Abstract
Background:
Depression and anxiety are common and underrecognized in end-stage renal disease (ESRD), are associated with poor outcomes and reduced health-related quality of life, and are potentially treatable. Simple, accurate screening tools are needed.
Objective:
We examined the operating characteristics of single questions for anxiety and depression from the Edmonton Symptom Assessment System (ESAS) in hemodialysis.
Design:
Cross-sectional study.
Setting:
Two outpatient hemodialysis units (1 tertiary, 1 community) in Hamilton, Canada.
Patients:
Adult prevalent hemodialysis patients.
Measurements:
ESAS and Hospital Anxiety and Depression Scale (HADS).
Methods:
Participants were asked the degree to which they experienced anxiety and depression using the ESAS. ESAS single questions for anxiety and depression were compared with the reference standard of the HADS using dialysis population specific cutoffs (HADS anxiety subscale ≥6 and HADS depression subscale ≥7). Logistic regression was used to create receiver operating characteristics (ROC) curves.
Results:
We recruited 50 participants with a mean age of 64 (SD = 12.4) years, of whom 52% were male and 96% were on ≥3× weekly hemodialysis. Using the reference standards, 28 (56%) had a diagnosis of anxiety and 27 (54%) had a diagnosis of depression. Areas under the ROC curves were 0.83 for anxiety and 0.81 for depression using ESAS scores of ≥2.
Limitations:
Sample size and the lack of a reference gold standard.
Conclusions:
The ESAS single questions for anxiety and depression have reasonable discrimination in a hemodialysis population. The use of more complex and time-consuming screening instruments could be reduced by adopting the ESAS questions for anxiety and depression in hemodialysis.
Keywords: screening, anxiety, depression, hemodialysis
Abrégé
Contexte :
La dépression et l’anxiété sont fréquentes quoique peu reconnues chez les patients souffrant d’insuffisance rénale terminale (IRT). Ces troubles sont associés à une évolution défavorable de la maladie et à une diminution de la qualité de vie liée à l’état de santé. Cependant, ils sont potentiellement traitables. Des outils de détection simples et précis sont requis.
Objectif :
Nous avons évalué la fonction d’efficacité de questions uniques sur l’anxiété et la dépression provenant du Système d’évaluation des symptômes d’Edmonton (ESAS) en contexte d’hémodialyse.
Type d’étude:
Étude transversale.
Cadre :
Deux unités d’hémodialyse ambulatoire (une en soins tertiaires, une en milieu communautaire) à Hamilton, au Canada.
Sujets:
Des patients adultes hémodialysés.
Mesures:
L’ESAS et l’Échelle d’anxiété et de dépression en milieu hospitalier (HADS).
Méthodologie:
Nous avons demandé aux participants, par l’entremise de l’ESAS, dans quelle mesure ils éprouvaient de l’anxiété et de la dépression. Les questions uniques de l’ESAS sur l’anxiété et la dépression ont été comparées à l’étalon de référence de la HADS en utilisant les seuils spécifiques à une population dialysée (sous-échelle de la HADS pour l’anxiété ≥ 6 et sous-échelle de la HADS pour la dépression ≥ 7). Une régression logistique a été utilisée pour établir les courbes de fonction d’efficacité de l’observateur (courbes ROC).
Résultats:
Nous avons recruté 50 patients (52 % d’hommes) âgés de 64 ans en moyenne (écart-type : 12,4 ans). La plupart des sujets (96 %) étaient dialysés au moins trois fois par semaine. Selon l’étalon de référence, 28 patients (56 %) vivaient de l’anxiété et 27 (54 %) souffraient de dépression. La surface sous la courbe ROC était de 0,83 pour l’anxiété et de 0,81 pour la dépression selon les scores ESAS ≥ 2.
Limites:
Le faible échantillon de sujets et le fait que l’étude ne comportait pas d’étalon-or.
Conclusion:
Les questions uniques de l’ESAS sur l’anxiété et la dépression ont discriminé adéquatement dans une population de patients hémodialysés. L’adoption du questionnaire ESAS sur l’anxiété et la dépression avec les patients hémodialysés pourrait limiter le recours à des outils de détection chronophages et complexes.
What was known before
Anxiety and depression are common and underrecognized in dialysis and are associated with adverse outcomes. A variety of screening instruments exist but whether or not the ESAS is accurate is uncertain.
What this adds
The ESAS single questions for anxiety and depression have utility as screening instruments in the dialysis population. As initial screening tools, they can be used to facilitate the diagnosis of mood disorders given their ease and simplicity.
Introduction
Anxiety and depression are common in the setting of end-stage renal disease (ESRD) with anxiety affecting 38% and depression 27% of individuals with kidney disease.1 Both mood disorders are commonly clinically underdiagnosed2 and suboptimally managed in patients with ESRD.3 The identification of patients with anxiety and/or depression in the setting of ESRD is important as these mood disorders are strongly associated with impaired health-related quality of life4,5 and adverse events such as peritonitis,6 hospitalization,7-9 cardiovascular events,10 dialysis withdrawal,11 and death.12,13
Simple screening tools that can be self-reported may help identify patients with significant anxiety and depressive symptoms who are eligible for treatment. This is particularly important given recent recommendations to incorporate patient-reported outcomes into clinical care14,15 and the availability of potentially effective therapies for anxiety and depression in dialysis such as cognitive behavioral therapy.16,17
Given the prevalence and impact of these symptoms, we undertook a study to determine how well a patient-reported outcome measure that incorporates a single question for each of anxiety and depression performs compared with a longer, validated screening instrument.
Methods
Study Cohort
We performed a cross-sectional study of prevalent adult hemodialysis patients from 2 hemodialysis units (1 tertiary, 1 community) in Hamilton, Canada. A consecutive sample of hemodialysis patients from morning and afternoon dialysis shifts were approached. Eligible participants were (1) 18 years of age or older, (2) receiving in-center hemodialysis ≥2× weekly for at least the last 90 days, and (3) provided informed consent. Patients were excluded if they were unable to complete the study instruments due to a cognitive impairment or an English language barrier.
Study Procedures
We collected participant demographics, comorbidities, and laboratory results at baseline. Participants completed the following instruments during dialysis at the first study visit: the Hospital Anxiety and Depression Scale (HADS)18 and the single anxiety and depression screening questions. The HADS19 was used as the reference standard for anxiety and depression instead of other mood disorder screening tools given similar operating characteristics and its ability to concurrently screen for both anxiety and depression in a simple manner. It consists of 14 questions, with 7 questions for anxiety (HADS-A subscale) and 7 questions for depression (HADS-D subscale), and responses ranging from 0 to 3, with higher scores indicating worse symptoms in the last week. The reference cutoffs20 for anxiety in dialysis is a HADS-A score of ≥6 or a HADS score of ≥12 and for depression in dialysis is a HADS-D score of ≥7 or a HADS score of ≥1221 or ≥14.
The screening questions for anxiety and depression were based off of the Edmonton Symptom Assessment System (ESAS) that originates from palliative care22 and has been extensively validated23 in cancer settings and contains 10 symptoms measured on an 11-point scale anchored at 0 (no symptoms) and 10 (worst possible symptoms). It was validated cross-sectionally24 and longitudinally25 in dialysis patients.
Ethics approval was obtained by the Hamilton Integrated Research Ethics Board.
Statistical Analysis
A sample size of 50 was chosen based on previous studies evaluating the operating characteristics of anxiety and depression screening tools.18 Assuming a 40% prevalence of anxiety, an area under the receiver operator curve (AUROC) of 0.8, and a 2-sided alpha of 0.05, a sample size of 50 patients would have greater than 97% power to detect a difference from an AUROC of 0.5. Assuming a 30% prevalence of depression, an AUROC of 0.8, and a 2-sided alpha of 0.05, a sample size of 50 patients would have greater than 95% power to detect a difference from an AUROC of 0.5.
Participant characteristics are described using means and standard deviations (SD) or medians and 25th to 75th percentiles for continuous variables and frequencies (%) for categorical variables. Differences between those who exceeded the HADS-A and HADS-D screening thresholds of 6 and 7, respectively, were compared with those who did not using a 2-sample t test for continuous variables and chi-squared test for categorical variables.
Using cutoffs of 1 to 10, the performances of the screening ESAS single questions for anxiety and depression were compared with HADS-A ≥6 and HADS-D ≥ 7 reference standards by calculating the screening question sensitivity, specificity, and AUROC. Sensitivity analyses were performed using the reference standards of HADS ≥ 12 for depression and HADS ≥ 14 for both anxiety and depression. The performance of ESAS screening questions and both HADS-A and HADS-D subscales were compared with the reference standard of a previously clinically documented diagnosis anxiety and depression. The relationship between ESAS single questions and HADS and both HADS-A and HADS-D subscales was assessed using Pearson correlation coefficients.
All statistical tests were performed at a P < .05 level of significance. All analyses were performed using STATA (Stata Statistical Software: Release 14; StataCorp LP, College Station, Texas).
Results
The study participants’ characteristics are provided in Table 1. Twenty-eight participants (56%) had a HADS-A ≥ 6 and 27 (54%) had a HADS-D ≥ 7. Individuals with a HADS-A ≥ 6 were more likely to be female and had higher serum phosphate (Supplemental Table 1). Individuals with a HADS-D ≥ 7 were older and had a higher serum phosphate (Supplemental Table 2). ESAS scores for anxiety and depression correlated moderately with HADS-A and HADS-D subscales with Pearson correlation coefficients of 0.50 (P = .0002) and 0.56 (P = .0000), respectively (Figures 1 and 2 for scatterplots). ESAS scores for anxiety and depression correlated moderately with HADS with Pearson correlation coefficients of 0.45 (P = .001) and 0.61 (P = .0000) respectively, not shown).
Table 1.
Study Cohort Characteristics.
| N = 50 | |
|---|---|
| Age, years (SD) | 64 (12.4) |
| Sex | |
| Male, n (%) | 26 (52%) |
| Female, n (%) | 24 (48%) |
| Dialysis treatments | |
| 2× weekly, n (%) | 2 (4%) |
| ≥3× weekly, n (%) | 48 (96%) |
| Duration of HD, hours (SD) | 3.6 (0.4) |
| Vascular access | |
| Fistula, n (%) | 24 (48%) |
| Graft, n (%) | 3 (6%) |
| Catheter, n (%) | 23 (46%) |
| URR, % (SD) | 69.5 (6.2) |
| Etiology of ESRD | |
| DN, n (%) | 19 (38%) |
| HTN, n (%) | 4 (8%) |
| GN, n (%) | 12 (24%) |
| Other, n (%) | 15 (30%) |
| Comorbidities | |
| Diabetes, n (%) | 24 (48%) |
| CAD, n (%) | 13 (26%) |
| PVD, n (%) | 9 (18%) |
| CVD, n (%) | 10 (20%) |
| OSA, n (%) | 11 (22%) |
| Anxiety, n (%) | 7 (14%) |
| Depression, n (%) | 7 (14%) |
| SSRI, n (%) | 6 (12%) |
| SNRI, n (%) | 2 (4%) |
| Hemoglobin, g/L (SD) | 106 (9.9) |
| Calcium, mmol/L (SD) | 2.28 (0.2) |
| Phosphate, mmol/L (SD) | 1.72 (0.5) |
| Albumin, g/L (SD) | 31.5 (2.8) |
| PTH, pmol/L (SD) | 60.3 (42.2) |
Note. Data are presented with mean (standard deviation) or number (percent). HD = hemodialysis; URR = urea reduction ratio; ESRD = end-stage renal disease; DN = diabetic nephropathy; HTN = hypertension; GN = glomerulonephritis; CAD = coronary artery disease; PVD = peripheral vascular disease; OSA = obstructive sleep apnea; SSRI = selective serotonin uptake inhibitor; SNRI = serotonin norepinephrine uptake inhibitor; PTH = parathyroid hormone ; CVD = cerebrovascular disease.
Figure 1.

Scatterplot for ESAS anxiety and HADS-A.
Note. r = 0.50. ESAS = Edmonton Symptom Assessment Scale; HADS-A = Hospital Anxiety Depression Scale anxiety subscale.
Figure 2.
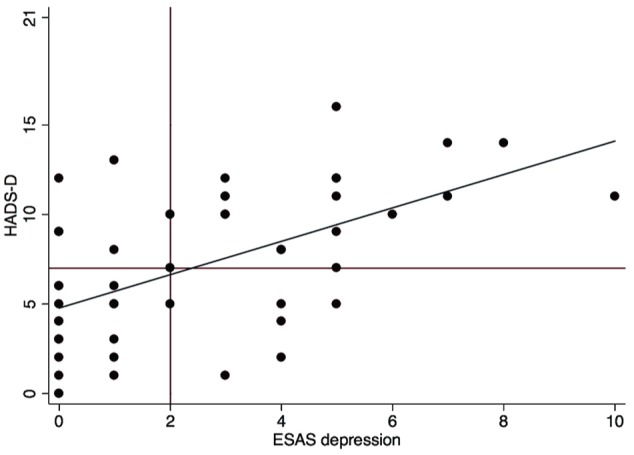
Scatterplot for ESAS depression and HADS-D.
Note. r = 0.56. ESAS = Edmonton Symptom Assessment Scale; HADS-D = Hospital Anxiety Depression Scale depression subscale.
Only 7 (14%) of study participants had a previous documented clinical diagnosis of anxiety and 7 (14%) had a previous documented clinical diagnosis of depression. Four of 7 and 3 of 7 individuals were currently on pharmacotherapy (including a selective serotonin reuptake inhibitor or serotonin norepinephrine reuptake inhibitor) for anxiety or depression, respectively. Two of seven individuals with a previous diagnosis of anxiety and 1 of 7 individuals with a previous diagnosis of depression did not screen positive using the HADS-A and HADS-D. Two of 3 of these false negatives were on pharmacotherapy.
The optimal ESAS screening cutoff to identify anxiety defined by HADS-A ≥ 6 was an ESAS score ≥ 2 with a sensitivity of 75%, specificity of 91%, and an AUROC of 0.83 (Figure 3). Using ESAS to screen for previously documented anxiety, the optimal cutoff was an ESAS score ≥ 1 with a sensitivity of 71%, specificity of 58%, and an AUROC of 0.76. The optimal HADS-A cutoff to identify previous documented anxiety was ≥ 5 with a sensitivity of 71%, a specificity of 47%, and an AUROC of 0.59. Using ESAS scores of ≥ 1 out of 10 to screen for anxiety defined by HADS-A ≥ 6, the prevalence of anxiety was 50% with a sensitivity of 66.7%, specificity of 78.6%, and AUROC of 0.82. A sensitivity analysis using HADS ≥ 14 as the reference standard at an ESAS cutoff of 2 did not improve the operating characteristics (Supplemental Figure 1).
Figure 3.

Receiver operating curve for ESAS anxiety and HADS-A.
Note. HADS-A > 6, ESAS cut point >2: sensitivity 0.75, specificity 0.91, AUROC 0.83. ESAS = Edmonton Symptom Assessment Scale; HADS-A = Hospital Anxiety Depression Scale anxiety subscale; ROC = receiver operating characteristics; AUROC = area under the receiver operator curve.
The optimal ESAS screening cutoff to identify depression defined by HADS-D ≥ 7 was an ESAS score of ≥2 with a sensitivity of 81%, specificity of 74%, and AUROC of 0.81 (Figure 4). Using ESAS to screen for previously documented depression, the optimal cutoff was an ESAS score ≥3 with a sensitivity of 71%, a specificity of 71%, and an AUROC of 0.71. The optimal HADS-D cutoff to identify previous documented depression was ≥9 with a sensitivity of 71%, specificity of 70%, and an AUROC of 0.71. Using ESAS scores of ≥1 out of 10 to screen for depression defined by HADS-D ≥ 7, the prevalence of depression was 72% with a sensitivity of 88.0%, a specificity of 76.0%, and an AUROC of 0.73. Sensitivity analyses using HADS ≥12 or ≥14 as the reference standards at ESAS cutoffs of 2 did not improve the operating characteristics (Supplemental Figures 2 and 3).
Figure 4.

Receiver operating curve for ESAS depression and HADS-D.
Note. HADS-D ≥ 7, ESAS cut point ≥2: sensitivity 0.81, specificity 0.74, AUROC 0.81. ESAS = Edmonton Symptom Assessment Scale; HADS-D = Hospital Anxiety Depression Scale depression subscale; ROC = receiver operating characteristics; AUROC = area under the receiver operator curve.
Discussion
In this observational study of 50 hemodialysis patients from both tertiary and community dialysis units, single questions for the screening of anxiety and depression from the ESAS showed reasonable discrimination compared with HADS reference standards. ESAS cutoffs of ≥2 had a sensitivity of 75% and specificity of 91% for anxiety and a sensitivity of 81% and specificity 74% for depression. ESAS scores for anxiety and depression moderately correlated with HADS-A and HADS-D subscales as well as total HADS scores suggesting that ESAS single questions for anxiety and depression may be suitable screening tools given their operating characteristics, ease of administration, either alone or as part of the entire ESAS as a global symptom screening strategy.
ESAS has been previously evaluated as screening tool for anxiety and depression with HADS-A and HADS-D reference standards of ≥826 in 216 individuals from 3 different settings including male cancer survivors, patients with advanced cancer and dyspnea, and individuals receiving outpatient palliative care. In that study, the prevalence of anxiety was 43.6% and the prevalence of depression was 36.5%, and optimal ESAS cutoffs for screening were ≥2 for both anxiety and depression with similar sensitivities but worse specificities compared with our study (sensitivity 86% and specificity 56% for anxiety, sensitivity 77% and specificity 55% for depression, AUROC not reported). Given the overlapping somatic symptoms of ESRD and depression and the possibility of interference, it is surprising that ESAS performed more accurately as a screening tool for depression in our study. It is unclear why the performance of ESAS for the screening of anxiety was also more accurate in our study but this may be due to interference by symptoms and/or stressors unique to malignancy but not ESRD. It is reassuring that we identified ESAS cutoffs that were the same in a non-ESRD and diverse patient population with superior operating characteristics.
It should be noted that we did not use any formal gold standard, including a structured clinical interview with the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria for anxiety and depression. Instead, we relied on a reference standard that has previously been validated for screening in dialysis and performed sensitivity analyses with dialysis specific reference cutoffs. The poor performance of both ESAS and HADS to screen for clinically documented anxiety or depression in our study is likely due to only 7 of 50 (14%) and 7 of 50 (14%) participants previously having these diagnoses, likely as a result of ascertainment bias due to underrecognition, inadequate documentation, and possibly fluctuating symptoms related to the natural history of these disorders and effective treatment.
Our study is limited by its relatively small size and homogeneous population from 2 hemodialysis units in Ontario, Canada. Our results cannot be extrapolated to home modalities or the nondialysis setting including chronic kidney disease or kidney transplant patients with potentially different biology of mental health disorders. We also excluded those with cognitive impairment or an English language barrier further limiting the generalizability of our findings. However, we did employ a broad approach for patient inclusion resulting in a study population representative of a typical dialysis unit in North America. Lastly, we included patients with a history of mood disorders as well as those undergoing treatment which may artificially bias the accuracy of screening tools.27
Conclusions
Given the shift in priorities to include patient-reported outcome measures in the clinical care of dialysis patients, simple and accurate screening questions are needed to identify symptoms and conditions that remain underdiagnosed and suboptimally treated. ESAS single questions for anxiety and depression may be appropriate as initial screening tools in hemodialysis given their functionality and operating characteristics either administered alone or as components of the ESAS followed by either additional longer, disease-specific screening tools (eg, HADS or other depending on pretest/posttest probabilities) and/or a clinical interview as the gold standard. Additional studies are needed to evaluate the ESAS single questions screening performances across centers and dialysis populations as well as set robust screening thresholds.
Supplemental Material
Supplemental material, Supplemental_material_version for Single Questions for the Screening of Anxiety and Depression in Hemodialysis by David Collister, Jennifer C. Rodrigues, Andrea Mazzetti, Kelsi Salisbury, Laura Morosin, Christian Rabbat, K. Scott Brimble and Michael Walsh in Canadian Journal of Kidney Health and Disease
Acknowledgments
D.C. is supported by a Kidney Research Scientist Core Education and National Training Program (KRESCENT) Postdoctoral Fellowship Award. M.W. is supported by a New Investigator Award from the Canadian Institutes of Health Research.
Footnotes
Ethics Approval and Consent to Participate: Ethics approval was obtained by the Hamilton Integrated Research Ethics Board.
Consent for Publication: All authors reviewed the final manuscript and provided consent for publication.
Availability of Data and Materials: Data and materials may be made available upon written request to the corresponding author.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by a grant from Cancer Care Ontario/Ontario Renal Network.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Murtagh FE, Addington-Hall J, Higginson IJ. The prevalence of symptoms in end-stage renal disease: a systematic review. Adv Chronic Kidney Dis. 2007;14(1):82-99. [DOI] [PubMed] [Google Scholar]
- 2. Lopes AA, Albert JM, Young EW, et al. Screening for depression in hemodialysis patients: associations with diagnosis, treatment, and outcomes in the DOPPS. Kidney Int. 2004;66(5):2047-2053. [DOI] [PubMed] [Google Scholar]
- 3. Cukor D, Coplan J, Brown C, Peterson RA, Kimmel PL. Course of depression and anxiety diagnosis in patients treated with hemodialysis: a 16-month follow-up. Clin J Am Soc Nephrol. 2008;3(6):1752-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Preljevic VT, Osthus TB, Os I, et al. Anxiety and depressive disorders in dialysis patients: association to health-related quality of life and mortality. Gen Hosp Psychiatry. 2013;35(6):619-624. [DOI] [PubMed] [Google Scholar]
- 5. Tentori F, Mapes DL. Health-related quality of life and depression among participants in the DOPPS: predictors and associations with clinical outcomes. Semin Dial. 2010;23(1):14-16. [DOI] [PubMed] [Google Scholar]
- 6. Troidle L, Watnick S, Wuerth DB, Gorban-Brennan N, Kliger AS, Finkelstein FO. Depression and its association with peritonitis in long-term peritoneal dialysis patients. Am J Kidney Dis. 2003;42(2):350-354. [DOI] [PubMed] [Google Scholar]
- 7. Hedayati SS, Grambow SC, Szczech LA, Stechuchak KM, Allen AS, Bosworth HB. Physician-diagnosed depression as a correlate of hospitalizations in patients receiving long-term hemodialysis. Am J Kidney Dis. 2005;46(4):642-649. [DOI] [PubMed] [Google Scholar]
- 8. Lacson E, Jr, Bruce L, Li NC, Mooney A, Maddux FW. Depressive affect and hospitalization risk in incident hemodialysis patients. Clin J Am Soc Nephrol. 2014;9(10):1713-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lopes AA, Bragg J, Young E, et al. Depression as a predictor of mortality and hospitalization among hemodialysis patients in the United States and Europe. Kidney Int. 2002;62(1):199-207. [DOI] [PubMed] [Google Scholar]
- 10. Boulware LE, Liu Y, Fink NE, et al. Temporal relation among depression symptoms, cardiovascular disease events, and mortality in end-stage renal disease: contribution of reverse causality. Clin J Am Soc Nephrol. 2006;1(3):496-504. [DOI] [PubMed] [Google Scholar]
- 11. Lacson E, Jr, Li NC, Guerra-Dean S, Lazarus M, Hakim R, Finkelstein FO. Depressive symptoms associate with high mortality risk and dialysis withdrawal in incident hemodialysis patients. Nephrol Dial Transplant. 2012;27(7):2921-2928. [DOI] [PubMed] [Google Scholar]
- 12. Farrokhi F, Abedi N, Beyene J, Kurdyak P, Jassal SV. Association between depression and mortality in patients receiving long-term dialysis: a systematic review and meta-analysis. Am J Kidney Dis. 2014;63(4):623-635. [DOI] [PubMed] [Google Scholar]
- 13. Palmer SC, Vecchio M, Craig JC, et al. Association between depression and death in people with CKD: a meta-analysis of cohort studies. Am J Kidney Dis. 2013;62(3):493-505. [DOI] [PubMed] [Google Scholar]
- 14. Obbarius A, van Maasakkers L, Baer L, et al. Standardization of health outcomes assessment for depression and anxiety: recommendations from the ICHOM Depression and Anxiety Working Group. Qual Life Res. 2017;26(12):3211-3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Breckenridge K, Bekker HL, Gibbons E, et al. How to routinely collect data on patient-reported outcome and experience measures in renal registries in Europe: an expert consensus meeting. Nephrol Dial Transplant. 2015;30(10):1605-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cukor D, Ver Halen N, Asher DR, et al. Psychosocial intervention improves depression, quality of life, and fluid adherence in hemodialysis. J Am Soc Nephrol. 2014;25(1):196-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kusztal M, Trafidlo E, Weyde W, Klinger M. Cognitive-behavioral group therapy is an effective treatment for major depression in hemodialysis (HD) patients. Kidney Int. 2010;77(7):646-647;author reply 647-648. [DOI] [PubMed] [Google Scholar]
- 18. Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52(2):69-77. [DOI] [PubMed] [Google Scholar]
- 19. Martin CR, Tweed AE, Metcalfe MS. A psychometric evaluation of the Hospital Anxiety and Depression Scale in patients diagnosed with end-stage renal disease. Br J Clin Psychol. 2004;43(pt 4):51-64. [DOI] [PubMed] [Google Scholar]
- 20. Preljevic VT, Osthus TB, Sandvik L, et al. Screening for anxiety and depression in dialysis patients: comparison of the Hospital Anxiety and Depression Scale and the Beck Depression Inventory. J Psychosom Res. 2012;73(2):139-144. [DOI] [PubMed] [Google Scholar]
- 21. Loosman WL, Siegert CE, Korzec A, Honig A. Validity of the Hospital Anxiety and Depression Scale and the Beck Depression Inventory for use in end-stage renal disease patients. Br J Clin Psychol. 2010;49(pt 4):507-516. [DOI] [PubMed] [Google Scholar]
- 22. Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7(2):6-9. [PubMed] [Google Scholar]
- 23. Nekolaichuk C, Watanabe S, Beaumont C. The Edmonton Symptom Assessment System: a 15-year retrospective review of validation studies (1991-2006). Palliat Med. 2008;22(2):111-122. [DOI] [PubMed] [Google Scholar]
- 24. Davison SN, Jhangri GS, Johnson JA. Cross-sectional validity of a modified Edmonton symptom assessment system in dialysis patients: a simple assessment of symptom burden. Kidney Int. 2006;69(9):1621-1625. [DOI] [PubMed] [Google Scholar]
- 25. Davison SN, Jhangri GS, Johnson JA. Longitudinal validation of a modified Edmonton symptom assessment system (ESAS) in haemodialysis patients. Nephrol Dial Transplant. 2006;21(11):3189-3195. [DOI] [PubMed] [Google Scholar]
- 26. Vignaroli E, Pace EA, Willey J, Palmer JL, Zhang T, Bruera E. The Edmonton Symptom Assessment System as a screening tool for depression and anxiety. J Palliat Med. 2006;9(2):296-303. [DOI] [PubMed] [Google Scholar]
- 27. Thombs BD, Arthurs E, El-Baalbaki G, Meijer A, Ziegelstein RC, Steele RJ. Risk of bias from inclusion of patients who already have diagnosis of or are undergoing treatment for depression in diagnostic accuracy studies of screening tools for depression: systematic review. BMJ. 2011;343:d4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_material_version for Single Questions for the Screening of Anxiety and Depression in Hemodialysis by David Collister, Jennifer C. Rodrigues, Andrea Mazzetti, Kelsi Salisbury, Laura Morosin, Christian Rabbat, K. Scott Brimble and Michael Walsh in Canadian Journal of Kidney Health and Disease


