Abstract
Background:
Soft tissue leiomyosarcomas are rare, accounting for almost 5%–10% of all soft tissue sarcomas; they account for almost 1% of all sarcomas. They are aggressive tumors where location, size, and management require a multidisciplinary approach. Since there are few series published, we here analyze epidemiological pattern, clinical and pathologic features of soft tissue leiomyosarcomas.
Methods:
We conducted a retrospective study of 29 consecutive cases of histologically proven soft tissue leiomyosarcoma extracted from the database of the Cancer Registry of the Center of Tunisia and the Department of Pathology of Farhat Hached University Hospital of Sousse of Tunisia, during a 10-year period (from January 1996 to December 2005). Epidemiologic details, clinico-pathological features, and treatment modalities were assessed with focus on patients’ 5-year overall survival, tumor relapse, and metastases.
Results:
Soft tissue leiomyosarcoma accounted for 17.5% of all soft tissue sarcomas diagnosed at our pathology department. Most of patients were of advanced age (median: 52 years), with extremes ranging from 12 and 87 years. There was a slight male predominance (sex-ratio = 1.07). Tumors were located mostly in the lower limbs (45%). Deep sites as retroperitoneum was found only in two cases. Tumor size was more than 5 cm in 83% of cases (average size = 9.4 cm). Five cases had metastasis on initial staging. For 24 patients, the disease was locally limited at the moment of diagnosis. Palliative chemotherapy was indicated for four patients and surgery was performed for 20 patients. Local recurrence occurred in 11 patients (55% of operated patients) and metastasis in 6 patients. Overall, 5-year survival was about 24%.
Conclusion:
Our study results highlight the scarcity of soft tissue leiomyosarcoma. Unfortunately, unusual tumor sites, disease’s advanced stages, and intralesional resection made the prognosis poorer than in other series. Clinical course of soft tissue leiomyosarcoma was highly marked by local recurrence and metastasis.
Keywords: Leiomyosarcoma, soft tissue, treatment, prognosis
Introduction
Leiomyosarcoma (LMS) of soft tissue is a relatively uncommon malignant tumor that has the phenotypic features of smooth muscle differentiation and may occur anywhere in the body. It is frequently encountered in subcutaneous or in deep soft tissues of limbs, head and neck, and retroperitoneum.1 However, cutaneous and great vessel remain uncommon as primitive origin. Data on soft part LMS were scanty. Most epidemiological studies of soft tissue sarcoma (STS) were performed in the Western countries, and only limited data highlight that in the Asian population. To our knowledge, soft tissue LMS has not been described in any large previous study with complete follow-up, in North Africa.
Materials and methods
This article reports a monocentric retrospective observational and descriptive study during a period of 10 years, based on a series of patients who were diagnosed with LMS in the Department of Pathology of Farhat Hached University Hospital in Sousse, Tunisia, and who were reported to the National Cancer Registry of the Tunisian Center.
We included patients with subcutaneous and deep-seated LMS of extremities, trunk wall, retroperitoneum, and head and neck region. All of them had nearly complete clinical data regarding tumor size and depth, imaging, treatment modalities, and outcome. Patients with cutaneous, mediastinal, gynecologic, genitourinary, intra-thoracic, and major vessel LMS were excluded.
All histological slides were reevaluated by two experienced pathologists. We have only focused on immunohistochemical expression of each of the three markers separately: smooth muscle actin (SMA), desmin, and h-Caldesmon. In all cases, the LMS diagnosis was confirmed based on morphology that showed smooth muscle differentiation and on positive immunohistochemical staining (IHC) for at least one muscle marker (α-smooth muscle, h-Caldesmon, and desmin) for all patients. Reclassified tumors, for which IHC staining was negative, were excluded from the study.
Histologic malignancy of the tumor was graded according to the French Federation of Comprehensive Cancer Centers (FNCLCC) grading system. The superficial or deep-seated location of the tumors was determined either microscopically or according to clinical records. All statistical analyses for the current study were performed using the SPSS Statistical Package.
Results
Our study recorded 29 patients diagnosed with LMS. They represented 17, 7% of all STSs in Central Tunisia (29 cases, diagnosed at that period (1995–2005)). There were 14 females and 15 males (sex ratio: 1.07). Patients’ ages were ranging from 12 to 87 years (median: 52 years). The most common primary site was the lower limbs (37%), followed by trunk (24.5%) and cephalic region (17.5%) (Table 1). Tumors measured from 2 to 20 cm (average size: 9.4 cm) and tumors of extremities were smaller than their retroperitoneal counterpart (10.5 vs 15 cm).
Table 1.
Initial location of tumor.
| Localization | Number of cases | Percentage |
|---|---|---|
| Lower limbs | ||
| Thigh | 7 | 37 |
| Leg | 3 | |
| Ankle | 1 | |
| Upperlimbs | 4 | 14 |
| Head and neck | ||
| Head | 4 | 17.5 |
| Neck | 1 | |
| Trunk | ||
| Thorax | 2 | 24.5 |
| Abdominal wall | 2 | |
| Inguinal | 3 | |
| Retroperitoneum | 2 | |
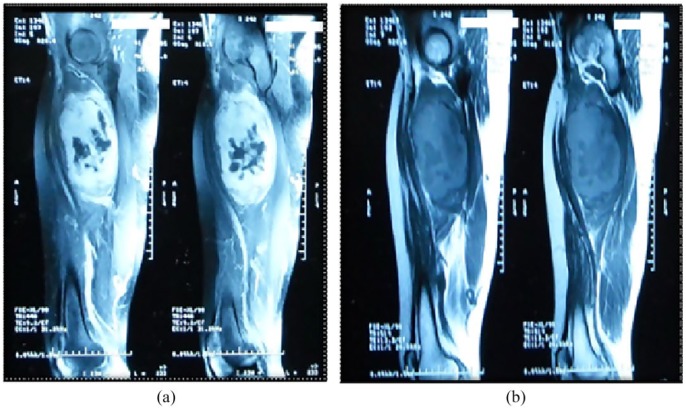
Radiologic assessment was based on computed tomographic (CT) scan in 15 cases and magnetic resonance imaging (MRI) in eight cases. CT findings showed a hypodense heterogeneous mass, well circumscribed (in five cases) and ill defined (in 10 cases). In addition to anatomic site, tumor depth, and size, imaging revealed simultaneous distant metastases at diagnosis, for five patients (Figure 1).
Figure 1.
MRI: A well-circumscribed mass of the thigh with cystic foci, that is (b) isointense to muscle in T1 sequence and (a) hyperintense on T2 fat-suppressed sequence.
Diagnosis of soft tissue LMS was made on core biopsy in 15 cases, but grading was made in only five cases (with undergrading in these same cases, because of the frequent absence of necrosis on core biopsy.
Microscopically, classic variant of LMS was found in 24 cases (83%), characterized by an ill-defined proliferation of smooth muscle cells with indistinct margins, arranged in intersecting fascicles (Figure 2). Nuclei were enlarged and atypical. Mitotic rate was high ranging from 5 to 32 mitoses/10 HPF (high power field) (Figure 3). Foci of hemorrhage and necrosis were seen in 10 cases (34%) and 17 cases (58%), respectively. Other variants of LMS were found, such as inflammatory LMS (one case), pleomorphic LMS (one case), and myxoid LMS (one case).
Figure 2.
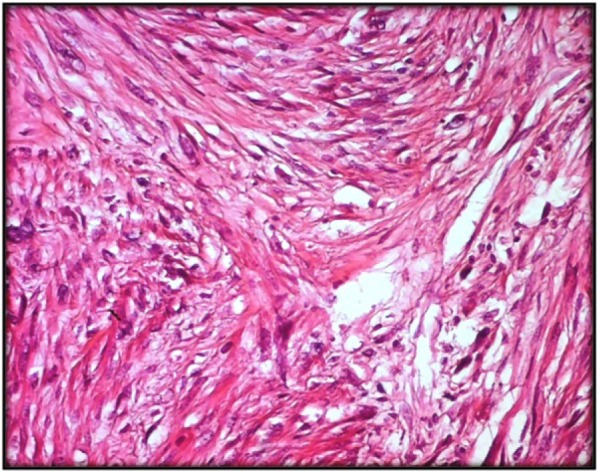
Classical form of soft tissue LMS made of intersecting fascicles of eosinophilic spindle cells with atypical nuclei (HE ×200).
Figure 3.
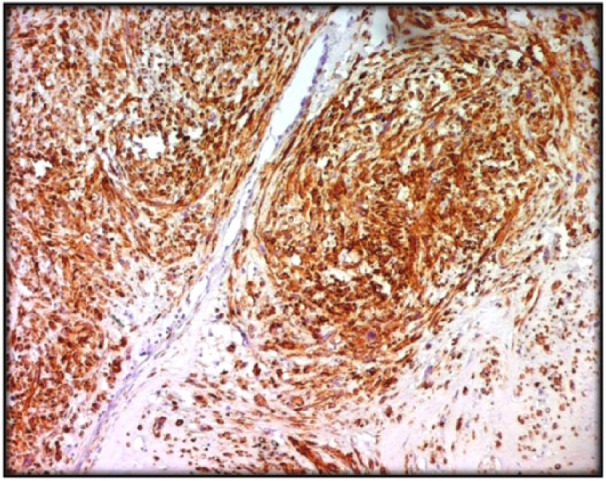
Diffuse expression of h-Caldesmon by tumoral cells (IHC ×100).
According to the FNCLCC grading system, grade 1 LMS was found in four cases (14%) and grade 2 LMS was found in 13 cases (45%). For 12 cases (41%), it was a grade 3 LMS. At immunohistochemistry (Figure 3), tumor cells were positive for SMA (79.3%), desmin (68.9%), and h-Caldesmon (58.6%). They were negative for PS100, CD34, and c-Kit in all cases.
About 72% of patients were diagnosed at an advanced stage of disease (Stage IIB, III, and IV). Five patients with synchronous metastases died rapidly (mean survival 1 month after diagnosis). A total of 24 patients were followed up (74%), and four patients had only palliative chemotherapy.
Surgical resection of tumor mass was performed in 20 cases (Table 2). Two patients underwent disarticulation secondary to a recurrence of a LMS in the knee in a 48-year-old male and in the leg in a 68-year-old female. Surgical margins were intralesional for seven patients and marginal in 11 cases. Wide resection margins were obtained in only two cases (>1 cm). Postoperative radiotherapy was indicated for only seven patients for whom surgical resection was complete with negative margins. The dose received varied from 45 to 55 Gy, with a median of 52 Gy. Adjuvant chemotherapy was indicated in six cases, using the following cytotoxic drug associations: ifosfamide–doxorubicin or ifosfamide–epirubicin.
Table 2.
Treatment modalities of soft tissue LMS.
| Number of patients | Percentage | |
|---|---|---|
| Surgery | 12 | 41 |
| Chemotherapy (CT) | 4 | 10 |
| Radiotherapy (RT) | 0 | 0 |
| Surgery + CT | 1 | 3 |
| Surgery + RT | 2 | 7 |
| Surgery + CT + RT | 5 | 17 |
| CT + RT | 0 | 0 |
LMS: leiomyosarcoma.
The 5-year overall survival rate was about 24%. The mean survival in four patients who had palliative chemotherapy was 10 months.
Concerning the 20 patients who had surgery, 3 of them were still alive 5 years after without recurrence. Local recurrence occurred in 11 patients (55%). Number of recurrences varied from one to five tumor sites per patient. Among these 11 patients, 4 were still alive 5 years after surgery. In the other seven patients, death was due to the complications of local recurrence such as cachexia, infection, and decubitus complications. We did not have cases of death due to treatment-related toxicity.
Finally, among the 20 operated patients, metachronous metastases occurred in six patients. The mean survival time in these patients was 16 months. No patients among those who had metachronous metastases survived 5 years (Table 3).
Table 3.
Patients’ clinical follow-up after surgery grouped by their survival status.
| Survival free: 3 patients | Local recurrence after surgery: 11 patients | Metastatic recurrence: 6 patients | |
|---|---|---|---|
| Age | (15–87) years | (12–84) years | (34–80) years |
| Median age | 34 years | 42.33 years | 60 years |
| Site | |||
| Upper limb | 2 | 0 | 2 |
| Lower limb | 0 | 5 | 3 |
| Abdominal wall | 1 | 1 | 0 |
| Thorax | 0 | 1 | 0 |
| Head | 0 | 1 | 1 |
| Retroperitoneum | 0 | 1 | 0 |
| Inguinal | 0 | 2 | 0 |
| Tumor size (cm) | 3–15 | 3–12 | 4.5–16 |
| Mean size | 9 | 8 | 9 |
| Grade | |||
| Grade 1 | 1 | 2 | 0 |
| Grade 2 | 2 | 3 | 2 |
| Grade 3 | 0 | 6 | 4 |
| Stage | |||
| Ia | 1 | 0 | 0 |
| Ib | 0 | 0 | 0 |
| IIa | 0 | 1 | 2 |
| IIb | 1 | 7 | 1 |
| III | 1 | 3 | 3 |
| IV | 0 | 0 | 1 |
| Treatment | |||
| Surgery | 3 | 11 | 6 |
| Radiotherapy | 1 | 4 | 2 |
| Chemotherapy | 1 | 4 | 1 |
| Margin status | |||
| Wide | 2 | 0 | 0 |
| Intralesional | 0 | 5 | 1 |
| Marginal | 1 | 6 | 5 |
| Follow-up time (months) | 84–148 | 4–172 | 7–29 |
| Median | 116 | 42.95 | |
| Outcome | |||
| Survival (5 years) | 3 | 4 | 0 |
| Deaths (5 years) | 0 | 7 | 6 |
| Mean survival time (months) | 153 | 62 | 16 |
For patients with tumors sized ⩽5 cm, the 5-year survival rate was 22%, patients with tumors sized ⩾5 cm, the 5-year survival rate was 25%, and the difference between those two groups was not statistically significant (p = 0.3). The 5-year survival rate depends on the tumor site: upper limbs, lower limbs, and trunk wall were 50%, 23%, and 29%, respectively. The 5-year survival rate according to tumor malignancy grade was, respectively, 25%, 36%, and 11% for grade 1, grade 2, and grade 3 LMS without a statistically significant difference (p = 0.83). Only clinical stage based on the association of TNM classification and the tumor malignancy grade was found to be the main risk factor for decreased survival rate with a statistically significant difference (p = 0.01). Resection margins were the only multivariable significant predictor of local recurrence.
Discussion
LMS is an uncommon malignant tumor arising from smooth muscle, mostly occurring in the uterus. However, it is among the most common types of soft tissue sarcoma. Soft tissue sarcoma represent 1% of all cancer. Soft tissue LMS account for approximately 5%–10% of all STSs.1 In our series, they represent 17.7% of all STS. The epidemiologic characteristics of soft tissue LMS in central Tunisia are quite similar to the international data. The incidence increases with age and they typically arise in middle-aged and elderly patients. There is a slight female predominance.2 No relevant risk factors were found, especially there was no history of trauma.
While histologically similar, soft tissue LMS have classically been subdivided into three groups for prognostic and treatment purposes: somatic soft tissues LMS, cutaneous LMS, and LMS of vascular origin.3 Soft tissue LMS can occur at any site, although they are more frequent in extremities. The commonest sites of involvement are lower limbs, upper limbs, and trunk followed by retroperitoneum and head and neck region.4
LMS of soft tissues often present as an enlarging painless mass. Clinically, there are no specific manifestations that distinguish them from other soft part sarcomas, although occasional symptoms like abdominal discomfort, pain, and/or weight loss may be observed with deeply seated tumors as retroperitoneal LMS. In our study, a painful mass was found in 89% of cases. At imaging, LMS are large masses, generally heterogeneous, demonstrating central low attenuation representing necrosis on CT scan. Calcifications are exceedingly rare.5 On MRI, LMS frequently display cystic foci. It is isointense to muscle in T1 sequence and predominantly hyperintense on T2 fat-suppressed sequence. Diagnosis is usually made on core-needle biopsies, it represents a safe technique, recommended for the diagnosis of musculoskeletal masses.6 Histologically, diagnosis is easily made in well- and moderately differentiated tumors. Diagnosis difficulties are encountered with poorly differentiated tumors or LMS variants (myxoid, pleomorphic), especially with small samples. Immunohistochemistry is therefore mandatory to make the diagnosis of LMS. At immunohistochemistry, LMS express desmin in 50% of cases, SMA is more often positive, but less specific. The h-Caldesmon is relatively a new marker, specific of LMS and expressed in 85% of cases.7 Age, location of tumor, size, histological grade, clinical stage, and surgical margins are crucial prognostic factors in defining patient outcomes with soft tissue LMS.
In a retrospective study of 410 patients with non-metastatic STS, Guillou et al.8 had found that large tumors (>10 cm) and deep-location and high-grade tumors (G2, G3) were predictive of metastasis in multivariate analysis. Cutaneous LMS have usually a favorable outcome, but local recurrences can occur. Sub-cutaneous LMS can give rise to metastasis in one-third of cases. Retroperitoneal LMS have a bad prognosis, with frequent metastasis and an overall 5-year survival rate of 30%. LMS of vascular origin have the worst prognosis with metastasis at the time of diagnosis observed with half of patients.9 Moreover, disruption of tumor by a previous incisional biopsy or incomplete excision was also significantly correlated with metastasis (p = 0.0001). It was also strongly correlated with large size and deep location.10
Our multivariate analysis also showed that only clinical stage was the significant prognostic factor for death. Our results are in accordance with previous studies made on LMS.11–13 Metastasis rate (initial or at recurrence) was 31% in our series. Previous analyses from other institutions reported rates that range from 29.4% to 44.7%.14,15 Also, adequate local treatment was the only statistically significant factor for local control in our multivariate analysis. It is well known that an adequate surgical margin is the most important factor for local control.13,16
In a recent single German institution study of Harati et al.,17 which has included 164 patients with somatic LMS of the soft tissues, high histologic grade (p = 0.001), size >5 cm (p = 0.002), and subfascial localization (p = 0.002) were associated with significantly diminished disease-specific survival (DSS) in univariate analysis. In multivariate analysis, only histologic grade was found to be an independent prognostic factor of DSS.
Our patients’ 5-year overall survival rate was about 24%, while it was 70.6% (95% confidence interval (CI): 60.9–78.3) in the German study.17 Indeed, 35.4% of the German patients received adjuvant radiotherapy after resection of their primary tumor, with a median overall dose of 60.0 Gray (range: 30.0–78.0). In addition, all patients of that series with local recurrences could undergo further resection of their initial local recurrence.
For most of patients with localized disease, surgery is the main treatment, sometimes combined with radiotherapy. Radiotherapy when performed post-operatively allows a better local control of the disease, but overall survival and recurrence-free survival are not modified.18 In our series, only seven patients had received adjuvant radiotherapy for whom surgical resection was complete with negative margins.
In another recent series of 225 patients with non-visceral LMS treated at a specialist sarcoma center “Scandinavian Sarcoma Group,” the local treatment was adequate in 154 of 206 patients (75%) who were without metastasis at presentation. In our series, the patients were at advanced stage (III and IV) at the moment of diagnosis in 44.8%. At 10 years, 84% of the 206 patients with localized disease at presentation were free from local recurrence, 66% remained metastasis free, and 49% were alive.19 This study has also shown that higher malignancy grade (p = 0.006), larger tumor size (p = 0.003), and deeper tumor location (p = 0.002) were significantly correlated with decreased metastasis-free survival; inadequate local treatment was correlated with local recurrence (p = 0.007); and high malignancy grade was correlated with decreased overall survival (p = 0.007), in multivariate analysis.
For patients with advanced stage of disease, there are other treatment options. The best option is to consider cytotoxic chemotherapy to reduce the size of the tumor and to make it more favorable for surgery. However, only a very limited number of agents are active on LMS. Doxorubicin and ifosfamide are widely accepted as the most effective molecules in STSs.20 In a retrospective study conducted by the French Sarcoma Group in 133 patients with unresectable or metastatic STS, the gemcitabine and docetaxel combination was tolerable and showed better response and survival rates in patients with LMS.21 Temozolomide as a single agent is also active in patients with advanced pretreated STS, especially those with unresectable or metastatic LMS.22
The discrepancy in terms of overall survival between our study and other publications seems to be due to the sample’s small size, the advanced clinical stage at the moment of diagnosis which reduces chances of surgical control and indications of adjuvant therapy. The high rate of recurrence (55%) in relation to a very high R1 surgery rate in our series with reduced second-line therapeutic choice had made the prognosis poorer.
In summary, LMS of soft tissues are aggressive tumors, with poor prognosis due to an advanced stage at time of diagnosis, which cannot allow an adequate surgical treatment. Survival rates are the lowest among all STSs. We have found that risk of local recurrence was dependent on adequacy of local treatment, and that 5-year overall survival was correlated with clinical stage. These findings challenged our own previous treatment policy and inspired us to review our institutional experience. The current results confirmed the importance of clinical stage and surgical margins in defining our patients’ outcome. We also underline the need for an early and adequate diagnosis of STSs, using needle-core biopsy. We also underline the need for centralization and multidisciplinary integration of the diagnosis and treatment of these tumors.
Footnotes
Conflict of interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: The director of the cancer registry agreed to use the registry data and validated the results.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed Consent: Not applicable.
Trial registration: Not applicable.
References
- 1. Coindre JM, Terrier P, Guillou L, et al. Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer 2001; 91(10): 1914–1926. [DOI] [PubMed] [Google Scholar]
- 2. el-Jabbour JN, Akhtar SS, Kerr GR, et al. Prognostic factors for survival in soft tissue sarcoma. Br J Cancer 1990; 62(5): 857–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Farshid G, Pradhan M, Goldblum J, et al. Leiomyosarcomas of somatic soft tissues: a tumor of vascular origin with multivariate analysis of outcome in 42 cases. Am J Surgpathol 2002; 26(1): 14–24. [DOI] [PubMed] [Google Scholar]
- 4. Eppsteiner R, DeYoung BR, Milhem MM, et al. Leiomyosarcoma of the head and neck. A population based analysis. Arch Otolaryngol Head Neck Surg 2011; 137(9): 921–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goldblum J, Folpe A, Weiss S, et al. Enzinger and Weiss’s soft tissue tumors. 6th ed. Philadelphia, PA: Elsevier, 2014. [Google Scholar]
- 6. Italiano A, Toulmonde M, Stoeckle E, et al. Clinical outcome of leiomyosarcomas of vascular origin: comparison with leiomyosarcomas of other origin. Ann Oncol 2010; 21(9): 1915–1921. [DOI] [PubMed] [Google Scholar]
- 7. Gustafson P, Willen H, Baldetorp B, et al. Soft tissue leiomyosarcoma: a population-based epidemiologic and prognostic study of 48 patients, including cellular DNA content. Cancer 1992; 70(1): 114–119. [DOI] [PubMed] [Google Scholar]
- 8. Guillou L, Coindre JM, Bonichon F, et al. Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft tissue sarcoma. J Clin Oncol 1997; 15(1): 350–362. [DOI] [PubMed] [Google Scholar]
- 9. Jensen ML, Jensen OM, Michalski W, et al. Intradermal and subcutaneous leiomyosarcoma: a clinicopathological and immunohistochemical study of 41 cases. J Cutan Pathol 1996; 23(5): 458–463. [DOI] [PubMed] [Google Scholar]
- 10. Liberal JM. Leiomyosarcoma: principles of management. Intractable Rare Dis Res 2013; 2(4): 127–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lintz F, Moreau A, Odri GA, et al. Critical study of resection margins in adult soft-tissue sarcoma surgery. Orthop Traumatol Surg Res 2012; 98(4 Suppl): S9–S18. [DOI] [PubMed] [Google Scholar]
- 12. Massi D, Beltrami G, Mela MM, et al. Prognostic factors in soft tissue leiomyosarcoma of the extremities: a retrospective analysis of 42 cases. Eur J Surg Oncol 2004; 30(5): 565–572. [DOI] [PubMed] [Google Scholar]
- 13. Rha SE, Byun JY, Jung SE, et al. CT and MRI of uterine sarcomas and their mimickers. AJR Am J Roentgenol 2003; 181(5): 1369–1374. [DOI] [PubMed] [Google Scholar]
- 14. Gladdy RA, Qin LX, Moraco N, et al. Predictors of survival and recurrence in primary leiomyosarcoma. Ann Surg Oncol 2013; 20(6): 1851–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Svarvar C, Bohling T, Berlin O, et al. Clinical course of nonvisceral soft tissue leiomyosarcoma in 225 patients from the Scandinavian Sarcoma Group. Cancer 2007; 109(2): 282–291. [DOI] [PubMed] [Google Scholar]
- 16. Schwartz D, Einck J, Hunt K, et al. The effect of delayed postoperative irradiation on local control of soft tissue of the extremities and torso. Int J Radiat Oncol Biol Phys 2002; 7(4): 348–359. [DOI] [PubMed] [Google Scholar]
- 17. Harati K, Daigeler A, Lange K, et al. Somatic leiomyosarcoma of the soft tissues: a single-institutional analysis of factors predictive of survival in 164 patients. World J Surg 2017; 41(6): 1534–1541. [DOI] [PubMed] [Google Scholar]
- 18. Watabnabe H, Kusakabe T, Hoshi N, et al. h-Caldesmon in leiomyosarcoma and tumors with smooth muscle cell-like differentiation: its specific expression in the smooth muscle cell tumor. Hum Pathol 1999; 30(4): 392–396. [DOI] [PubMed] [Google Scholar]
- 19. Svarvar C, Bohling T, Berlin O, et al. Clinical course of nonvisceral soft tissue leiomyosarcoma in 225 patients from the Scandinavian Sarcoma Group. Cancer 2007; 109(2): 282–291. [DOI] [PubMed] [Google Scholar]
- 20. Welker JA, Henshaw RM, Jelinek J, et al. The percutaneous needle biopsy is safe and recommended in the diagnosis of musculoskeletal masses outcomes analysis of 155 patients at a sarcoma referral center. Cancer 2000; 89(12): 2677–2686. [DOI] [PubMed] [Google Scholar]
- 21. Bay JO, Ray-Coquard I, Fayette J, et al. Docetaxel and gemcitabine combination in 133 advanced soft-tissue sarcomas: a retrospective analysis. Int J Cancer 2006; 119(3): 706–711. [DOI] [PubMed] [Google Scholar]
- 22. Garcia Del Muro X, Lopez-Pousa A, Martin J, et al. A phase II trial of temozolomide as a 6-week, continuous, oral schedule in patients with advanced soft tissue sarcoma: a study by the Spanish Group for Research on Sarcomas. Cancer 2005; 104(8): 1706–1712. [DOI] [PubMed] [Google Scholar]