated with an excellent safety profile, and is an efficacious clinic-based treatment for BPH involving an intraprostatic injection that requires only a few minutes to administer, with no catheter nor anesthesia requirements.
Keywords: BPH, fexapotide triflutate, LUTS, urology
Introduction
Benign prostatic hyperplasia (BPH) is progressive age-related hyperplasia of prostate glandular and stromal tissues principally in the transition zone of the prostate. BPH is one of the most commonly diagnosed pathological conditions of men 50 years of age or older. It is present microscopically in the majority of men after the age of 70 years, and clinically significant BPH affects 50% or more of men in the elderly age groups >70 years old.1–4 BPH is a histological term and the symptoms with which BPH correlates are referred to as male lower urinary tract symptoms (LUTS). LUTS is more complex than simply BPH and has many extraprostatic aspects involving bladder, urethra, smooth muscle, nerves, metabolic abnormalities and other factors. Categories and terms of benign outlet obstruction, benign prostatic obstruction (BPO), benign prostatic enlargement (BPE), and overactive bladder (OAB) are recognized by urologists.5–8 LUTS is also subcategorized according to storage and voiding symptoms, and individual symptoms such as urgency, frequency, and nocturia, for example are often considered separately. Prostate volume (PV) increases over time9 and BPH/BPE symptoms usually get worse over time.3 Left untreated, BPH may lead to complications such as acute urinary retention (AUR), urinary tract infections (UTIs), bladder calculi, renal deterioration and may require surgery for any of those causes or for symptom control. The typical man with bothersome BPH-related nocturia, urgency, frequency, hesitation, and dysuria experiences a significant impact on his activities of daily life, and suffers from the distressing effects of poor sleep patterns and inhibition of many activities. Due to the complexity of LUTS and other reasons, such as demographic trends, new and effective treatments for symptomatic BPH/BPE have become an increasingly important focus for research and development. A broad range of newer devices and techniques have been in development in recent years, designed to meet the challenges of the demographic trend in middle aged and older men. This report reviews the progress in the development of fexapotide triflutate (FT) which is a first-in-class new molecular approach to managing BPH symptoms.
Management of LUTS due to BPH includes diagnostic evaluation to exclude prostate cancer (PCa) or benign conditions that are not attributable to prostate hyperplasia, treatment options with conventional BPH medications [α blockers (ABs); 5-α reductase inhibitors (5ARIs)], and interventional therapies when necessary, such as transurethral minimally invasive surgical treatment (MIST) or invasive surgery [transurethral resection (TURP) or laser ablation].7,10–15 Newer MIST approaches have included the use of transurethral water vapor (Rezum);16 insertion of prostatic retraction devices (Urolift);17 transurethral water injections.18 These new MIST techniques have many promising advantages over older MIST techniques such as stents, microwave (TUMT), needle ablation (TUNA), ultrasound (HIFU), and various thermotherapies and invasive ablation modalities. Oral medications and surgical treatments are associated with adverse events, some of which may be unacceptable to individual patients. Medications are more often than not stopped due to reasons including combinations of intolerable side effects, diminished efficacy over time, poor risk–benefit ratio, interactions with other drugs, or cost.19–23 Overall, compliance with LUTS/BPH medication is poor with more than 50% of patients discontinuing treatment within 1 year of initiation. TURP, historically acknowledged as the ‘gold standard’, is highly effective in terms of urinary functional improvement, but TURP and other surgical techniques are associated with anesthetic risk, postoperative morbidities of voiding discomfort, bleeding, incontinence, frequent retrograde ejaculation, and sexual dysfunction.24
Overview of the need for newer medical treatments for BPH
Current therapeutic approaches to LUTS due to BPH/BPE depend on the severity of the symptoms and apart from watchful waiting include the following: initially, relaxing the smooth muscle of the bladder or the prostate with medications, or shrinking the prostate gland with medications is instituted; for more severe symptoms, minimally invasive interventions are available with ablation or coagulation of tissue in the transition zone of the prostate or surgically widening the prostatic urethral lumen with stents or stent-like devices; and for the most intractable symptoms surgical debulking of the transition zone is required.
Surgical debulking of the prostate’s transition zone
TURP is regarded as the gold standard for the treatment of BPH with typically marked and long lasting improvements in symptoms [International Prostate Symptom Score (IPSS) improvement of 15 points] and peak urinary flow rates (improvement ⩾7.5 ml/s).11,12 Other surgical procedures for the treatment of BPH include holmium laser enucleation of the prostate, transurethral incision of the prostate, transurethral vaporization of the prostate, transurethral laser vaporization, transurethral laser coagulation, and open prostatectomy.7,10–15 TURP requires hospitalization and has risks of both perioperative and postoperative complications, including excessive bleeding, TUR syndrome (causing hyponatremia), UTI, AUR, and (rarely) death; and long-term complications including incontinence, urethral stricture, bladder neck contracture, and sexual dysfunction in the form of retrograde ejaculation or erectile dysfunction.24,25 Of men receiving a TURP, ~15% do not respond to the treatment.26 Of those who do respond, retreatment rates are typically low (1–2%/year). TURP has become less frequently used as medical treatments and MIST have become more available. These surgical procedures are typically indicated for patients with complications due to BPH/BPE or severe symptoms and in whom drug treatment and conservative management options have failed or are not appropriate.7,10–15
Transition zone prostatic tissue ablation
A number of MIST procedures involving transurethral thermal ablation of tissue in the transition zone have been developed for office-based or shorter-term in-hospital treatment of BPH. These include TUMT, TUNA, HIFU, and others.7,10–15 The mean improvement in symptom score for MIST is ~ 9–11 points and mean improvement in peak urinary flow rate between 3 and 5 ml/sec. PV is typically not reduced by the ablative necrosis of the treated tissue. Long-term benefit has not been established and a significant percentage of patients treated subsequently receive TURP or require retreatment. MIST requires some form of sedation or anesthesia and post-procedure catheterization is often needed for 2–7 days. It also usually requires expensive dedicated equipment and specialized training in order to perform the procedures. Post-procedure and long-term complications can include retrograde ejaculation, significant hematuria, UTI, AUR, incontinence, bladder neck contracture, and urethral stricture. In addition to ablation MIST, other MIST treatments, such as stents, use mechanical approaches to treat LUTS. The newer techniques of Rezum16 and Urolift17 have reported substantial improvements in their results in terms of much less morbidity and greater patient convenience.
Medications to reduce the overall size of the prostate
The two approved 5ARIs are finasteride (Proscar™) and dutasteride (Avodart™).19,26–30 5ARI drugs have the effect of reducing the size of the prostate gland by 20–30% by inhibiting the conversion of the main male androgenic hormone testosterone to dihydrotestosterone (DHT) by the two isoenzymes 5α-reductase types I and II, and thereby lowering the intraprostatic levels of DHT. This class of drugs are intended for permanent daily use and require ⩾6 months to achieve modest symptomatic improvement (mean IPSS improvement of 3–4 points and peak urinary flow rate mean improvement of 1.5–2 ml/sec). Long-term use (>4 years) has been found to reduce the risk of AUR and the need for surgery by up to 50%. This class of drugs is indicated for men with LUTS who have larger prostates (>30 g) or prostate-specific antigen (PSA) >1.4 ng/ml, and who are considered to be at high risk of progression. Side effects are mainly related to sexual function and include decreased libido, impotence, decreased ejaculate, and more rarely breast enlargement. PCa chemoprevention studies raised the additional concern of the development of high grade PCa over time,29 a question as of yet unresolved. Prior 5ARI treatment was not associated with increased PCa mortality in a retrospective study.31 These drugs must be taken daily to maintain effect with relatively low real-world compliance rates, probably as a result of a combination of relatively low efficacy and low tolerability due to the side effects.19,27
Medications to relax the smooth muscle of the bladder and prostate
ABs affect α-adrenoceptors causing the smooth muscles in the bladder neck and prostate to relax, resulting in an improvement in urine flow rate and a reduction in symptoms of BPH.19,26–30 Approved ABs for the treatment of BPH include, for example, tamsulosin, alfuzosin, and silodosin as well as other generic ABs originally developed to treat hypertension, such doxazosin and terazosin. Onset of action is within 24 h to a few days, with moderate symptomatic improvement (mean IPSS improvement of 4–5 point, and mean peak flow improvement of 2 ml/sec). About one-third of men do not respond to such treatment. ABs do not affect prostate size and have no effect on the risk of AUR or the need for surgery. They are typically indicated for men with bothersome moderate-to-severe LUTS. Side effects include cardiovascular effects, such as hypotension, dizziness, and syncope, retrograde ejaculation (silodosin in particular), and intraoperative floppy iris syndrome (tamsulosin). Like 5ARIs, these drugs must be taken daily to maintain effect and there is poor long-term compliance.19–23 Combination therapy consists of an α-blocker (tamsulosin) and a 5ARI (dutasteride)28 designed to produce both the improved outcomes of a 5ARI and the symptomatic improvement of an α-blocker but it also has the predictable combined side effects of each of the individual medications.
Other medications
Tadalafil (Cialis™) is a phosphodiesterase type 5 (PDE-5) inhibitor drug approved for BPH in patients with erectile dysfunction and produces modest benefit for these patients.32 Intraprostatic injection of botulinum toxin type A had many reports of initial successes but did not succeed in larger trials.33,34
FT
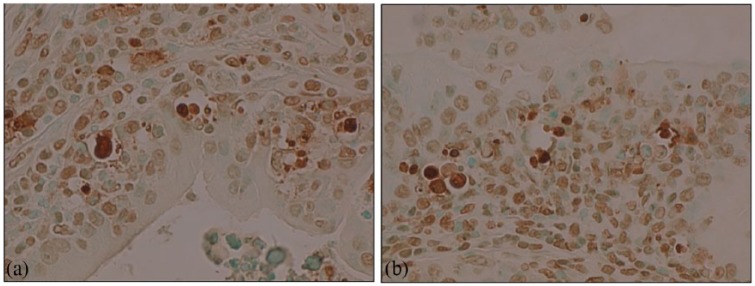
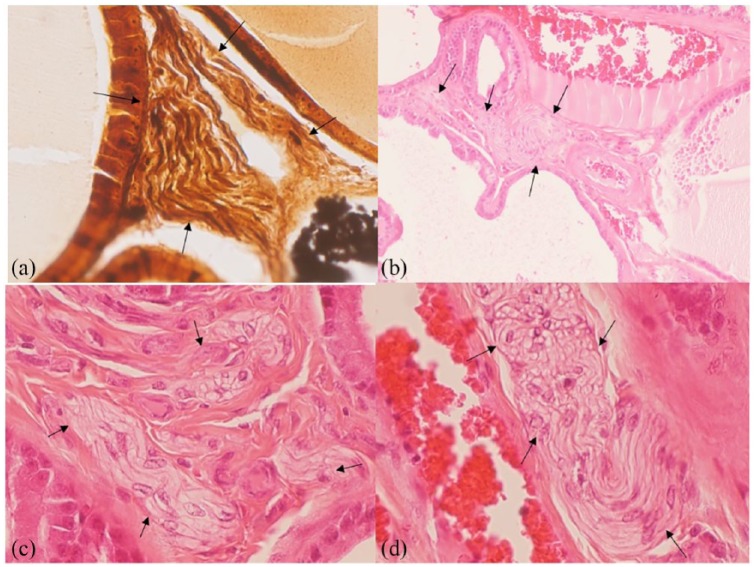
FT35–39 (also referred to as NX-1207) is a new molecular entity which stimulates caspase pathways (activation of caspases 7, 8, and 10, caspase recruitment domains 6, 11, and 14, and DIABLO), tumor necrosis factor pathways (activation of TNF1, TNFSF6, TNFSF8, TNFSF9, CD70 ligands, and TNFRSF19L, TNFRSF25, TRAF2, TRAF3, TRAF4, TRAF6 receptors), and B-cell lymphoma (BCL) pathways (activation of BIK, HRK, BCL2L10 and BCL3) in prostate glandular epithelial cells. FT selectively causes loss of cell membrane integrity, mitochondrial metabolic arrest, depletion of RNA, DNA lysis and aggregation, and cell fragmentation and cell loss (Figures 1, 2, and 3) with subsequent decompression of the urethral lumen.
Figure 1.
(a). Normal rat prostate gland, hematoxylin-eosin, X 400 (Figure courtesy of Nymox Corp.)
(b). Rat injected intraprostatically with FT 1 mg/ml, showing apoptotic cell loss after 72 h. Hematoxylin-eosin, X400 (Figure courtesy of Nymox Corp.)
(c, d). Rat injected intraprostatically with FT 1 mg/ml, showing more advanced extensive apoptotic cell loss after 72 h. Hematoxylin-eosin, X400 (Figure courtesy of Nymox Corp.)
(e). Rat prostate 12 months after FT single injection 1 mg/ml, showing near total loss of prostatic glandular epithelial cell population and marked shrinkage of gland. Hematoxylin-eosin, X20 (Figure courtesy of Nymox Corp.)
FT, fexapotide triflutate.
Figure 2.
(a, b). Rat prostate after FT single injection 1 mg/ml, showing positive staining with TUNEL, X400 original magnification (Figures courtesy of Nymox Corp.).
FT, fexapotide triflutate.
Figure 3.
(a). LNCAP prostate cancer cells in vitro 48 h post-treatment with FT 2.5 mg/ml, TUNEL stain, viewed under UV light, X600. Green fluorescence indicates cells undergoing apoptosis (Figures courtesy of Nymox Corp.).
(b). Electron micrograph of apoptotic cell in vitro 48 h after FT 2.5 mg/ml treatment illustrating prominent nuclear bleb formation (Figure courtesy of Nymox Corp.).
FT, fexapotide triflutate; LNCAP, lymph node carcinoma of the prostate; UV, ultraviolet.
Animal histopathology studies of FT in rats and dogs have demonstrated apoptosis in the prostate glandular cells with complete sparing of adjacent structures including the rectum, bladder, periprostatic tissues and urethra. The apoptosis can be shown histologically within 24 h and is present after a single injection for up to several weeks. The architecture of the injected glands becomes distorted as cells die and eventually the majority of cells in the injected areas have been depleted [Figure 1(a–e)]. Typical shrunken, hyperconvoluted and fragmented nuclei, with apoptotic bodies, and blebbing of tissues is seen. The Figures show progressive grades of severity of the changes [Figure 1(a–d)]. The nerve structures within and around the prostate are histologically normal after FT treatment [Figure 4a–d)].
Figure 4.
Rat prostate after FT administration with near total loss of glandular epithelial cell populations, showing normal surviving nerve fibers (arrows) in fields with total loss of prostate glandular epithelium.
(a). Rat prostate 3 months after FT 2 mg/ml administration once weekly for 1st month. Bielschowsky, X400 (Figure courtesy of Nymox Corp.).
(b, c). Rat prostate 12 months after FT 1 mg/ml administration once weekly for 1st month. Hematoxylin-eosin, B) X100; C) X400 (Figure courtesy of Nymox Corp.).
(d). Rat prostate 12 months after FT 2 mg/ml administration once weekly for 1st month. Hematoxylin-eosin X400 (Figure courtesy of Nymox Corp.).
FT, fexapotide triflutate.
FT mode of administration
Sterile FT is formulated in two vials, consisting of the lyophilized active pharmaceutical ingredient and the second vial of diluent consisting of phosphate-buffered saline (PBS) at physiologic pH (7.4). FT is administered by transrectal intraprostatic injection under ultrasound guidance (Figure 5) using a #22-gauge needle. Of the dose, half (5 ml) is injected into each of left and right transition zones of the prostate. The procedure time requirement is approximately 3–5 min and does not require a urethral catheter, intravenous or general anesthetic, and apart from a standard transrectal ultrasound (TRUS) requires no specialized equipment or instrumentation.36,37
Figure 5.
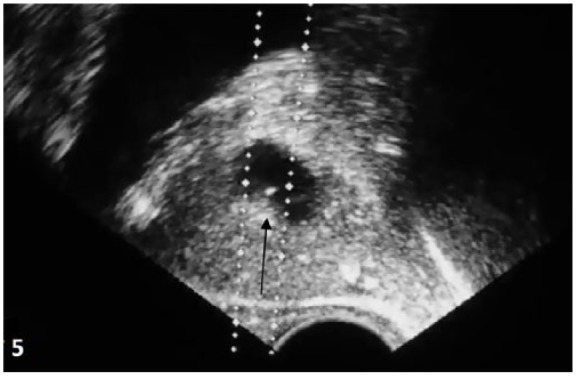
Ultrasound image showing FT injection (arrow) in parasagittal view.
FT, fexapotide triflutate.
FT pharmacodynamics and pharmacokinetics
Preclinical animal studies established that intraprostatic administration of FT caused cell loss in the prostate, leading to nonregressive prostate shrinkage. The effect of FT on prostatic tissue was examined in vivo by intraprostatic infusion of FT under open surgical direct visualization into the prostates of 2 and 3 month old Sprague–Dawley rats. Prostates were examined grossly and histologically after sacrifice at 24 h, 72 h, 1 week, 4 weeks, 12 weeks and 12 months. Prostatic glandular elements were reduced at 24 h, and the gland size was reduced at the earlier mentioned time intervals from 72 h to 12 months. Rats given intraprostatic injections of FT in PBS pH 7.4 showed on average 30–50% decrease in PV when compared with controls given PBS alone. These volumetric changes were present at 72 h and persisted at 12 months. The rat volume changes are based on a volume of injection corresponding to the complete volume of the prostate (as opposed to focal injection in the human transition zone of volume approximating to 10–30% of the prostate gland volume). A similar effect on prostate weight was found in beagle dogs given intraprostatic FT by needle injection through the abdominal wall (data on file, Nymox Corporation).
Pharmacokinetic measurements of FT in plasma post-injection have shown no detectable levels in plasma of FT in any patients, at any time interval.35–37 This lack of exposure to nonprostate tissues reinforces the selectivity that is evidenced in the animal safety studies and the human safety data discussed below.
Clinical trial experience with fexapotide
Overall, eight multicenter United States (US) BPH studies have been completed, including two phase I–II studies, two phase II studies and four phase III studies with long-term follow up in all studies. The initial studies were single administration open-label 1 month; and the phase II studies were blinded and of 3 and 6 months duration. Phase III studies NX02-0017 and NX02-0018 (0017/0018) were randomized, double-blind, parallel-group studies initially comparing FT with placebo 10 ml (vehicle alone) at 1 year and extending in the same protocols with double-blind follow up for 2–6.5 years post-randomization. Studies were conducted at 72 US sites (85 US sites approved and initiated; 80 with patient screening and assessments; 72 with patient enrollments) from 2009 to 2017, with protocols approved by institutional review boards (clinicaltrials.gov identifiers: NCT00918983, NCT00945490, NCT01438775, NCT01846793). Informed consent was obtained from all individual participants included in the study. Patients were enrolled based on BPH symptom score (IPSS), TRUS prostate volume (PV), and urinary peak flow rate (Qmax; Table 1). Patients were centrally randomized in a 3:2 ratio FT:placebo by a computer-generated randomization schedule executed by nonstudy personnel at an independent randomization service provider with no contact except by interactive voice response system. Patients, investigators, all site and nonsite study personnel, monitors, and outcome assessors were blinded as to randomization. The following were captured at baseline and 10 days, 1, 3, 6, 9, 12 months: IPSS, Qmax (3, 6, 12 months), PV (12 months), BPH impact index, sexual function questionnaire, and safety parameters. IPSS, BPH treatments and urological events were prospectively captured at long-term follow up. Crossover studies: 351 patients after completion of 0017/0018 (maintaining initial treatment double-blind) were randomized (n = 344 treated) at intervals of 0.5–39.1 months after the first year [mean 20.4 (standard deviation 7.15) months post-randomization] into two open-label FT reinjection 6 month studies NX02-0020 and NX02-0022 (0020/0022) with additional protocol-based long-term follow up. The 0020/0022 studies were required for reinjection safety/efficacy data and for patient access to FT after 1 year. Blinded patients could elect no further treatment; oral conventional BPH medications; surgical treatment; or FT treatment. Long-term prespecified comparator outcomes included objective measures of incidence of surgery and incidence of AUR, as well as self-reported IPSS and nocturia scores (IPSS question 7).35–39 Safety outcomes (including sepsis, new incidence of PCa, etc.) were preplanned. 0017/0018 enrolled 498 (1212 screened) and 497 (1224 screened) patients, respectively. Baseline characteristics, demographics, and patient disposition are summarized previously35 and disposition is summarized in the CONSORT diagram (Table 2). ITT lost to follow up percentages were 1.4–1.6% at 12 months and 7.1–7.3% at long-term follow up (mean 43 months). At long-term follow up 2.9% of patients had unrelated serious adverse events or death, or otherwise inability to provide a valid questionnaire response (e.g. dementia or other exclusion criteria).
Table 1.
Inclusion/exclusion criteria for studies NX02-0017 and NX02-0018.
|
Inclusion criteria
Male, ⩾45 years of age, with signed informed consent. No clinically significant deviation from normal in medical history, physical examination, clinical laboratory determinations and ECG. History of BPH ⩾1 year; AUASI ⩾15; PV ⩾30 ml (30 g) and ⩽ 70 ml (70 g) as determined by TRUS ⩽6 mos prior, Qmax <15 ml/s. No BPH medications prior to baseline assessments (AB ⩾2 weeks, and 5-ARI ⩾6 months stopped prior), and for trial duration. |
|
Exclusion criteria
Use of any of the following concomitant medications: immunosuppressants, anticoagulants, α-blockers, 5-ARIs, antipsychotics, chemotherapy, medication prescribed for dementia, male hormonal replacement, and medication prescribed for overactive bladder. Use of any new prescription or over-the-counter medications and herbal preparations within 1 week prior to visit 2. Documented urinary tract infection more than once in the past 12 months. PSA ⩾10 ng/ml. For patients with PSA ⩾4 ng/ml and <10 ng/ml a negative prostatic biopsy within prior 12 months. Presence of a symptomatic median lobe of the prostate. Post-void residual urine volume >200 ml. Lower urinary tract instrumentation of any type within 30 days of visit 1. |
5-ARI, α reductase inhibitor; AB, α-blockers; AUASI, American Urological Association Symptom Index; BPH, benign prostatic hyperplasia; ECG, electrocardiograph; mos, months; PSA, prostate-specific antigen; PV, prostate volume; TRUS, transrectal ultrasonography.
Table 2.
CONSORT diagram of patient enrollment, allocations, treatment, follow up, and disposition.

|
AE, adverse event; FT, fexapotide triflutate; LTFU, lost to follow up; SAE, serious adverse event.
Side effects and safety of FT
FT is highly selective for prostate glandular epithelium when it is injected into prostate. The nerve and vascular elements are unaffected, which has been demonstrated in animal and safety studies. FT does not circulate outside of prostate to any discernible level after intraprostatic administration. The pharmacokinetic studies in patients and in animals showed no detectable plasma level after FT administration into the prostate.35–38 After intravenous administration in animals the drug is undetectable in plasma after 1–2 min. Therefore, FT is highly selective for prostate glandular epithelium and has no contact with other tissues outside of prostate, and the absence of systemic or other significant side effects is predictable. In clinical trials to date, there have been no side effects that have been found that are not found in placebo administrations. As with any instance involving a needle entering the gland the #22-gauge needle injection is associated with: mild dysuria, trace hematuria, and occasionally mild hematospermia, most or all of which are transient and require no treatment, as well as momentary discomfort from the injection itself, which is not described as painful by patients. FT treatment involves prophylactic antibiotic administration to help prevent infectious complications of the transrectal injection. Patients who receive FT or placebo also report side effects from the antibiotics, such as transient loose stools and transient antibiotic-related dyspepsia or mild transient abdominal discomfort.
The serious infection rate post-FT administration was virtually 0% in phase III studies per protocol. The #22-gauge needle injection of 5 ml bilaterally is very different from a prostate biopsy in terms of infection risk: much larger needle gun devices for biopsy (1) are much larger overall gauge; (2) involve removal of cores of tissue; and (3) involve 16 or more passages of the biopsy needle. In the phase III FT program with careful surveillance and broad spectrum prophylactic antibiotic treatment in n = 977 treatments of FT and placebo there were no cases per protocol of serious infection in FT-treated patients related to the injection. There were also no increases in uncomplicated UTIs related to FT administration. The notable virtual absence of serious infectious complications compared with prevalent needle biopsy data is attributable to (1) the less invasive nature of the needle passage (a smaller needle passed twice versus a larger needle or trocar passed ⩾14 times); (2) the atraumatic nature of the procedure (which is a simple injection and not a biopsy removal of tissue); (3) the patient trial selection which has excluded all patients with altered immunity or at-risk of infectious complications (e.g. on immunosuppressive regimens for other conditions); (4) thorough routine follow up monitoring post-injection including routine examination with urinalysis, and patient education regarding signs of infection such as fever; and (5) antibiotic prophylaxis including intramuscular (IM) broad spectrum prophylaxis at the time of FT administration (e.g. with gentamicin or carbapenem or equivalents). The FT study data on infection prophylaxis will be the subject of a separate report in preparation.
Laboratory data in FT-treated patients
Laboratory results were similar in FT and placebo groups. Incidence of culture confirmed a UTI <30 days post-treatment was not increased (0.6% in FT and 0.5% in placebo) and there were no cases of urosepsis in FT-treated patients. After 12 months, all statistical analyses of hematological and clinical chemistry parameters showed no significant changes, and mean serum PSA values were not significantly altered. Semen analyses of a subgroup of n = 41 FT samples and n = 32 placebo consecutively available samples were overall unchanged from baseline and no FT changes versus placebo were found. Anti-FT antibodies were not detected in n = 1072 samples (n = 574 FT n = 498 controls). Pharmacokinetic (PK) analyses of n = 106 consecutive plasma samples drawn at 1, 5, 10 and 20 min post-FT injections have shown no extraprostatic systemic signal.35,38,39
PV and PSA values were not expected to be statistically significantly reduced in BPH patients compared with placebo after a single FT injection after 1 year due to: (1) the anticipated 5–10% shrinkage of the gland localized to the transition zone from the FT administration added to (2) the 5% annual growth of the gland would not give a change large enough to be adequately powered in these studies for statistical comparison.35,38,39 However, the PV in FT single administration treated patients was statistically reduced from baseline in the phase III studies after 1 year (−2.06% p = 0.0003), which was not found in placebo controls.
Prostate cancer incidence in FT-treated patients
At 4 years follow up, newly diagnosed PCa after the first 12 months in FT-treated patients (1.1%, 4/349) was reduced compared with placebo (5.3%, 5/95; p = 0.0116; Table 3). These values are based on thorough follow up at urology specialty clinics and consist of diagnosed PCa with biopsy for cause, but do not refer to prospective randomized protocol-based requisite biopsy follow ups where the incidence would be expected to be somewhat higher. Nevertheless, the reduction is greater than placebo and much lower than reported control values in long-term BPH studies in the literature.35,38,39
Table 3.
Prostate cancer and spontaneous AUR incidence in phase III FT studies.a
| FT 2.5 mg |
Placebo |
p value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Patients with prostate cancer | 349b | 1.1 (4/349) | 95c | 5.3 (5/95) | 0.0116d |
| Incidence of AUR | 277e | 1.08 (3/277) | 142f | 5.63 (8/142) | 0.0058d |
NX02-0017, NX02-0018, NX02-0020, and NX02-0022.
Patients who received FT at least once in 0017, 0018, 0020, or 0022 with ⩾4 years LF.
Patients who received placebo in 0017/0018 and not CO to FT, with ⩾4 years LF.
Chi-squared test.
All patients in CO studies with ⩾3 years follow up.
Placebo patients in studies 0017/0018 not CO to FT, with ⩾3 years follow up.
Biopsies for cause n = 44 (9.7%); FT n = 27 (7.7%), placebo n = 17 (17.9%). Cancer detection rate on biopsies FT 4/27 (14.8%); placebo 5/17 (29.4%).
AUR, acute urinary retention; CO, crossover; FT, fexapotide triflutate; LF, long term follow up.
Incidence of spontaneous AUR in FT-treated patients
Incidence of spontaneous AUR within 3 years in the FT group was reduced (1.08%) compared with the placebo population (5.63%; p = 0.0058; Table 3).
Sexual effects of FT
There were no transient or persistent FT-related sexual side effects.35,38,39 For prior BPH treatment-naïve patients enrolled in the studies, FT versus placebo control patients’ long-term sexual questionnaire data (problem assessment scale of the brief male sexual function inventory) showed an improvement in self-reported sexual function in FT-treated patients (+0.64) compared with a worsening in placebo-treated patients (−0.88, p = 0.0049).
IPSS scores after FT treatment
In shorter-term smaller initial phase I–II and phase II trials, FT treatment produced 6–9 points improvement at 3–6 months after a single treatment. In the long-term phase III studies the effect of a single administration after a mean of 3.6 years was overall 5.7 points improvement from baseline (versus 4 for placebo, p < 0.0001). In phase III reinjection studies, the mean benefit from baseline was 8.02 points after a mean duration post-initial randomization of 26.2 months. The latter improvement from two injections is a reasonable minimal estimate for the long-term benefit of repeated treatments of FT.
The IPSS improvement from baseline has an early onset, with mean change −5.8 points after 10 days (versus placebo −4.6, p = 0.0032). The mean improvement over time versus placebo requires >1 year to be statistically apparent. It is well established that placebo patient-related outcomes (PROs) after interventions or sham interventions for BPH are consistently in the 5–6-point IPSS range40–43 so that for a molecular treatment, PRO data well past 1 year is needed to assess PRO efficacy. That is not the case for surgical treatments where improvements in IPSS in the 9–12-point range are possible. This is not a unique situation; for example, 5ARIs require ⩾6 months to show efficacy versus placebo, and there are many examples in other fields such as oncology. It has also been pointed out that since BPH is chronic, long-term data therefore is required in any event.
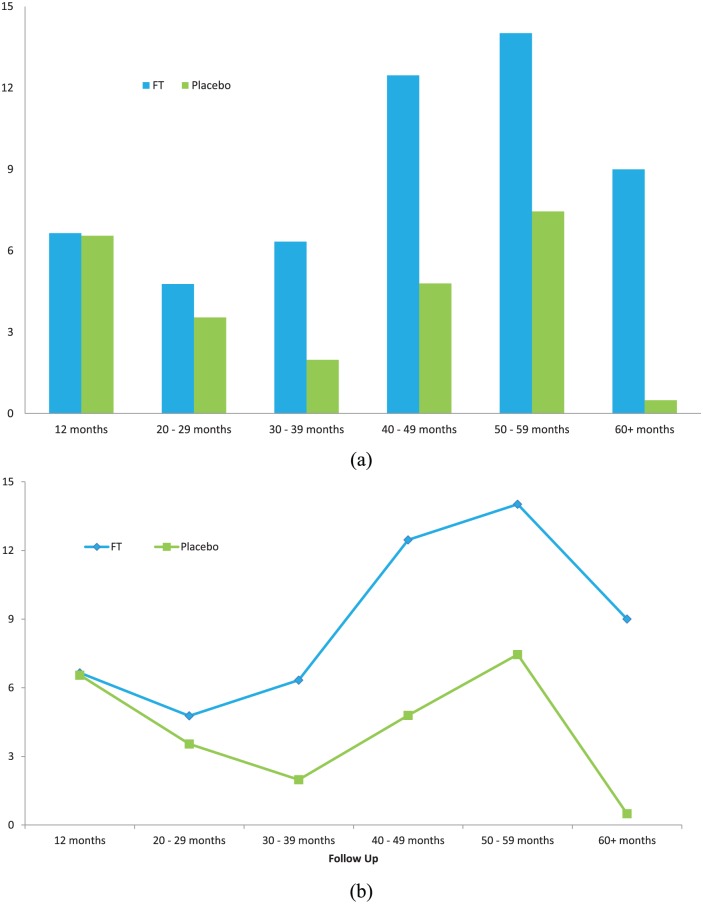
In the long-term FT studies, patients were followed up by protocol as a cross-section population with post-treatment intervals of 2–6.5 years and mean follow up of 3.6 years. The IPSS improvement means were compared using highly conservative methods (all failures or dropouts due to treatment effects or lack of efficacy were included) and were statistically significant in both of the two large phase III studies as well as in the two studies pooled together. The lost to follow up percentage was extremely low: 2% after 1 year and 7% at long-term follow up. Additional analyses were done with stratifications of the follow-up population by time interval post-treatment (Figure 6). The time curve analyses (including the first year of data in the analyses) were consistently significant compared with placebo, indicating a significant benefit of FT overall, using all the available data including carry forward of all failures. These additional analyses included for example, time-weighted mean improvements from baseline over time (Figure 6); statistical trend comparisons versus time, Student’s t tests of means of interval means, and others.
Figure 6.
Time-weighted mean values of IPSS improvement (reduction) from baseline plotted versus time in (a) bar graph; and (b) time curve showing mean of time-weighted IPSS improvement from baseline versus time. The overall time-weighted mean IPSS improvement for the pivotal phase III trials (including all first year values) was statistically significant versus placebo [FT mean 8.87 SD 3.68, placebo mean 4.13 SD 2.67; p = 0.0287 95% confidence interval (CI) .6043–8.8757]. For this comparison, double-blind follow-up IPSS values were plotted according to follow-up interval (time from randomization to follow up) and weighted by duration (each value multiplied by time in years). All failures (e.g. withdrawals due to lack of efficacy, BPH surgical treatments) were carried forward (i.e. included in subsequent time interval mean efficacy values).38
BPH, benign prostatic hyperplasia; CI, confidence interval; FT, fexapotide triflutate;
IPSS, International Prostate Symptom Score; SD, standard deviation.
Responder analysis for IPSS improvement at long-term follow up was statistically significant at cutoffs of <0, < −1, < −2, and < −3 IPSS [i.e. proportion of patients with either 1, 2, 3, or 4 points or more improvement (reduction) from baseline]. Subgroup analyses for IPSS improvement showed that nearly all adequately powered subgroups analyzed at long-term follow up showed statistical significance, including subgroups according to age; ethnicity; prior use of BPH treatment; BPH history; baseline IPSS; baseline PV; baseline urinary peak flow rate.
Long-term IPSS improvement in first-line (treatment-naïve) patients (FT mean −6.6 points, placebo −4.0, median −6.2 versus −3.0, p < 0.0001) and in prior BPH-treatment patients were both found; treatment-naïve patients have greater improvement than patients who have had prior BPH treatments before entering the trials.
Incidence of surgical treatments for BPH after FT treatment
After 1 year in the phase III studies, patients (still double blinded as to initial randomization) were offered open-label FT at their discretion if they still qualified by inclusion/exclusion criteria, or they could pursue other treatments if they so wished or needed. These FT reinjections were required for regulatory purposes to demonstrate safety; and also, were needed for initial accrual purposes in order to offer treatment to patients who had 40% potential chance of randomization to blinded placebo. Many parameters were tested in these patients, mainly focused on long-term PRO outcomes and long-term incidence of surgical interventions.
Incidence of surgical interventions in FT-reinjected patients at 2 and at 3 years post-initial treatment was significantly reduced in all FT groups at both time points. Incidence of BPH surgery within 3 years of randomization was 5.22% in placebo-treated patients who had crossed over to FT versus 16.44% in placebo-treated patients who did not receive FT (p = 0.0048). Similar significant reductions were found for incidence of AUR, and in other groups of patients receiving FT.
Comparison of outcomes in blinded placebo-treated patients with subsequent FT treatment versus blinded placebo-treated patients with subsequent oral BPH medication treatments
Placebo-treated patients who received subsequent oral BPH medications had a rate of surgical intervention within 3 years of 30.3% compared with 5.22% for placebo patients who elected to have FT (p < 0.0001). The same trend was found in all other crossover FT groups compared with subsequent treatment with conventional oral medications. The IPSS changes long term were similarly better in FT-treated patients (mean improvement 8.02 points after mean duration post-randomization 26.2 months, compared with 2.79 points improvement in placebo-treated patients who subsequently used oral BPH medications, p < 0.0001).
Comparison of outcomes in blinded FT-treated patients with subsequent oral medication treatment or surgical interventions versus blinded placebo-treated patients with subsequent oral BPH medication or surgical interventions
At long-term follow up, comparison was made of IPSS scores in all FT and control patients who subsequently used conventional oral medications for any reason. The FT-treated patients had statistically higher IPSS improvements compared with placebo-treated patients (−8.28 versus −4.74, p < 0.0094). A similar outcome was found in all patients who either received oral medications or who had surgical interventions for their BPH (−10.6 versus −7.40, p < 0.0308).
Comment and discussion
Despite the high prevalence of symptomatic BPH (estimated at up to 25–50% or more of the elderly male population), there remains a need for effective treatments without the side effects and risks associated with conventional treatments. The risk–benefit is not adequate for many or most men, who may start 5ARI or ABs or combination therapy, but more often than not stop treatment due to side effects or insufficient efficacy. Surgical interventions are effective but involve pain, anesthesia, catheterization, recovery, risks, and frequent sexual sequelae, and are generally a last resort for serious complications or uncontrollable symptoms. There is a need for office-based noninterventional molecular treatments that are effective and well tolerated. FT is a first-in-class well tolerated and new molecular approach to office-based treatment of BPH.
TRUS-guided transrectal 22-gauge needle administration of 10 ml FT in two bilateral transition zone 5 ml injections without anesthesia, sedation or catheter placement is not the same as prostatic biopsy with 16 cores removed with a larger bore biopsy gun, neither in terms of discomfort nor risk of infection. The injection of sterile drug solution with antibiotic coverage in the FT program resulted in no cases of sepsis after FT administration under the phase III protocols, and the rate of related UTI in the first month was not increased. Patients do not find the injection painful and most have reported more discomfort from the routine TRUS than from the injection. Considering that 351 patients from phase III studies, NX02-0017/0018, decided at their individual discretion to return for elective reinjection of FT in studies NX02-0020/0022, it is clear that the administration of FT during a routine TRUS was considered well tolerated. Feedback from earlier trials36,37 and questionnaires (NX02-0016, clinicaltrials.gov identifier: NCT00759135) had earlier indicated that the injection was not considered painful by patients (unpublished data).
Adverse events and laboratory results have been similar in FT and placebo groups in all studies, indicating thus far, no known molecular side effects from FT. The only reported related adverse experiences in both drug and placebo groups were from prophylactic antibiotic treatments and from the transrectal needle insertion; in both of these categories the events reported were mainly mild, transient and of infrequent requirement of additional treatment. There were no cases of urosepsis in FT-treated patients, and the rate of UTIs was not increased compared with placebo or the expected untreated incidence rate. There were no significant sexual side effects reported, and semen analysis showed no significant changes from baseline. Anti-FT antibodies were undetectable. PK analysis showed no extraprostatic signal at any time point post-injection, indicating that FT has no measurable contact with tissues outside the prostate.
Although 10 ml of sterile saline injected into prostate is termed a placebo, it is probably more accurate to refer to it as an active injection of vehicle. The strongest evidence for that is the peak flow data which are considered by most scholars to be objective: vehicle-only injected patients had a mean improvement in peak flow rate of 1.9 ml/sec up to 1 year post-injection. That level of improvement is comparable to α-blocker treatments and exceeds 5ARI improvement levels. Considering also that the 1 year persistence of IPSS improvements in vehicle-only injected patients is also above α-blocker and 5ARI levels, it is fair to suggest that vehicle-only injection of 10 ml into the prostate is active. Longer-term the effect diminishes, and the benefit of FT comes into clearer view. The published literature indicates that 1-year outcomes in BPH studies are less valid than previously assumed, insofar as strong placebo responses and effects are constantly encountered up to 1 year and perhaps longer.40–43 The literature further indicates that long-term (>1 year) data capture and analyses are required to validate BPH treatments; given also that BPH is a condition that requires chronic treatment.19 Vehicle injections into the prostate have been postulated to be active controls in the reported literature due to a ‘mass effect’ or to a change in prostate parenchyma or capsule, which may also in part explain the pronounced effect up to 1 year in all published trials of this nature.43
Reduction in PCa incidence rate in FT-treated BPH patients was statistically significant. Patients who received FT had an incidence after 4 years of 1.1% compared with a non-crossover placebo incidence of 5.3% (p = 0.0116). FT 2.5 mg and 15 mg single targeted injections to biopsy-proven PCa foci have been found to reduce clinical and biopsy progression and treatment outcome parameters in T1c PCa patients in a multi-year study of 147 patients plus crossovers (Nymox, clinicaltrial.gov identifier: NCT01620515; data on file). Although the present BPH studies used TRUS targeted to the transition zone, it is probable that the 10 ml injections also entered to some extent the peripheral prostate zones where PCa is usually found. Significant PCa reduction in the FT BPH studies suggests that FT may have an inhibitory effect on clinically undetected low-grade PCa microfoci or precursor cells and lesions in quadrant locations where the FT BPH injections were bioavailable. Further study will be required to clarify the mechanism(s) of action.
Questions for future studies
The efficacy and safety of FT combination treatments, of FT used in different patient study populations, comparative studies versus other treatments, and the variability of dosing schedules remain to be answered by future investigations. Patients who have intractable severe LUTS but are poor surgical candidates are another important group where investigation may be warranted. Further studies will be needed to determine the impact of FT in relation to the gold standard of TURP.
Conclusion
For many men suffering from BPH, there remains an unmet need for office-based treatments for BPH that are effective and that have fewer side effects and better safety profiles than existing approved molecular and surgical treatments. Large long-term prospective randomized US studies of FT have shown statistically significant long-term improvement in BPH symptoms and objective outcomes including significant reduction in both spontaneous AUR as well as the subsequent incidence of BPH surgery. Based on a total of >1700 patient treatments including FT and placebo in US trials to date since 2002, FT has been shown to be well tolerated with an excellent safety profile. FT is a well-tolerated and efficacious clinic-based treatment for BPH involving an intraprostatic injection that requires only a few minutes to administer, with no catheter nor anesthesia requirements. FT injection represents a novel, first-in-class BPH treatment modality.
Footnotes
Funding: Funded by Nymox Pharmaceutical Corporation, Nassau, Bahamas.
Conflict of interest statement: Authors Shore and Tutrone are consultants/speakers for NeoTract. Author Shore is a consultant for NxThera. Authors Shore, Tutrone and Roehrborn are consultants for Nymox. Author Tutrone owns stock in Nymox. Author Roehrborn has a financial interest or other relationship with NxThera, NeoTract, Procept, ZenFlow and Meditate.
ORCID iD: Neal Shore  https://orcid.org/0000-0001-5767-0548
https://orcid.org/0000-0001-5767-0548
Contributor Information
Neal Shore, Carolina Urologic Research Center, 823 82nd PKWY, Myrtle Beach, SC 29572, USA.
Ronald Tutrone, Chesapeake Urology Research Associates, Baltimore, MD, USA.
Claus G. Roehrborn, University of Texas Southwestern Medical Center, Dallas, TX, USA
References
- 1. Bushman W. Etiology, epidemiology, and natural history of benign prostatic hyperplasia. Urol Clin North Am 2009; 36: 403–415. [DOI] [PubMed] [Google Scholar]
- 2. Jacobsen SJ, Girman CJ, Lieber MM. Natural history of benign prostatic hyperplasia. Urology 2001; 58: 5–16; discussion 16. [DOI] [PubMed] [Google Scholar]
- 3. Fitzpatrick JM. The natural history of benign prostatic hyperplasia. BJU Int 2006; 97(Suppl. 2): 3–6. [DOI] [PubMed] [Google Scholar]
- 4. Chute CG, Panser LA, Girman CJ, et al. The prevalence of prostatism: a population-based survey of urinary symptoms. J Urol 1993; 150: 85–89. [DOI] [PubMed] [Google Scholar]
- 5. Chapple CR, Wein AJ, Abrams P. Lower urinary tract symptoms revisited: a broader clinical perspective. Eur Urol 2008; 54: 563–569. [DOI] [PubMed] [Google Scholar]
- 6. Sexton CC, Coyne KS, Kopp ZS. The overlap of storage, voiding and postmicturition symptoms and implications for treatment seeking in the USA, UK and Sweden: EpiLUTS. BJU Int 2009; 103(Suppl. 3): 12–23. [DOI] [PubMed] [Google Scholar]
- 7. Gravas S, Bach T, Bachmann A, et al. Guidelines on the management of non-neurogenic male lower urinary tract symptoms (LUTS), including benign prostatic obstruction (BPO). European Association of Urology; (2015), http://uroweb.org/wp-content/uploads/EAU-Guidelines-Non-NeurogenicMale-LUTS-Guidelines-2015-v2.pdf. [DOI] [PubMed] [Google Scholar]
- 8. Gratzke C, Bachmann A, Descazeaud A. EAU guidelines on the assessment of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol 2015; 67: 1099–1109. [DOI] [PubMed] [Google Scholar]
- 9. Loeb S, Kettermann A, Carter H, et al. Prostate volume changes over time: results from the Baltimore longitudinal study of aging. J Urol 2009; 182: 1458–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oelke M, Bachmann A, Descazeaud A, et al. EAU Guidelines on the treatment and follow-up of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol 2013; 64: 118–140. [DOI] [PubMed] [Google Scholar]
- 11. McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol 2011; 185: 1793–1803. [DOI] [PubMed] [Google Scholar]
- 12. NICE National Clinical Guideline Centre for Acute and Chronic Conditions. Lower urinary tract symptoms. (2010) The management of lower urinary tract symptoms in men. London: Clinical Guideline. [Google Scholar]
- 13. Speakman M, Kirby R, Doyle S, et al. Burden of male lower urinary tract symptoms (LUTS) suggestive of benign prostatic hyperplasia (BPH) - focus on the UK. BJU Int 2015; 115: 508–519. [DOI] [PubMed] [Google Scholar]
- 14. Abrams P, Chapple C, Khoury S, et al. Evaluation and treatment of lower urinary tract symptoms in older men. J Urol 2013; 189: S93–S101. [DOI] [PubMed] [Google Scholar]
- 15. Lukacs B, Cornu JN, Aout M, et al. Management of lower urinary tract symptoms related to benign prostatic hyperplasia in real-life practice in France: a comprehensive population study. Eur Urol 2013; 64: 493–501. [DOI] [PubMed] [Google Scholar]
- 16. McVary KT, Gange SN, Gittelman MC, et al. Minimally invasive prostate convective water vapor energy (WAVE) ablation: a multicenter, randomized, controlled study for treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol 2016; 195: 1529–1538. [DOI] [PubMed] [Google Scholar]
- 17. Roehrborn C, Gange S, Shore N, et al. Four year results from the largest, prospective, randomized study of prostatic urethral lift (PUL). Eur Urol 2016; 15(Suppl. 3): e1077. [Google Scholar]
- 18. Roehrborn C, Gilling P. PNFLBA-03. The water study clinical results – a phase III blinded randomized parallel group trial of aquablation vs. transurethral resection of the prostate with blinded outcome assessment for moderate to severe LUTS in men with benign prostatic hyperplasia. J Urol 2017; 197: e603–e604. [Google Scholar]
- 19. Cindolo L, Pirozzi L, Fanizza C, et al. Drug adherence and clinical outcomes for patients under pharmacological therapy for lower urinary tract symptoms related to benign prostatic hyperplasia: population-based cohort study. Eur Urol 2015; 68: 418–425. [DOI] [PubMed] [Google Scholar]
- 20. Nichol MB, Knight TK, Wu J, et al. Evaluating use patterns of and adherence to medications for benign prostatic hyperplasia. J Urol 2009; 181: 2214–2222. [DOI] [PubMed] [Google Scholar]
- 21. Cindolo L, Pirozzi L, Sountoulides P, et al. Patient’s adherence on pharmacological therapy for benign prostatic hyperplasia (BPH)-associated lower urinary tract symptoms (LUTS) is different: is combination therapy better than monotherapy? BMC Urol 2015; 15: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fwu CW, Eggers P, Kirkali Z, et al. Change in sexual function in men with lower urinary tract symptoms/benign prostatic hyperplasia associated with long-term treatment with doxazosin, finasteride and combined therapy. J Urol 2014; 191: 1828–1834. [DOI] [PubMed] [Google Scholar]
- 23. Koh JS, Cho KJ, Kim HS, et al. Twelve-month medication persistence in men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Int J Clin Pract 2014; 68: 197–202. [DOI] [PubMed] [Google Scholar]
- 24. Rassweiler J, Teber D, Kuntz R, et al. Complications of transurethral resection of the prostate (TURP): incidence, management, and prevention. Eur Urol 2006; 50: 969–979. [DOI] [PubMed] [Google Scholar]
- 25. Zong HT, Peng XX, Yang CC, et al. The impact of transurethral procedures for benign prostate hyperplasia on male sexual function: a meta-analysis. J Androl 2012; 33: 427–434. [DOI] [PubMed] [Google Scholar]
- 26. Madersbacher S, Marszalek M, Lackner J, et al. The long-term outcome of medical therapy for BPH. Eur Urol 2007; 51: 1522–1533. [DOI] [PubMed] [Google Scholar]
- 27. Souverein PC, van Riemsdijk MM, de la Rosette J, et al. Treatment of benign prostatic hyperplasia and occurrence of prostatic surgery and acute urinary retention: a population-based cohort study in the Netherlands. Eur Urol 2005; 47: 505–510. [DOI] [PubMed] [Google Scholar]
- 28. Roehrborn CG, Barkin J, Siami P, et al. Clinical outcomes after combined therapy with dutasteride plus tamsulosin or either monotherapy in men with benign prostatic hyperplasia (BPH) by baseline characteristics: 4-year results from the randomized, double-blind combination of Avodart and Tamsulosin (CombAT) trial. BJU Int 2011; 107: 946–954. [DOI] [PubMed] [Google Scholar]
- 29. Theoret MR, Ning YM, Zhang JJ, et al. The risks and benefits of 5α-reductase inhibitors for prostate-cancer prevention. N Engl J Med 2011; 365: 97–99. [DOI] [PubMed] [Google Scholar]
- 30. Kaplan SA. Medical therapy for benign prostatic hyperplasia: new terminology, new concepts, better choices. Rev Urol 2006. a; 8: 14–22. [PMC free article] [PubMed] [Google Scholar]
- 31. Azoulay L, Eberg M, Benayoun S, et al. 5-alpha reductase inhibitors and the risk of cancer-related mortality in men with prostate cancer. JAMA Oncol 2015; 1: 314–320. [DOI] [PubMed] [Google Scholar]
- 32. Hazimouratadis K. A review of the use of tadalafil in the treatment of benign prostatic hyperplasia in men with and without erectile dysfunction. Ther Adv Urol 2014; 6: 135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hsu YC, Wang HJ, Chuang YC. Intraprostatic botulinum neurotoxin type A injection for benign prostatic hyperplasia – A spotlight in reality. Toxins (Basel) 2016; 8: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shim SR, Cho YJ, Shin IS, et al. Efficacy and safety of botulinum toxin injection for benign prostatic hyperplasia: a systematic review and meta-analysis. Int Urol Nephrol 2015; 48: 19–30. [DOI] [PubMed] [Google Scholar]
- 35. Shore N, Tutrone R, Efros M, et al. Fexapotide triflutate: results of long-term safety and efficacy trials of a novel injectable therapy for symptomatic prostate enlargement. World J Urol 2018; 36: 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shore N, Cowan B. The potential for NX-1207 in benign prostatic hyperplasia: an update for clinicians. Ther Adv Chronic Dis 2011; 2: 377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shore N. NX-1207: a novel investigational drug for the treatment of benign prostatic hyperplasia. Expert Opin Investig Drugs 2010; 19: 305–310. [DOI] [PubMed] [Google Scholar]
- 38. Tutrone R, Bidair M, Grunberger I, et al. Phase 3 clinical studies and biology of fexapotide triflutate office injectable for BPH. Presented at the American Urological Association Annual Meeting, 20 May 2018, San Francisco, CA. [Google Scholar]
- 39. Tutrone R, Goldberg K, Grunberger I, et al. Fexapotide triflutate: first in Class Injectable for BPH. Presented at the American Urological Association New York Section Annual Meeting, 6 November 2017, Havana, Cuba. [Google Scholar]
- 40. Eredics K, Madersbacher S, Schauer I. A relevant mid-term (12 months) placebo effect on lower urinary tract symptoms and maximum flow rate in male LUTS/BPH – a meta-analysis. Urology. Epub ahead of print 12 May 2017. DOI: 10.1016/j.urology.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 41. Fässler M, Meissner K, Kleijnen J, et al. A systemic review found no consistent difference in effect between more and less intensive placebo interventions. J Clin Epidemiol 2015; 68: 442–451. [DOI] [PubMed] [Google Scholar]
- 42. van Leeuwen J, Castro R, Busse M, et al. The placebo effect in the pharmacologic treatment of patients with lower urinary tract symptoms. Eur Urol 2006; 50: 440–453. [DOI] [PubMed] [Google Scholar]
- 43. Welliver C, Kottwitz M, Feustel P, et al. Clinically and statistically significant changes seen in sham surgery arms of randomized, controlled benign prostatic hyperplasia surgery trials. J Urol 2015; 194:1682–1687. [DOI] [PubMed] [Google Scholar]