Short abstract
Sex-based disparities have been identified in respiratory physiology, and in many chronic lung diseases including asthma, chronic obstructive pulmonary disease, and cystic fibrosis. The observed sex differences in lung disease prevalence and incidence have been linked to changes in circulating levels of sex hormones that start after puberty and that have been shown to affect physiological and immunological functions. While the exact roles of male and female sex hormones in these processes have not been fully elucidated, it is now evident that these can target many lung cell types and affect several functions of the respiratory system. In this mini-review, we have summarized seminal studies aimed to understand the effects of the most relevant male and female sex hormones (estrogens, progesterone, and androgens) and their receptors on lung function. Moreover, we have reviewed the known influences of sex hormones and of the hypothalamic–pituitary–gonadal axis in lung disease and immunity. Understanding the roles of sex hormones in the regulation of lung function and inflammation is the first step for the potential development of more effective therapeutic options to prevent and treat lung disease in men and women.
Impact statement
Sex-differences in the incidence and severity of inflammatory lung diseases have been recognized for years. Women of reproductive age are more likely to suffer from chronic lung disease, with higher mortality rates than men. Physiological changes in hormone levels such as those occurring during the menstrual cycle, pregnancy, and menopause have been associated with lung function changes and asthma symptoms. Despite this, the roles of sex hormones in the mechanisms associated with lung diseases have not been fully elucidated. This review summarizes basic and clinical studies of sex hormones as potential modulators of lung function and inflammation. The information obtained from sex-specific research on lung physiology and pathology will potentially help in the development of sex-specific therapeutics for inflammatory lung disease that may account for the hormonal status of the patient.
Keywords: Sex differences, lung immunity, lung disease, asthma, hormone, inflammation
Introduction
Inflammatory lung diseases affect a large proportion of the world’s population, and are a substantial health problem and a significant economic burden. It is estimated that these diseases affect more than 510 million people worldwide, with worsening of symptoms reported markedly higher in women as compared to men.1 A critical barrier in our ability to effectively treat women with lung disease and in preventing negative lung health effects is the gap of knowledge of sex-specific mechanisms of inflammation in the lung, and the contributions of sex hormones to these mechanisms. In the past decade, several clinical and basic science studies have been conducted in order to elucidate the specific roles that male and female sex hormones play in lung inflammatory processes.2,3 Here, we discuss the most recent literature available describing sex differences in inflammatory lung disease prevalence and outcomes, with a particular emphasis on asthma and COPD, which show a clear sexual dimorphism in prevalence and incidence.4,5 We also summarize the results from multiple studies focused on understanding the roles of sex hormones as modulators of lung function and inflammation using different experimental models.
Sex differences in lung diseases
Various lung disorders and cancers display gender disparities in their prevalence, severity, and/or outcome. For example, cystic fibrosis (CF), a genetic disorder associated with frequent respiratory infections, has been shown to have poorer outcomes and higher mortality in females than males, particularly in response to Pseudomonas aeruginosa infection.6 Intriguingly, idiopathic pulmonary arterial hypertension is also more predominant in females than males, while idiopathic pulmonary fibrosis disproportionately affects males.7–9 With regard to lung cancer, the leading cause of cancer deaths worldwide, the most recent reports indicate that while the disease is still more likely to occur in men than in women in the United States (1 in 15 men vs. 1 in 17 women), the rates of lung cancer in women are rising faster, and have recently exceeded rates for men in the younger populations.10,11 In addition, specific types of cancers such as malignant mesothelioma and adenocarcinoma are more common in men and in women, respectively.12 While studies continue to report statistical data on sex differences in the prevalence for these diseases, very few have explored the effects of sex hormones in their development and course. However, for inflammatory lung diseases such as asthma and chronic obstructive pulmonary disease (COPD), researchers have begun to explore in more detail the effects of male and female hormones in the mechanisms associated with their onset and progression.13
Asthma and COPD are the most prevalent inflammatory diseases of the lung, affecting a significant portion of the world’s population. The most recent Global Burden and Disease Study reported that in 2015 more than 174 million people had COPD and more than 358 million people suffered from asthma, resulting in 3.2 million and 0.4 million deaths, respectively.14 While both diseases impose a substantial public health burden in terms of impaired quality of life and mortality in men and women, a clear sexual dimorphism exists in their risk, prevalence, and severity.15–17 While asthma symptoms in children are more prevalent in boys than in girls, studies in adult populations more frequently report negative lung health outcomes for women than men, suggesting an involvement of female sex hormones in mediating these effects.18 The severity of asthma in men also increases later in life when testosterone levels decrease, suggesting a potential role of androgens in mediating asthma pathophysiology.19
Asthma is a chronic inflammatory disease that displays a notorious sexual dimorphism across the lifespan. Many epidemiological studies of childhood asthma have shown that prepubertal boys have more asthma than girls, especially at younger ages.20 In the United States, it is estimated that 9.2% of boys and 7.4% of girls under 18 years old currently suffer from asthma.21 Interestingly, these patterns are reversed after puberty, where asthma prevalence rates for women are almost twice as those for men (10.4% vs. 6.2% for women and men over 18 years of age, respectively).21 These statistics have led investigators to generate the hypothesis that hormonal changes starting in puberty contribute to asthma development. In this regard, studies showing variations in asthma symptoms and hospitalization rates throughout the menstrual cycle in adult women, and a decline in asthma incidence in women after menopause also support this hypothesis.22,23 This notion is further supported by studies showing that girls who undergo menarche at an earlier age have a higher risk of developing asthma after puberty than girls in which menarche occurs later.24
With regard to puberty, data on asthma prevalence indicated that in 2016 about 11.2% of the U.S. population ages 12–14 had asthma.25 A recent study in children and adolescents reported that the prevalence of asthma in adolescents (aged 13–14 years) was significantly higher than that in schoolchildren aged 6–7 years.26 This study also found that the severity of asthma in girls was higher than that in boys aged 13–14 years. In older adults, two phenotypes are usually observed: patients with longstanding asthma who develop additional airflow limitation, and those who develop late-onset asthma. In these patients, symptoms are frequently superimposed with age-related respiratory problems, making associations with hormonal factors difficult to isolate.27
For many years, COPD was considered a disease that primarily affected men.28 However, recently there has been a shocking increase in the number of women diagnosed with COPD, partially due to a rise in smoking rates in females over the past few decades.29,30 The most recent reports have also indicated that females have more severe disease with early onset and are more sensitive to tobacco exposure than men.30 While females tend to smoke less than men, they have a faster annual decay in lung function than male smokers, making them more prone to develop COPD.31 Interestingly, it has been shown that cigarette smoke exposure alters estrogen signaling in airway smooth muscle, which could contribute to increased airway contractility in women.32 In addition, women smokers are about twice as likely as men smokers to develop COPD, suggesting a role of female hormones in the mechanisms leading to COPD development.33 Despite these data, clinical studies conducted to date have not reported the effects of the menstrual cycle or hormone replacement therapy on COPD, nor sufficient evidence to support the effect of sex and gender in the overall response to COPD treatment.34,35 Overall, more research is needed to elucidate the mechanisms underlying the observed sex differences in COPD susceptibility and progression.
Sex hormones and lung disease
Both clinical studies and experimental evidence from mouse models have shown that female hormones such as estrogens and their metabolites (e.g. estradiol, estrone, estriol) can trigger lung inflammatory and allergic reactions, and male hormones such as testosterone usually play the opposite role.36,37 While most studies cited below have concentrated on the effects of estradiol, in this manuscript, we use the term “estrogen” to refer to all compounds. In the next few sections, we discuss recent work on the roles of hypothalamic, pituitary, and gonadal hormones in lung function, with a particular emphasis on lung inflammatory cells.
The HPG axis and the lung: Is there a crosstalk?
The hypothalamic–pituitary–gonadal (HPG) axis includes the hypothalamus, the pituitary gland, and the gonads. To control functions such as reproduction, neurohormones such as gonadotropin-releasing hormone (GnRH) are released from the hypothalamus and target the pituitary gland, which releases hormones including luteinizing hormone (LH) and follicle-stimulating hormone (FSH), that stimulate the gonads to produce sex hormones such as estrogen, progesterone, testosterone, activin, inhibin, and follistatin. While pathological or environmentally induced changes in the function of this axis may produce hormonal alterations that affect reproduction, they can also cause local and systemic effects on different systems such as the respiratory or immune system. In addition, sex steroid hormone receptors, and GnRH, LH, and activin receptors have been identified in many tissues including the lung, suggesting that alterations in serum hormones would potentially affect normal lung function.38 In this regard, clinical studies have shown that several lung function parameters actually vary throughout the women’s menstrual cycle phases,39 and during pregnancy.40,41 Studies conducted in mice have also hypothesized that estrogen usually acts as a pro-inflammatory agent, while androgens act as negative regulators of lung inflammation.42,43 While many studies have focused on sex steroid receptor-associated signaling pathways, the exact mechanisms underlying these effects remain unknown.44–49
With regard to lung disease and normal HPG axis function, an interesting fact is the observed reduction in asthma severity in menopausal women between ages 50 and 65.50 During the post-menopausal period, GnRH levels rise due to a dramatic decrease in sex hormone synthesis.51 This loss of hypothalamic feedback inhibition causes the increase of GnRH and gonadotropins levels following ovarian senescence.52 However, in a research study, post-menopausal women with asthma exhibited lower serum FSH and LH levels when compared to non-asthmatic post-menopausal women.53 This trend was also observed in women with preexisting lung disease.40 Despite these data, additional studies need to be performed to unveil the relationships between the HPG axis and respiratory function in the healthy and asthmatic lung. Additionally, studies have shown that neuroendocrine hormones external to the HPG axis also regulate lung diseases such as asthma and its pathogenesis.54 There is now evidence that the HPA (hypothalamic-pituitary-adrenal) axis, which largely controls glucocorticoid secretion, could be an important mediator of these effects, particularly due to the strong relationship between stress and immunity and the known roles of glucocorticoids in mediating the mechanisms of lung development and inflammation.55 A study of long-term systemic administration of exogenous glucocorticoids in subjects with lung disease showed inhibition of the HPA axis and decreased lung function.56 It has also been shown that secretion dysfunction of cortical hormones can aggravate asthma severity and make patients become more dependent on exogenous glucocorticoids, creating a vicious cycle that manifests in repeated asthma attacks.57
Little is known on the effects of lung disease on reproductive function in men and women, although a few studies have reported that asthma can actually affect pregnancy and fertility, mainly due to side effects of systemic inflammation.58–60 Overall, while the evidence on the physiological crosstalk between neuroendocrine and pulmonary systems has shown influences of hormones secreted by both the HPG and HPA on lung function, inflammatory processes, and lung disease, more research is needed to elucidate the mechanisms linking these processes.
Sex hormones and sex hormone receptors in the lung: Expression and function
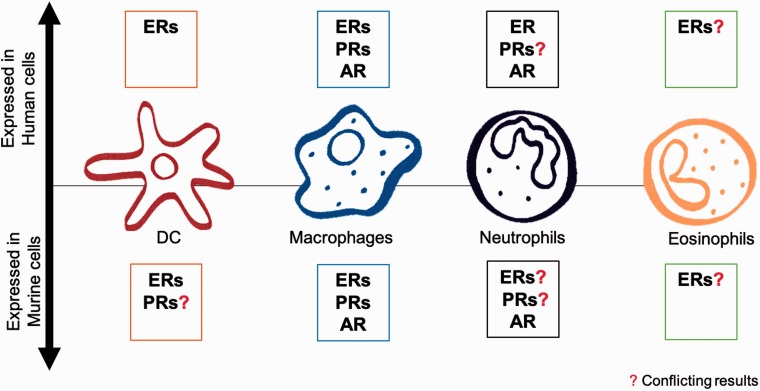
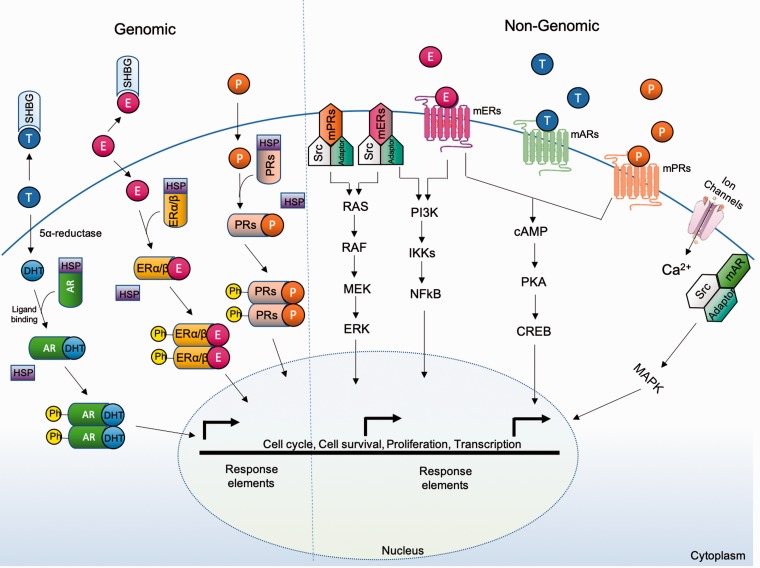
Male and female sex hormones mainly exert their effects through interactions with their receptors, whose expression has been detected in murine and human lung immune cells (Figure 1). These are: the estrogen receptors (ERs), the androgen receptor (AR), and the progesterone receptors (PRs). Their actions involve either direct control of transcription through binding of hormone-receptor complexes to gene promoter regions (genomic effects), or indirect regulation through activation of intracellular signaling cascades mediated by G proteins (non-genomic effects) that also result in transcriptional regulation (Figure 2).61
Figure 1.
Expression of sex hormone receptors in lung immune cells. (A color version of this figure is available in the online journal.)
Figure 2.
Summary of sex hormone receptors intracellular signaling pathways. (A color version of this figure is available in the online journal.)
A number of studies have suggested that sex hormones contribute to the overall lung inflammatory response to exogenous insults such as pollutants and infectious agents, as well as lung disease.62 Table 1 summarizes the known effects of sex hormones (estrogen, progesterone, and testosterone) on the expression of cytokines and inflammatory mediators in the lung. Table 2 summarizes the known influences of sex hormones on lung disease. While most of these effects have been attributed to gonadal hormones, it should be mentioned that the lung is able to synthesize sex hormones locally, and mediate local production of reactive oxygen species and/or undergo oxidative stress that can also contribute to inflammation, although more research in this subject needs to be conducted. This notion is supported by work in normal and cancerous lung cells indicating the expression of key enzymes in important metabolic pathways leading to estrogen synthesis, such as aromatase (CYP19A1), hydroxysteroid (17-beta) dehydrogenase type 1 (HSD17β1), steroid sulfatase (STS), and estrogen sulfotransferase (EST).63
Table 1.
Influence of sex hormones on cytokines and inflammatory mediators in the lung.
| Sex hormones | Up-regulation | Down-regulation/ Inhibition | Sources |
|---|---|---|---|
| Estrogen | IL-1β, IL-6, type I IFN,TNF-α, NF-κB, TLR8 | TGF-β1, IL-10 | 64,65 |
| Progesterone | IL-10, IL-1β, IL-5, IL-6, IL-22, TNFα, IL-4, Src/ p21, Erk, IL-9, IL-13 | NF-κB, TGF-β1, CTGF, TAGLN, PAI-1, IFN-γ | 45,51,66,67,68,69,70 |
| Testosterone | IL-2, IFNγ, Hbb-b1, Hbb-y, Hbq1 | IL-33, TSLP, IL-4, IL-5, IL-13, Angptl4, Cyp1a1 | 40,47,71,72,73,74 |
Table 2.
Influences of sex hormones on lung disease.
| Hormone effects |
||||
|---|---|---|---|---|
| Disease | Estrogen | Progesterone | Testosterone | Sources |
| Asthma | +/− | + | +/− | 2,24,67,75–80 |
| COPD | − | + | + | 28,81–84 |
| Cystic fibrosis | − | − | nd | 85–90 |
| Allergic rhinitis | − | − | +/− | 91–93 |
| Lung cancer | − | + | − | 94–97 |
Note: (+) positive effect, (−) negative effect, (+/v) conflictive data, (nd) no data.
Estrogen
The literature on estrogen effects on lung function and disease remains inconclusive.98 While some animal studies have shown that increases in circulating estrogen levels result in reduced innate immunity, others have reported pro-inflammatory effects.46 This may be partially due to the pleiotropic expression of the ERs and the multiple genomic and non-genomic effects of estrogen in various cell types.99 Some studies also reported that the expression of proinflammatory cytokines such as IL-1β, IL-6, type I interferon (IFN), tumor necrosis factor alpha (TNFα), and NF-κB in lung cells is strongly induced by estrogen.64 In the case of NF-κB, ERα can obstruct its intracellular trafficking mechanisms, thus inhibiting the expression of inflammatory genes and modulating immune function.100 On the other hand, a recent study reported that transforming growth factor beta1 (TGF-β1) represses the expression of ERα in bronchial epithelial cells.65
There are two classes of ERs: the nuclear ERs: ERα (ESR1) and ERβ (ESR2), and the membrane ERs: mERs (G protein-coupled ER 1, GPER/GPER30).101 Estrogen directly binds to the nuclear ERs, triggering receptor dimerization and binding to estrogen response elements (EREs) in the promoter region of target genes. Indirectly, ERs can also interact and form complexes with transcription factors in the nucleus.102 ERs are expressed in various types of immune cells, including lymphocytes, macrophages, and dendritic cells (DCs).103,104 In human lung tissue, ERα and ERβ have been found differentially expressed in asthmatic and non-asthmatic airway smooth muscle (ASM). Interestingly, ERβ expression is significantly greater in asthmatic ASM in both males and females.105 Moreover, ERβ inhibits platelet-derived growth factor (PDGF)-stimulated ASM proliferation.44
There is experimental evidence that the ERs are also involved in lung development. ERα modulates alveolar size and number, and induces alveolar regeneration after their loss in adult ovariectomized mice.106 Also, ER-β positively regulates the extracellular matrix initiating normal elastic tissue recoil in the lungs.107 More recently, it has been shown that the ERs are also under the control of microRNAs (miRNAs). For example, miRNA-221/222 has been reported to target ERα.108 In addition, ERα is upregulated by miR-625-5p and miRNA-22-3p in pediatric patients with dust mite-induced asthma.109 Interestingly, our recent work indicates that both sex and hormonal status can influence lung miRNA expression in ozone-induced lung inflammation.110 We have shown that miR-221/222 are differentially expressed in females exposed to ozone in proestrus, which is the stage of the mouse estrous cycle where estrogen levels are high. Other studies have also shown that estrogen down-regulates lung miRNA expression leading to up-regulation of target proto-oncogenes, cellular proliferation, and lung tumor development following cigarette-smoke exposure.111 Collectively, these studies have suggested that estrogens regulate multiple functions in the lung via interactions with the ERs and subsequent control of transcription, potentially influencing the outcomes of developmental, inflammatory, and disease processes. More research is needed to unveil the implicated mechanisms and identify potential points of intervention for lung disease.
Progesterone
While the regulatory effects of estrogen have been considerably researched, less is known about the role of progesterone, although both hormones may contribute to sex differences in lung immunity. Progesterone exerts its effect through the activation of the PRs. There are two PR isoforms conserved in murine and humans: PR-A and PR-B. These isoforms exhibit distinctive transcriptional patterns on progesterone response promoters.112 PR-B is the principal activator of gene transcription, while PR-A represses PR-B and ERs transcription.71,113 It has been shown that agonist-activated PR-B associates with ERs causing the activation of the Src/p21(ras)/Erk pathway.66
In terms of lung inflammation, studies have found that progesterone decreases contractility and increases relaxation of bronchial smooth muscle.67 Compared to estrogen and testosterone, it has the most powerful vasodilator effect in pulmonary arteries of both male and female rats.114 Moreover, progesterone stimulates the development of T-helper 1 cells and inflammatory cytokines such as of IL-10, IL-1β, IL-5, IL-6, IL-22, TNFα, and IL-4 in an allergic model of lung inflammation.68,69 In a study using a mouse model of influenza, treatment with progesterone reduced inflammation and improved lung function restoring tissue homeostasis.69 In humans, serum progesterone is positively associated with peak expiratory flow rate during the luteal phase of the menstrual cycle when the progesterone levels are high.115 Interestingly, PRs are expressed in the fibrotic areas of patients with usual interstitial pneumonia, but the mechanisms by which PRs exert these effects remain unknown.116
Testosterone
It has been known for decades that androgens control multiple physiological functions in the lung.117 Protective roles of testosterone have been proposed based on the associations of puberty Tanner stages with decreased symptoms observed in boys with asthma.118 Testosterone also causes bronchial tissue relaxation and reduces the response to histamine.119 In animal studies, testosterone has been shown to attenuate airway inflammation induced by Alternaria alternata and house dust mite extracts exposure, by diminishing a population of cells called lung group 2 innate lymphoid cells (ILC2), and by decreasing expression of IL-33 and thymic stromal lymphopoietin (TSLP), which are ILC2-stimulating cytokines.72
The AR regulates the activity and effects of male sex steroids, and is mainly expressed in the bronchial epithelium of the murine lung.73 The AR is a nuclear receptor that is activated by binding to testosterone or to its more active metabolite, 5α-dihydrotestosterone (DHT) in the cytoplasm, and then translocating into the nucleus where it acts as a DNA-binding transcription factor that controls gene expression. Because the AR is very similar to the PRs, it has been shown that, in higher dosages, PRs can actually antagonize the AR.120 The AR has also been reported to affect expression of lung gene expression and modulate function of lung immune cells. For example, mechanisms mediated by AR signaling result in decreased Th17 and Th2 cells via reduction of IL-4 production in the lung.74 Overall, studies exploring the roles of androgens in allergic airway inflammation have mainly demonstrated their protective and anti-inflammatory effects, but more studies are needed to elucidate the specific contributions of AR signaling in these mechanisms.
Influence of sex hormones in lung immune cells
Studies have shown that sex hormones can affect airway tone and inflammation, and exert effects in different lung cell types, including airway smooth muscle,121 and immune cells,48,122,123 although these effects are still under investigation. In the next paragraphs, we discuss the available literature on the effects of sex hormones on the key cell players of lung immunity. These include: lung macrophages, neutrophils, DCs, and eosinophils.
Macrophages
Alveolar macrophages (AMs) provide one of the first lines of defense in the lower airway and have emerged as important mediators of inflammation and tissue remodeling. AMs are professional phagocytes highly specialized in removal of foreign materials. It has been discovered that female mice treated with ovalbumin (OVA) have greater amounts of AMs in lung tissue than males, and that these cells increase allergic airway inflammation.124 Also, it has been suggested that sex hormones may regulate macrophage polarization states. Studies have shown that estrogen enhances the resolution phase (M2) gene expression, shortens the duration of the pro-inflammatory stage (M1), and thereby contributes to sex differences observed in asthma.125 On the other hand, progesterone but not estradiol can inhibit the release of microparticles, which are membrane-bound vesicles that display proinflammatory properties, by stimulated AMs.126 Studies associated with the role of testosterone in AMs are limited; however, researchers determined that testosterone reduces the production of TNFα and expression of nitric oxide in AMs.127,128
Neutrophils
Neutrophils are considered to be central to the pathogenesis of most forms of acute lung injury.129 We have previously shown that females displayed significantly higher neutrophil number than males in an ozone-induced lung inflammation model.130 Moreover, in a murine model of CF, scientists found a higher mortality rate and slower bacterial clearance in females than males, but this effect was reverted when neutropenia was induced in estrogen-treated ovariectomized mice. In addition, neutrophils treated with estrogen enhanced oxidative burst, but reduced bacterial killing in the lung independent of progesterone.131 Lastly, testosterone has been shown to decrease neutrophilic lung inflammation in an allergic asthma mouse model.132
DCs
DCs induce T-cell responses after migration to lymphoid tissue. There are a higher number of DCs migrating from the lungs to the lymph nodes in female mice sensitized with OVA than males. However, the effect of sex hormones on lung DCs has not been well studied. In studies not associated with the lung, estrogen has been shown to stimulate the functional DCs formation and DCs stimulation of T cells.133
Eosinophils
Eosinophils are key cellular participants in the development of allergic airway disease and asthma.134 In an OVA model, researchers demonstrated that females showed more severe eosinophilia than males. However, the effects were reversed after gonadectomy.135 Another study showed that estrogen treatment significantly enhanced eosinophil adhesiveness; however, treatment with both estrogen and progesterone induced degranulation.136 Treatment with only progesterone exacerbates eosinophilic airway inflammation and bronchial hyperreactivity. Contrarily, testosterone significantly reduced eosinophil adhesiveness and viability.70
Conclusion
From the literature reviewed above, it is evident that sex hormones play major roles in pulmonary diseases and lung immunity. This minireview emphasizes the significance of sex-specific research and the importance of taking into consideration sex and hormonal status as factors when studying respiratory physiology and disease. Overall, while opposite effects for male and female hormones have been generally found with regard to inflammatory mechanisms, studies remain inconclusive as to whether specific sex hormones exert pro- or anti-inflammatory effects in the lung. We believe that rather than direct actions of individual hormones, a balance of the contributions of multiple hormones and their receptors, as well as interactions of several pathways activated by these are likely to be responsible for the physiological outcomes and sex disparities observed in lung diseases. A better understanding of the roles of sex hormones in the control of lung function and inflammation will likely help in the development of more effective sex-specific preventative and therapeutic options for these diseases.
Authors’ contributions
All authors participated in the design, writing, and editing of the manuscript.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
This research was supported by funding from NIH K01HL133520 (PS), R03HL141618 (PS), Center for Research on Women and Newborn Health (PS) and the American Physiological Society Porter Physiology Development Program (NF).
References
- 1.Centers for Disease Control and Prevention. Asthma's Impact on the Nation, www.cdc.gov/asthma/impacts_nation/asthmafactsheet.pdf (accessed 19 November 2018)
- 2.Card JW, Zeldin DC. Hormonal influences on lung function and response to environmental agents: lessons from animal models of respiratory disease. Proc Am Thorac Soc 2009; 6:588–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Assaggaf H, Felty Q. Gender, estrogen, and obliterative lesions in the lung. Int J Endocrinol 2017; 2017:8475701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinkerton KE, Harbaugh M, Han MK, Jourdan Le Saux C, Van Winkle LS, Martin WJ, Kosgei RJ, Carter JE, Sitkin N, Smiley-Jewell SM, George M. Women and lung disease. Sex differences and global health disparities. Am J Respir Crit Care Med 2015; 192:11–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liptzin DR, Landau LI, Taussig LM. Sex and the lung: observations, hypotheses, and future directions. Pediatr Pulmonol 2015; 50:1159–69 [DOI] [PubMed] [Google Scholar]
- 6.Harness-Brumley CL, Elliott AC, Rosenbluth DB, Raghavan D, Jain R. Gender differences in outcomes of patients with cystic fibrosis. J Womens Health 2014; 23:1012–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thenappan T, Shah SJ, Rich S, Gomberg-Maitland M. A USA-based registry for pulmonary arterial hypertension: 1982-2006. Eur Respir J 2007; 30:1103–10 [DOI] [PubMed] [Google Scholar]
- 8.Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, Barst RJ, Benza RL, Liou TG, Turner M, Giles S, Feldkircher K, Miller DP, McGoon MD. Pulmonary arterial hypertension: baseline characteristics from the REVEAL registry. Chest 2010; 137:376–87 [DOI] [PubMed] [Google Scholar]
- 9.Raghu G, Chen SY, Yeh WS, Maroni B, Li Q, Lee YC, Collard HR. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001-11. Lancet Respir Med 2014; 2:566–72 [DOI] [PubMed] [Google Scholar]
- 10.Jemal A, Miller KD, Ma J, Siegel RL, Fedewa SA, Islami F, Devesa SS, Thun MJ. Higher lung cancer incidence in young women than young men in the United States. N Engl J Med 2018; 378:1999–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Cancer Society. Key statistics for lung cancer, https://cancerstatisticscenter.cancer.org
- 12.Hyland RA, Ware S, Johnson AR, Yates DH. Incidence trends and gender differences in malignant mesothelioma in New South Wales, Australia. Scand J Work Environ Health 2007; 33:286–92 [DOI] [PubMed] [Google Scholar]
- 13.Tam A, Morrish D, Wadsworth S, Dorscheid D, Man SF, Sin DD. The role of female hormones on lung function in chronic lung diseases. BMC Womens Health 2011; 11:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med 2015; 5:691–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grønseth R, Erdal M, Tan WC, Obaseki DO, Amaral AFS, Gislason T, Juvekar SK, Koul PA, Studnicka M, Salvi S, Burney P, Buist AS, Vollmer WM, Johannessen A. Unemployment in chronic airflow obstruction around the world: results from the BOLD study. Eur Respir J 2017; 50:1700499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Torres JP, Cote CG, López MV, Casanova C, Díaz O, Marin JM, Pinto-Plata V, de Oca MM, Nekach H, Dordelly LJ, Aguirre-Jaime A, Celli BR. Sex differences in mortality in patients with COPD. Eur Respir J 2009; 33:528–35 [DOI] [PubMed] [Google Scholar]
- 17.Falagas ME, Mourtzoukou EG, Vardakas KZ. Sex differences in the incidence and severity of respiratory tract infections. Respir Med 2007; 101:1845–63 [DOI] [PubMed] [Google Scholar]
- 18.Yung JA, Fuseini H, Newcomb DC. Hormones, sex, and asthma. Ann Allergy Asthma Immunol 2018; 120:488–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canguven O, Albayrak S. Do low testosterone levels contribute to the pathogenesis of asthma? Med Hypoth 2011; 76:585–8 [DOI] [PubMed] [Google Scholar]
- 20.La Grutta S, Tornatore M, Montalbano L, Antona R, Ferrante G, Malizia V. Gender differences in asthmatic children. Eur Resp J 2014; 44:P4117 [Google Scholar]
- 21.Centers for Disease Control and Prevention. Most Recent Asthma Data, www.cdc.gov/asthma/most_recent_data.htm (accessed 19 November 2018)
- 22.Postma DS. Gender differences in asthma development and progression. Gend Med 2007; 4(Suppl B):S133–46 [DOI] [PubMed] [Google Scholar]
- 23.Brenner BE, Holmes TM, Mazal B, Camargo CA. Relation between phase of the menstrual cycle and asthma presentations in the emergency department. Thorax 2005; 60:806–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salam MT, Wenten M, Gilliland FD. Endogenous and exogenous sex steroid hormones and asthma and wheeze in young women. J Allergy Clin Immunol 2006; 117:1001–7 [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Most Recent Asthma Data, www.cdc.gov/asthma/most_recent_data.htm (accessed 19 November 2018)
- 26.Mehravar F, Rafiee S, Bazrafshan B, Khodadost M. Prevalence of asthma symptoms in Golestan schoolchildren aged 6-7 and 13-14 years in Northeast Iran. Front Med 2016; 10:345–50 [DOI] [PubMed] [Google Scholar]
- 27.Hanania NA, King MJ, Braman SS, Saltoun C, Wise RA, Enright P, Falsey AR, Mathur SK, Ramsdell JW, Rogers L, Stempel DA, Lima JJ, Fish JE, Wilson SR, Boyd C, Patel KV, Irvin CG, Yawn BP, Halm EA, Wasserman SI, Sands MF, Ershler WB, Ledford DK. Asthma in Elderly workshop participants. Asthma in the elderly: current understanding and future research needs – a report of a National Institute on Aging (NIA) workshop. J Allergy Clin Immunol 2011; 128:S4–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camp PG, O'Donnell DE, Postma DS. Chronic obstructive pulmonary disease in men and women: myths and reality. Proc Am Thorac Soc 2009; 6:535–8 [DOI] [PubMed] [Google Scholar]
- 29.Chapman KR. Chronic obstructive pulmonary disease: are women more susceptible than men? Clin Chest Med 2004; 25:331–41 [DOI] [PubMed] [Google Scholar]
- 30.Sørheim IC, Johannessen A, Gulsvik A, Bakke PS, Silverman EK, DeMeo DL. Gender differences in COPD: are women more susceptible to smoking effects than men? Thorax 2010; 65:480–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gan WQ, Man SF, Postma DS, Camp P, Sin DD. Female smokers beyond the perimenopausal period are at increased risk of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Respir Res 2006; 7:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sathish V, Freeman MR, Long E, Thompson MA, Pabelick CM, Prakash YS. Cigarette smoke and estrogen signaling in human airway smooth muscle. Cell Physiol Biochem 2015; 36:1101–15 [DOI] [PubMed] [Google Scholar]
- 33.Barnes PJ. Sex differences in chronic obstructive pulmonary disease mechanisms. Am J Respir Crit Care Med 2016; 193:813–4 [DOI] [PubMed] [Google Scholar]
- 34.Lahm T. Sex differences in COPD. Eur Respir J 2009; 34:288–9 author reply 9. [DOI] [PubMed] [Google Scholar]
- 35.Robles PG, Brooks D, Goldstein R, Salbach N, Mathur S. Gender-associated differences in pulmonary rehabilitation outcomes in people with chronic obstructive pulmonary disease: a systematic review. J Cardiopulm Rehabil Prev 2014; 34:87–97 [DOI] [PubMed] [Google Scholar]
- 36.Wulfsohn NL, Politzer WM, Henrico JS. Testosterone therapy in bronchial asthma. South Afr Med J 1964; 38:170–2 [PubMed] [Google Scholar]
- 37.Fuseini H, Newcomb DC. Mechanisms driving gender differences in asthma. Curr Allergy Asthma Rep 2017; 17:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atwood CS, Bowen RL. A multi-hit endocrine model of intrinsic adult-onset asthma. Ageing Res Rev 2008; 7:114–25 [DOI] [PubMed] [Google Scholar]
- 39.Matteis M, Polverino F, Spaziano G, Roviezzo F, Santoriello C, Sullo N, Bucci MR, Rossi F, Polverino M, Owen CA, D'Agostino B. Effects of sex hormones on bronchial reactivity during the menstrual cycle. BMC Pulm Med 2014; 14:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Townsend EA, Miller VM, Prakash YS. Sex differences and sex steroids in lung health and disease. Endocr Rev 2012; 33:1–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.LoMauro A, Aliverti A. Respiratory physiology of pregnancy: physiology masterclass. Breathe 2015; 11:297–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carey MA, Card JW, Bradbury JA, Moorman MP, Haykal-Coates N, Gavett SH, Graves JP, Walker VR, Flake GP, Voltz JW, Zhu D, Jacobs ER, Dakhama A, Larsen GL, Loader JE, Gelfand EW, Germolec DR, Korach KS, Zeldin DC. Spontaneous airway hyperresponsiveness in estrogen receptor-alpha-deficient mice. Am J Respir Crit Care Med 2007; 175:126–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayashi T, Adachi Y, Hasegawa K, Morimoto M. Less sensitivity for late airway inflammation in males than females in BALB/c mice. Scand J Immunol 2003; 57:562–7 [DOI] [PubMed] [Google Scholar]
- 44.Ambhore NS, Katragadda R, Raju Kalidhindi RS, Thompson MA, Pabelick CM, Prakash YS, Sathish V. Estrogen receptor beta signaling inhibits PDGF induced human airway smooth muscle proliferation. Mol Cell Endocrinol 2018; 476:37–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kunzmann S, Ottensmeier B, Speer CP, Fehrholz M. Effect of progesterone on Smad signaling and TGF-β/Smad-regulated genes in lung epithelial cells. PLoS One 2018; 13:e0200661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keselman A, Fang X, White PB, Heller NM. Estrogen signaling contributes to sex differences in macrophage polarization during asthma. J Immunol 2017; 199:1573–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laffont S, Blanquart E, Savignac M, Cénac C, Laverny G, Metzger D, Girard JP, Belz GT, Pelletier L, Seillet C, Guery JC. Androgen signaling negatively controls group 2 innate lymphoid cells. J Exp Med 2017; 214:1581–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keselman A, Heller N. Estrogen signaling modulates allergic inflammation and contributes to sex differences in asthma. Front Immunol 2015; 6:568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sathish V, Martin YN, Prakash YS. Sex steroid signaling: implications for lung diseases. Pharmacol Ther 2015; 150:94–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zein JG, Erzurum SC. Asthma is different in women. Curr Allergy Asthma Rep 2015; 15:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raj MN, Suresh V, Mukka A, Reddy A, Sachan A, Mohan A, Vengamma B, Srinivas Rao PVLN. Evaluation of activity of hypothalamo-pituitary-gonadal axis in postmenopausal women suffering from severe acute illness. Ind J Med Res 2016; 143:66–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carr B. Williams textbook of endocrinology. Philadelphia, PA: WB Saunders Co, 1998 [Google Scholar]
- 53.Della Torre F, Cassani L, Segale M. Asma ed orticaria nell'anziano: ruolo degli ormoni ipofiso-gonadici in menopausa. Rassegna Geriatrica 1988; 24:165–71 [Google Scholar]
- 54.Dery RE, Ulanova M, Puttagunta L, Stenton GR, James D, Merani S, Mathison R, Davison J, Befus AD. Frontline: inhibition of allergen-induced pulmonary inflammation by the tripeptide feG: a mimetic of a neuro-endocrine pathway. Eur J Immunol 2004; 34:3315–25 [DOI] [PubMed] [Google Scholar]
- 55.Gibbs J, Ince L, Matthews L, Mei J, Bell T, Yang N, Saer B, Begley N, Poolman T, Pariollaud M, Farrow S, DeMayo F, Hussell T, Worthen GS, Ray D, Loudon A. An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nat Med 2014; 20:919–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Casale TB, Nelson HS, Stricker WE, Raff H, Newman KB. Suppression of hypothalamic-pituitary-adrenal axis activity with inhaled flunisolide and fluticasone propionate in adult asthma patients. Ann Allergy Asthma Immunol 2001; 87:379–85 [DOI] [PubMed] [Google Scholar]
- 57.Jiang YQ, Zhou ZX, Ji YL. Effects of the recombinant allergen rDer f 2 on neuro-endocrino-immune network in asthmatic mice. Cent Eur J Immunol 2014; 39:294–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Furlow B. Respiratory disease and fertility are linked-but why? Lancet Respir Med 2014; 2:28. [DOI] [PubMed] [Google Scholar]
- 59.Gade EJ, Thomsen SF, Lindenberg S, Kyvik KO, Lieberoth S, Backer V. Asthma affects time to pregnancy and fertility: a register-based twin study. Eur Respir J 2014; 43:1077–85 [DOI] [PubMed] [Google Scholar]
- 60.Cazzola M, Segreti A, Calzetta L, Rogliani P. Comorbidities of asthma: current knowledge and future research needs. Curr Opin Pulm Med 2013; 19:36–41 [DOI] [PubMed] [Google Scholar]
- 61.Filardo EJ, Thomas P. Minireview: G protein-coupled estrogen receptor-1, GPER-1: its mechanism of action and role in female reproductive cancer, renal and vascular physiology. Endocrinology 2012; 153:2953–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Silveyra P, Rivera L, Fuentes N. Understanding the intersection of environmental pollution, pneumonia, and inflammation: does gender play a role? In: Chroneos Z (ed) Contemporary topics of pneumonia. Croatia: InTechOpen Books, 2017, pp.3–34
- 63.Słowikowski BK, Lianeri M, Jagodziński PP. Exploring estrogenic activity in lung cancer. Mol Biol Rep 2017; 44:35–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol 2015; 294:63–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith LC, Moreno S, Robertson L, Robinson S, Gant K, Bryant AJ, Sabo-Attwood T. Transforming growth factor beta1 targets estrogen receptor signaling in bronchial epithelial cells. Respir Res 2018; 19:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Migliaccio A, Piccolo D, Castoria G, Di Domenico M, Bilancio A, Lombardi M, Gong W, Beato M, Auricchio F. Activation of the Src/p21ras/Erk pathway by progesterone receptor via cross-talk with estrogen receptor. EMBO J 1998; 17:2008–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Perusquía M, Hernández R, Montaño LM, Villalón CM, Campos MG. Inhibitory effect of sex steroids on guinea-pig airway smooth muscle contractions. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 1997; 118:5–10 [DOI] [PubMed] [Google Scholar]
- 68.de Oliveira AP, Peron JP, Damazo AS, Franco AL, Domingos HV, Oliani SM, Oliveira-Filho RM, Vargaftig BB, Tavares-de-Lima W. Female sex hormones mediate the allergic lung reaction by regulating the release of inflammatory mediators and the expression of lung E-selectin in rats. Respir Res 2010; 11:115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hall OJ, Limjunyawong N, Vermillion MS, Robinson DP, Wohlgemuth N, Pekosz A, Mitzner W, Klein SL. Progesterone-based therapy protects against influenza by promoting lung repair and recovery in females. PLoS Pathog 2016; 12:e1005840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hellings PW, Vandekerckhove P, Claeys R, Billen J, Kasran A, Ceuppens JL. Progesterone increases airway eosinophilia and hyper-responsiveness in a murine model of allergic asthma. Clin Exp Allergy 2003; 33:1457–63 [DOI] [PubMed] [Google Scholar]
- 71.Lamont KR, Tindall DJ. Androgen regulation of gene expression. Adv Cancer Res 2010; 107:137–62 [DOI] [PubMed] [Google Scholar]
- 72.Cephus JY, Stier MT, Fuseini H, Yung JA, Toki S, Bloodworth MH, Zhou W, Goleniewska K, Zhang J, Garon SL, Hamilton RG, Poloshukin VV, Boyd KL, Peebles RS, Jr, Newcomb DC. Testosterone attenuates group 2 innate lymphoid cell-mediated airway inflammation. Cell Rep 2017; 21:2487–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mikkonen L, Pihlajamaa P, Sahu B, Zhang FP, Jänne OA. Androgen receptor and androgen-dependent gene expression in lung. Mol Cell Endocrinol 2010; 317:14–24 [DOI] [PubMed] [Google Scholar]
- 74.Fuseini H, Yung JA, Cephus JY, Zhang J, Goleniewska K, Polosukhin VV, Peebles RS, Jr, Newcomb DC. Testosterone decreases house dust mite-induced type 2 and IL-17A-mediated airway inflammation. J Immunol 2018; 201:1843–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carey MA, Card JW, Voltz JW, Arbes SJ, Germolec DR, Korach KS, Zeldin DC. It's all about sex: gender, lung development and lung disease. Trends Endocrinol Metab 2007; 18:308–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schatz M, Camargo CA. The relationship of sex to asthma prevalence, health care utilization, and medications in a large managed care organization. Ann Allergy Asthma Immunol 2003; 91:553–8 [DOI] [PubMed] [Google Scholar]
- 77.Vrieze A, Postma DS, Kerstjens HA. Perimenstrual asthma: a syndrome without known cause or cure. J Allergy Clin Immunol 2003; 112:271–82 [DOI] [PubMed] [Google Scholar]
- 78.Chhabra SK. Premenstrual asthma. Ind J Chest Dis Allied Sci 2005; 47:109–16 [PubMed] [Google Scholar]
- 79.Dimitropoulou C, White RE, Ownby DR, Catravas JD. Estrogen reduces carbachol-induced constriction of asthmatic airways by stimulating large-conductance voltage and calcium-dependent potassium channels. Am J Respir Cell Mol Biol 2005; 32:239–47 [DOI] [PubMed] [Google Scholar]
- 80.Matsubara S, Swasey CH, Loader JE, Dakhama A, Joetham A, Ohnishi H, Balhorn A, Miyahara N, Takeda K, Gelfand EW. Estrogen determines sex differences in airway responsiveness after allergen exposure. Am J Respir Cell Mol Biol 2008; 38:501–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mousavi SA, Kouchari MR, Samdani-Fard SH, Gilvaee ZN, Arabi M. Relationship between serum levels of testosterone and the severity of chronic obstructive pulmonary disease. Tanaffos 2012; 11:32–5 [PMC free article] [PubMed] [Google Scholar]
- 82.Martinez FJ, Curtis JL, Sciurba F, Mumford J, Giardino ND, Weinmann G, Kazerooni E, Murray S, Criner GJ, Sin DD, Hogg J, Ries AL, Han M, Fishman AP, Make B, Hoffman EA, Mohsenifar Z, Wise R; National Emphysema Treatment Trial Research Group. Sex differences in severe pulmonary emphysema. Am J Respir Crit Care Med 2007; 176:243–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ino T, Aviado DM. Cardiopulmonary effects of progestational agents in emphysematous rats. Chest 1971; 59:659–66 [DOI] [PubMed] [Google Scholar]
- 84.Tyler JM. The effect of progesterone on the respiration of patients with emphysema and hypercapnia. J Clin Invest 1960; 39:34–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gurwitz D, Corey M, Francis PW, Crozier D, Levison H. Perspectives in cystic fibrosis. Pediatr Clin North Am 1979; 26:603–15 [DOI] [PubMed] [Google Scholar]
- 86.Davis PB. The gender gap in cystic fibrosis survival. J Gend Specif Med 1999; 2:47–51 [PubMed] [Google Scholar]
- 87.Rosenfeld M, Davis R, FitzSimmons S, Pepe M, Ramsey B. Gender gap in cystic fibrosis mortality. Am J Epidemiol 1997; 145:794–803 [DOI] [PubMed] [Google Scholar]
- 88.Johannesson M, Lúdvíksdóttir D, Janson C. Lung function changes in relation to menstrual cycle in females with cystic fibrosis. Respir Med 2000; 94:1043–6 [DOI] [PubMed] [Google Scholar]
- 89.Coakley RD, Sun H, Clunes LA, Rasmussen JE, Stackhouse JR, Okada SF, Fricks I, Young SL, Tarran R. 17beta-Estradiol inhibits Ca2+−dependent homeostasis of airway surface liquid volume in human cystic fibrosis airway epithelia. J Clin Invest 2008; 118:4025–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ajonuma L. Progesterone prevents ovarian hyperstimulation syndrome by suppressing cystic fibrosis transmembrane conductance regulator (CFTR) expression. Fertil Steril 2011; 96:S19–20 [Google Scholar]
- 91.Senthilselvan A, Rennie D, Chénard L, Burch LH, Babiuk L, Schwartz DA, Dosman JA. Association of polymorphisms of toll-like receptor 4 with a reduced prevalence of hay fever and atopy. Ann Allergy Asthma Immunol 2008; 100:463–8 [DOI] [PubMed] [Google Scholar]
- 92.PausJenssen ES, Cockcroft DW. Sex differences in asthma, atopy, and airway hyperresponsiveness in a university population. Ann Allergy Asthma Immunol 2003; 91:34–7 [DOI] [PubMed] [Google Scholar]
- 93.Fagan JK, Scheff PA, Hryhorczuk D, Ramakrishnan V, Ross M, Persky V. Prevalence of asthma and other allergic diseases in an adolescent population: association with gender and race. Ann Allergy Asthma Immunol 2001; 86:177–84 [DOI] [PubMed] [Google Scholar]
- 94.Recchia AG, Musti AM, Lanzino M, Panno ML, Turano E, Zumpano R, Belfiore A, Ando S, Maggliolini M. A cross-talk between the androgen receptor and the epidermal growth factor receptor leads to p38MAPK-dependent activation of mTOR and cyclinD1 expression in prostate and lung cancer cells. Int J Biochem Cell Biol 2009; 41:603–14 [DOI] [PubMed] [Google Scholar]
- 95.Ishibashi H, Suzuki T, Suzuki S, Niikawa H, Lu L, Miki Y, Moriya T, Hayashi S, Handa M, Kondo T, Sasano H. Progesterone receptor in non-small cell lung cancer – a potent prognostic factor and possible target for endocrine therapy. Cancer Res 2005; 65:6450–8 [DOI] [PubMed] [Google Scholar]
- 96.Márquez-Garbán DC, Chen HW, Fishbein MC, Goodglick L, Pietras RJ. Estrogen receptor signaling pathways in human non-small cell lung cancer. Steroids 2007; 72:135–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Albain KS, Belani CP, Bonomi P, O'Byrne KJ, Schiller JH, Socinski M. PIONEER: a phase III randomized trial of paclitaxel poliglumex versus paclitaxel in chemotherapy-naive women with advancedstage non-small-cell lung cancer and performance status of 2. Clin Lung Cancer 2006; 7:417–9 [DOI] [PubMed] [Google Scholar]
- 98.Shim B, Pacheco-Rodriguez G, Kato J, Darling TN, Vaughan M, Moss J. Sex-specific lung diseases: effect of oestrogen on cultured cells and in animal models. Eur Respir Rev 2013; 22:302–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cheskis BJ, Greger JG, Nagpal S, Freedman LP. Signaling by estrogens. J Cell Physiol 2007; 213:610–7 [DOI] [PubMed] [Google Scholar]
- 100.Ghisletti S, Meda C, Maggi A, Vegeto E. 17beta-estradiol inhibits inflammatory gene expression by controlling NF-kappaB intracellular localization. Mol Cell Biol 2005; 25:2957–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol 2011; 7:715–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Björnström L, Sjöberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol 2005; 19:833–42 [DOI] [PubMed] [Google Scholar]
- 103.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 2016; 16:626–38 [DOI] [PubMed] [Google Scholar]
- 104.Straub RH. The complex role of estrogens in inflammation. Endocr Rev 2007; 28:521–74 [DOI] [PubMed] [Google Scholar]
- 105.Aravamudan B, Goorhouse KJ, Unnikrishnan G, Thompson MA, Pabelick CM, Hawse JR, Prakash YS, Sathish V. Differential expression of estrogen receptor variants in response to inflammation signals in human airway smooth muscle. J Cell Physiol 2017; 232:1754–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Massaro D, Massaro GD. Estrogen regulates pulmonary alveolar formation, loss, and regeneration in mice. Am J Physiol Lung Cell Mol Physiol 2004; 287:L1154–9 [DOI] [PubMed] [Google Scholar]
- 107.Massaro D, Massaro GD. Estrogen receptor regulation of pulmonary alveolar dimensions: alveolar sexual dimorphism in mice. Am J Physiol Lung Cell Mol Physiol 2006; 290:L866–70 [DOI] [PubMed] [Google Scholar]
- 108.Cochrane DR, Cittelly DM, Howe EN, Spoelstra NS, McKinsey EL, LaPara K, Elias A, Yee D, Richer JK. MicroRNAs link estrogen receptor alpha status and Dicer levels in breast cancer. Horm Cancer 2010; 1:306–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dong X, Xu M, Ren Z, Gu J, Lu M, Lu Q, Zhong N. Regulation of CBL and ESR1 expression by microRNA-22‑3p, 513a-5p and 625-5p may impact the pathogenesis of dust mite-induced pediatric asthma. Int J Mol Med 2016; 38:446–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fuentes N, Roy A, Mishra V, Cabello N, Silveyra P. Sex-specific microRNA expression networks in an acute mouse model of ozone-induced lung inflammation. Biol Sex Differ 2018; 9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cohen A, Burgos-Aceves MA, Smith Y. A potential role for estrogen in cigarette smoke-induced microRNA alterations and lung cancer. Transl Lung Cancer Res 2016; 5:322–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shao R, Egecioglu E, Weijdegård B, Ljungström K, Ling C, Fernandez-Rodriguez J, Billig H. Developmental and hormonal regulation of progesterone receptor A-form expression in female mouse lung in vivo: interaction with glucocorticoid receptors. J Endocrinol 2006; 190:857–70 [DOI] [PubMed] [Google Scholar]
- 113.Giangrande PH, McDonnell DP. The A and B isoforms of the human progesterone receptor: two functionally different transcription factors encoded by a single gene. Recent Prog Horm Res 1999; 54:291–313 discussion 313–4 [PubMed] [Google Scholar]
- 114.English KM, Jones RD, Jones TH, Morice AH, Channer KS. Gender differences in the vasomotor effects of different steroid hormones in rat pulmonary and coronary arteries. Horm Metab Res 2001; 33:645–52 [DOI] [PubMed] [Google Scholar]
- 115.Mannan S, Begum N. Correlation of serum level of progesterone with peak expiratory flow rate (PEFR) in different phases of menstrual cycle. Anwer Khan Mod Med Coll J 2012; 3:6–9 [Google Scholar]
- 116.Mehrad M, Trejo Bittar HE, Yousem SA. Sex steroid receptor expression in idiopathic pulmonary fibrosis. Hum Pathol 2017; 66:200–5 [DOI] [PubMed] [Google Scholar]
- 117.Verma MK, Miki Y, Sasano H. Sex steroid receptors in human lung diseases. J Steroid Biochem Mol Biol 2011; 127:216–22 [DOI] [PubMed] [Google Scholar]
- 118.Fu L, Freishtat RJ, Gordish-Dressman H, Teach SJ, Resca L, Hoffman EP, Wang Z. Natural progression of childhood asthma symptoms and strong influence of sex and puberty. Ann Am Thorac Soc 2014; 11:939–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kouloumenta V, Hatziefthimiou A, Paraskeva E, Gourgoulianis K, Molyvdas PA. Non-genomic effect of testosterone on airway smooth muscle. Br J Pharmacol 2006; 149:1083–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kadel S, Kovats S. Sex hormones regulate innate immune cells and promote sex differences in respiratory virus infection. Front Immunol 2018; 9:1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Prakash YS. Emerging concepts in smooth muscle contributions to airway structure and function: implications for health and disease. Am J Physiol Lung Cell Mol Physiol 2016; 311:L1113–L40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Leffler J, Stumbles PA, Strickland DH. Immunological processes driving IgE sensitisation and disease development in males and females. Int J Mol Sci 2018; 19:pii: E1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rao CK, Moore CG, Bleecker E, Busse WW, Calhoun W, Castro M, Chung KF, Erzurum SC, Israel E, Curran-Everett D, Wenzel SE. Characteristics of perimenstrual asthma and its relation to asthma severity and control: data from the Severe Asthma Research Program. Chest 2013; 143:984–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Melgert BN, Oriss TB, Qi Z, Dixon-McCarthy B, Geerlings M, Hylkema MN, Ray A. Macrophages: regulators of sex differences in asthma? Am J Respir Cell Mol Biol 2010; 42:595–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Villa A, Rizzi N, Vegeto E, Ciana P, Maggi A. Estrogen accelerates the resolution of inflammation in macrophagic cells. Sci Rep 2015; 5:15224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pisetsky DS, Spencer DM. Effects of progesterone and estradiol sex hormones on the release of microparticles by RAW 264.7 macrophages stimulated by Poly(I:C). Clin Vaccine Immunol 2011; 18:1420–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rettew JA, Huet-Hudson YM, Marriott I. Testosterone reduces macrophage expression in the mouse of toll-like receptor 4, a trigger for inflammation and innate immunity. Biol Reprod 2008; 78:432–7 [DOI] [PubMed] [Google Scholar]
- 128.Savita, Rai U. Sex steroid hormones modulate the activation of murine peritoneal macrophages: receptor mediated modulation. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 1998; 119:199–204 [DOI] [PubMed] [Google Scholar]
- 129.Lee WL, Downey GP. Neutrophil activation and acute lung injury. Curr Opin Crit Care 2001; 7:1–7 [DOI] [PubMed] [Google Scholar]
- 130.Cabello N, Mishra V, Sinha U, DiAngelo SL, Chroneos ZC, Ekpa NA, Cooper TK, Caruso CR, Silveyra P. Sex differences in the expression of lung inflammatory mediators in response to ozone. Am J Physiol Lung Cell Mol Physiol 2015; 309:L1150–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Abid S, Xie S, Bose M, Shaul PW, Terada LS, Brody SL, Thomas PJ, Katzenellenbogen JA, Kim SH, Greenberg DE, Jain R. 17β-estradiol dysregulates innate immune responses to Pseudomonas aeruginosa respiratory infection and is modulated by estrogen receptor antagonism. Infect Immun 2017; 85:e00422–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fuseini H, Yung JA, Cephus JY, Zhang J, Goleniewska K, Polosukhin VV, Peebles RS, Jr, Newcomb DC. Testosterone decreases house dust mite-induced type 2 and IL-17A-mediated airway inflammation. J Immunol 2018; 201:1843–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Laffont S, Seillet C, Guéry JC. Estrogen receptor-dependent regulation of dendritic cell development and function. Front Immunol 2017; 8:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Calhoun WJ, Sedgwick J, Busse WW. The role of eosinophils in the pathophysiology of asthma. Ann N Y Acad Sci 1991; 629:62–72 [DOI] [PubMed] [Google Scholar]
- 135.Riffo-Vasquez Y, Ligeiro de Oliveira AP, Page CP, Spina D, Tavares-de-Lima W. Role of sex hormones in allergic inflammation in mice. Clin Exp Allergy 2007; 37:459–70 [DOI] [PubMed] [Google Scholar]
- 136.Hamano N, Terada N, Maesako K, Numata T, Konno A. Effect of sex hormones on eosinophilic inflammation in nasal mucosa. Allergy Asthma Proc 1998; 19:263–9 [DOI] [PubMed] [Google Scholar]