Abstract
Background:
BRAF (v-raf murine sarcoma viral oncogene homolog B1) V600E mutant colorectal cancer is associated with short survival. Recently, clinical trials have been conducted to improve outcomes of second or later lines of chemotherapy. However, there is a paucity of reference data pertaining to outcomes of second-line chemotherapy and prognostic factors that are relevant only to BRAF mutant patients.
Patients and methods:
We retrospectively reviewed metastatic colorectal cancer patients with BRAF V600E mutation who underwent second-line chemotherapy between January 2007 and March 2017. We evaluated treatment outcomes and performed prognostic analyses.
Results:
A total of 52 patients were included. The median progression-free survival and overall survival (OS) were 2.5 [95% confidence interval (CI) = 1.91–4.11] and 6.5 (95% CI = 4.30–9.63) months, respectively. Overall response and disease control rates were 7% and 48%, respectively. All the regimens which elicited a partial response included BRAF inhibitors in combination with anti-epidermal growth factor receptor (EGFR) antibodies. Therefore, the overall response was 0% after exclusion of patients treated with study drugs. Multivariate analysis for OS revealed that the Glasgow Prognostic Score (GPS), elevated lactate dehydrogenase, and poor performance status were independent prognostic factors. In particular, survival curves according to the GPS stratified the patients into distinct risk groups. The median OSs in patients with GPS of 0, 1, and 2 were 9.9, 5.0, and 1.9 months, respectively.
Conclusions:
Outcomes of second-line chemotherapy for metastatic colorectal cancer patients with BRAF V600E mutation were extremely poor. GPS may be useful in future clinical trials.
Keywords: BRAF V600E mutation, Glasgow Prognostic Score, metastatic colorectal cancer, second-line chemotherapy
Introduction
BRAF (v-raf murine sarcoma viral oncogene homolog B1) mutations are present in 5–10% of patients with metastatic colorectal cancer (mCRC).1–3 The mutation resulting in valine to glutamic acid substitution in codon 600 in BRAF kinase domain (V600E) is most commonly observed. It leads to constitutive activation of the BRAF kinase and the downstream mitogen-activated protein kinase (MAPK) pathway, which is a key mediator of tumor proliferation. The BRAF V600E mutation is more frequently seen in female or older patients or in patients with high-level microsatellite instability (MSI-H) and those with right-sided tumor or mucinous histology.4 It is well-known that BRAF V600E mutant colorectal cancer is less likely to respond to standard chemotherapy and is associated with short survival.1,5–7 In a pooled analysis of four trials evaluating first-line chemotherapy, overall survival (OS) was extremely poor in patients with BRAF V600E mutation [hazard ratio (HR) = 1.91, 95% confidence interval (CI) = 1.66–2.19, median OS = 11.4 versus 17.2 months).1
Several approaches have been attempted to improve the treatment outcomes for mCRC patients with BRAF V600E mutation. Loupakis et al. prospectively evaluated FOLFOXIRI (5-FU/leucovorin+oxaliplatin+irinotecan) plus bevacizumab and reported encouraging outcomes in the BRAF V600E mutant population (median OS = 24.1 months).8 In addition, the TRIBE phase III trial showed that patients with BRAF mutations gained greater benefits from FOLFOXIRI plus bevacizumab than from FOLFIRI plus bevacizumab (HR = 0.54, 95% CI = 0.24–1.20).9 Consequently, FOLFOXIRI plus bevacizumab is considered to be an option for first-line therapy and is recommended by the European Society of Medical Oncology (ESMO) guidelines as the preferred choice of regimen.10
Following the successful development of targeted therapies against malignant melanoma, targeted therapies for BRAF mutation have also been studied in the context of mCRC. BRAF inhibitor monotherapy is less effective for colorectal cancer compared with melanoma.11,12 Dual or triple regimens in combination with BRAF inhibitors and anti-epidermal growth factor receptor (EGFR) have been under development exclusively in the setting of second- or later lines of chemotherapy. Early results have shown more favorable antitumor response compared with that of BRAF inhibitor monotherapy. Currently, a randomized, phase III study to evaluate encorafenib, a BRAF inhibitor plus cetuximab with or without binimetinib, and a MEK inhibitor versus investigator’s choice regimens is ongoing for BRAF V600E mutant mCRC in second- or third- line settings (ClinicalTrials.gov identifier: NCT02928224).
In clinical trials, prognostic factors play an essential role. Randomized trials require stratification factors that have a prognostic impact on survival. Furthermore, identification of patients who are at an increased risk of early mortality is also important to ensure chemotherapy-related safety. However, there have been no reports that exclusively included only BRAF mutant mCRC patients in the exploration of prognostic factors. Prognostic factors applicable to the overall mCRC patient population may not be valid for BRAF mutant patients since BRAF mutation itself is a powerful prognostic factor. In the midst of drug development through clinical trials, identification of prognostic factors among BRAF mutant mCRC can be useful for patient stratification and for the exclusion of patients who are unfit for clinical trials. In addition, there are only a few reports focused on second- or later-line chemotherapy for colorectal cancer with BRAF V600E mutation and there is a lack of reference data pertaining to treatment outcomes.13,14 Therefore, we retrospectively examined the effectiveness of second-line chemotherapy and evaluated prognostic factors for patients with BRAF V600E mutant mCRC.
Patients and methods
Identification of patients
This was a retrospective study of mCRC patients with BRAF mutation who received second-line chemotherapy. We reviewed a computerized database of patients with colorectal cancer who had unresectable metastatic lesions and who received chemotherapy at the Aichi Cancer Center Hospital between January 2007 and March 2017. Patients with confirmed BRAF V600E mutation were included in the analysis. Among these patients, we identified the patients who underwent second-line chemotherapy. This study was approved by the Institutional Review Board at the Aichi Cancer Center Hospital (No. 2015-1-049). Written informed consent for clinical treatment and this study was obtained from all patients.
Molecular testing
DNA extracted from surgical or tumor biopsy specimens were amplified by polymerase chain reaction (PCR) assays. From 2007 to 2014, the cycleave PCR methods were applied using core kits (TAKARA, Co., Japan) to detect KRAS and BRAF V600E mutations.15 Since 2014, reverse sequence-specific oligonucleotide with PCR (PCR-rSSO) has been applied using multiplex kits, GENOSEARCH MuPACK (MBL, Japan) to detect KRAS/NRAS and BRAF V600E mutation, or GENOSEARCH BRAF (MBL, Japan) to detect BRAF mutations including non-V600E mutations.16 Microsatellite instability (MSI) status was determined by PCR from cancerous and normal tissues or by immunohistochemistry for mismatch repair proteins.
Statistical analyses
Patient characteristics were determined at the initiation of second-line chemotherapy. Adjuvant therapies were not counted as treatment lines and first-line chemotherapy was defined as the first systemic treatment administered in the metastatic setting. We defined right-sided colon cancer as that of the cecum and the ascending colon up to the splenic flexure. The Glasgow Prognostic Score (GPS) was calculated as follows: the presence of both elevated CRP level (>1.0 mg/dL) and hypoalbuminemia (<3.5 g/dL) was awarded a score of 2, the presence of only one of these abnormalities was awarded a score of 1, and the presence of neither of these was scored as 0.17
Survival was calculated using the Kaplan–Meier method. The median follow-up period was calculated from the follow up among the individuals with censored cases. Progression-free survival (PFS) was defined as the time between the initiation of second-line chemotherapy and radiographic progression or death from any cause. OS was defined as the time between the initiation of second-line chemotherapy and death from any cause. Tumor response was assessed by RECIST version 1.1 if patients had measurable lesions.
Univariate and multivariate analyses were performed using Cox proportional hazards models. Factors associated with p values <0.1 in univariate analysis were included in multivariate analyses and a forward stepwise method selection procedure was used. All tests were two-sided, and p < 0.05 was considered statistically significant.
Results
Patient characteristics
Of 80 BRAF mutant mCRC patients, four patients harbored BRAF non-V600E mutation, three patients received best supportive care (BSC) alone, and 73 patients with BRAF V600E mutation received systemic chemotherapy. Of the 64 patients who experienced failure of first-line chemotherapy, BSC alone was provided for 12 patients. Accordingly, a total of 52 patients met our inclusion criteria (Figure 1). The baseline patient characteristics are listed in Table 1. The median age was 59 years (range: 28–86). Consistent with previous studies, more than half of the patients were female (60%) and had right-sided primary (58%) and peritoneal metastases (69%). The proportion of patients with histologically poorly differentiated, signet ring cells or mucinous components was relatively high (37%). Seven patients (13%) had an Eastern Cooperative Oncology Group performance status (ECOG PS) ⩾2. An total of 24 (48%), 13 (26%), and 13 (26%) patients had GPS of 0, 1, and 2, respectively. The GPS could not be evaluated for two patients owing to missing data. Although most patients had undergone doublet regimens with or without biologics agents as first-line chemotherapy, five (10%) patients had received FOLFOXIRI regimen. One patient received a triple-combination regimen that included a BRAF inhibitor and one patient had received anti-PD-1 antibody. Quite a few patients (46%) developed early progression (first-line chemotherapy PFS ≤6 months). KRAS status was routinely evaluated along with BRAF status, and all patients had the KRAS wild type. MSI status was evaluated for 12 patients. In 2 out of these 12 patients, MSI-H was determined.
Figure 1.
Study flow chart.
Table 1.
Patient characteristics.
| Patients (n = 52) | |
|---|---|
| Age, years | |
| Median (range) | 59 (28–86) |
| Gender | |
| Male | 21 (40%) |
| Female | 31 (60%) |
| ECOG PS | |
| 0–1 | 45 (87%) |
| 2 | 5 (10%) |
| 3 | 2 (4%) |
| Histology | |
| Well or moderately differentiated | 33 (63%) |
| Poorly differentiated (Por), Signet-cell (Sig) or Mucinous (Muc) | 19 (37%) |
| MSI status | |
| MSI-High | 2 (4%) |
| Microsatellite stable | 10 (19%) |
| Unknown | 40 (77%) |
| Primary tumor site | |
| Right-sided | 30 (58%) |
| Left-sided | 22 (42%) |
| Site of metastasisa | |
| Liver | 30 (58%) |
| Lung | 19 (37%) |
| Peritoneum | 36 (69%) |
| Lymph node | 28 (54%) |
| Number of metastasis | |
| 1 | 10 (19%) |
| ⩾2 | 41 (81%) |
| Resection of primary tumor | |
| Yes | 37 (71%) |
| No | 15 (29%) |
| Adjuvant therapy | |
| Yes | 15 (29%) |
| No | 37 (71%) |
| WBC (/mm2 ) | |
| <10,000 | 43 (83%) |
| ⩾10,000 | 9 (17%) |
| LDH (IU/L) | |
| <240 | 28 (54%) |
| ⩾240 | 24 (46%) |
| ALP (IU/L) | |
| <300 | 17 (33%) |
| ⩾300 | 35 (67%) |
| Glasgow Prognostic Score | |
| 0 | 24 (48%) |
| 1 | 13 (26%) |
| 2 | 13 (26%) |
| Unknown | 2 (4%) |
| First-line chemotherapy | |
| Doublet with or without biologics | 43 (83%) |
| FOLFOXIRI with or without biologics | 15 (10%) |
| Others | 4 (8%) |
| PFS in first-line chemotherapy | |
| <6 months | 24 (46%) |
| ⩾6 months | 28 (54%) |
aThere is some overlap.
ALP, alkaline phosphatase; ECOG PS, Eastern Cooperative Oncology Group performance status; FOLFOXIRI, 5-FU/leucovorin+oxaliplatin+irinotecan; LDH, lactate dehydrogenase; MSI, microsatellite instability; PFS, progression-free survival; WBC, white blood cells.
Regimes used as second-line chemotherapy are listed in Table 2. Treatment regimens were classified into three strategies. A total of 28 (54%) patients received cytotoxic doublet or triplet regimens with conventional approved biologics. Four (8%) patients were treated with study drugs including BRAF inhibitors. The remaining 20 (38%) patients were treated with other regimens. No interaction was observed between treatment strategies and the prognostic factors described below. A total of 40 (77%) patients received oxaliplatin- or irinotecan-based regimens. Fifteen (29%) patients received adjuvant therapy and six (12%) developed recurrence either during adjuvant therapy or within 6 months after the last administration.
Table 2.
Regimens for second-line chemotherapy.
| Regimens | Cytotoxic doublet or triplet with conventional approved biologics (n = 28) | Other regimens (n = 20) | Study drugs including BRAF inhibitors ( n = 4) | p value |
|---|---|---|---|---|
| Cytotoxic doublet plus bevacizumab (n = 23)Cytotoxic doublet + anti-EGFR (n = 3) FOLFOXIRI + bevacizumab (n = 2) |
Anti-EGFR + irinotecan (n = 4) Anti-EGFR monotherapy (n = 4) Cytotoxic doublet (n = 4) Irinotecan + bevacizumab (n = 2) Antimicrotubule agents (n = 1) Capecitabine + bevacizumab (n = 1) Irinotecan monotherapy (n = 1) FL + bevacizumab (n = 1) Regorafenib (n = 1) Trifluridine/tipiracil (n = 1) |
Anti-EGFR + BRAF inhibitor + MEK inhibitor (n = 3) Anti-EGFR + BRAF inhibitor (n = 1) |
||
|
ECOG PS
0-1/2 |
26/2 | 15/5 | 4/0 | 0.17 |
|
LDH
<240/⩾240 |
16/12 | 9/11 | 3/1 | 0.58 |
|
GPS
0/1/2 |
15/7/5 | 7/4/8 | 2/2/0 | 0.27 |
Anti-EGFR, anti-epidermal growth factor receptor; ECOG PS, Eastern Cooperative Oncology Group performance status; FL, 5-FU/leucovorin; FOLFOXIRI, 5-FU/leucovorin+oxaliplatin+irinotecan; GPS, Glasgow Prognostic Score; LDH, lactate dehydrogenase.
Treatment results
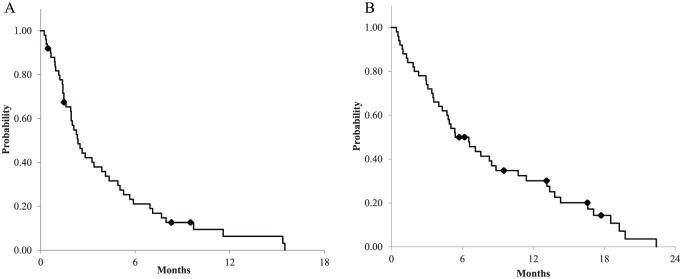
The median PFS and OS were 2.5 (95% CI = 1.91–4.11) and 6.5 (95% CI = 4.30–9.63) months, respectively, with a median follow-up period of 11.3 months (Figure 2). The 1-year survival rate was 30.1 (95% CI = 17.0–41.6)%. For patients treated with the most frequently used therapy, doublet regimens plus bevacizumab, the median PFS and OS were 4.3 (95% CI = 1.94–5.88) and 8.8 (95% CI = 5.39–13.37) months, respectively. Among the 44 patients who had measurable lesions, the overall response and disease control rates were 7% and 48%, respectively (Figure 3). All regimens that elicited a partial response were comprised of BRAF inhibitors in combination with anti-EGFR anti bodies, which provided response rates of 75% (three of four) and disease control rates of 100%. Therefore, the overall response and disease control rates were 0 and 43%, after exclusion of four patients who were treated with study drugs in clinical trials.
Figure 2.
Survival curve for overall patients: (A) progression-free survival; and (B) overall survival.
Figure 3.
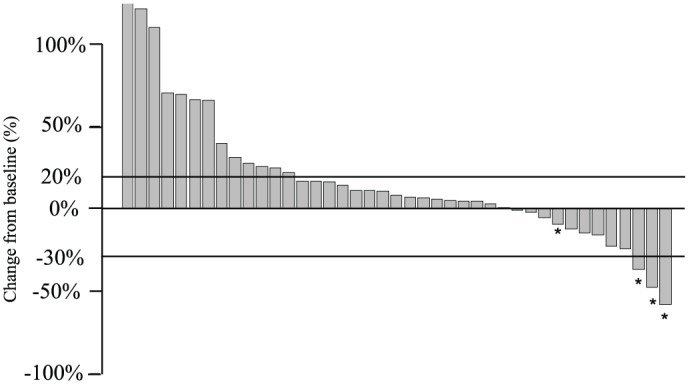
Change in tumor size. Waterfall plot showing the best percentage change from baseline in tumor measurement using RECIST version 1.1.
*Patients who were treated with study drugs in clinical trials.
Prognostic factors
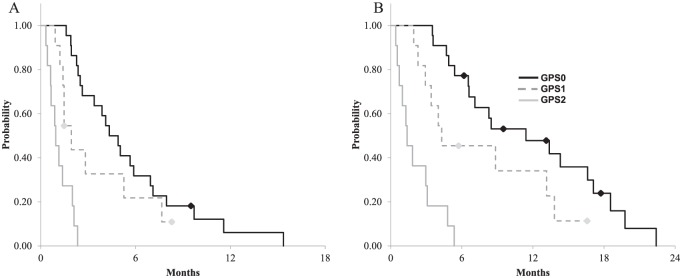
Results of univariate and multivariate analysis for OS are presented in Table 3. On univariate analysis, four factors showed a significant association with survival: the GPS, high white blood cell (WBC) count, elevated lactate dehydrogenase (LDH), and ECOG-PS. Multivariate analysis revealed that the GPS, elevated LDH, and ECOG-PS were independent prognostic factors for OS. Meanwhile, the well-known negative prognostic factors such as right-sided tumor location were not prognostic. The median PFSs in patients with GPS of 0, 1, and 2 were 4.7, 1.7, and 0.9 months, respectively. The median OSs in patients with GPS of 0, 1, and 2 were 10.0, 5.0, and 1.3 months, respectively (Figure 4). After exclusion of patients with ECOG-PS ⩾2, univariate analysis for OS showed that prognostic value of GPS was still maintained (GPS = 1, HR = 2.24, 95% CI = 1.02–4.90 and GPS = 2, HR = 10.27, 95% CI = 3.49–30.2).
Table 3.
Univariate and multivariate analyses for OS.
| Factors | Univariate HR |
p | Multivariate HR |
p | |
|---|---|---|---|---|---|
| Age | ⩾60 | 0.78 | 0.42 | ||
| Gender | Female | 0.89 | 0.70 | ||
| ECOG-PS | ⩾2 | 24.1 | <0.01 | 7.41 | <0.01 |
| Histology | Por, Sig, Muc | 1.18 | 0.60 | ||
| Primary tumor location | Right-sided | 0.80 | 0.46 | ||
| Peritoneal metastasis | Present | 0.94 | 0.84 | ||
| Number of metastasis | ⩾2 | 1.29 | 0.55 | ||
| Colostomy | No | 1.71 | 0.09 | ||
| WBC | ⩾10,000 | 2.23 | 0.03 | ||
| LDH | ⩾240 | 2.56 | <0.01 | 2.61 | <0.01 |
| ALP | ⩾300 | 1.43 | 0.27 | ||
| GPS (versus 0) | 1 | 2.21 | 0.05 | 2.42 | 0.03 |
| 2 | 15.3 | <0.01 | 8.82 | <0.01 | |
| First-line chemotherapy regimen | Triplet | 2.30 | 0.08 | ||
| PFS in first-line chemotherapy | <6 months | 1.84 | 0.05 | ||
ALP, alkaline phosphatase; ECOG PS, Eastern Cooperative Oncology Group performance status; GPS, Glasgow Prognostic Score; HR, hazard ratio; LDH, lactate dehydrogenase; Muc, Mucinous; Por, poorly differentiated; PFS, progression-free survival; Sig, signet cell; WBC, white blood cells.
Figure 4.
Survival curve according to Glasgow Prognostic Score (GPS): (A) progression-free survival according to GPS; (B) overall survival according to GPS.
Subsequent therapies
Among the 50 patients who discontinued their second-line chemotherapy, most patients stopped the treatment due to disease progression (87%). Among the fifty patients, twenty-five (50%) received third-line chemotherapy and the remaining (50%) received BSC alone. The third-line chemotherapy regimens were as follows: fluoropyrimidine-based regimens (seven patients), trifluridine/tipiracil (6), anti-EGFR antibodies plus irinotecan (5), anti-EGFR antibody monotherapy (4), regorafenib (2), and pilot studies of antimicrotubule agents (1).
Discussion
In this study, we identified GPS at the initiation of second-line treatment as a meaningful prognostic factor in mCRC patients with BRAF V600E mutation. To the best of our knowledge, this is the first study to explore significant prognostic factors and demonstrate the usefulness of the GPS for BRAF mutant mCRC. It is noteworthy that survival curves according to the GPS separated clearly. Patients with a GPS of 2 had a median PFS of 0.9 months and a median OS of 1.3 months, which indicates that these patients are unfit for enrolment in clinical trials. GPS is also useful in that the score (0 versus 1) serves as a stratification factor. Thus, GPS seems to be an especially efficient factor for use in clinical trials. GPS, an inflammation-based score, has been shown to be a prognostic indicator in various types of cancers.18–22 For colorectal cancer, GPS has been found to be a useful independent predictor of tumor stage.23–25 Although the modified GPS (mGPS), in which hypoalbuminemia alone is classified as mGPS of 0, was proposed later,26 it remains unclear whether GPS or mGPS is a more valuable marker. In our analysis, the survival curves separated more clearly according to GPS than mGPS (data not shown).
In our study, the 52 patients who underwent second-line chemotherapy had a dismal prognosis; the median PFS and OS were 2.5 (95% CI = 1.91–4.11) and 6.5 (95% CI = 4.30–9.63) months, respectively. Moreover, the overall response was 0% after exclusion of the four patients who were treated with study drugs in clinical trials. Several clinical trials in the second-line setting performed subgroup analyses of BRAF mutant patients and the reported median PFS and OS were 1.8–3.5 and 4.4–6.7 months,14 respectively, which is in line with our results. Morris et al. retrospectively analyzed metastatic colorectal cancer with BRAF mutation and reported that the median PFS in patients who received second-line therapy was 2.5 months.13 Seligmann et al. assessed three randomized trials and compared survival outcomes between BRAF mutant and wild-type patients.27 In that study, BRAF mutation conferred a markedly worse survival after first-line therapy, while there was no difference in first-line PFS and disease control rate. The authors proposed that patients with BRAF mutation were likely to develop rapid tumor growth after chemotherapy failure. It is evident that there is an urgent need to explore a breakthrough approach in second- or later-line chemotherapy for BRAF V600E-mutated mCRC patients.
Selective BRAF inhibition by small molecules has been considered to be a promising strategy. Preclinical studies have demonstrated that BRAF inhibition, in addition to the blockage of other signal pathways, is necessary to prohibit tumor growth.28,29 Based on these findings, dual or triple regimens with BRAF inhibitors in combination with EGFR antibodies, MEK inhibitors, or PI3K inhibitors have been tested. Dabrafenib (BRAF inhibitor) plus panitumumab plus trametinib (MEK inhibitor) yielded an overall response rate of 18% and a disease control rate of 67%.30 Phase II trials comparing encorafenib (BRAF inhibitor) plus cetuximab and encorafenib plus cetuximab plus alpelisib (PI3K inhibitor) showed an overall response rate of 22% and 27%, respectively. The median PFSs were 4.2 and 5.4 months for the doublet and triplet regimen (HR = 0.69, 95% CI = 0.43–1.11), respectively.31 The SWOG S1406 trial, a randomized phase II trial of irinotecan and cetuximab with or without vemurafenib, showed that PFS was extended with the addition of vemurafenib (HR = 0.42, 95% CI = 0.26–0.66) with a median PFS of 4.4 versus 2.0 months. The objective response rate was also higher in the irinotecan and cetuximab with vemurafenib group (16% versus 4%).32 Compared with BRAF inhibitor monotherapy, these results were encouraging. Indeed, only the patients who received study drugs including BRAF inhibitors achieved a partial response in our study, which indicates that regimens which include BRAF inhibitors and EGFR antibodies are promising.
Of interest, well-known negative prognostic factors such as histological type or primary tumor location were associated with BRAF mutation, but were not prognostically relevant when the analysis was limited to patients with BRAF mutation. Lee et al. demonstrated that right-sided tumor was associated with worse prognosis but was not an independent prognostic factor when adjusted for other variables including BRAF mutation status, which seems to be consistent with our results.33 It may be because BRAF mutation is a powerful prognostic factor. From our findings, we believe that it is essential to identify independent prognostic factors among BRAF mutant patients. In a recent study, patients with colorectal cancer with BRAF mutation were divided into two groups (BM1 and BM2) using clustering based on genetic profile analysis.34 It is suggested that the two subgroups were dependent on different signal pathways, and that the differences affected the sensitivity to chemotherapeutic agents and the prognosis of the two subgroups differed significantly. These results reinforce the presence of heterogeneity among BRAF mutations, which is in line with our findings that the survival outcomes differed according to baseline patient characteristics such as GPS.
There are a few limitations to our study. First, this is a retrospective analysis from a single institution and included a small sample size. Prognostic factors identified in our study should be validated in further research. Biomarker analysis is also important to reveal the biological background behind our findings. Second, our study enrolled various patients including patients with poor condition who are usually ineligible for participation in clinical trials. However, the prognostic value of GPS was intact even after exclusion of patients with ECOG-PS ⩾2. Third, the treatment regimens differed considerably because the standard second-line chemotherapy for metastatic colorectal cancer with BRAF V600E mutation remains unclear. In the currently ongoing phase III trial (ClinicalTrials.gov identifier: NCT02928224), investigator’s choice regimens (FOLFIRI plus cetuximab or irinotecan plus cetuximab) are adopted as the control arm. Moreover, our study was a prognostic, not a predictive, analysis. All the regimens used in our study have been shown to be effective for metastatic colorectal cancer in previous studies and therefore can be included in the analysis. Fourth, MSI status was evaluated for only a few patients (12 of 52, 23%) since routine MSI examination is not covered by Japanese national insurance. The frequency of colorectal cancer with deficient mismatch repair (MMR) is lower in Japan and other Asian countries compared with that in Western countries.35–37 In a study by Fujiyoshi et al., MSI-H tumor was detected in 3.7% of Japanese mCRC patients and the frequency in BRAF mutant patients was 16.0%.37 However, it was reported that no significant survival differences were observed irrespective of MSI status.1 MSI has shown to be an effective biomarker for immune check inhibitors38,39 and the screening system is rapidly spreading in Japan. Despite these limitations, our study is the first to report the detailed outcomes of second-line chemotherapy and identify prognostic factors for BRAF mutant mCRC.
In summary, the results of second-line chemotherapy for mCRC with BRAF V600E mutation were extremely poor. Clinical trials of new agents and further development of treatment strategies are urgently required. GPS, elevated LDH and ECOG-PS were shown to be independent prognostic factors for OS. In particular, it is suggested that GPS is useful in future clinical trials to serve as a stratification factor (GPS 0 versus 1) and to identify patients who are unfit for trials (GPS 2).
Acknowledgments
We sincerely appreciate all the patients who received treatment and were enrolled in this study.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Seiichiro Mitani: Eli Lilly & Co. (Personal Fees)Hiroya Taniguchi: Takeda Pharmaceutical Co., Taiho, and Chugai Pharmaceutical Co. (Personal Fees) Takeda Pharmaceutical Co. (Grant) Toshiki Masuishi: Eli Lilly & Co. (Personal Fees)Yukiya Narita: Bristol-Myers Squibb, Taiho, Ono Pharmaceutical Co., and Nihon Kayaku (Personal Fees) Shigenori Kadowaki: Eli Lilly & Co., Taiho, Chugai Pharmaceutical Co., Ono Pharmaceutical Co., Merck Serono, Bayer, Eisai and Yakult Honsha (Personal Fees), Boehringer Ingelheim, Eli Lilly & Co., and Taiho (Grant) Kei Muro: Eli Lilly & Co., Chugai Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ono Pharmaceutical Co., Ltd., Taiho, Bayer (Personal Fees) Gilead Sciences, Ono Pharmaceutical Co., Ltd., Merck Sharp & Dohme, Shionogi, Kyowa Hakko Kirin, Daiichi Sanyo (Grant)
ORCID iD: Seiichiro Mitani  https://orcid.org/0000-0003-0033-8741
https://orcid.org/0000-0003-0033-8741
Contributor Information
Seiichiro Mitani, Department of Clinical Oncology, Aichi Cancer Center Hospital, Nagoya, Japan.
Hiroya Taniguchi, Department of Clinical Oncology, Aichi Cancer Center Hospital, 1-1 Kanokoden, Chikusa-ku, Nagoya, Aichi 464-8681, Japan.
Keiji Sugiyama, Department of Clinical Oncology, Aichi Cancer Center Hospital, Nagoya, Japan.
Toshiki Masuishi, Department of Clinical Oncology, Aichi Cancer Center Hospital, Nagoya, Japan.
Kazunori Honda, Department of Clinical Oncology, Aichi Cancer Center Hospital, Nagoya, Japan.
Yukiya Narita, Department of Clinical Oncology, Aichi Cancer Center Hospital, Nagoya, Japan.
Shigenori Kadowaki, Department of Clinical Oncology, Aichi Cancer Center Hospital, Nagoya, Japan.
Takashi Ura, Department of Clinical Oncology, Aichi Cancer Center Hospital, Nagoya, Japan.
Masashi Ando, Department of Clinical Oncology, Aichi Cancer Center Hospital, Nagoya, Japan.
Masahiro Tajika, Department of Endoscopy, Aichi Cancer Center Hospital, Nagoya, Japan.
Yasushi Yatabe, Department of Pathology and Molecular Diagnosis, Aichi Cancer Center Hospital, Nagoya, Japan.
Kei Muro, Department of Clinical Oncology, Aichi Cancer Center Hospital, Nagoya, Japan.
References
- 1. Venderbosch S, Nagtegaal ID, Maughan TS, et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin Cancer Res 2014; 20: 5322–5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Peeters M, Oliner KS, Price TJ, et al. Analysis of KRAS/NRAS mutations in a phase III study of panitumumab with FOLFIRI compared with FOLFIRI alone as second-line treatment for metastatic colorectal cancer. Clin Cancer Res 2015; 21: 5469–5479. [DOI] [PubMed] [Google Scholar]
- 3. Yokota T, Ura T, Shibata N, et al. BRAF mutation is a powerful prognostic factor in advanced and recurrent colorectal cancer. Br J Cancer 2011; 104: 856–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen D, Huang JF, Liu K, et al. BRAFV600E mutation and its association with clinicopathological features of colorectal cancer: a systematic review and meta-analysis. PLoS One 2014; 9: e90607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Van Cutsem E, Kohne CH, Lang I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 2011; 29: 2011–2019. [DOI] [PubMed] [Google Scholar]
- 6. Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 2013; 369: 1023–1034. [DOI] [PubMed] [Google Scholar]
- 7. Price TJ, Hardingham JE, Lee CK, et al. Impact of KRAS and BRAF gene mutation status on outcomes from the phase III AGITG MAX trial of capecitabine alone or in combination with bevacizumab and mitomycin in advanced colorectal cancer. J Clin Oncol 2011; 29: 2675–2682. [DOI] [PubMed] [Google Scholar]
- 8. Loupakis F, Cremolini C, Salvatore L, et al. FOLFOXIRI plus bevacizumab as first-line treatment in BRAF mutant metastatic colorectal cancer. Eur J Cancer 2014; 50: 57–63. [DOI] [PubMed] [Google Scholar]
- 9. Cremolini C, Loupakis F, Antoniotti C, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol 2015; 16: 1306–1315. [DOI] [PubMed] [Google Scholar]
- 10. Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016; 27: 1386–1422. [DOI] [PubMed] [Google Scholar]
- 11. Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011; 364: 2507–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kopetz S, Desai J, Chan E, et al. Phase II pilot study of vemurafenib in patients with metastatic BRAF-mutated colorectal cancer. J Clin Oncol 2015; 33: 4032–4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morris V, Overman MJ, Jiang ZQ, et al. Progression-free survival remains poor over sequential lines of systemic therapy in patients with BRAF-mutated colorectal cancer. Clin Colorectal Cancer 2014; 13: 164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Seymour MT, Brown SR, Middleton G, et al. Panitumumab and irinotecan versus irinotecan alone for patients with KRAS wild-type, fluorouracil-resistant advanced colorectal cancer (PICCOLO): a prospectively stratified randomised trial. Lancet Oncol 2013; 14: 749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yokota T, Shibata N, Ura T, et al. Cycleave polymerase chain reaction method is practically applicable for V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS)/V-raf murine sarcoma viral oncogene homolog B1 (BRAF) genotyping in colorectal cancer. Transl Res 2010; 156: 98–105. [DOI] [PubMed] [Google Scholar]
- 16. Bando H, Yoshino T, Shinozaki E, et al. Simultaneous identification of 36 mutations in KRAS codons 61 and 146, BRAF, NRAS, and PIK3CA in a single reaction by multiplex assay kit. BMC Cancer 2013; 13: 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McMillan DC, Crozier JE, Canna K, et al. Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancer. Int J Colorectal Dis 2007; 22: 881–886. [DOI] [PubMed] [Google Scholar]
- 18. Kinoshita A, Onoda H, Imai N, et al. The Glasgow Prognostic Score, an inflammation based prognostic score, predicts survival in patients with hepatocellular carcinoma. BMC Cancer 2013; 13: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen C, Sun P, Dai QS, et al. The Glasgow Prognostic Score predicts poor survival in cisplatin-based treated patients with metastatic nasopharyngeal carcinoma. PLoS One 2014; 9: e112581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Crumley AB, McMillan DC, McKernan M, et al. Evaluation of an inflammation-based prognostic score in patients with inoperable gastro-oesophageal cancer. Br J Cancer 2006; 94: 637–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gioulbasanis I, Pallis A, Vlachostergios PJ, et al. The Glasgow Prognostic Score (GPS) predicts toxicity and efficacy in platinum-based treated patients with metastatic lung cancer. Lung Cancer 2012; 77: 383–388. [DOI] [PubMed] [Google Scholar]
- 22. Kobayashi T, Teruya M, Kishiki T, et al. Inflammation-based prognostic score, prior to neoadjuvant chemoradiotherapy, predicts postoperative outcome in patients with esophageal squamous cell carcinoma. Surgery 2008; 144: 729–735. [DOI] [PubMed] [Google Scholar]
- 23. Furukawa K, Shiba H, Haruki K, et al. The Glasgow prognostic score is valuable for colorectal cancer with both synchronous and metachronous unresectable liver metastases. Oncol Lett 2012; 4: 324–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maeda K, Shibutani M, Otani H, et al. Prognostic value of preoperative inflammation-based prognostic scores in patients with stage IV colorectal cancer who undergo palliative resection of asymptomatic primary tumors. Anticancer Res 2013; 33: 5567–5573. [PubMed] [Google Scholar]
- 25. Dreanic J, Maillet M, Dhooge M, et al. Prognostic value of the Glasgow Prognostic Score in metastatic colorectal cancer in the era of anti-EGFR therapies. Med Oncol 2013; 30: 656. [DOI] [PubMed] [Google Scholar]
- 26. Proctor MJ, Talwar D, Balmar SM, et al. The relationship between the presence and site of cancer, an inflammation-based prognostic score and biochemical parameters. Initial results of the Glasgow inflammation outcome study. Br J Cancer 2010; 103: 870–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Seligmann JF, Fisher D, Smith CG, et al. Investigating the poor outcomes of BRAF-mutant advanced colorectal cancer: analysis from 2530 patients in randomised clinical trials. Ann Oncol 2017; 28: 562–568. [DOI] [PubMed] [Google Scholar]
- 28. Corcoran RB, Ebi H, Turke AB, et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov 2012; 2: 227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mao M, Tian F, Mariadason JM, et al. Resistance to BRAF inhibition in BRAF-mutant colon cancer can be overcome with PI3K inhibition or demethylating agents. Clin Cancer Res 2013; 19: 657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Corcoran RB, André T, Yoshino T, et al. Efficacy and circulating tumor DNA (ctDNA) analysis of the BRAF inhibitor dabrafenib (D), MEK inhibitor trametinib (T), and anti-EGFR antibody panitumumab (P) in patients (pts) with BRAF V600E–mutated (BRAFm) metastatic colorectal cancer (mCRC). Ann Oncol 2016; 27(Suppl. 6): 4550. [Google Scholar]
- 31. Tabernero J, Geel RV, Guren TK, et al. Phase 2 results: Encorafenib (ENCO) and cetuximab (CETUX) with or without alpelisib (ALP) in patients with advanced BRAF-mutant colorectal cancer (BRAFm CRC). J Clin Oncol 2016; 34(Suppl. 15): abstract 3544. [Google Scholar]
- 32. Kopetz S, McDonough SL, Morris VK, et al. Randomized trial of irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG 1406). J Clin Oncol 2017; 35(Suppl. 4): abstract 520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee MS, Advani SM, Morris J, et al. Association of primary site and molecular features with prgression-free survival and overall survival of metastatic colorectal cancer after anti-epidermal growth factor receptor therapy. J Clin Oncol 2016; 34(Suppl. 15): abstract 3506. [Google Scholar]
- 34. Barras D, Missiaglia E, Wirapati P, et al. BRAF V600E mutant colorectal cancer subtypes based on gene expression. Clin Cancer Res 2017; 23: 104–115. [DOI] [PubMed] [Google Scholar]
- 35. Nakaji Y, Oki E, Nakanishi R, et al. Prognostic value of BRAF V600E mutation and microsatellite instability in Japanese patients with sporadic colorectal cancer. J Cancer Res Clin Oncol 2017; 143: 151–160. [DOI] [PubMed] [Google Scholar]
- 36. Asaka S, Arai Y, Nishimura Y, et al. Microsatellite instability-low colorectal cancer acquires a KRAS mutation during the progression from Dukes’ A to Dukes’ B. Carcinogenesis 2009; 30: 494–499. [DOI] [PubMed] [Google Scholar]
- 37. Fujiyoshi K, Yamamoto G, Takenoya T, et al. Metastatic pattern of stage IV colorectal cancer with high-frequency microsatellite instability as a prognostic factor. Anticancer Res 2017; 37: 239–247. [DOI] [PubMed] [Google Scholar]
- 38. Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015; 372: 2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 2017; 18: 1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]