Abstract
Background:
The prevalence of exercise-induced ischemia in the asymptomatic limb of patients with unilateral claudication based on history and treadmill evaluation, and with unilateral ipsilateral peripheral artery disease (i.e ankle-to-brachial systolic pressure index <0.90) is unknown.
Methods:
We detected exercise-induced ischemia in the asymptomatic limb of patients with apparently unilateral claudication. Among 6059 exercise-oximetry tests performed in 3407 nondiabetic and 961 diabetic patients. We estimated the intensity of ischemia in the both limb (buttocks and calves) using the lowest minimum value of the decrease from rest of oxygen pressure (DROP; limb changes minus chest changes from rest), with significant ischemia defined as DROP lower than −15 mmHg.
Results:
We found 152 tests performed in 142 nondiabetic patients and 40 tests performed in 38 diabetic patients. The asymptomatic limb showed significant ischemia in 46.7% and 37.5% of the tests. Strictly unilateral exercise-induced claudication with apparently unilateral peripheral artery disease was rare (<4% of all tests). However, among these highly selected tests, significant ischemia was found in the asymptomatic limb in more than one-third of cases.
Conclusion:
The asymptomatic limb of patients with peripheral artery disease should not be considered a normal limb.
Keywords: claudication, lower limb, exercise, treadmill testing, transcutaneous oxygen pressure, diabetes mellitus, walking impairment
Introduction
Lower extremity peripheral artery disease (PAD) affects up to a quarter of elderly persons and its prevalence increases with age.1–3 Ankle-to-brachial index (ABI) at rest is defined as the ratio of ankle-to-brachial artery systolic blood pressures.4 An ABI below 0.90 is widely considered to indicate the presence of PAD because PAD patients can remain asymptomatic for many years. Generally, intermittent claudication resulting from exercise-induced muscle ischemia is the first symptom of PAD.5 Intermittent claudication results in functional impairment that alters the quality of life.6,7 Comparing the functional impairments of muscle function resulting from chronic ischemia between people with PAD and healthy people without PAD is not optimal because the clinical profiles and risk factors of both groups differ. Thus, previous studies have analyzed the biochemical or biomechanical consequences of PAD in patients with so-called ‘unilateral PAD’ and have used the contralateral limb as a control.8,9 In these studies of patients with unilateral claudication, the absence of symptoms and a normal ABI in the contralateral limb are generally considered diagnostic for the absence of exercise-induced ischemia in the contralateral control limb. PAD is a systemic disease and as a result, it is unlikely to be strictly unilateral, particularly when it results from atherosclerosis, the common cause of PAD.6
Although a PubMed search with the expression (‘asymptomatic myocardial ischemia’ OR ‘silent myocardial ischemia’) retrieves more than a thousand references, little is known on asymptomatic exercise-induced ischemia in the asymptomatic lower limb of patients with unilateral symptomatic PAD. The fact that PAD is rather an asymmetrical disease10 does not prove that the asymptomatic limb is devoid of significant exercise-induced ischemia. To date, no study has ever: (1) determined the prevalence of strictly unilateral claudication among patients referred for treadmill investigation of lower extremity exertional symptoms; or (2) estimated the prevalence and severity of exercise-induced ischemia in the asymptomatic limb of these patients with apparently unilateral PAD based on both unilateral symptoms and unilateral abnormal ABI recordings. We quantified exercise-induced ischemia in the asymptomatic limb of patients reporting unilateral claudication based on history-taking and treadmill evaluation and who had a unilateral ipsilateral low ABI (<0.90). Because arterial stiffness in diabetes increases the risk of ABI overestimation or incompressibility, we separately studied diabetic and nondiabetic patients.11,12 After excluding patients without exercise-induced ischemia in the symptomatic limb, we aimed to estimate the proportion of patients with asymptomatic ischemia in the apparently unaffected limb and to estimate the severity of ischemia in these asymptomatic limbs. Since diabetes is considered to potentially alter pain sensation in PAD patients, the analysis of patients was performed in diabetic and nondiabetic patients respectively.
Materials and methods
Studied population
A retrospective analysis was performed on treadmill tests performed between 1999 and August 2017. Since the end of 1999, all patients managed at our institution for claudication suspected to originate from lower extremity arterial disease undergo a treadmill test with exercise oximetry. We have used exercise oximetry as a tool to ascertain the presence of regional blood flow impairment during exercise. Exercise oximetry is of particular interest in exertional limb pain because the technique is independent of arterial stiffness or cardiac arrhythmia. It is also independent of the observer and overall, allows for the recording of both proximal and distal ischemia on both limbs simultaneously and throughout exercise and recovery.
Characteristics of patients, including age, sex, height and weight for calculating body mass index, usual treatments (among which the use of insulin and oral antidiabetic treatment allowed the separation of patients treated for diabetes from nondiabetic patients), ABI, symptoms via history and treadmill tests, and the results of the exercise oximetry were systematically collected from a database that has been fully authorized by the French National Liberty and Computer Authority (CNIL: Commission Nationale Informatique et Liberté). All patients were aware of the recording of their data and were offered the opportunity to refuse the use of their data for research purposes. The present study conformed to the principles of the Declaration of Helsinki and the study protocol was approved by the Ethics Committee of the University Hospital in Angers (reference no. 2016-86). As a retrospective analysis of routine clinical procedures and according to French law, this present study did not require individual patient’s approval.
Symptoms obtained by history
Before the treadmill test, we asked the patient to fill a French translation of the Edinburgh claudication questionnaire (ECQ).13,14 Self-reported symptoms were defined as ‘symptoms by history’ and analyzed limb by limb regardless of whether symptoms were proximal or distal.15
Resting-ABI measurements
If data were unavailable from a recent patient file, most patients underwent pressure measurements at rest by a specialized nurse to calculate the resting ABI with a handheld Doppler, according to recommendations, except for patients with arrhythmia, ankle ulcers, or limb amputation, and for those for whom the strict lying position was infeasible.4 The resting ABI was calculated by dividing the highest pressure of the limb (dorsalis pedis or posterior tibial pressures) by the highest arm pressure.4 For both limbs, the presence of PAD was based on a resting ABI ⩽ 0.90. An ABI ⩾ 1.30 on one or both limbs was considered poorly compressible resting ABI resulting from arterial stiffness.
Treadmill tests and symptoms on the treadmill
Usual walking speed was measured in the corridor between the waiting room and the treadmill test room. Patients walked on the treadmill under electrocardiographic monitoring and medical supervision. All treadmill tests used a grade (incline) of 10%. Most patients walked at 3.2 km/h. Patients that were unable to walk 10 m in less than 15 s at their usual walking speed in the corridor had their maximum speed fixed to 2 km/h. The protocol was stopped due to limiting symptoms, or in case of repeated ventricular arrhythmia, or abnormal ST segment depression. In the absence of limiting symptoms, the tests were stopped after 20 min (tests until 2009) or changed to 15 min for an incremental procedure (tests since 2010). Lower limb symptoms on the treadmill, when present, were encoded by the physician that performed the test using the ECQ and analyzed side by side, regardless of the localization (proximal or distal) of symptoms.
Exercise oximetry
All exercise-oximetry tests were performed according to a standard procedure described elsewhere.16–18 We used TCM400 (Radiometer, Denmark) until 2016 and PF6000 (Perimed, Sweden) since 2017, both using E5250 probes (Radiometer, Denmark). Exercise transcutaneous oximetry is strictly noninvasive and can be used simultaneously on both limbs and at the proximal and distal level. As such, although a surface technique, it allows for the accurate detection of exercise-induced regional blood flow impairment and has shown high accuracy compared to arteriography and computed tomography angiography.16,19,20 In brief, we systematically used five probes; one on each calf, one on each buttock, and one on the chest. The decrease from rest of oxygen pressure (DROP; changes in the limb minus changes in the chest from the pretest resting period) was calculated automatically by dedicated software for each limb probe. The minimal buttock or calf value of DROP (DROPmin) observed during exercise and recovery was used for the analysis. Normally, DROP remains close to zero in healthy people and in the absence of ischemia. In lower limb exercise-induced ischemia, DROP decreases during exercise. Lower DROPmin values are indicative of more severe ischemia. DROPmin values lower than −15 mmHg indicate the presence of significant regional exercise-induced ischemia at the buttock and calf level.16,19,20 An illustration of the procedure is shown in Figure 1 in a patient with unilateral calf ischemia.
Figure 1.
Illustration of the recording procedure. The Gray square is the walking period. In the upper left corner is a zoom on one probe fixed to the calf. Middle panels are results in absolute values on the 8 probes. Note from the lower panel that only the left calf reached significance. Also note on the lower panel, that the DROP min may occur either during (buttocks and left thigh) or following (calves and right thigh) the walking period.
Patient selection criteria
We selected adult patients (>18 years old) who complained of unilateral exertional limb pain (excluding asymptomatic patients or patients with bilateral symptoms). We defined symptomatic PAD as an ABI < 0.90 ipsilateral to symptoms reported by history. We excluded patients with nonavailable ABI, arterial stiffness (ABI > 1.30), or noncompressible ABI on one or both limbs. Thereafter, we selected only patients with symptomatic PAD on one side alone. Subsequently, we selected only the patients with reproducible symptoms in the treadmill test on the same side as obtained by history (patients reporting bilateral pain, contralateral, or no pain on the treadmill were not selected). Finally, since some patients could have been limited by nonvascular comorbid conditions and then show no ischemia on the symptomatic side, we selected only patients that had significant ischemia on exercise oximetry on the symptomatic side. Indeed we previously showed that up to 20% of patients with claudication and an abnormal ABI can have normal oximetry results as a consequence of nonvascular limitation.21 Thus, the highly selected remaining population had apparent unilateral PAD (based on ABI value), strictly unilateral symptoms (via history and treadmill evaluation and on the similar side), and confirmed exercise-induced ischemia on the symptomatic side (based on exercise oximetry). In these patients, we estimated the intensity of ischemia on the asymptomatic limb using the lowest DROPmin value of each limb (buttock or calf) and calculated the proportion of tests still revealing a significant exercise-induced ischemia (DROPmin < −15 mmHg) on the asymptomatic (both by history and on treadmill) and unaffected (normal ABI) limb.
Statistical analysis
Results are expressed as mean ± standard deviation or number of observations (percentage). Comparison of results observed in diabetic and non-diabetic patients was performed with the unpaired Student’s t test and Chi-squared test. Statistical analyses were performed using SYSTAT for Windows, version 15.0.1 (SPSS Inc., France). For all statistical tests, a two-tailed probability value of p < 0 .05 was used to indicate statistical significance.
Results
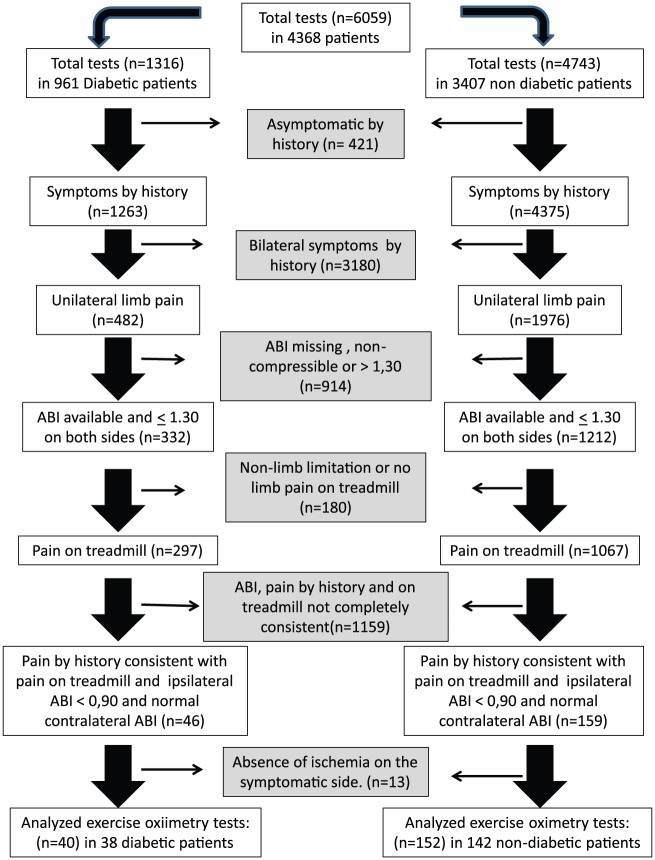
Among the 6059 tests performed on patients referred to the laboratory, 1316 were performed for 961 diabetic patients and 4743 were performed for 3407 nondiabetic patients. The flowchart of patient selection is presented in Figure 2. As shown in Figure 2, we had a large number of missing ABI values due to the following reasons: Doppler not available, patients unable to stay in the lying position, cardiac arrhythmia, amputation or ankle ulcer, or data unavailability (n = 853) or noncompressible ABI (n = 61), at least on one side. Note also on Figure 2 that, after removal of patients complaining of bilateral symptoms, most of the 1544 patients with available ABI that complained of unilateral pain demonstrated bilateral pain on the treadmill; few reported no limb pain or nonlimb pain, resulting in the further removal of 1159. Finally, we found only 180 patients (192 tests) that fulfilled the selection criteria, that is, patients with apparent strictly unilateral claudication (by history and on treadmill) and unilateral PAD (ABI abnormal on symptomatic side only) and with confirmed exercise-induced ischemia on the symptomatic side. This represents only 3% of all 6059 tests performed in our patients. Although this proportion may be underestimated due to the number of missing ABI values, this proportion is at best 12.4% of the 1544 tests performed in patients with unilateral claudication based on history alone and with available ABI. The average age of these strictly selected patients was comparable for the diabetic and nondiabetic patients. Most patients were men (83.8% and 81.6% for diabetic and nondiabetic patients, respectively), with only seven women in the diabetic group. The characteristics of these patients are shown in Table 1. A typical example of the exercise oximetry is presented in Figure 3. Results presented in Table 2 show that calf pain was the most frequent localization of the unilateral pain in the selected patients both by history and by treadmill testing; however, we also noted a relatively high prevalence of proximal pain (buttock or thigh). Table 3 shows the number of positive DROPs in the symptomatic and asymptomatic limbs
Figure 2.
Flow diagram of the patients in this analysis.
Table 1.
Baseline characteristics of the 180 studied patients.
| Nondiabetic patients | Diabetic patients | p value | |
|---|---|---|---|
| Age (years) | 59.9 ± 10.6 | 62.1 ± 9.7 | 0.240 |
| Male sex | 119 (83.8) | 31 (81.6) | 0.744 |
| Weight (kg) | 74.4 ± 15.7 | 81.1 ± 13.7 | 0.012 |
| Height (cm) | 169 ± 8 | 168 ± 7 | 0.335 |
| Body mass index (kg/m²) | 25.9 ± 5.3 | 28.6 ± 4.0 | <0.001 |
| Active smoking | 48 (33.8) | 11 (29.0) | 0.577 |
| Antiplatelet agent (n) | 120 (84.5) | 32 (84.2) | 0.964 |
| Antihypertensive drugs (n) | 79 (55.6) | 25 (65.8) | 0.260 |
| Cholesterol-lowering drug (n) | 89 (62.7) | 30 (79.0) | 0.60 |
| Beta blockers (n) | 37 (26.1) | 17 (44.7) | 0.026 |
| Systolic brachial pressure (mmHg) | 142 ± 20 | 141 ± 22 | 0.786 |
| Diastolic brachial pressure (mmHg) | 77 ± 8 | 76 ± 9 | 0.762 |
Results are presented as mean ± standard deviation or number of observations (%). p values are provided for each parameter. Significant differences between groups are marked with bold characters.
Figure 3.
Typical example of the exercise oximetry of one of the 180 selected patients (upper panel).
This non-diabetic 54-year-old female patient demonstrated unilateral right proximo-distal claudication via history and treadmill evaluation. ABI was 0.6 for the right limb and 1.0 for the left limb. An angioscan showed severe stenoses of the internal and external right iliac arteries(left middle panel) treated by angioplasty (right middle panel), and mild to moderate femoro-popliteal bilateral lesions As illustrated in the upper panel, exercise-oximetry showed significant buttock and severe thigh and calf ischemia and a significant asymptomatic contralateral calf ischemia. The grey rectangle corresponds to the walking period. In this patient, a recording was also available 3 months after angioplasties of iliac stenoses. Although not selected in the 192 finally selected tests, this second recording is of interest to show the effect of internal and external right angioplasty on exercise oximetry results (lower panel). Interestingly, the left calf ischemia seemed less pronounced than before revascularization, possibly as a result of regular training in this active patient.
Table 2.
ABI values at rest, pain on the symptomatic limb via history and treadmill evaluation, and exercise oximetry results of the 192 treadmill tests.
| Nondiabetic patients | Diabetic patients | p value | ||
|---|---|---|---|---|
| Pain localization by history | Buttock (n) | 48 (31.6) | 13 (32.5) | 0.911 |
| Thigh (n) | 58 (38.2) | 7 (17.5) | 0.014 | |
| Calf (n) | 124 (81.6) | 32 (80.0) | 0.820 | |
| Foot (n) | 8 (5.3) | 1 (2.5) | 0.462 | |
| Ankle-to-brachial index | Asymptomatic limb | 1.03 ± 0.09 | 1.03 ± 0.09 | 0.465 |
| Symptomatic limb | 0.67 ± 0.14 | 0.69 ± 0.15 | 0.935 | |
| Treadmill protocol | Standard procedure (n) | 147 (96.7) | 40 (100.0) | 0.245 |
| Maximal walking time (min) | 7.8 ± 6.9 | 7.0 ± 5.5 | 0.458 | |
| Exercise DROPmin |
Symptomatic side (mmHg) | −36 ± 13 | −37 ± 15 | 0.608 |
| Asymptomatic side (mmHg) | −18 ± 10 | −16 ± 10 | 0.510 | |
| Symptomatic buttock (mmHg) | −18 ± 12 | −16 ± 11 | 0.394 | |
| Symptomatic calf (mmHg) | −33 ±14 | −36 ± 16 | 0.270 | |
| Asymptomatic buttock (mmHg) | −15 ± 10 | −13 ± 9 | 0.347 | |
| Asymptomatic calf (mmHg) | −13 ± 8 | −14 ± 9 | 0.857 | |
| Ischemia in the asymptomatic limb (n) | 71 (46.7) | 15 (37.5) | 0.297 | |
| Pain localization on treadmill. | Buttock (n) | 63 (41.4) | 10 (25.0) | 0.657 |
| Thigh (n) | 14 (9.2) | 4 (10.0) | 0.879 | |
| Calf (n) | 135 (88.8) | 34 (85.0) | 0.508 | |
| Foot (n) | 1 (0.7) | 0 | 0.607 | |
Results are presented as mean ± standard deviation or number of observations (%). Ischemia was defined as DROPmin less than −15 mmHg. The standard procedure was a constant load (3.2 km/h, 10% slope) without (until 2009) or with (since 2010) subsequent incremental load in the absence of limitations during the constant load phase.
ABI, ankle-to-brachial index; DROP, decrease from rest of oxygen pressure; DROPmin, minimal buttock or calf value of DROP.
Table 3.
Presence of ischemia on the symptomatic limb and asymptomatic limb during exercise oximetry: results of the 180 treadmill tests.
| Positive DROP symptomatic limb |
||||
|---|---|---|---|---|
| Buttock | Calf | Calf and buttock | ||
| Asymptomatic limb | Neither calf nor buttock | 60 | 6 | 32 |
| Calf | 13 | 0 | 9 | |
| Buttock | 9 | 2 | 16 | |
| Buttock and calf | 8 | 1 | 24 | |
Ischemia was defined as DROPmin less than −15 mmHg.
DROP, decrease from rest of oxygen pressure; DROPmin, minimal buttock or calf value of DROP.
As expected from the strict selection process, among these patients, ischemia was relatively severe in the symptomatic limb but no difference was found between diabetic and nondiabetic patients. The contralateral asymptomatic limb showed significant ischemia in 46.7% and 37.5% of nondiabetic and diabetic patients, respectively, with the average DROPmin being half the DROPmin observed in the symptomatic limb in both nondiabetic and diabetic patients.
Discussion
The first novel finding of the present study is the very low prevalence of strictly unilateral PAD (based on symptoms obtained by history and treadmill testing, and the consistent ABI) in our patients. The second novel finding is the high prevalence of exercise-induced ischemia in the asymptomatic limb of these already highly selected patients, which was detected in 44.8% of our patients.
The performance of ABI in diagnosing PAD and the importance of ABI as a marker of perioperative vascular surgery risk or long-term prognosis even in asymptomatic patients are well known.6,22,23 In the selection process shown in Figure 1, we noted a relatively high prevalence of missing ABI and poorly or noncompressible ankle arteries compared to other studies in PAD patients at risk of arterial calcifications.24 This may be partly due to the incomplete recording of patient data and also to the fact that in our initial experience, many patients were referred for atypical claudication or claudication of doubtful vascular origin (ABI ⩽ 0.90). In case of resting ABI > 0.90, the American Heart Association practice guidelines for the management of patients with PAD stated that in patients with suspected PAD, post-exercise ABI or other investigations should be used.6 Nevertheless limits to the use of post-exercise ABI exist. Cohoon and colleagues underscored the problem of interpreting post-exercise ABI due to discrepancies between various definitions of post-exercise normal values.25 Further, ABI is not the optimal tool for detecting proximal exercise-induced ischemia.26 Thus, it may be helpful to use a technique other than ABI to detect asymptomatic ischemia. Using thallium scintigraphy, Duet and colleagues27 showed that 38% of apparently asymptomatic diabetic patients had perfusion defects but the eventual neuropathy resulting from diabetes may have masked the pain induced by exercise ischemia. Using the same technique in 36 diabetic male patients who had no evidence of PAD, Lin and colleagues found a significant impairment of perfusion reserve after exercise compared with 24 healthy age-matched nondiabetic men.28 Of interest is the fact that no apparent difference was found between diabetic and nondiabetic patients despite higher body mass index (BMI), weight, and prevalence of taking beta blockers. With technetium-99 m-labeled methoxy-isobutyl-isonitrile (99 mTc-MIBI) scintigraphy, Kuśmierek and colleagues showed that the stress and rest perfusion indices of thighs and calves were impaired in 22 asymptomatic patients with early atherosclerotic compared to findings in healthy people.29 Muscle metabolism and oxygen saturation can also be studied by magnetic resonance imaging30,31 or near-infrared spectroscopy, respectively.32–34 The former technique cannot be used routinely and the latter showed low accuracy for the detection of proximal exercise-induced ischemia.35 Overall, these studies underline the importance of detecting asymptomatic ischemia but none assessed the presence of ischemia in the asymptomatic limb of symptomatic patients as in the present study. Although a surface technique, transcutaneous oximetry can be used routinely to evaluate the regional mismatch of oxygen consumption and oxygen delivery and has been validated against angiography.16–18,36,37 Although resting absolute values lack reliability, DROP results are highly reliable in intra-test and test–retest recordings.38
Regarding our selection process, it is also noteworthy that we excluded 77% of the tests performed on patients with available ABI who showed pain on treadmill evaluation. Most tests were excluded on the basis of the ABI being abnormal on the asymptomatic side and also because treadmill testing did not reproduce the symptoms obtained via history as previously shown.39 Finally, one-third of the remaining patients showed no exercise-related ischemia in the symptomatic limb which could be interpreted as low sensitivity to exercise oximetry. In fact, we previously showed that these patients were generally limited by nonvascular comorbid conditions.40
The results of the present study are to be considered with respect to studies that attempt to analyze the effect of arterial claudication on biomechanical or biochemical factors. Because of chronic ischemia, impaired walking gait and muscle metabolism have largely been studied in symptomatic PAD patients with claudication.31,41–45 Little is known about asymptomatic PAD patients. Low ABI, even in asymptomatic patients, is associated not only with slow walking velocity, poor standing balance score, smaller calf muscle area, and higher calf muscle fat percentage but also with slower walking speed and fewer blocks walked per week.23,46 As underscored in these two just-mentioned studies, the absence of symptoms possibly results from PAD patients having low or reduced daily physical activity. Using the asymptomatic limb of patients with unilateral PAD appears an optimal option to evaluate the consequences of arterial impairment.8,9,47 Nevertheless, it should be considered that many patients have asymptomatic ischemia in this contralateral control limb.
Limitations
One limitation of the present study is that we cannot exclude that comorbid conditions may have interfered with the relationship between symptoms and ischemia, specifically diabetic neuropathy. The prevalence of diabetic patients in the final group was not different from that observed in the initial population and we would like to underline that the prevalence of asymptomatic ischemia is not higher in diabetic patients compared to non-diabetic patients. This probably relies on the fact that DROP value is independent from absolute resting value and then insensitive to potential microvascular dysfunction.
Another limitation is the long period of observation (17 years), with respect to changes that have occurred in our methodology over this period. Specifically, in patients with nonlimiting limb pain during the constant load phase (3.2 km/h, 10% slope) of the test, we stopped the test after 20 min until 2009, and since 2010, the test protocol changes to 15 min, with the speed and grade of the treadmill increased progressively. In this case, significant ischemia may occur as a result of increased oxygen demand leading to the exclusion of patients. However, 632 of 6059 (10.4%) tests involved an incremental phase in which 191 tests led to no lower limb symptoms.
In our study, the number of women was low as observed in our previous studies. There is no specific explanation for this but the over-representation of men in studies of PAD patients is a frequent observation. This was not a major issue in the global analysis but led to our excluding a sex-specific analysis that would have little meaningfulness and power, specifically in diabetic patients.
It is possible that some of the 13 patients excluded based on the absence of exercise-induced ischemia on the symptomatic limb might have had undiagnosed isolated thigh or foot ischemia since we did not perform systematic oximetry measurements in the foot and thigh in the present study. We recently added thigh probes to our routine buttock and calf recording; however, our findings showed that isolated thigh or foot ischemia, without buttock or calf associated ischemia, is rare.40
Lastly, the fact that patients with very slow usual walking speeds were subjected to a specific slow procedure on the treadmill could raise concerns. Indeed, this specific procedure has not been validated and could have been insufficient for inducing ischemia on the treadmill. However, this slow procedure resulted in significant ischemia in the symptomatic limb in the finally included patients. We believe that subjecting patients with slow walking speeds to the standard (3.2 mk/h) walking speed would have led to a large number of tests with nonlimb-limiting symptoms. Further, we would like to emphasize that this slow procedure was used in only 352 (5.8%) of the total 6059 tests (2.6% of the 192 studied tests).
Conclusion
Unilateral claudication in PAD patients is rare, especially if a strict selection of patients is based on (1) unilateral claudication as evidenced by history and treadmill testing; (2) unilateral consistently abnormal ABI; and (3) the presence of exercise-induced ischemia in the symptomatic limb. This finding is consistent with the concept of atherosclerosis being a systemic disease. However, among these highly selected patients, although ischemia is less severe in the asymptomatic than in the symptomatic limb, it still reaches a significant level in more than one-third of the cases. In view of our results, we advocate that when studying ‘unilateral PAD’, the contralateral limb should be referred to, at best, as the ‘less-affected limb’ and not as the ‘normal limb’ as previously suggested.48 Exercise oximetry seems useful for identifying the absence of ischemia or the presence of minimal ischemia in the asymptomatic limb, compared to the symptomatic ischemic limb. The test is a promising tool to optimally select the patients with true unilateral ischemia and then better analyze the pathophysiological consequences of arterial claudication. In clinical practice, one often encounters patients who undergo revascularization of one limb for severe claudication, only to find that the other side also needs treatment once the patient is treated on one side. Whether or not exercise oximetry could better account for this risk and help to better select the patients with unilateral symptoms that would require bilateral rather that unilateral treatment remains to be determined.
Acknowledgments
The authors thank Isabelle Laporte and Mathieu Feuilloy for their technical assistance. We would like to thank Editage (www.editage.com) for English language editing.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Pierre Abraham  https://orcid.org/0000-0001-7053-2801
https://orcid.org/0000-0001-7053-2801
Contributor Information
Samir Henni, Department of Vascular Investigation, University of Angers Hospital, France; UMR Mitovasc CNRS6015-INSERM 1083, University of Angers, France.
Pascal Bauer, Cardiology and Angiology, University Hospital Giessen, Germany.
Tanguy Le Meliner, Department of Vascular Investigation, University of Angers Hospital, France.
Jeanne Hersant, Department of Vascular Investigation, University of Angers Hospital, France.
Xavier Papon, Department of Vascular and thoracic Surgery, University of Angers Hospital, France.
Mickael Daligault, Department of Vascular and thoracic Surgery, University of Angers Hospital, France.
Jean-Marie Chretien, Department of Biostatistics and Data Management, University of Angers Hospital, France.
Myriam Ammi, Department of Vascular and thoracic Surgery, University of Angers Hospital, France.
Jean Picquet, Department of Vascular and thoracic Surgery, University of Angers Hospital, France; UMR Mitovasc CNRS6015-INSERM 1083, University of Angers, France.
Pierre Abraham, Laboratoire d’Explorations Vasculaires; Centre Hospitalier Universitaire, 49033 Angers Cedex 01, France.
References
- 1. Hiatt WR. Medical treatment of peripheral arterial disease and claudication. N Engl J Med 2001; 344: 1608–1621. [DOI] [PubMed] [Google Scholar]
- 2. Kroger K, Stang A, Kondratieva J, et al. Prevalence of peripheral arterial disease - results of the Heinz Nixdorf recall study. Eur J Epidemiol 2006; 21: 279–285. [DOI] [PubMed] [Google Scholar]
- 3. Criqui MH, Fronek A, Barrett-Connor E, et al. The prevalence of peripheral arterial disease in a defined population. Circulation 1985; 71: 510–515. [DOI] [PubMed] [Google Scholar]
- 4. Aboyans V, Criqui MH, Abraham P, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation 2012; 126: 2890–2909. [DOI] [PubMed] [Google Scholar]
- 5. Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 2007; 45(Suppl. S): S5–S67. [DOI] [PubMed] [Google Scholar]
- 6. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006; 113: e463–e654. [DOI] [PubMed] [Google Scholar]
- 7. McDermott MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA 2001; 286: 1599–1606. [DOI] [PubMed] [Google Scholar]
- 8. Wurdeman SR, Myers SA, Johanning JM, et al. External work is deficient in both limbs of patients with unilateral PAD. Med Eng Phys 2012; 34: 1421–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koutakis P, Pipinos II, Myers SA, et al. Joint torques and powers are reduced during ambulation for both limbs in patients with unilateral claudication. J Vasc Surg 2010; 51: 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Herraiz-Adillo A, Soriano-Cano A, Martinez-Hortelano JA, et al. Simultaneous inter-arm and inter-leg systolic blood pressure differences to diagnose peripheral artery disease: a diagnostic accuracy study. Blood Press 2017: 1–8. [Google Scholar]
- 11. Hyun S, Forbang NI, Allison MA, et al. Ankle-brachial index, toe-brachial index, and cardiovascular mortality in persons with and without diabetes mellitus. J Vasc Surg 2014; 60: 390–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tehan PE, Bray A, Chuter VH. Non-invasive vascular assessment in the foot with diabetes: sensitivity and specificity of the ankle brachial index, toe brachial index and continuous wave Doppler for detecting peripheral arterial disease. J Diabetes Complications 2016; 30: 155–160. [DOI] [PubMed] [Google Scholar]
- 13. Lacroix P, Aboyans V, Boissier C, et al. Validation of a French translation of the Edinburgh claudication questionnaire among general practitioners’ patients. Arch Mal Coeur Vaiss 2002; 95: 596–600. [PubMed] [Google Scholar]
- 14. Fowkes FG, Housley E, Cawood EH, et al. Edinburgh artery study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol 1991; 20: 384–392. [DOI] [PubMed] [Google Scholar]
- 15. Abraham P. Pain description in patients with isolated proximal (without distal) exercise-related lower limb arterial ischemia. J Physiol 2004; 9: 261–265. [DOI] [PubMed] [Google Scholar]
- 16. Audonnet M, Signolet I, Colas-Ribas C, et al. Exercise transcutaneous oximetry of the buttocks-external validation with computed tomography angiography. Circ J 2017; 81: 1123–1128. [DOI] [PubMed] [Google Scholar]
- 17. Abraham P, Picquet J, Bouye P, et al. Transcutaneous oxygen pressure measurements (tcpO2) at ankle during exercise in arterial claudication. Int Angiol 2005; 24: 80–88. [PubMed] [Google Scholar]
- 18. Abraham P, Picquet J, Vielle B, et al. Transcutaneous oxygen pressure measurements on the buttocks during exercise to detect proximal arterial ischemia: comparison with arteriography. Circulation 2003; 107: 1896–1900. [DOI] [PubMed] [Google Scholar]
- 19. Abraham P. Transcutaneous oxygen pressure measurements (tcpO2) at ankle during exercise in arterial claudication. Am J Physiol Heart Circ Physiol 2005; 24: 80–88. [PubMed] [Google Scholar]
- 20. Abraham P. Transcutaneous oxygen pressure measurements on the buttocks during exercise to detect proximal arterial ischemia: comparison with arteriography. Am J Physiol Regul Integr Comp Physiol 2003; 107: 1896–1900. [DOI] [PubMed] [Google Scholar]
- 21. Signolet I, Henni S, Colas-Ribas C, et al. Prevalence and causes of normal exercise oximetry in the calf in patients with peripheral artery disease and limiting calf claudication. Eur J Vasc Endovasc Surg 2016; 51: 572–578. [DOI] [PubMed] [Google Scholar]
- 22. Flu WJ, van Kuijk JP, Voute MT, et al. Asymptomatic low ankle-brachial index in vascular surgery patients: a predictor of perioperative myocardial damage. Eur J Vasc Endovasc Surg 2010; 39: 62–69. [DOI] [PubMed] [Google Scholar]
- 23. McDermott MM, Guralnik JM, Ferrucci L, et al. Asymptomatic peripheral arterial disease is associated with more adverse lower extremity characteristics than intermittent claudication. Circulation 2008; 117: 2484–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leskinen Y, Salenius JP, Lehtimaki T, et al. The prevalence of peripheral arterial disease and medial arterial calcification in patients with chronic renal failure: requirements for diagnostics. Am J Kidney Dis 2002; 40: 472–479. [DOI] [PubMed] [Google Scholar]
- 25. Cohoon KP, Mahe G, Liedl DA, et al. Discrepancies in prevalence of peripheral arterial disease between lower extremities at rest and postexercise. Int J Angiol 2017; 26: 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gernigon M, Marchand J, Ouedraogo N, et al. Proximal ischemia is a frequent cause of exercise-induced pain in patients with a normal ankle to brachial index at rest. Pain Physician 2013; 16: 57–64. [PubMed] [Google Scholar]
- 27. Duet M, Virally M, Bailliart O, et al. Whole-body (201)Tl scintigraphy can detect exercise lower limb perfusion abnormalities in asymptomatic diabetic patients with normal Doppler pressure indices. Nucl Med Commun 2001; 22: 949–954. [DOI] [PubMed] [Google Scholar]
- 28. Lin CC, Ding HJ, Chen YW, et al. Usefulness of thallium-201 muscle perfusion scan to investigate perfusion reserve in the lower limbs of Type 2 diabetic patients. J Diabetes Complications 2004; 18: 233–236. [DOI] [PubMed] [Google Scholar]
- 29. Kusmierek J, Dabrowski J, Bienkiewicz M, et al. Radionuclide assessment of lower limb perfusion using 99mTc-MIBI in early stages of atherosclerosis. Nucl Med Rev Cent East Eur 2006; 9: 18–23. [PubMed] [Google Scholar]
- 30. Taylor DJ, Amato A, Hands LJ, et al. Changes in energy metabolism of calf muscle in patients with intermittent claudication assessed by 31P magnetic resonance spectroscopy: a phase II open study. Vasc Med 1996; 1: 241–245. [DOI] [PubMed] [Google Scholar]
- 31. Kemp GJ, Hands LJ, Ramaswami G, et al. Calf muscle mitochondrial and glycogenolytic ATP synthesis in patients with claudication due to peripheral vascular disease analysed using 31P magnetic resonance spectroscopy. Clin Sci (Lond) 1995; 89: 581–590. [DOI] [PubMed] [Google Scholar]
- 32. Koutsiaris AG. Deep tissue near infrared second derivative spectrophotometry for the assessment of claudication in peripheral arterial disease. Clin Hemorheol Microcirc 2017; 65: 275–284. [DOI] [PubMed] [Google Scholar]
- 33. Manfredini F, Malagoni AM, Mandini S, et al. Near-infrared spectroscopy assessment following exercise training in patients with intermittent claudication and in untrained healthy participants. Vasc Endovascular Surg 2012; 46: 315–324. [DOI] [PubMed] [Google Scholar]
- 34. Komiyama T, Shigematsu H, Yasuhara H, et al. Near-infrared spectroscopy grades the severity of intermittent claudication in diabetics more accurately than ankle pressure measurement. Br J Surg 2000; 87: 459–466. [DOI] [PubMed] [Google Scholar]
- 35. Bouye P, Jacquinandi V, Picquet J, et al. Near-infrared spectroscopy and transcutaneous oxygen pressure during exercise to detect arterial ischemia at the buttock level: comparison with arteriography. J Vasc Surg 2005; 41: 994–999. [DOI] [PubMed] [Google Scholar]
- 36. Koch C, Chauve E, Chaudru S, et al. Exercise transcutaneous oxygen pressure measurement has good sensitivity and specificity to detect lower extremity arterial stenosis assessed by computed tomography angiography. Medicine (Baltimore) 2016; 95: e4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bouye P, Picquet J, Jaquinandi V, et al. Reproducibility of proximal and distal transcutaneous oxygen pressure measurements during exercise in stage 2 arterial claudication. Int Angiol 2004; 23: 114–121. [PubMed] [Google Scholar]
- 38. Henni S, Sempore YW, Le Meliner T, et al. Intra-test and test-retest reliability of exercise oximetry in arterial claudication. Microvasc Res 2018; 117: 44–49. [DOI] [PubMed] [Google Scholar]
- 39. Audat G, Harbonnier M, Ouedraogo N, et al. Comparison of reported symptoms to those produced by treadmill testing in patients with claudication suspected of arterial origin. Int Angiol 2014; 33: 379–383. [PubMed] [Google Scholar]
- 40. Henni S, Colas-Ribas C, Signolet I, et al. Multiprobe devices for exercise transcutaneous oxymetry in patients complaining claudication: interest and limits of unusual probe positions. Int Angiol 2016; 35: 557–564. [PubMed] [Google Scholar]
- 41. Gommans LNM, Smid AT, Scheltinga MRM, et al. Prolonged stance phase during walking in intermittent claudication. J Vasc Surg 2017; 66: 515–522. [DOI] [PubMed] [Google Scholar]
- 42. Myers SA, Pipinos II, Johanning JM, et al. Gait variability of patients with intermittent claudication is similar before and after the onset of claudication pain. Clin Biomech (Bristol, Avon) 2011; 26: 729–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gardner AW, Montgomery PS, Ritti-Dias RM, et al. The effect of claudication pain on temporal and spatial gait measures during self-paced ambulation. Vasc Med 2010; 15: 21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen SJ, Pipinos I, Johanning J, et al. Bilateral claudication results in alterations in the gait biomechanics at the hip and ankle joints. J Biomech 2008; 41: 2506–2514. [DOI] [PubMed] [Google Scholar]
- 45. Baum O, Torchetti E, Malik C, et al. Capillary ultrastructure and mitochondrial volume density in skeletal muscle in relation to reduced exercise capacity of patients with intermittent claudication. Am J Physiol Regul Integr Comp Physiol 2016; 310: R943–R951. [DOI] [PubMed] [Google Scholar]
- 46. McDermott MM, Fried L, Simonsick E, et al. Asymptomatic peripheral arterial disease is independently associated with impaired lower extremity functioning: the women’s health and aging study. Circulation 2000; 101: 1007–1012. [DOI] [PubMed] [Google Scholar]
- 47. England JD, Regensteiner JG, Ringel SP, et al. Muscle denervation in peripheral arterial disease. Neurology 1992; 42: 994–999. [DOI] [PubMed] [Google Scholar]
- 48. Bhat HK, Hiatt WR, Hoppel CL, et al. Skeletal muscle mitochondrial DNA injury in patients with unilateral peripheral arterial disease. Circulation 1999; 99: 807–812. [DOI] [PubMed] [Google Scholar]