Abstract
Background:
Our aim was to systematically review the relationship between iron and incident cognitive decline or dementia from midlife onwards.
Methods:
Systematic review of eligible studies using Medline, Embase and PsycINFO® for the period from 1 January 1986 to 2 December 2016 (CRD42016023800), where study populations had a mean age of over 50 years and were free of cognitive impairment or dementia at baseline. Two authors independently extracted data according to eligibility criteria and assessed study characteristics, quality and outcomes. Disagreement was resolved by discussion.
Results:
A total of 1185 relevant records were identified with 12 full-text articles eligible for review. Six studies were excluded, leaving six texts to be included. Sample size ranged from 90 to 7173, with an average follow up of approximately 11.5 years. Baseline iron measures included brain iron (n = 2), iron-related biomarkers in blood and plasma (n = 2), and iron intake estimates from dietary records (n = 2). Outcomes were dementia incidence (n = 2) and longitudinal outcomes on neuropsychological tests (n = 4). Bias was evident across studies in one or more of the following: recruitment, iron exposure, outcome assessments, potential confounders, missing data or attrition.
Conclusions:
Diversity across the small number of identified studies precludes conclusions regarding the role of iron in cognitive decline or dementia. Our review highlights substantial gaps in the evidence base and the need for more comprehensive, higher quality studies in this area.
Keywords: cognitive ageing, dementia, incidence, iron, review
Introduction
Older age is associated with increased risk of iron deficiency (ID), elevated body iron stores and increased brain iron levels.1,2 Complex regulatory mechanisms fine-tune iron metabolism and homeostasis for both heme and nonheme iron. Iron has a key role in multiple pathways, for example, as a constituent part of proteins needed for oxygen transport, oxidative phosphorylation, myelin production, and production and breakdown of neurotransmitters.3–5 Consequently there are multiple plausible mechanisms by which iron deficiency or overload may elevate the risk of age-related cognitive decline and dementia. A detailed mechanistic review is beyond the scope of this article, however for recent reviews in this area, see Hare and colleagues3 and Waldvogel-Abramowski and colleagues4
Iron deficiency
In older adults, absolute ID is caused by insufficient dietary iron intake, gastrointestinal malabsorption, or increased blood losses attributable to gastrointestinal pathologies.2 Potential cognitive impacts related to inadequate iron might stem from cerebral hypoxia,6 insufficient neurotransmitter synthesis5 or poor myelin integrity.7
There is also a growing body of evidence that anaemia in older age (defined by haemoglobin level <13 g/dl in men and <12 g/dl in women) is associated with poor cognition, cognitive decline and dementia.8 ID anaemia accounts for around 16% of total anaemia cases.9
Iron overload
Body iron levels may be elevated in middle-aged and older adults due to consumption of highly bioavailable forms of iron (supplemental iron and red meat) and of fruit, an enhancer of nonheme-iron absorption (vitamin C).10 In two meta-analyses of prospective cohort studies, high body iron stores and consumption of heme iron were associated with increased risk of coronary heart disease (21 studies)11 and type 2 (T2) diabetes (11 studies),12 which may highlight a further pathway between iron overload and cognitive impairment and dementia.13–15
Increased brain iron
The accumulation of iron in the brain is an established hallmark of ageing.16 Excess iron stores may increase pro-oxidant reactions and generation of free radicals,1,3 which could contribute to neurodegeneration. In cross-sectional studies, magnetic resonance imaging (MRI) measures of iron are associated with amyloid load,17 smaller hippocampal volume18 and poorer cognitive performance.15,19 Hippocampal iron content has also been correlated with cognitive performance and disease duration in Alzheimer’s disease20,21 and MRI-measured iron in the basal ganglia in cognitively normal adults predicted greater rates of cognitive decline and cortical atrophy.22,23 Brain iron deposits have also been positively associated with white matter hyperintensities, suggesting a potential link between brain iron and vascular pathology.15,24
The proportion of the population at risk for cognitive decline and dementia is rapidly increasing, and there is no effective pharmacological cure or preventive intervention. The promotion of clinical understanding through collation of evidence for modifiable risk factors is an important preventive strategy. Iron has been raised as a potentially modifiable risk factor for cognitive decline or dementia but the area is complex. There are multiple ways of measuring both iron and cognition, and to date there is no critical appraisal of the evidence depth, breadth or quality. Our aim was to systematically review and evaluate the current evidence base that relates to the relationship between iron and incident cognitive decline or dementia.
Methods
Search strategy and selection criteria
The databases MEDLINE, Embase and PsycINFO were searched over a 30-year period from 1986 to 2 December 2016. Because this is a first review of the literature in this area, search terms were chosen to be as broad as possible to prevent inadvertent exclusion of particular cognitive assessment or particular iron measures. Search terms included dementia, alzheimers disease, vascular dementia, multi-infarct dementia, cognit* and iron, heme, haem, ferritin and serum transferrin. See supplementary text for details. Reference lists were screened and experts in the field were consulted. In addition, searches of the Cochrane Library, the ISRCTN Register and the ClinicalTrials.gov website were carried out to look for ongoing or completed relevant trials.
All identified abstracts, or titles, where abstracts were unavailable, were independently read by two reviewers (DH, RP) and a list of potential evidence for inclusion was compiled by the two analysts (DH, RP). These lists were then compared and any differences resolved by discussion. Full text copies of the selected texts were independently read and assessed for relevance by both analysts and in accordance with the following inclusion and exclusion criteria.
Inclusion criteria
Prospective longitudinal studies of primary research (cohorts or clinical trials) reporting on the following:
An assessment of exposure to iron (assessed via biomarkers, e.g. blood, cerebral spinal fluid or neuroimaging) or external sources, for example, diet or iron supplementation.
Evidence, or clear implication, that participants were free of cognitive decline or dementia at baseline assessment.
Use of formal assessment of cognitive function.
Reporting outcomes of cognitive decline or incident dementia.
Adult populations with a mean age over 50 years
Exclusion criteria:
Publications relating only to populations selected on the basis of clinical comorbidity when that comorbidity may also affect cognitive function, for example, requirement for renal dialysis.
Non English publications (in the absence of resources available for translation).
Inclusion of nonadults.
Papers identified as relevant were then independently assessed for quality and data relating to study characteristics, assessment of cognitive function and iron exposure were extracted by each analyst. A formal quality scoring scheme was not used as these lack discriminatory power, however each paper was assessed against the key factors given in Critical Appraisal Skills Programme checklists for evaluating trials and longitudinal studies (Critical Appraisal Skills Programme: http://www.casp-uk.net/ accessed 15th February 2017).
The review was carried out to best practice and in accordance with PRISMA guidelines.25 Protocol registration no. CRD42016023800.
Results
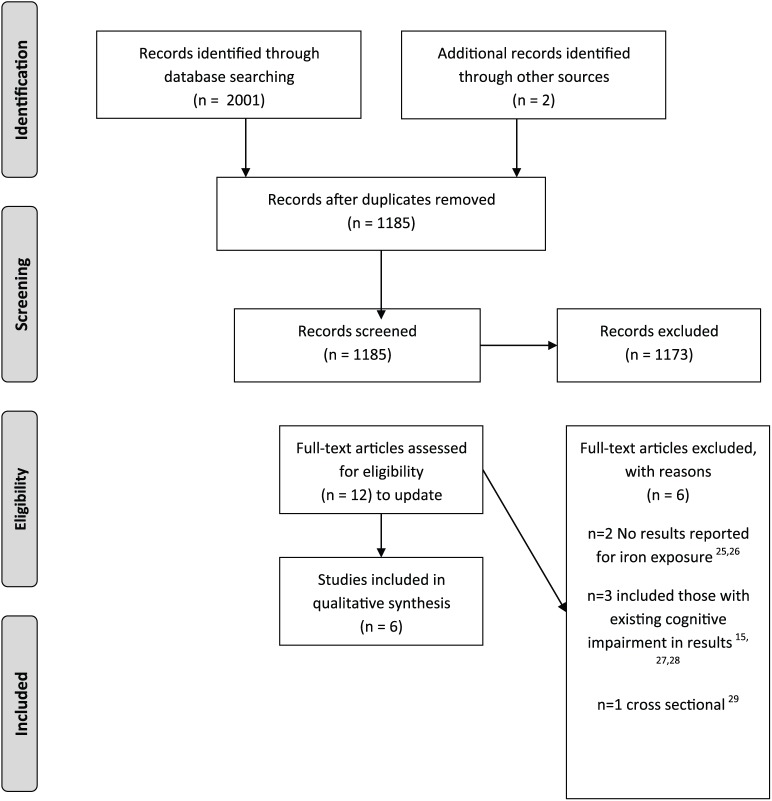
A total of 2001 records were identified from the searches and a further two from reference screening/expert recommendation; 1185 records remained once duplicates were removed. These were screened and 12 full text articles representing 12 separate studies were reviewed. Six studies were included with the remaining six excluded because they lacked results related to iron exposure (n = 2),26,27 included prevalent cases of cognitive impairment (n = 3),15,28,29 or had a cross-sectional design (n = 1).30 See Figure 1 for the PRISMA study flow chart.
Figure 1.
Study selection flowchart.
Source: http://prisma-statement.org/
Study characteristics
Of the studies that were included, four reported results from North American populations (United States of America); two using data from the National Health and Nutrition Examination Survey (NHANES),31,32 one from a Detroit-based cohort study,22 and one using participant data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) cohort.33 The remaining studies were from Australia, a longitudinal cohort study based in Canberra and Queanbeyan,34 and the Netherlands, a cohort analysis of clinical trial data from the Folic Acid and Carotid Intima-Media Thickness (FACIT) trial.35 For further study characteristics, see Table 1.
Table 1.
Study characteristics.
| Author and study | Design | Participants and setting | Baseline n | Follow up n | Follow-up years | Reason losses to follow up | Baseline age or mean (SD) | Baseline% female |
|---|---|---|---|---|---|---|---|---|
| Mainous et al.32
NHANES 40 |
Longitudinal cohort study | The National Health and Nutritional Examination Survey (NHANES survey): USA | 6558 | 6427 | 18 | No | 40+ | ~ 45–63% across measures |
| Schiepers et al.35
FACIT |
Longitudinal cohort study | The Folic Acid and Carotid Intima-Media Thickness (FACIT) Trial: The Netherlands | 818 | 800 | 3 | Yes | Mean 60.3 (5.6) | 28.4% |
| Cherbuin et al.34
PATH |
Longitudinal cohort study | The PATH through Life study: Canberra, Australia | 2096 | 1354 | 8 | Yes | 60–64 Mean 62.54 (1.53) |
51.7% |
| Daugherty et al.22
DETROIT |
Longitudinal cohort study | Detroit cohort study: Detroit, USA | 125 | 78 | 2 | Yes | 19–77 Mean 52.53 (14.91) |
70% |
| Min et al.31
NHANES 60 |
Longitudinal cohort study | NHANES survey linked to mortality data (1999–2006): USA | 4688 | 4639 | 5–12 | N/A: loss due to mortality | 60–89 | 51% |
| Ayton et al.33
ADNI |
Longitudinal cohort study | The Alzheimer’s Disease Neuroimaging Initiative (ADNI) cohort: USA | 90 | 43 | 7 | No | Mean 75.7 (5.5) | 51% |
N/A, not applicable.
Measures of iron
Studies used widely differing measures of baseline iron (Table 2). Of the six identified studies, two used measures of brain iron22,33 and four used measures of peripheral iron.31,32,34,35 The NHANES 40-plus cohort reported on serum transferrin,32 the NHANES 60-plus cohort and the Australian cohort study used dietary iron,34 the FACIT trial collected total iron-binding capacity, transferrin saturation, ferritin and nontransferrin bound iron.35 Of those that collected data on brain iron, the ADNI cohort used ferritin in cerebrospinal fluid (CSF)33 and the Detroit study used imaging (MRI).22 The Detroit cohort and the FACIT trial cohort were the only studies to report assessment of iron at follow up. The FACIT trial collected serum iron, ferritin and nontransferrin bound iron and the Detroit study repeated their baseline MRI scan.22,35
Table 2.
Measurements of iron.
| Author | Peripheral or brain iron measures | Measure of iron at baseline | Measure of iron at follow up |
|---|---|---|---|
| Mainous et al.32
NHANES 40 |
Peripheral | Serum transferrin | N/A |
| Schiepers et al.35
FACIT |
Peripheral | Total serum iron, serum concentration of transferrin*, Transferrin saturation$, serum ferritin‡, nontransferrin-bound iron§ | Same as baseline with the exception of nontransferrin-bound iron |
| Cherbuin et al.34
PATH |
Peripheral | Dietary iron calculated from the Commonwealth Scientific Industrial Research Organisation (CSIRO FFQ) | N/A |
| Daugherty et al.22
DETROIT |
Brain | In vivo estimation with MRI adds regions of interest hippocampus, caudate and putamen | In vivo estimation with MRI |
| Min et al.31
NHANES 60 |
Peripheral | Dietary iron (calculated from 24 h recall) | N/A |
| Ayton et al.33
ADNI |
Brain | Ferritin in cerebrospinal fluid | N/A |
Transferrin: iron transport protein.
Transferrin saturation: the ratio of serum iron to serum concentration of transferrin.
Ferritin: indicator of total body iron stores.
Nontransferrin-bound iron: free iron in the circulation not bound to transferrin.
MRI, magnetic resonance imaging; N/A, not applicable; FFQ, food frequency questionnaire.
Cognitive outcome measures
The NHANES cohorts reported on dementia outcomes (Table 3). Data on dementia and Alzheimer’s disease outcomes were collected from death certificates, hospital and nursing home records for the 40-plus cohort.32 Diagnoses of Alzheimer’s disease for the 60-plus cohort were collected from death certificates only;31 ; neither carried out cognitive testing. The Detroit cohort,22 FACIT trial cohort,35 ADNI cohort33 and Australian cohort34 used standard cognitive assessment tools at baseline and follow up.
Table 3.
Measurement of cognitive function.
| Author | Baseline assessment cognitive function |
Follow-up assessment cognitive function |
|---|---|---|
| Mainous et al.32
NHANES 40 |
N/A | N/A |
| Schiepers et al.35
FACIT |
Five cognitive domains assessed. Domains constructed by using either the average of individual test z scores or for two constructs, single tests only Memory (visual word learning test; immediate recall, maximal immediate recall, delayed recall) Sensorimotor speed (concept shifting tests ‘empty’, ‘letters’, ‘numbers’, colour Stroop, ‘reading words’) Complex speed (concept shifting, ‘letters & numbers’, colour Stroop, ‘naming ink’) Information processing speed (letter digit substitution test) Verbal fluency (verbal fluency test) |
Five cognitive domains assessed. Domains constructed by using either the average of individual test z scores or for two constructs, single tests only Memory (visual word learning test; immediate recall, maximal immediate recall, delayed recall) Sensorimotor speed (concept shifting tests, ‘empty’, ‘letters’, ‘numbers’, colour Stroop, ‘reading words’) Complex speed (concept shifting, ‘letters & numbers’, colour Stroop, ‘naming ink’) Information processing speed (letter digit substitution test) Verbal fluency (verbal fluency test) |
| Cherbuin et al.34
PATH |
Participants were selected for clinical assessment if they had any of the following: a MMSE cutoff ⩽25, a score below 5th percentile on the CVLT, on SDMT or the Purdue pegboard with both hands or RT Clinical assessment included neuropsychological testing using the following: trials A&B; clock drawing; Boston naming task; constructional praxis (CERAD); the RAVLT, recall of constructional praxis for nonverbal memory |
Participants were selected for clinical assessment if they had any of the following: a MMSE cutoff ⩽25, a score below 5th percentile on the CVLT, on SDMT or the Purdue pegboard with both hands or RT Clinical assessment included neuropsychological testing using the following: trials A&B; clock drawing; Boston naming task; constructional praxis (CERAD); the RAVLT, recall of constructional praxis for non-verbal memory |
| Daugherty et al.22
DETROIT |
Latent cognitive constructs were tested for: working memory (listening span, verbal N-back task), nonverbal working memory (spatial recall, visual N-back tasks), episodic memory (logical memory subset of the Wechsler memory scales revised) | Latent cognitive constructs were tested for: working memory (listening span, verbal N-back task), nonverbal working memory (spatial recall, visual N-back tasks), episodic memory (logical memory subset of the Wechsler memory scales revised) |
| Min et al.31
NHANES 60 |
N/A | N/A |
| Ayton et al.33
ADNI |
The ADAS Cog, RAVLT | ADAS Cog, RAVLT |
ADAS Cog, Alzheimer’s disease Assessment Scale; CERAD, Consortium to Establish a Registry for Alzheimer’s disease; CVLT, California Verbal Learning Test; N/A, not applicable; RAVLT, Rey Auditory Verbal Learning Test; RT, reaction time; SDMT, Digit Symbol Modalities Test; MMSE, mini-mental state exam.
Association between iron measures and cognitive outcomes as reported by the included studies
Overall the results were variable across studies (Table 4):
Table 4.
Results reported by included studies.
| Author | N | Results | Additional comment | Confounders |
|---|---|---|---|---|
| Mainous et al.32
NHANES 40 |
6427 | Unadjusted (transferrin alone) transferrin >75% compared with <75% Dementia HR 1.11 (95% CI 0.79–1.56) AD HR 1.47 (95% CI 0.82–2.63) |
High cholesterol and high transferrin saturation levels consistently had the highest risk of developing AD compared with the reference group (low cholesterol and low transferrin). Having only high cholesterol or only high transferrin did not increase risk for AD. Unadjusted relative risk of developing AD when both TS and cholesterol were at the 75th percentile was 3.19 (95% CI 1.31–7.75). In adjusted models when only one marker was elevated, there was no significant increased risk for AD. The risk of AD increased as both markers increased | Age, sex, race, education, BMI, exercise, diabetes, hypertension, vitamin C level |
| Schiepers et al.35
FACIT |
800 | When analyses were stratified by blood donor status, higher serum iron predicted less decline in nondonors in sensorimotor speed (parameter estimate = 0.005, p = 0.010) in word fluency (parameter estimate = 0.006, p = 0.012) | Cognitive performance declined over 3 years for sensorimotor speed, complex speed and information processing. Memory improved and word fluency did not change. Ferritin concentrations increased significantly but serum iron decreased, although not significantly. No significant longitudinal associations were found between any of the iron parameters and cognitive functioning. The HFEC28Y mutation (produces elevated iron levels) was not associated with cross-sectional or longitudinal cognitive performance, indicating lifelong exposure to elevated iron does not affect cognitive function in later life. The potential effect modification by treatment with folic acid in the trial was assessed prior to longitudinal analyses and no interaction between folic acid supplementation and iron status was found. All subsequent analyses were adjusted for covariates | Age, sex, education (formal schooling), alcohol consumption, current smoking, BMI, physical activity, apolipoprotein E, genotype C-reactive protein, haemoglobin, blood donor status, group status (for folic acid treatment) |
| Cherbuin et al.34
PATH |
1354 | For each 1 mg increase in iron at baseline, risk of MCI but not MCD increased over 8 years of follow up For transition from normal cognition to MCI, HR 1.54 (95% CI 1.03–2.29), p = 0.03 For transition from normal cognition to MCD, HR 1.10 (95% CI 0.87–1.37), p = 0.418. |
Unadjusted For each 1 mg increase in iron at baseline the risk of MCI but not MCD increased over 8 years For transition from normal cognition to MCI, HR 1.50 (95% CI 1.07–2.10), p = 0.019 For transition from normal cognition to MCD, HR 1.05 (95% CI 0.95–1.16), p = 0.309 Trend towards sex interaction such that higher iron intake associated with decreased risk of MCI incidence in women, HR 0.81 (95% CI 0.61–1.07), p =0.144) and increased risk of MCI incidence in men, HR 1.08 (95% CI 0.94–1.18), p =0.287 |
age, sex education, alcohol intake, smoking, BMI, stroke, diabetes, hypertension (including medication use) APOE Ɛ 4status depressive symptoms physical activity |
| Daugherty et al.22
DETROIT |
78 | Higher baseline iron in the caudate nucleus was associated with less improvement in working memory, β –0.18 (p = 0.01) | No association between putamen or hippocampal baseline iron or change in iron with working memory. No association between brain iron at baseline or change in brain iron and episodic memory | Age and sex retained in final models |
| Min et al.31
NHANES 60 |
4639 | Increased risk of AD mortality found for those with low haemoglobin (HR 8.4, 95% CI 1.4–50.8) | There were 49 AD-related deaths at follow up. No relationship between AD death and dietary iron intake, but those with AD mortality had significantly lower haemoglobin levels. Those who had AD had lower haemoglobin (14.0g/dl compared with 14.1g/dl, p = 0.0089 t test). Significant reduction in AD mortality risk with increases in haemoglobin for those with low levels of both folate and vitamin B12 |
Age, sex, ethnicity, education, smoking history, BMI, diabetes or hypertension, dietary intake of iron |
| Ayton et al.33
ADNI |
43 | Baseline ferritin was associated with cognitive decline in a three-way interaction with time and presence of APOE Ɛ4 ADAS Cog β 0.11 (SE 0.04), p = 0.01 RAVLT β –1.58 (SE 0.54), p = 0.004 Examined by presence of APOE Ɛ4 APOE Ɛ4 positive: ADAS Cog β 0.09 (SE 0.04), p = 0.02 RAVLT β –1.49 (SE 0.4), p < 0.001 APOE Ɛ4 negative: ADAS Cog β –0.04 (SE 0.016), p = 0.02 RAVLT NS |
All models included sex and years of education. The covariates CSF APOE, factor H and haemoglobin were not predictive in any models. No unadjusted results. Models initially included all covariates before minimal models were obtained empirically via empirical testing of model fit | Age, sex, BMI, education, CSF tau/amyloid β, CSF APOE, CSF ferritin, CSF factor H (a measure of inflammation) |
AD, Alzheimer’s disease; ADAS Cog, Alzheimer’s disease Assessment Scale; APOE, apolipoprotein E; BMI, body mass index; CI, confidence interval; CSF, cerebrospinal fluid; HR, hazard ratio; NS, nonsignificant; RAVLT, RAVLT, Rey Auditory Verbal Learning Test; SE, standard error; MCD, mild cognitive disorders; MCI, mild cognitive impairment.
In the NHANES 40-plus cohort (n = 6427), an association was found between co-occurring high levels of transferrin (TS) and cholesterol, and increased risk of Alzheimer’s disease [both TS and cholesterol at the 75th percentile, hazard ratio (HR) 3.19, 95% confidence interval (CI) 1.31–7.75]; but no association between transferrin alone and Alzheimer’s disease32 (transferrin >75% compared with < 75% Alzheimer’s disease: HR 1.47, 95% CI 0.82–2.63).
In the FACIT trial (n = 800) the potential of folic acid supplementation to modify the effects of iron measures on cognitive change was tested prior to examining associations between serum iron parameters and longitudinal cognitive functioning. No associations were found. When stratifying for status as a blood donor, higher iron status was associated with less decline in sensorimotor (parameter estimate = 0.005, p = 0.010) and word fluency tasks (parameter estimate = 0.006, p = 0.012).35
-
The Australian PATH cohort study (n = 1354) found an increased risk of cognitive impairment with higher levels of iron intake (HR 1.54, 95% CI 1.03–2.29 for transition from normal cognition to mild cognitive impairment (MCI), per 1 mg iron) and the potential for an interaction with sex such that higher intake may be associated with lower risk in women but higher risk in men.34
The PATH study reported a mean iron intake of 18 mg per day, more than double the daily intake of 8 mg recommended by the Australian National Health and Medical Research Council (NHMRC) for those in their sixties.
The Detroit cohort (n = 78) (examining the hippocampus, caudate and putamen) reported no association between baseline iron or change in iron and episodic memory or nonverbal working memory, with the exception of baseline iron in the caudate nucleus which was associated with less improvement in verbal working memory, β –0.18 (p = 0.01).22
The NHANES 60-plus cohort (n = 4639) found no association between Alzheimer’s disease related death and dietary iron intake (p = 0.1022) but an increased risk of Alzheimer’s disease mortality found for those with low haemoglobin (HR 8.4, 95% CI 1.4–50.8).31
The study using the ADNI cohort (n = 43) reported an increased risk of decline associated with higher levels of ferritin, but suggested that the result may be confined to those with the apolipoprotein (APOE) Ɛ4 allele. For those who were APOE Ɛ4 positive, Alzheimer’s disease Assessment Scale (ADAS Cog) β 0.09 [standard error (SE) 0.04], p = 0.02, Rey Auditory Verbal Learning Test (RAVLT) β –1.49 (SE 0.4), p < 0.001 compared with APOE Ɛ4 negative, ADAS Cog β –0.04 (SE 0.016), p = 0.02, RAVLT nonsignificant.33
Study quality
All studies reported a clear research question (Table 5). However, studies utilized diverse designs with the potential for bias in recruitment, measures of iron exposure, outcome assessment, potential confounders, missing data or attrition. The applicability of three studies was limited by recruitment from specific population groups; one from an ongoing longitudinal study of ageing,22 one from the ongoing ADNI study33 and one from a clinical trial population.35 The other three studies used representative population samples from Australia34 and the USA.31,32 However, Mainous and colleagues32 (n = 6427) selected a cohort aged from 40 years upwards, thus potentially minimizing the potential to observe meaningful cognitive change. Four of the studies reported on populations with a mean age over 60 years, that is those more likely to manifest cognitive change,31, 33–35 and recruited large numbers (NHANES 60 n = 4639, PATH N = 1354, FACIT n = 800), with the exception of the ADNI study (n = 43). None focused solely on older adults and one, the Daugherty imaging study, included a wide age range from 19 to 77 years (n = 78), which would have included a varied population; in particular, pre- and postmenopausal women.22 Furthermore, full details of statistical methods and adjustment for potential confounders was limited and inconsistent, and three studies lacked adequate adjustment for the confounding variables likely to influence cognitive function.22,31,32
Table 5.
Assessment of study quality.
| Author | Recruitment bias | Exposure bias | Bias in outcome measurement | Bias in assessment of confounders | Bias in follow up, patient loss | Bias in follow up, length | Overall quality assessment |
|---|---|---|---|---|---|---|---|
| Mainous et al.32
NHANES 40 |
Moderate. NHANES was designed to be representative of the US population with additional oversampling of key population subgroups including those aged 65 and over. However, baseline included those in midlife (40 years+) which limits opportunity to detect association between exposure and dementia incidence over 20 years | Moderate. Baseline measures only and longer term exposure not considered | Moderate. Dementia/AD measured through health, hospital and nursing home records plus death certificates. Misclassification possible and cases living in the community may have been overlooked | Moderate. Multiple key confounders are included but assessment methods were not noted except for vitamin C usual intake not necessarily captured by 24 h dietary history | Low. 98% follow-up rate for NHANES. Follow-up data collected by interview or proxy if participant unable to respond or deceased | Moderate. Potential for some participants to have contributed fairly short follow up meaning reverse causality cannot be excluded | Moderate |
| Schiepers et al.35
FACIT |
High. Not a representative sample (54% blood donors). Participants had to meet clinical trial inclusion criteria, e.g. a plasma total homocysteine ⩾13 and ⩽26 µmol/liter; 50% also received folic acid supplementation | Moderate. Baseline and follow-up measures are used but longer term exposure not considered | Moderate. Cognitive tests from a neuropsychological battery were combined into researcher defined composite domain scores rather than taking a data-driven factor analytic approach to determine the underlying constructs assessed | Low. Multiple key confounders are included. BMI was specified as calculated through height and weight but not clear if this was measured objectively or by self report | Low. Minimal loss to follow up | Moderate. Reverse causality possible in 2-year follow up | Moderate |
| Cherbuin et al.34
PATH |
Low. Recruitment of a random selection from the electoral role membership of which is mandatory in Australia | Moderate. Baseline measures only and longer term exposure not considered and those with missing data were excluded. Iron status assessed through self-report FFQ which does not have the reliability of serum markers for iron status | Moderate. Neuropsychological battery and clinical assessment of dementia used to assess cognitive function but those with missing data were excluded | Moderate. Key confounders are included but those with missing data were excluded. BMI was calculated using self-report height and weight | Moderate. There were 2551 recruited and 1275 included after exclusion of missing data etc. | Moderate. Follow up is sufficient to detect incident change, however exclusion of those with missing data may have caused bias | Moderate |
| Daugherty et al.22
DETROIT |
Moderate. Recruitment was part of an ongoing longitudinal study of ageing | Moderate. Baseline and follow-up measures are used but longer term exposure not considered | Moderate. Working and episodic memory measures were taken from a larger neuropsychological battery assessment. No theoretical justification was given as to why these measures were chosen | Moderate A selection of key confounding variables were included. In particular, time-varying confounders were measured by objective blood markers and included in the model as a latent metabolic factor, thereby excluding variable-specific error | High; ~37% of sample lost to follow up | Moderate. Reverse causality possible in 2-year follow up, however reverse effects models were empirically tested | Moderate |
| Min et al.31
NHANES 60 |
Low. NHANES was designed to be representative of the US population with additional oversampling of key population subgroups including those aged 65 and over | Moderate. Baseline measures only and longer term exposure not considered. Measurement methods for dietary iron not clear | Moderate. Death certificate data may not be accurate or complete. At follow up those without AD mortality may nonetheless have had AD | High. Insufficient details of analysis methods for iron to allow evaluation. Small cell sizes for each iron quartiles in subjects with AD mortality | Moderate. Those without measures of haemoglobin, or red-cell folate/B12 and those with any other missing data were excluded | Moderate. Potential for some participants to have contributed fairly short follow up, meaning reverse causality cannot be exclude | Moderate |
| Ayton et al.33
ADNI |
Moderate. Selection was from the mild cognitive impairment and control groups of the Alzheimer’s Disease Neuroimaging Initiative (ADNI) data sets. ADNI recruited from multiple sites across North America | Moderate. Baseline measures only and longer term exposure not considered | Moderate. A standard neuropsychological test and cognitive screening test were used and data collected annually. However, the ADAS Cog (screening test) may not be a sensitive measure of cognitive change in those who are cognitively normal. Some bias may also have been possible as participant data were included until death or loss to follow up | Low. Key confounders including a measure of inflammation | Moderate. However, all participants were included until loss | Moderate. Some participants left the study prior to the end of the 7-year follow up, however overall follow up was long enough to measure change in cognitive function | Moderate |
AD, Alzheimer’s disease; ADAS Cog, Alzheimer’s disease Assessment Scale; BMI, body mass index; NHANES, National Health and Nutrition Examination Survey; FFQ, food frequency questionnaire.
The measurement of iron was similarly varied. None of the studies attempted to take account of prior iron exposure and iron status at follow up was assessed only in the FACIT trial35 and the Detroit Aging study.22 Exposure measures were selective, with no overlap and no combined dietary and biomarker assessments. Only two studies measured brain iron levels.22,33
Measurement of cognitive function was also limited, both in breadth and sensitivity. Outcome assessments were selected from standard test batteries for four of the studies.33–36 The two studies using the NHANES database collected outcome data from death certificates31,32 and healthcare records,32 thus raising the possibility of misclassification and under reporting due to missed cases in the community, lack of high-quality diagnoses in nursing home environments and less than rigorous death certificate coding.
The interpretation of study results is also limited by the varying research questions and statistical focus in the different studies. For example, the publication by Mainous and colleagues aimed to look at cholesterol in combination with transferrin rather than using iron measures alone,32 and the publication by Min and colleagues focused on haemoglobin, folate and vitamin B12, reporting on iron only in passing as a covariate and as part of the baseline characteristics.31 None of the studies focused on a potential U-shaped relationship with iron and cognitive outcome. Participant numbers, and subsequently the numbers per cell in analyses were also particularly small in some studies; in the study by Min and colleagues only 49 participants from 4688 had died from Alzheimer’s disease at follow up.31 Daugherty included only 125 participants22 and in the study by Ayton and colleagues there were only 90 participants in the cognitively normal group.33 Finally, participant dropout and missing data31,34 further limit the interpretation of the results.
Discussion
To our knowledge, this is the first systematic review of the evidence relating to iron, cognitive decline and dementia. Overall, the available studies were inconsistent in terms of length of follow up, composition of study population, percentage female or male, age of study sample, iron assessment, outcome measure and results. Two studies assessed the influence of dietary iron in relatively similar age groups, one (the USA NHANES 60-plus cohort31) found no association with dementia. The other (the Australian PATH study34) found an increased risk of cognitive decline associated with greater iron intake, possibly more relevant in men.
Several of the studies did report a stronger relationship in a particular subgroup, although the subgroups also varied. The older NHANES cohort found a relationship between higher transferrin and increased risk of Alzheimer’s disease in those with higher cholesterol.32 In the ADNI cohort the relationship between higher cerebrospinal ferritin and cognitive decline was strongest in those who were also APOE Ɛ4 positive,33 and in the FACIT trial cohort, higher serum iron levels were associated with less decline in sensorimotor speed and word fluency in those who did not donate blood.35
A recent comprehensive review of iron deficiency and risk of cardiovascular disease included 13 longitudinal studies of serum iron, 15 studies of ferritin and 14 of transferrin saturation and total iron binding capacity, but also observed heterogeneity and inconclusive findings across studies. Although they concluded that ‘extreme conditions of iron deficiency as well as iron overload were associated with increased cardiovascular disease risk’.37
Limitations associated with our review and its conclusions stem mostly from the paucity of studies rather than the methods used for the review process. It is possible that in restricting the language to English and in excluding study populations with serious clinical comorbidities, potentially relevant evidence has been overlooked. However, including populations with specific comorbidities where the comorbidity itself may influence iron metabolism, risk of mortality and risk of cognitive decline would have been to address a different research question. The lack of evidence, and the varying study designs and populations used within the available evidence base, present a more serious limitation.
Limitations in the assessment of iron
None of the studies used a fully comprehensive assessment of iron status. This is important because cerebrospinal fluid and blood reporters of iron only have a modest association,29 blood iron markers do not predict MRI measured brain iron content38 and, in older adults in particular, levels of the peripheral iron reporters, ferritin and haemoglobin, may also signify peripheral inflammation such as anaemia of chronic inflammation,39 or indeed noniron-deficient anaemia. In the case of the latter, tissue deposition of iron may occur even when presenting with low iron markers in the blood. For example, the iron-trafficking disease, aceruloplasminemia, results in tissue iron deposition and lower blood iron markers because iron cannot efficiently be exported out of the cell.40 If cellular iron trafficking, rather than iron exposure, is the lesion that results in brain iron elevation in Alzheimer’s disease, it is possible that depletion of iron in the blood accompanies iron elevation in tissues. Furthermore, only two of the studies assessed dietary iron and no studies evaluated the impact of iron supplementation. No studies directly followed a potential link between iron intake and iron blood or neuroimaging biomarkers and subsequent cognitive function, and none attempted to evaluate iron impact by baseline iron level or by clinically relevant categories such as iron deficiency anaemia or iron deficiency no anaemia. We were unable to identify any trials of dietary iron exposure, or of iron supplementation, in normal or iron-deficient adults in mid or late life and risk of cognitive decline.
Limitations in the assessment of outcome
Cognition is complex and multifaceted and it is possible that iron status differentially affects change or decline across the spectrum of ability domains. Clearly, in the cognitively healthy a more comprehensive and psychometrically driven approach to assessing cognitive change is necessary if the impact of iron status on cognitive decline is to be more fully understood. The use of medical and death certificate records for dementia diagnoses in the NHANES analyses presents a further limitation since their accuracy depends upon robust and comprehensive assessment and record keeping.
Gaps in the evidence base and future work
Our systematic review of the current evidence base relating to iron and cognitive function and dementia found a sparse and limited field of evidence. Much more work is needed to evaluate the links between iron intake, brain deposition, ageing and cognitive function over time. In particular, a detailed focus is needed on regional brain iron and related cognitive abilities with multiple follow ups, MRI measures or historical health measures taking closer account of potential causal and associative relationships, including key confounding measures. Obvious examples include ensuring that analyses with ferritin (also an acute phase protein) are, at minimum, adjusted for other inflammatory markers, and taking account of obesity and its association with iron dysregulation. Multimodal approaches should also be used, such as using CSF iron biomarkers in combination with MRI measures of brain iron. Larger, longer and more thorough epidemiological studies are also needed to fully explore the potential pathways as is a greater emphasis on collection of biomarkers to allow more accurate evaluation of the homeostatic iron picture in its entirety, taking account of the varied iron types and storage mechanisms. This would also be an important part of the safety considerations prior to conducting any randomized clinical trials of iron supplementation or iron chelation. In particular, we recommend that future studies in this area focus on reducing risk of bias derived from varied participant populations; that is, select those of a particular age range and stratify by sex. Studies should collect at least two measures of cognitive function over a minimum follow up of 12 months to allow assessment of change; when possible, two matching iron measures should be taken at the same interval, ideally using imaging and linking region of interest to the cognitive domain under assessment. This should be supplemented by peripheral measures, including inflammatory markers; when possible, additional data on usual diet, and clinical and cardiovascular history should be obtained.
Conclusion
The current evidence base is sparse and limited. The diversity of the existing evidence limits interpretation and applicability by either healthcare providers or researchers and precludes any conclusions relating to the relationship between iron and cognition. Much more work is needed before we can develop our understanding of the role of iron in cognitive function.
Supplemental Material
Supplemental material, SUPPLEMENTARY_MATERIAL for More evidence is needed. Iron, incident cognitive decline and dementia: a systematic review by Diane E. Hosking, Scott Ayton, Nigel Beckett, Andrew Booth and Ruth Peters in Therapeutic Advances in Chronic Disease
Footnotes
Author contributions: RP: study concept; RP, DH, AB: study design; RP, DH, SA: data analysis; RP, DH, SA, NB: data interpretation. All authors contributed to manuscript preparation and revision, and approved the final version. No sponsor had any role in the design, methods, subject recruitment, data collection, analysis or preparation of the paper.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grant CE110001029 from the Australian Research Council (DH) and the Dementia Collaborative Research Centre for Early Diagnosis and Prevention; a member of the NHMRC National Institute for Dementia Research (RP).
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Ruth Peters  https://orcid.org/0000-0003-0148-3617
https://orcid.org/0000-0003-0148-3617
Supplemental Material: Supplemental material for this article is available online.
Contributor Information
Diane E. Hosking, Centre for Research into Ageing Health and Wellbeing, Australian National University, Canberra, Australia
Scott Ayton, The Florey Institute of Neuroscience and Mental Health, University of Melbourne, Melbourne, Australia.
Nigel Beckett, Care of the Elderly, Imperial College London, London, UK.
Andrew Booth, School of Health and Related Research, University of Sheffield, Sheffield, UK.
Ruth Peters, Lifecourse Ageing Research Centre, Neuroscience Research Australia, University of New South Wales, Sydney NSW 2031, Australia.
References
- 1. Fairweather-Tait SJ, Wawer AA, Gillings R, et al. Iron status in the elderly. Mech Ageing Dev 2014; 136–137: 22–28. DOI: 10.1016/j.mad.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Busti F, Campostrini N, Martinelli N, et al. Iron deficiency in the elderly population, revisited in the hepcidin era. Front Pharmacol 2014; 5: 83 DOI: 10.3389/fphar.2014.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hare D, Ayton S, Bush A, et al. A delicate balance: iron metabolism and diseases of the brain. Front Aging Neurosci 2013; 5 Review. DOI: 10.3389/fnagi.2013.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Waldvogel-Abramowski S, Waeber G, Gassner C, et al. Physiology of iron metabolism. Transfus Med Hemother 2014; 41: 213–221. DOI: 10.1159/000362888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ward RJ, Zucca FA, Duyn JH, et al. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol 2014; 13: 1045–1060. DOI: 10.1016/s1474-4422(14)70117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Petranovic D, Batinac T, Petranovic D, et al. Iron deficiency anaemia influences cognitive functions. Med Hypotheses 2008; 70: 70–72. DOI: 10.1016/j.mehy.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 7. Bourre JM. Effects of nutrients (in food) on the structure and function of the nervous system: update on dietary requirements for brain. Part 1: micronutrients. J Nutr Health Aging 2006; 10: 377–385. [PubMed] [Google Scholar]
- 8. Andro M, Le Squere P, Estivin S, et al. Anaemia and cognitive performances in the elderly: a systematic review. Eur J Neurol 2013; 20: 1234–1240. DOI: 10.1111/ene.12175. [DOI] [PubMed] [Google Scholar]
- 9. Patel KV. Epidemiology of anemia in older adults. Semin Hematol 2008; 45: 210–217. DOI: 10.1053/j.seminhematol.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fleming DJ, Tucker KL, Jacques PF, et al. Dietary factors associated with the risk of high iron stores in the elderly Framingham Heart Study cohort. Am J Clin Nutr 2002; 76: 1375–1384. [DOI] [PubMed] [Google Scholar]
- 11. Hunnicutt J, He K, Xun P. Dietary iron intake and body iron stores are associated with risk of coronary heart disease in a meta-analysis of prospective cohort studies. J Nutr 2013. DOI: 10.3945/jn.113.185124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bao W, Rong Y, Rong S, et al. Dietary iron intake, body iron stores, and the risk of type 2 diabetes: a systematic review and meta-analysis. BMC Med 2012; 10: 119 DOI: 10.1186/1741-7015-10-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gudala K, Bansal D, Schifano F, et al. Diabetes mellitus and risk of dementia: a meta-analysis of prospective observational studies. J Diabetes Investig 2013; 4: 640–650. DOI: 10.1111/jdi.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wolters FJ, Segufa RA, Darweesh SKL, et al. Coronary heart disease, heart failure, and the risk of dementia: a systematic review and meta-analysis. Alzheimers Dement 2018. DOI: 10.1016/j.jalz.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 15. Penke L, Valdés Hernandéz MC, Maniega SM, et al. Brain iron deposits are associated with general cognitive ability and cognitive aging. Neurobiol Aging 2012; 33: 510–517.e512. DOI: 10.1016/j.neurobiolaging.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 16. Hallgren B, Sourander P. The effect of age on the non-haemin iron in the human brain. J Neurochem 1958; 3: 41–51. [DOI] [PubMed] [Google Scholar]
- 17. van Bergen JM, Li X, Hua J, et al. Colocalization of cerebral iron with Amyloid beta in Mild Cognitive Impairment. Sci Rep 2016; 6: 35514 DOI: 10.1038/srep35514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rodrigue KM, Daugherty AM, Haacke EM, et al. The role of hippocampal iron concentration and hippocampal volume in age-related differences in memory. Cereb Cortex (New York, NY) 2013; 23: 1533–1541. DOI: 10.1093/cercor/bhs139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sullivan EV, Adalsteinsson E, Rohlfing T, et al. Relevance of iron deposition in deep gray matter brain structures to cognitive and motor performance in healthy elderly men and women: exploratory findings. Brain Imaging Behav 2009; 3: 167–175. DOI: 10.1007/s11682-008-9059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bei D, Ke-Min C, Hua-Wei L, et al. Correlation of iron in the hippocampus with MMSE in patients with Alzheimer’s disease. J Magn Reson Imaging 2009; 29: 793–798. DOI: doi: 10.1002/jmri.21730. [DOI] [PubMed] [Google Scholar]
- 21. Zhu W-z, Zhong W-d, Wang W, et al. Quantitative MR phase-corrected imaging to investigate increased brain iron deposition of patients with Alzheimer Disease. Radiology 2009; 253: 497–504. DOI: 10.1148/radiol.2532082324. [DOI] [PubMed] [Google Scholar]
- 22. Daugherty AM, Haacke EM, Raz N. Striatal iron content predicts its shrinkage and changes in verbal working memory after two years in healthy adults. J Neurosci 2015; 35: 6731–6743. DOI: 10.1523/jneurosci.4717-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Daugherty AM, Raz N. Accumulation of iron in the putamen predicts its shrinkage in healthy older adults: a multi-occasion longitudinal study. Neuroimage 2016; 128: 11–20. DOI: 10.1016/j.neuroimage.2015.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. del C., Valdés Hernández M, Allan J, Glatz A, et al. Exploratory analysis of dietary intake and brain iron accumulation detected using magnetic resonance imaging in older individuals: the Lothian Birth Cohort 1936. J Nutr Health Aging 2015; 19: 64–69. DOI: 10.1007/s12603-014-0523-3. [DOI] [PubMed] [Google Scholar]
- 25. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339 DOI: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alizadeh BZ, Njajou OT, Millán MR, et al. HFE variants, APOE and Alzheimer’s disease: Findings from the population-based Rotterdam Study. Neurobiol Aging 2009; 30: 330–332. DOI: 10.1016/j.neurobiolaging.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 27. Gamaldo AA, Ferrucci L, Rifkind J, et al. Relationship between mean corpuscular volume and cognitive performance in older adults. J Am Geriatr Soc 2013; 61: 84–89. DOI: 10.1111/jgs.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mueller C, Zhou W, VanMeter A, et al. The heme degradation pathway is a promising serum biomarker source for the early detection of Alzheimer’s Disease. J Alzheimers Dis 2010; 19: 1081–1091. DOI: 10.3233/JAD-2010-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ayton S, Faux NG, Bush AI. Ferritin levels in the cerebrospinal fluid predict Alzheimer’s disease outcomes and are regulated by APOE. Nat Commun 2015; 6: 6760 DOI: 10.1038/ncomms7760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Andreeva VA, Galan P, Arnaud J, et al. Midlife iron status is inversely associated with subsequent cognitive performance, particularly in perimenopausal women. J Nutr 2013; 143: 1974–1981. [DOI] [PubMed] [Google Scholar]
- 31. Min J, Min K. The folate-vitamin B12 interaction, low hemoglobin, and the mortality risk from Alzheimer’s Disease. J Alzheimers Dis 2016; 52: 705–712. [DOI] [PubMed] [Google Scholar]
- 32. Mainous A, Eschenbach S, Wells B, et al. Cholesterol, transferrin saturation, and the development of dementia and Alzheimer’s disease: results from an 18-year population-based cohort. Fam Med 2005; 31: 36–42. [PubMed] [Google Scholar]
- 33. Ayton S, Faux NG, Bush AI. Association of cerebrospinal fluid ferritin level with preclinical cognitive decline in apoe-ε4 carriers. JAMA Neurol 2017; 74: 122–125. DOI: 10.1001/jamaneurol.2016.4406. [DOI] [PubMed] [Google Scholar]
- 34. Cherbuin N, Kumar R, Sachdev PS, et al. Dietary mineral intake and risk of mild cognitive impairment: the PATH through life project. Front Aging Neurosci 2014; 6: 4 DOI: 10.3389/fnagi.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schiepers OJG, van Boxtel MPJ, de Groot RHM, et al. Serum iron parameters, HFE C282Y genotype, and cognitive performance in older adults: results from the FACIT study. J Gerontol 2010; 65A: 1312–1321. DOI: 10.1093/gerona/glq149. [DOI] [PubMed] [Google Scholar]
- 36. Daugherty AM, Raz N. Appraising the role of iron in brain aging and cognition: promises and limitations of MRI methods. Neuropsychol Rev 2015; 25: 272–287. DOI: 10.1007/s11065-015-9292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lapice E, Masulli M, Vaccaro O. Iron deficiency and cardiovascular disease: an updated review of the evidence. Curr Atheroscler Rep 2013; 15: 358 DOI: 10.1007/s11883-013-0358-0. [DOI] [PubMed] [Google Scholar]
- 38. Pirpamer L, Hofer E, Gesierich B, et al. Determinants of iron accumulation in the normal aging brain. Neurobiol Aging 2016; 43: 149–155. DOI: 10.1016/j.neurobiolaging.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 39. Nemeth E, Valore EV, Territo M, et al. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood 2003; 101: 2461–2463. DOI: 10.1182/blood-2002-10-3235. [DOI] [PubMed] [Google Scholar]
- 40. Miyajima H, Takahashi Y, Kono S. Aceruloplasminemia, an inherited disorder of iron metabolism. Biometals 2003; 16: 205–213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, SUPPLEMENTARY_MATERIAL for More evidence is needed. Iron, incident cognitive decline and dementia: a systematic review by Diane E. Hosking, Scott Ayton, Nigel Beckett, Andrew Booth and Ruth Peters in Therapeutic Advances in Chronic Disease