Short abstract
Mechanical ventilation is an essential intervention for intensive care unit patients with acute lung injury. However, the use of controlled mechanical ventilation in both animal and human models causes ventilator-induced diaphragm dysfunction, wherein a substantial reduction in diaphragmatic force-generating capacity occurs, along with structural injury and atrophy of diaphragm muscle fibers. Although diaphragm dysfunction, noted in most mechanically ventilated patients, is correlated with poor clinical outcome, the specific pathophysiology underlying ventilator-induced diaphragm dysfunction requires further elucidation. Numerous factors may underlie this condition in humans as well as animals, such as increased oxidative stress, calcium-activated calpain and caspase-3, the ubiquitin–proteasome system, autophagy–lysosomal pathway, and proapoptotic proteins. All these alter protein synthesis and degradation, thus resulting in muscle atrophy and impaired contractility and compromising oxidative phosphorylation and upregulating glycolysis associated with impaired mitochondrial function. Furthermore, infection combined with mechanical stretch may induce multisystem organ failure and render the diaphragm more sensitive to ventilator-induced diaphragm dysfunction. Herein, several major cellular mechanisms associated with autophagy, apoptosis, and mitochondrial biogenesis—including toll-like receptor 4, nuclear factor-κB, Src, class O of forkhead box, signal transducer and activator of transcription 3, and Janus kinase—are reviewed. In addition, we discuss the potential therapeutic strategies used to ameliorate ventilator-induced diaphragm dysfunction and thus prevent delay in the management of patients under prolonged duration of mechanical ventilation.
Impact statement
Mechanical ventilation (MV) is life-saving for patients with acute respiratory failure but also causes difficult liberation of patients from ventilator due to rapid decrease of diaphragm muscle endurance and strength, which is termed ventilator-induced diaphragmatic damage (VIDD). Numerous studies have revealed that VIDD could increase extubation failure, ICU stay, ICU mortality, and healthcare expenditures. However, the mechanisms of VIDD, potentially involving a multistep process including muscle atrophy, oxidative loads, structural damage, and muscle fiber remodeling, are not fully elucidated. Further research is necessary to unravel mechanistic framework for understanding the molecular mechanisms underlying VIDD, especially mitochondrial dysfunction and increased mitochondrial oxidative stress, and develop better MV strategies, rehabilitative programs, and pharmacologic agents to translate this knowledge into clinical benefits.
Keywords: Acute lung injury, mitochondria, nuclear factor-κB, endotoxemia, toll-like receptor 4, ventilator-induced diaphragm dysfunction
Clinical prevalence of ventilator-induced diaphragm dysfunction
Although mechanical ventilation (MV) is life saving for patients with acute lung injury (ALI), it causes weaning failures in approximately 20% of patients due to rapid deterioration of diaphragm muscle endurance and strength; this condition is called ventilator-induced diaphragm dysfunction (VIDD).1–4 In most of the intensive care unit (ICU) patients (80%), diaphragmatic dysfunction can occur on admission or during subsequent stay.4–6 Accumulating clinical evidence has revealed that VIDD aggravates ventilator-associated pneumonia, extubation failure, in-hospital mortality, ventilator dependence, and health costs.4–6 VIDD has a pathophysiology similar to ventilator-induced lung injury (VILI), which is characterized by diffuse inflammation and increased oxidative stress, ultimately leading to impaired gas exchange.7 However, the mechanism underlying VIDD—potentially involving a multistep process including oxidative stress, muscle weakness (arising from caspase-3, calpain, ubiquitin–proteasome system (UPS) activation, and autophagy-lysosomal pathway (ALP)), structural damage, and myofiber remodeling8–10—requires further elucidation. Therefore, a detailed knowledge of the molecular mechanisms underlying VIDD is crucial for designing potential strategies and reducing prolonged MV use, ICU stay, and thus ICU mortality.
Pathophysiology of VIDD
Diaphragmatic atrophy
A study on VIDD in humans demonstrated a substantial reduction (∼53%–57%) in the diameters of both slow-twitch and fast-twitch muscle fibers in 14 adults who met the brain-dead criteria for 18–69 h.11 Notably, in these patients, the pectoralis muscle was unaffected during the same period of dysfunction, suggesting that the rapid weakness was related to the diaphragm. Recent studies on ICU patients revealed that diaphragm thickness measured by ultrasound is associated with a lower daily probability of successful weaning, prolonged ICU stay, and high risk of ventilator-associated complications.4,6,12 In previous studies, the clinical impact of diaphragm atrophy was demonstrated, revealing that MV caused rapid onset of sarcomeric disarray, disuse atrophy, and impaired contractility in the diaphragm.11–14 Studies have indicated that diaphragm dysfunction causes diaphragm atrophy, whereas excess inspiratory efforts aggravate VILI and damage the diaphragm.13–15
Contractile dysfunction
Diaphragm strength is crucial in weaning patients from MV and transferring them to long-term care facilities; it also determines ICU mortality.16,17 A recent study indicated that during their ICU stay, approximately 80% of mechanically ventilated ICU patients demonstrated various patterns of ICU-acquired diaphragm weakness after the initial use of MV.4 In addition, diaphragm inactivity is found two times as frequent as limb inactivity in critically ill patients.16 Multimodal evaluation of the diaphragm (magnetic stimulation of the phrenic nerve, ultrasound measurement of the diaphragm excursion and thickening fraction, and maximal inspiratory pressure) in ICU patients revealed that diaphragm dysfunction is often in patients with ICU-acquired inactivity and is associated with a higher rate of weaning failure and ICU mortality.6
Oxidative stress
MV-induced oxidative stress in the diaphragm may impair diaphragm contractility and is a crucial signaling event leading to proteolytic pathway activation.13,18–21 During ALI, reactive oxygen species (ROS) are the primary oxidants in the diaphragm; they occur in mitochondria, sarcolemma, sarcoplasmic reticula, transverse tubes, and cytosol within 6 h of MV.10,18,19,22 Oxidative loads inactivate skeletal muscles through the interaction of different oxidant production pathways: (1) superoxide radical generation in mitochondria, (2) generation of hydroxyl radicals because of elevated cellular reactive iron levels, (3) NO production by nitric oxide synthase (NOS), and (4) ROS generation by xanthine oxidase.23,24 ROS disassembles proteins from the muscle fibers by activating calcium-activated proteases, such as caspase-3 and calpain, and degrades muscle proteins through the UPS.23,24 Elevation in intracellular calcium is a prerequisite for calpain activation. Oxidative stress can augment calpain cleavage of Z line-related proteins, such as titin and nebulin in diaphragmatic myofibers by dampening the activity of plasma membrane Ca2+-ATPase.25,26 These modifications may desensitize muscle fiber to calcium and increase intracellular calcium accumulation. Caspase-3, a cysteine protease upregulated by oxidative loads, can increase calpain activity and upregulate proapoptotic proteins to elicit intrinsic apoptosis correlated with mitochondrial abnormalities.27,28 Furthermore, the UPS deteriorates monomeric muscle fibers, which are liberated from actomyosin complexes after caspase-3 and calpain breakdown.29 However, there are contradictory findings regarding the mechanisms of developing diaphragm dysfunction in a clinical study of ventilated critically ill patients undergoing surgery.30 van den Berg et al.30 found that diaphragm muscle biopsies from these patients exhibited substantial atrophy and reduced contractility triggered by a redox imbalance, but lacked upregulated oxidative markers and impaired mitochondrial biogenetics. The authors explained the inconsistencies between their results and those from ventilated animals and brain-dead organ donors were attributed to different clinical features. Further clinical investigations are warranted to clarify the causative role of oxidative stress involved in the pathogenesis of VIDD.
Changes in proteolytic protein synthesis
The major proteases in the skeletal muscle include (1) lysosomal enzymes, (2) calcium-related proteases, and (3) the UPS.3,31 The lysosomal pathway is specific for the degradation of cytosolic proteins and organelles, including mitochondria and peroxisomes, whereas the last two are responsible for myofilament protein degradation.31 The connection of ubiquitin to protein substrates necessitates E1 enzymes, E2 carrier protein, and (in many cases) specific E3 enzymes.9,32 The 26S proteasome complex, consisting of a core 20S proteasome combined with a pair of 19S regulators, is activated after ubiquitin binding to protein substrates and labels them for degeneration. However, if the protein substrate is monoubiquitinated or diubiquitinated, then it is degraded through internalization and lysosomal transport. The activation of muscle-specific ubiquitin E3 ligases F-box protein atrogin-1 and muscle RING-finger proteins-1 (MuRF-1) is pivotal for the degradation of monomeric myofibrillar proteins in the diaphragms of animals and patients with MV; both proteins are modulated by transcription factor nuclear factor (NF)-κB or class O of forkhead box (FoxO).33 Moreover, according to a murine study, MuRF-1 plays a crucial role in regulating ALI-associated muscle atrophy.34
Intracellular signaling pathways
Relationships among VIDD, sepsis, autophagy, and apoptosis
Animal studies have indicated that infection is a primary cause of impaired diaphragm dysfunction.18,35,36 Sepsis was demonstrated to be a crucial risk factor for diaphragm dysfunction in ICU patients.37 A study on diaphragm contractility in mechanically ventilated ICU patients indicated that the union of infection and ventilator-mediated diaphragm weakness might induce sufficient diaphragm abnormalities to adversely affect patient outcomes, including higher mortality and longer weaning periods.17 Sepsis-enhanced diaphragmatic weakness and VIDD seem to have several molecular mechanisms, including elevated oxidative loads and mitochondrial dysregulation (mitochondrial biogenesis inhibition and increased mitochondrial permeability) within the diaphragm myofibrils, indicating that sepsis may be an accessory contributor for VIDD.17,18,38–40 Moreover, sepsis and MV-mediated oxidative stress may increase generation of inflammatory cytokines, including high mobility group box (HMGB) 1, interleukin (IL)-6, macrophage inflammatory protein (MIP)-2, and tumor necrosis factor (TNF)-α.18,20,27,41–43 These inflammatory mediators can suppress diaphragmatic contractility and exacerbate sepsis-induced systemic translocation via mechanisms including reduced protein synthesis, atrogin-1 and MuRF-1 induction, and signal transducer and activator of transcription (STAT) 3-myostatin pathway activation.41,44
Autophagy is a catabolic process marked by the expulsion of intracellular components, such as mitochondria, in the muscle fibers, and it is designated to regulate cell proliferation and death (or survival), innate and adaptive immune responses, and mitochondrial turnover.26,45,46 Mitochondria are a major source of diaphragmatic free radicals, a vital upstream mediator that starts the signaling pathways leading to diaphragm muscle atrophy during endotoxemia or mechanical stretch.47,48 The activity of electron transport chain isoform complexes II, III, and IV was reduced in mitochondria separated from the diaphragms of rats with 12 h of MV.49 Autophagy may prevent or promote the progress of pulmonary diseases by exerting its diverse functions. Although basal autophagy is crucial for regulating cell survival, uncontrolled autophagy enhances abnormalities, such as intrinsic apoptosis, muscle weakness, and mitochondrial morphological damage in the diaphragm of patients with sepsis.32,46 Animal investigations on VIDD have shown that MV augmented diaphragmatic weakness through excessive ROS generation by enhancing proteolysis and microtubule-related protein light chain (LC) 3.47,50,51 LC3-I becomes autophagosome-bound LC3-II by conjugating to phosphatidylethanolamine and upregulation of LC3-II expression is a biomarker of increased autophagosome formation.45,47 LC3-II accumulation in the diaphragm after MV could be majorly because of pathological abnormalities of autophagosome breakdown, rather than activation of the ALP.52 In particular, recent studies have demonstrated that increased autophagy is boosted by oxidative stress, resulting in selective degradation of the endogenous antioxidant catalase by eliminating peroxisomes and mitochondria, thus further increasing both ROS generation and autophagy.50,51
MV-induced elevation in mitochondrial ROS level is associated with oxidation of lipid and protein, inducing breakdown of mitochondrial structures.19,28,53 Liberation of cytochrome c from mitochondria to cytosol subsequently mediates apoptotic cell death.28,53 Sepsis may affect mitochondria by (1) generating free radicals and reactive nitrogen species, thus increasing lipid peroxidation and protein oxidation within mitochondria; (2) impairing perfusion of mitochondria, leading to tissue hypoxia, and triggering the cell death pathway; and (3) altering hormones and downregulating genes transcribing mitochondrial proteins.54 Moreover, myonuclear apoptosis can be induced by (1) mitochondrial ROS and elevated cellular calcium levels, (2) Fas ligand- and TNF-α receptor-mediated pathways, and (3) sarcoplasmic (endoplasmic) reticulum (SR) stress-induced activation of caspase-3 and calpain.21,28,47 Mitochondrial biogenesis is modulated principally at the transcriptional level and needs coordinated expression of both mitochondrial- and nuclear-encoded proteins, involving mitochondrial transcription factor A, nuclear respiratory factors 1 and 2, peroxisome proliferator-activated receptor coactivator (PGC)-1α, and 5ʹ-adenosine monophosphate-activated protein kinase.19,26,54,55 The downregulation of oxidative phosphorylation but upregulation of glycolysis, reduction in mitochondrial membrane potential, cytochrome c leak into the cytosol, and constitutive opening of mitochondrial pores have all been associated with apoptosis pathways.54,55
The NF-κB signaling pathway, a primary transcription factor for inflammatory cytokines, may increase diaphragm atrophy through transcriptional regulation of the manifestations of atrogin-1 and MuRF-1.13,21,27 In a murine endotoxemia study, transgenic (muscle-specific inhibitor IκBα super-repressor) mice induced with endotoxemia demonstrated that skeletal muscle fiber-specific inhibition of canonical NF-κB signaling prevents lipopolysaccharide (LPS)-induced diaphragmatic injury.27 In addition, NF-κB is pivotal in modulation of autophagy and the apoptotic pathway correlated with mitochondrial abnormalities in the diaphragm.21,28,47 Toll-like receptor (TLR)4 is the most thoroughly investigated receptors of the TLR family and is important for the recognition of damage-associated molecular patterns, involving HMGB1, extracellular matrix components, and LPS.43,56,57 Recent murine studies on inflammatory myopathies and Duchenne muscular dystrophy in the diaphragm have shown that HMGB1 could be an early inducer of skeletal muscle dysfunction by activating the TLR4 signaling pathway.57–59 Studies have revealed that stimulation of TLR4 pathway by LPS enhances the expression of cytokines, such as TNF-α, MIP-2, and IL-6 in skeletal muscles.37,44 Increases in free intracellular calcium levels in the calcium-dependent calpain and caspase-3 system have been identified in animal models of endotoxemia to amplify proinflammatory cytokines associated with diaphragm contractility.29,60
Murine studies on endotoxemia have exhibited that TLR4 regulates diaphragm inflammation and autophagy by activating the p38 mitogen-activated protein kinase (MAPK) or NF-κB pathways.57,59,61 In a myogenic cell line and murine study of endotoxemia, TLR4 activation augmented autophagosome generation in a p38 MAPK-dependent pathway.61 Sepsis-induced systemic inflammation may sensitize diaphragm stretch-related injuries by increasing sarcolemma membrane fragility. It may also increase disturbances in different steps of the muscular energy supply chain, including hypoxic ischemia and cytopathic ischemia, and it may directly impair contractile proteins through inflammatory cytokines.6,16,18 In a murine study of sepsis, TLR4 homozygous knockout could inhibit the ALP and attenuate mitochondrial ultrastructural changes by suppressing TLR4/NF-κB signaling.62
Relationships among VIDD, hyperoxia, and mitochondrial dysfunction
The management of ALI often requires the support of MV with high levels of oxygen to maintain adequate oxygenation of the brain and other vital organs. However, concurrent MV and hyperoxia may interact to worsen ALI and result in generation of inflammatory cytokines, including MIP-2, TNF-α, and plasminogen activator inhibitor (PAI)-1.63,64 Src, a critical nonreceptor protein tyrosine kinase serving in intracellular signal transduction, mediates leukocytes influx and acute inflammatory reactions triggered by oxidative stress.33,65 Mechanical stretch of C2C12 myoblasts upregulates p38 MAPK activity by activating Src-induced TNF-α-converting enzyme.66 In a murine model of VILI, Src plays a principal role in the activation of ROS formation and lung inflammation.67 Moreover, in a murine Duchenne muscular dystrophy model, persistent Src activation, which enhanced autophagy through phosphoinositide 3-OH kinase/serine/threonine kinase/protein kinase B (Akt) phosphorylation, was noted.68
In skeletal muscle in mdx mice, Src and Rac1 were shown to play important roles in eliciting ROS production via NADPH oxidase 2.68,69 The increased ROS in skeletal muscle unloading may activate the NF-κB and FoxO signaling pathways.13,70 FoxO1 is a mammalian FoxO transcription factor responsible for the regulation of cellular proliferation, apoptosis, and cell-cycle arrest.26,71–73 FoxOs activate atrophic proteins (i.e. atrogin-1 and MuRF-1) and autophagy-related proteins (B cell lymphoma (Bcl)-2 19-kilodalton interacting protein 3, cathepsin L, and LC3).20,26,50,51 In the unstimulated state, FoxO is phosphorylated by Akt, which inhibits FoxO transcriptional activity.22,73 However, MV suppresses FoxO1 phosphorylation mediated by Akt and translocates FoxO1 to the nucleus to trigger autophagy-related gene transcription in animal and human diaphragms.33,61 Although the activation of atrogin-1 and MuRF-1 is substantially upregulated by MV with hyperoxia, their expression is reduced by suppression of Src-dependent FoxO1 signaling.33 Moreover, FoxOs augment apoptotic signaling by upregulating the activity of Fas ligand and stimulating the members of the Bcl-2 family (e.g. the apoptosis facilitator gene, Bcl2-interacting mediator (Bim), which controls mitochondrial membrane permeability), as evidenced by loss of cytochrome c release and membrane potential in an isolated mitochondrial study.26,28,72,74 In particular, accumulated lipid level in human diaphragm during MV implicates accelerated glycolysis, which generate fatty acids converted from intermediate substances but suppresses fatty acid breakdown due to impaired mitochondrial function.19 In addition, p62, a biomarker of protein turnover, binds with polyubiquitinated proteins and LC3, functioning as a cargo receptor for the autolysosome degradation process.45–47 The role of mitochondrial dynamics and biogenesis in ALI is complex. In some studies, reduced mitochondrial membrane potential, mitochondrial fragmentation, and DNA damage are demonstrated in the diaphragms of both animals and patients subjected to MV.24,44,47–49,55 However, no alteration of mitochondrial bioenergetics and morphology is reported in a recent study of ICU patients.30 Further clinical trials are required to delineate this discrepancies.
Other signaling pathways
A recent work suggested that infection elicits cytokine production, resulting in cell-surface neutral sphingomyelinase receptors upregulation. The muscle ceramide metabolism is altered by the activation of these receptors, increasing mitochondrial ROS formation and triggering the generation of oxidative stress.60
Murine studies on endotoxemia have revealed that myostatin may induce muscle atrophy mediated by Smad3, atrogin-1, and FoxO3 signaling, and blocking the Akt/mammalian target of the rapamycin (mTOR) pathway. Furthermore, the muscle fibrosis-related PAI-1 and the mitochondrial biogenesis-related PGC-1α were inhibited by Smad3 activtion.55,75–77
Intracellular calcium overload in the diaphragm can trigger proinflammatory cytokine production and proteolysis in sepsis. Diaphragm contractility was recovered after curbing the release of HMGB1 by calcium antagonists in the septic diaphragm of mice.29
A murine model of cecal ligation puncture-induced sepsis demonstrated that skeletal muscle calcium-dependent phospholipase (cPL) A2 is upregulated by cytokines and connected with mitochondrial superoxide formation, and that cPLA2-induced ROS production induces calpain activation in skeletal muscle fibers.60
STAT3 and Janus kinase (JAK), constituting a signaling cascade activated by hormones, growth factors, and inflammatory cytokines via ligand–receptor interaction, can be rapidly phosphorylated or upregulated in a ventilated, inactive diaphragm.44 STAT3, an upstream inducer of mitochondria-derived ROS generation in the nucleus, can activate the expression of proteins, including Bim, uncoupling protein, and Cox5A, which decrease efficiency of ATP formation and mitochondrial membrane potential. Suppressing the JAK/STAT pathway substantially ameliorates oxidative loads, diaphragm inactivity, and proteolysis in rats.26,44,78,79
In both human and murine models of VIDD, MV promptly modulates the ryanodine receptor on the SR membrane through oxidation, S-nitrosylation, and Ser-2844 phosphorylation, causing the instability in the receptor complex and leading to calcium leakage.25 The dysregulated calcium homeostasis can result in impaired muscle contractility and reduction of muscle mass through activation of caspase-3, calpain, and oxidative stress,.26,35 Persistent elevated level of cytosolic calcium can also upregulate MAPKs, protein kinase C, and histone deacetylase (HDAC) 4. Calcium-dependent upregulation of protein phosphatases, including calcineurin, can also occur.80
Potential therapeutic strategies
Prolonged MV use can increase the risk of ventilator-associated pneumonia and lung fibrosis with restrictive ventilatory impairment; this not only causes physical and mental suffering but also affects quality of life and increases financial burden on patients and their families.40,81 Therefore, identifying effective clinical parameters and molecular mechanisms to facilitate liberating patients from long-term ventilator use is crucial.
Clinical care
Treating electrolyte imbalances and endocrine disorders, including hypoalbuminemia, hypophosphatemia, hypocalcemia, hypomagnesemia, hyperglycemia, severe untreated renal failure, and hypothyroidism, as well as avoiding neuromuscular blocking reagent overuse and sustained corticosteroid administration is of primary importance.39,40 Several animal and clinical studies have suggested that adjusting sedation and ventilation mode to keep appropriate levels of inspiratory muscle effort and reduce patient-ventilator asynchrony may minimize diaphragm atrophy.12,82 Furthermore, recent investigations have demonstrated that phrenic nerve stimulation may serve in increasing diaphragmatic activity during MV.15,83 A randomized clinical trial (ClinicalTrials.gov identifier: NCT03096639) is investigating the use of a temporary diaphragm pacing therapy system to facilitate liberating ventilated patients who have failed at least two weaning attempts.83
Antioxidants
In addition to diminishing oxidative loads, antioxidants may modulate the expression of proteolysis-related genes; for example, administration of high-dose vitamin E to animals alleviates the expression of several proteases, such as caspase-3 and calpain.24,84 Mounting evidence demonstrates that the use of antioxidants, such as N-acetylcysteine and trolox can ameliorate the detrimental effects on respiratory muscle function induced by controlled or prolonged MV.45,85,86 Treatment of animals with SS-31, a mitochondria-targeting antioxidant selectively functioned on the inner mitochondrial membrane, prevented rat diaphragms from prolonged MV-induced muscle atrophy by countering oxidative stress and protease activation.24,82
Theophylline
The molecular mechanisms of theophylline include (1) dilating airway smooth muscles by inhibiting phosphodiesterase-3 activity and antagonizing adenosine A1 and A2 receptors; (2) functioning as an anti-inflammatory agent by augmenting the effect of IL-10 and blocking the translocation of proinflammatory transcription factor NF-κB; and (3) enhancing HDAC2 activity (which is reduced by oxidative stress) to decrease peroxynitrite radical generation.84 In a rodent study, theophylline alleviated diaphragm atrophy and recovered the decrease of transdiaphragmatic pressure resulting from resistive loaded breathing in newborns.87 It also facilitated diaphragmatic perfusion by improving cardiac output and providing vasodilation in diaphragmatic arterioles.88 A retrospective cohort study disclosed that low-dose theophylline substantially improved contractility in the diaphragm without significant adverse drug reactions in patients admitted to medical ICU with VIDD.89
Other pharmacological agents
The calcium sensitizer levosimendan, a positive inotropic agent, has been applied to chronic obstructive pulmonary disease patients. It strengthens the muscle contractility of the diaphragm by increasing the calcium sensitivity of the contractile proteins.90,91 The agent may exert and energetically promote diaphragm contractility and mean that less calcium is required to maintain force generation.90,91 Furthermore, levosimendan can augment contractile function of the diaphragm in healthy humans performing inspiratory loading tasks.92 Further research using levosimendan to regain respiratory muscle function in mechanically ventilated patients is currently underway (ClinicalTrials.gov identifier NCT01727434).
The mTOR pathway is essential for the regulation of adipogenesis and muscle protein synthesis.93,94 Controlled MV increases lipid accumulation and deteriorates diaphragm contractility. These detrimental effects are partially blocked by the mTOR inhibitor rapamycin.95
Novel therapy
Considerable research on the use of stem cell therapy for ALI treatment is underway.64,96 Induced pluripotent stem cells (iPSCs) derived from human fibroblasts by delivering four reprogramming factors can differentiate into patient-specific progenitor cells and tissues for any of the three germ layers and facilitate personalized therapy in future clinical application.97 Li et al.33 demonstrated that hyperoxia-augmented VIDD can be attenuated by iPSC therapy through suppressing the Src-FoxO1 signaling pathway. The authors conducted a preclinical investigation by using iPSCs and iPSC-conditioned media to study the mechanisms and beneficial effects of stem cell therapy on combinatorial MV and hyperoxia-induced oxidative stress, proteolysis, apoptosis, autophagy, and functional impairment simulating the clinical scenario. Our results indicate that stem cell therapy may provide a novel therapeutic option for VIDD.33
Conclusion
Without adequate preventive and therapeutic strategies, a resting and inactive diaphragm muscle after prolonged MV may experience fast morphological and functional alterations, including accelerated protein degradation, muscle atrophy, and impaired contractile force. Numerous pathogenic mechanisms underlying the destructive effects of MV on diaphragmatic structure and contractility have been demonstrated in animal and human models of VIDD. However, these studies, performed in healthy animals, did not consider the effects of risk factors, such as sepsis and multisystem organ failure, in ICU patients. Because of the presence of multiple confounding factors, reaching a definitive diagnosis of VIDD in ICU patients is not easy; nevertheless, physicians should be careful of the occurrence of VIDD when a ventilated patient demonstrates poor progress during weaning trials in despite of clinical improvements in underlying diseases.39,40,98,99 Furthermore, the effort adaptive ventilation should be used as soon as possible to attenuate the deleterious effects of MV on the diaphragm. Further research on the mechanistic framework of this condition is required to understand the molecular mechanisms underlying VIDD (Figure 1), particularly mitochondrial dysfunction and increased mitochondrial ROS emission, and for developing further improved MV strategies, rehabilitative programs, and pharmacological agents to translate this knowledge into clinical benefits.
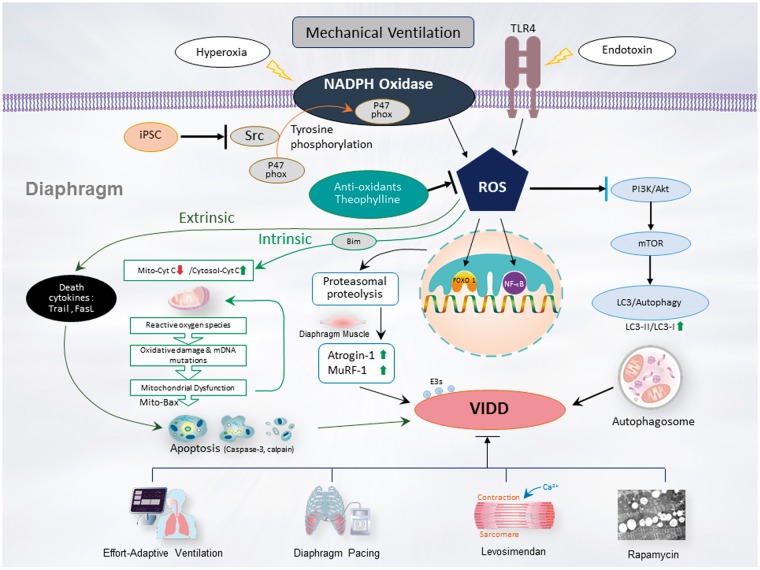
Figure 1.
Schematic of the signaling pathway implicated in VIDD development. Endotoxin- or hyperoxia-induced augmentation of mechanical stretch-mediated ROS generation and diaphragm injury are associated with diaphragm proteolysis, apoptosis, mitochondrial dysfunction, autophagy, as well as activation of the caspase-3, calpain, and ubiquitin–proteasome pathways. Diaphragm weakness can be attenuated by administering iPSCs, antioxidants, theophylline, levosimendan, or rapamycin, or through partial support MV or diaphragm pacing through PI3K/Akt, Src, and TLR4 pathway inhibition. Akt: serine/threonine kinase/protein kinase B; Bax: Bcl2-associated X; Bim: Bcl2-interacting mediator; Bnip3: Bcl-2 nineteen-kilodalton interacting protein 3; FoxO1: Class O of forkhead box1; iPSCs: Induced pluripotent stem cells; LC3: light chain 3; mTOR: mammalian target of rapamycin; MuRF-1: muscle ring finger-1; NADPH: nicotinamine adenine dinucleotide phosphate; NF-κB: nuclear factor kappa B; PI3-K: phosphoinositide 3-OH kinase; ROS: reactive oxygen species; TLR4: toll-like receptor 4; VIDD: ventilator-induced diaphragm dysfunction. (A color version of this figure is available in the online journal.)
ACKNOWLEDGEMENTS
The authors thank Chang-Hung Tien, Center for Big Data Analytics and Statistics, Chang Gung Memorial Hospital, Linkou and Wallace Academic Editing, for their help with the editing.
Authors’ contributions
LF-L collected the references. LF-L and YY-L reviewed the references and wrote the manuscript.
DECLARATION OF CONFLICTING INTERESTS
All authors have read the journal’s policy on disclosure of potential conflicts of interest and declared that no competing interests exist.
FUNDING
The study was supported by the Ministry of Science and Technology (107–2314-B-182A-143-MY3). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Dreyfuss D, Saumon G. Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med 1998; 157:294–323 [DOI] [PubMed] [Google Scholar]
- 2.Jubran A. Critical illness and mechanical ventilation: effects on the diaphragm. Respir Care 2006; 51:1054–61 discussion 62–4 [PubMed] [Google Scholar]
- 3.Petrof BJ, Jaber S, Matecki S. Ventilator-induced diaphragmatic dysfunction. Curr Opin Crit Care 2010; 16:19–25 [DOI] [PubMed] [Google Scholar]
- 4.Demoule A, Molinari N, Jung B, Prodanovic H, Chanques G, Matecki S, Mayaux J, Similowski T, Jaber S. Patterns of diaphragm function in critically ill patients receiving prolonged mechanical ventilation: a prospective longitudinal study. Ann Intensive Care 2016; 6:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dot I, Perez-Teran P, Samper MA, Masclans JR. Diaphragm dysfunction in mechanically ventilated patients. Arch Bronconeumol 2017; 53:150–6 [DOI] [PubMed] [Google Scholar]
- 6.Jung B, Moury PH, Mahul M, de Jong A, Galia F, Prades A, Albaladejo P, Chanques G, Molinari N, Jaber S. Diaphragmatic dysfunction in patients with ICU-acquired weakness and its impact on extubation failure. Intensive Care Med 2016; 42:853–61 [DOI] [PubMed] [Google Scholar]
- 7.Reiss LK, Uhlig U, Uhlig S. Models and mechanisms of acute lung injury caused by direct insults. Eur J Cell Biol 2012; 91:590–601 [DOI] [PubMed] [Google Scholar]
- 8.McClung JM, Van Gammeren D, Whidden MA, Falk DJ, Kavazis AN, Hudson MB, Gayan-Ramirez G, Decramer M, DeRuisseau KC, Powers SK. Apocynin attenuates diaphragm oxidative stress and protease activation during prolonged mechanical ventilation. Crit Care Med 2009; 37:1373–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vassilakopoulos T. Ventilator-induced diaphragm dysfunction: the clinical relevance of animal models. Intensive Care Med 2008; 34:7–16 [DOI] [PubMed] [Google Scholar]
- 10.Zergeroglu MA, McKenzie MJ, Shanely RA, Van Gammeren D, DeRuisseau KC, Powers SK. Mechanical ventilation-induced oxidative stress in the diaphragm. J Appl Physiol 2003; 95:1116–24 [DOI] [PubMed] [Google Scholar]
- 11.Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, Zhu J, Sachdeva R, Sonnad S, Kaiser LR, Rubinstein NA, Powers SK, Shrager JB. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med 2008; 358:1327–35 [DOI] [PubMed] [Google Scholar]
- 12.Goligher EC, Dres M, Fan E, Rubenfeld GD, Scales DC, Herridge MS, Vorona S, Sklar MC, Rittayamai N, Lanys A, Murray A, Brace D, Urrea C, Reid WD, Tomlinson G, Slutsky AS, Kavanagh BP, Brochard LJ, Ferguson ND. Mechanical ventilation-induced diaphragm atrophy strongly impacts clinical outcomes. Am J Respir Crit Care Med 2018; 197:204–13 [DOI] [PubMed] [Google Scholar]
- 13.Jaber S, Petrof BJ, Jung B, Chanques G, Berthet JP, Rabuel C, Bouyabrine H, Courouble P, Koechlin-Ramonatxo C, Sebbane M, Similowski T, Scheuermann V, Mebazaa A, Capdevila X, Mornet D, Mercier J, Lacampagne A, Philips A, Matecki S. Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med 2011; 183:364–71 [DOI] [PubMed] [Google Scholar]
- 14.Grosu HB, Lee YI, Lee J, Eden E, Eikermann M, Rose KM. Diaphragm muscle thinning in patients who are mechanically ventilated. Chest 2012; 142:1455–60 [DOI] [PubMed] [Google Scholar]
- 15.Reynolds SC, Meyyappan R, Thakkar V, Tran BD, Nolette MA, Sadarangani G, Sandoval RA, Bruulsema L, Hannigan B, Li JW, Rohrs E, Zurba J, Hoffer JA. Mitigation of ventilator-induced diaphragm atrophy by transvenous phrenic nerve stimulation. Am J Respir Crit Care Med 2017; 195:339–48 [DOI] [PubMed] [Google Scholar]
- 16.Jung B, Nougaret S, Conseil M, Coisel Y, Futier E, Chanques G, Molinari N, Lacampagne A, Matecki S, Jaber S. Sepsis is associated with a preferential diaphragmatic atrophy: a critically ill patient study using tridimensional computed tomography. Anesthesiology 2014; 120:1182–91 [DOI] [PubMed] [Google Scholar]
- 17.Supinski GS, Callahan LA. Diaphragm weakness in mechanically ventilated critically ill patients. Crit Care 2013; 17:R120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maes K, Stamiris A, Thomas D, Cielen N, Smuder A, Powers SK, Leite FS, Hermans G, Decramer M, Hussain SN, Gayan-Ramirez G. Effects of controlled mechanical ventilation on sepsis-induced diaphragm dysfunction in rats. Crit Care Med 2014; 42:e772–82 [DOI] [PubMed] [Google Scholar]
- 19.Picard M, Jung B, Liang F, Azuelos I, Hussain S, Goldberg P, Godin R, Danialou G, Chaturvedi R, Rygiel K, Matecki S, Jaber S, Des Rosiers C, Karpati G, Ferri L, Burelle Y, Turnbull DM, Taivassalo T, Petrof BJ. Mitochondrial dysfunction and lipid accumulation in the human diaphragm during mechanical ventilation. Am J Respir Crit Care Med 2012; 186:1140–9 [DOI] [PubMed] [Google Scholar]
- 20.Schellekens WJ, van Hees HW, Vaneker M, Linkels M, Dekhuijzen PN, Scheffer GJ, van der Hoeven JG, Heunks LM. Toll-like receptor 4 signaling in ventilator-induced diaphragm atrophy. Anesthesiology 2012; 117:329–38 [DOI] [PubMed] [Google Scholar]
- 21.Smuder AJ, Hudson MB, Nelson WB, Kavazis AN, Powers SK. Nuclear factor-kappaB signaling contributes to mechanical ventilation-induced diaphragm weakness*. Crit Care Med 2012; 40:927–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McClung JM, Kavazis AN, Whidden MA, DeRuisseau KC, Falk DJ, Criswell DS, Powers SK. Antioxidant administration attenuates mechanical ventilation-induced rat diaphragm muscle atrophy independent of protein kinase B (PKB Akt) signalling. J Physiol 2007; 585:203–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Powers SK, Duarte J, Kavazis AN, Talbert EE. Reactive oxygen species are signalling molecules for skeletal muscle adaptation. Exp Physiol 2010; 95:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powers SK, Hudson MB, Nelson WB, Talbert EE, Min K, Szeto HH, Kavazis AN, Smuder AJ. Mitochondria-targeted antioxidants protect against mechanical ventilation-induced diaphragm weakness. Crit Care Med 2011; 39:1749–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matecki S, Dridi H, Jung B, Saint N, Reiken SR, Scheuermann V, Mrozek S, Santulli G, Umanskaya A, Petrof BJ, Jaber S, Marks AR, Lacampagne A. Leaky ryanodine receptors contribute to diaphragmatic weakness during mechanical ventilation. Proc Natl Acad Sci U S A 2016; 113:9069–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang H, Shrager JB. The signaling network resulting in ventilator-induced diaphragm dysfunction. Am J Respir Cell Mol Biol 2018; 59:417–27 [DOI] [PubMed] [Google Scholar]
- 27.Okazaki T, Liang F, Li T, Lemaire C, Danialou G, Shoelson SE, Petrof BJ. Muscle-specific inhibition of the classical nuclear factor-kappaB pathway is protective against diaphragmatic weakness in murine endotoxemia. Crit Care Med 2014; 42:e501–9 [DOI] [PubMed] [Google Scholar]
- 28.Tang H, Lee M, Budak MT, Pietras N, Hittinger S, Vu M, Khuong A, Hoang CD, Hussain SN, Levine S, Shrager JB. Intrinsic apoptosis in mechanically ventilated human diaphragm: linkage to a novel Fos/FoxO1/Stat3-Bim axis. FASEB J 2011; 25:2921–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiu YW, Chen D, Xu MY, Li ST. Beneficial effects of dantrolene on sepsis-induced diaphragmatic dysfunction are associated with downregulation of high-mobility group box 1 and calpain-caspase-3 proteolytic pathway. J Surg Res 2016; 200:637–47 [DOI] [PubMed] [Google Scholar]
- 30.van den Berg M, Hooijman PE, Beishuizen A, de Waard MC, Paul MA, Hartemink KJ, van Hees HWH, Lawlor MW, Brocca L, Bottinelli R, Pellegrino MA, Stienen GJM, Heunks LMA, Wüst RCI, Ottenheijm CAC. Diaphragm atrophy and weakness in the absence of mitochondrial dysfunction in the critically ill. Am J Respir Crit Care Med 2017; 196:1544–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Powers SK, Wiggs MP, Sollanek KJ, Smuder AJ. Ventilator-induced diaphragm dysfunction: cause and effect. Am J Physiol Regul Integr Comp Physiol 2013; 305:R464–77 [DOI] [PubMed] [Google Scholar]
- 32.Powers SK, Smuder AJ, Fuller D, Levine S. CrossTalk proposal: mechanical ventilation-induced diaphragm atrophy is primarily due to inactivity. J Physiol 2013; 591:5255–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li LF, Chang YL, Chen NH, Wang CY, Chang GJ, Lin MC, Chang CH, Huang CC, Chuang JH, Yang YP, Chiou SH, Liu YY. Inhibition of Src and forkhead box O1 signaling by induced pluripotent stem-cell therapy attenuates hyperoxia-augmented ventilator-induced diaphragm dysfunction. Transl Res 2016; 173:131–47 [DOI] [PubMed] [Google Scholar]
- 34.Files DC, D'Alessio FR, Johnston LF, Kesari P, Aggarwal NR, Garibaldi BT, Mock JR, Simmers JL, DeGorordo A, Murdoch J, Willis MS, Patterson C, Tankersley CG, Messi ML, Liu C, Delbono O, Furlow JD, Bodine SC, Cohn RD, King LS, Crow MT. A critical role for muscle ring finger-1 in acute lung injury-associated skeletal muscle wasting. Am J Respir Crit Care Med 2012; 185:825–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaber S, Jung B, Matecki S, Petrof BJ. Clinical review: ventilator-induced diaphragmatic dysfunction–human studies confirm animal model findings! Crit Care 2011; 15:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chacon-Cabrera A, Rojas Y, Martinez-Caro L, Vila-Ubach M, Nin N, Ferruelo A, Esteban A, Lorente JA, Barreiro E. Influence of mechanical ventilation and sepsis on redox balance in diaphragm, myocardium, limb muscles, and lungs. Transl Res 2014; 164:477–95 [DOI] [PubMed] [Google Scholar]
- 37.Demoule A, Jung B, Prodanovic H, Molinari N, Chanques G, Coirault C, Matecki S, Duguet A, Similowski T, Jaber S. Diaphragm dysfunction on admission to the intensive care unit. Prevalence, risk factors, and prognostic impact-a prospective study. Am J Respir Crit Care Med 2013; 188:213–9 [DOI] [PubMed] [Google Scholar]
- 38.Petrof BJ. Diaphragmatic dysfunction in the intensive care unit: caught in the cross-fire between sepsis and mechanical ventilation. Crit Care 2013; 17:R181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Supinski GS, Morris PE, Dhar S, Callahan LA. Diaphragm dysfunction in critical illness. Chest 2018; 153:1040–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dres M, Goligher EC, Heunks LMA, Brochard LJ. Critical illness-associated diaphragm weakness. Intensive Care Med 2017; 43:1441–52 [DOI] [PubMed] [Google Scholar]
- 41.Demoule A, Divangahi M, Yahiaoui L, Danialou G, Gvozdic D, Labbe K, Bao W, Petrof BJ. Endotoxin triggers nuclear factor-kappaB-dependent up-regulation of multiple proinflammatory genes in the diaphragm. Am J Respir Crit Care Med 2006; 174:646–53 [DOI] [PubMed] [Google Scholar]
- 42.Jiang J, Yang B, Han G, Yang M, Li S. Early administration of cisatracurium attenuates sepsis-induced diaphragm dysfunction in rats. Inflammation 2015; 38:305–11 [DOI] [PubMed] [Google Scholar]
- 43.Tadie JM, Bae HB, Jiang S, Park DW, Bell CP, Yang H, Pittet JF, Tracey K, Thannickal VJ, Abraham E, Zmijewski JW. HMGB1 promotes neutrophil extracellular trap formation through interactions with Toll-like receptor 4. Am J Physiol Lung Cell Mol Physiol 2013; 304:L342–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang H, Smith IJ, Hussain SN, Goldberg P, Lee M, Sugiarto S, Godinez GL, Singh BK, Payan DG, Rando TA, Kinsella TM, Shrager JB. The JAK-STAT pathway is critical in ventilator-induced diaphragm dysfunction. Mol Med 2015; 20:579–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Azuelos I, Jung B, Picard M, Liang F, Li T, Lemaire C, Giordano C, Hussain S, Petrof BJ. Relationship between autophagy and ventilator-induced diaphragmatic dysfunction. Anesthesiology 2015; 122:1349–61 [DOI] [PubMed] [Google Scholar]
- 46.Nakahira K, Pabon Porras MA, Choi AM. Autophagy in pulmonary diseases. Am J Respir Crit Care Med 2016; 194:1196–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mofarrahi M, Sigala I, Guo Y, Godin R, Davis EC, Petrof B, Sandri M, Burelle Y, Hussain SN. Autophagy and skeletal muscles in sepsis. PLoS One 2012; 7:e47265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Picard M, Azuelos I, Jung B, Giordano C, Matecki S, Hussain S, White K, Li T, Liang F, Benedetti A, Gentil BJ, Burelle Y, Petrof BJ. Mechanical ventilation triggers abnormal mitochondrial dynamics and morphology in the diaphragm. J Appl Physiol 2015; 118:1161–71 [DOI] [PubMed] [Google Scholar]
- 49.Kavazis AN, Talbert EE, Smuder AJ, Hudson MB, Nelson WB, Powers SK. Mechanical ventilation induces diaphragmatic mitochondrial dysfunction and increased oxidant production. Free Radic Biol Med 2009; 46:842–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smuder AJ, Sollanek KJ, Nelson WB, Min K, Talbert EE, Kavazis AN, Hudson MB, Sandri M, Szeto HH, Powers SK. Crosstalk between autophagy and oxidative stress regulates proteolysis in the diaphragm during mechanical ventilation. Free Radic Biol Med 2018; 115:179–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hussain SN, Mofarrahi M, Sigala I, Kim HC, Vassilakopoulos T, Maltais F, Bellenis I, Chaturvedi R, Gottfried SB, Metrakos P, Danialou G, Matecki S, Jaber S, Petrof BJ, Goldberg P. Mechanical ventilation-induced diaphragm disuse in humans triggers autophagy. Am J Respir Crit Care Med 2010; 182:1377–86 [DOI] [PubMed] [Google Scholar]
- 52.Tang H, Lee M, Khuong A, Wright E, Shrager JB. Diaphragm muscle atrophy in the mouse after long-term mechanical ventilation. Muscle Nerve 2013; 48:272–8 [DOI] [PubMed] [Google Scholar]
- 53.Gogvadze V, Orrenius S, Zhivotovsky B. Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochim Biophys Acta 2006; 1757:639–47 [DOI] [PubMed] [Google Scholar]
- 54.Singer M. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence 2014; 5:66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cloonan SM, Choi AM. Mitochondria in lung disease. J Clin Invest 2016; 126:809–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boyd JH, Divangahi M, Yahiaoui L, Gvozdic D, Qureshi S, Petrof BJ. Toll-like receptors differentially regulate CC and CXC chemokines in skeletal muscle via NF-kappaB and calcineurin. Infect Immun 2006; 74:6829–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Giordano C, Mojumdar K, Liang F, Lemaire C, Li T, Richardson J, Divangahi M, Qureshi S, Petrof BJ. Toll-like receptor 4 ablation in mdx mice reveals innate immunity as a therapeutic target in Duchenne muscular dystrophy. Hum Mol Genet 2015; 24:2147–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grundtman C, Bruton J, Yamada T, Ostberg T, Pisetsky DS, Harris HE, Andersson U, Lundberg IE, Westerblad H. Effects of HMGB1 on in vitro responses of isolated muscle fibers and functional aspects in skeletal muscles of idiopathic inflammatory myopathies. FASEB J 2010; 24:570–8 [DOI] [PubMed] [Google Scholar]
- 59.Zong M, Bruton JD, Grundtman C, Yang H, Li JH, Alexanderson H, Palmblad K, Andersson U, Harris HE, Lundberg IE, Westerblad H. TLR4 as receptor for HMGB1 induced muscle dysfunction in myositis. Ann Rheum Dis 2013; 72:1390–9 [DOI] [PubMed] [Google Scholar]
- 60.Supinski GS, Alimov AP, Wang L, Song XH, Callahan LA. Calcium-dependent phospholipase A2 modulates infection-induced diaphragm dysfunction. Am J Physiol Lung Cell Mol Physiol 2016; 310:L975–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Doyle A, Zhang G, Abdel Fattah EA, Eissa NT, Li YP. Toll-like receptor 4 mediates lipopolysaccharide-induced muscle catabolism via coordinate activation of ubiquitin-proteasome and autophagy-lysosome pathways. FASEB J 2011; 25:99–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li LF, Liu YY, Chen NH, Chen YH, Huang CC, Kao KC, Chang CH, Chuang LP, Chiu LC. Attenuation of ventilation-induced diaphragm dysfunction through toll-like receptor 4 and nuclear factor-kappaB in a murine endotoxemia model. Lab Invest 2018; 98:1170–83 [DOI] [PubMed] [Google Scholar]
- 63.Liu YY, Liao SK, Huang CC, Tsai YH, Quinn DA, Li LF. Role for nuclear factor-kappaB in augmented lung injury because of interaction between hyperoxia and high stretch ventilation. Transl Res 2009; 154:228–40 [DOI] [PubMed] [Google Scholar]
- 64.Li LF, Liu YY, Yang CT, Chien Y, Twu NF, Wang ML, Wang CY, Huang CC, Kao KC, Hsu HS, Wu CW, Chiou SH. Improvement of ventilator-induced lung injury by IPS cell-derived conditioned medium via inhibition of PI3K/Akt pathway and IP-10-dependent paracrine regulation. Biomaterials 2013; 34:78–91 [DOI] [PubMed] [Google Scholar]
- 65.Oyaizu T, Fung SY, Shiozaki A, Guan Z, Zhang Q, dos Santos CC, Han B, Mura M, Keshavjee S, Liu M. Src tyrosine kinase inhibition prevents pulmonary ischemia-reperfusion-induced acute lung injury. Intensive Care Med 2012; 38:894–905 [DOI] [PubMed] [Google Scholar]
- 66.Niu A, Wen Y, Liu H, Zhan M, Jin B, Li YP. Src mediates the mechanical activation of myogenesis by activating TNFalpha-converting enzyme. J Cell Sci 2013; 126:4349–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu YY, Li LF, Fu JY, Kao KC, Huang CC, Chien Y, Liao YW, Chiou SH, Chang YL. Induced pluripotent stem cell therapy ameliorates hyperoxia-augmented ventilator-induced lung injury through suppressing the Src pathway. PLoS One 2014; 9:e109953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pal R, Palmieri M, Loehr JA, Li S, Abo-Zahrah R, Monroe TO, Thakur PB, Sardiello M, Rodney GG. Src-dependent impairment of autophagy by oxidative stress in a mouse model of Duchenne muscular dystrophy. Nat Commun 2014; 5:4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lam GY, Huang J, Brumell JH. The many roles of NOX2 NADPH oxidase-derived ROS in immunity. Semin Immunopathol 2010; 32:415–30 [DOI] [PubMed] [Google Scholar]
- 70.Levine S, Biswas C, Dierov J, Barsotti R, Shrager JB, Nguyen T, Sonnad S, Kucharchzuk JC, Kaiser LR, Singhal S, Budak MT. Increased proteolysis, myosin depletion, and atrophic AKT-FOXO signaling in human diaphragm disuse. Am J Respir Crit Care Med 2011; 183:483–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pardo PS, Lopez MA, Boriek AM. FOXO transcription factors are mechanosensitive and their regulation is altered with aging in the respiratory pump. Am J Physiol Cell Physiol 2008; 294:C1056–66 [DOI] [PubMed] [Google Scholar]
- 72.Smuder AJ, Sollanek KJ, Min K, Nelson WB, Powers SK. Inhibition of forkhead boxO-specific transcription prevents mechanical ventilation-induced diaphragm dysfunction. Crit Care Med 2015; 43:e133–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 2004; 14:395–403 [DOI] [PubMed] [Google Scholar]
- 74.Sugiyama T, Shimizu S, Matsuoka Y, Yoneda Y, Tsujimoto Y. Activation of mitocondrial voltage-dependent anion channel by a pro-apoptotic BH3-only protein Bim. Oncogene 2002; 21:4944–56 [DOI] [PubMed] [Google Scholar]
- 75.Goodman CA, McNally RM, Hoffmann FM, Hornberger TA. Smad3 induces atrogin-1, inhibits mTOR and protein synthesis, and promotes muscle atrophy in vivo. Mol Endocrinol 2013; 27:1946–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stana F, Vujovic M, Mayaki D, Leduc-Gaudet JP, Leblanc P, Huck L, Hussain SNA. Differential regulation of the autophagy and proteasome pathways in skeletal muscles in sepsis. Crit Care Med 2017; 45:e971–9 [DOI] [PubMed] [Google Scholar]
- 77.Tang H, C LK, Lee M, Gao Y, Xia H, Olguin F, Fraga DA, Ayers K, Choi S, Kim M, Tehrani A, Sowb YA, Rando TA, Shrager JB. Smad3 initiates oxidative stress and proteolysis that underlies diaphragm dysfunction during mechanical ventilation. Sci Rep 2017; 7:14530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liang F, Li T, Azuelos I, Giordano C, Liang H, Hussain SN, Matecki S, Petrof BJ. Ventilator-induced diaphragmatic dysfunction in MDX mice. Muscle Nerve 2018; 57:442–8 [DOI] [PubMed] [Google Scholar]
- 79.Smith IJ, Godinez GL, Singh BK, McCaughey KM, Alcantara RR, Gururaja T, Ho MS, Nguyen HN, Friera AM, White KA, McLaughlin JR, Hansen D, Romero JM, Baltgalvis KA, Claypool MD, Li W, Lang W, Yam GC, Gelman MS, Ding R, Yung SL, Creger DP, Chen Y, Singh R, Smuder AJ, Wiggs MP, Kwon OS, Sollanek KJ, Powers SK, Masuda ES, Taylor VC, Payan DG, Kinoshita T, Kinsella TM. Inhibition of Janus kinase signaling during controlled mechanical ventilation prevents ventilation-induced diaphragm dysfunction. FASEB J 2014; 28:2790–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tu MK, Levin JB, Hamilton AM, Borodinsky LN. Calcium signaling in skeletal muscle development, maintenance and regeneration. Cell Calcium 2016; 59:91–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hung MC, Lu HM, Chen L, Lin MS, Chen CR, Yu CJ, Wang JD. Cost per QALY (quality-adjusted life year) and lifetime cost of prolonged mechanical ventilation in Taiwan. PLoS One 2012; 7:e44043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hudson MB, Smuder AJ, Nelson WB, Wiggs MP, Shimkus KL, Fluckey JD, Szeto HH, Powers SK. Partial support ventilation and mitochondrial-targeted antioxidants protect against ventilator-induced decreases in diaphragm muscle protein synthesis. PLoS One 2015; 10:e0137693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reynolds S, Ebner A, Meffen T, Thakkar V, Gani M, Taylor K, Clark L, Sadarangani G, Meyyappan R, Sandoval R, Rohrs E, Hoffer JA. Diaphragm activation in ventilated patients using a novel transvenous phrenic nerve pacing catheter. Crit Care Med 2017; 45:e691–4 [DOI] [PubMed] [Google Scholar]
- 84.Kim WY, Lim CM. Ventilator-induced diaphragmatic dysfunction: diagnosis and role of pharmacological agents. Respir Care 2017; 62:1485–91 [DOI] [PubMed] [Google Scholar]
- 85.Agten A, Maes K, Smuder A, Powers SK, Decramer M, Gayan-Ramirez G. N-Acetylcysteine protects the rat diaphragm from the decreased contractility associated with controlled mechanical ventilation. Crit Care Med 2011; 39:777–82 [DOI] [PubMed] [Google Scholar]
- 86.Smuder AJ, Gonzalez-Rothi EJ, Kwon OS, Morton AB, Sollanek KJ, Powers SK, Fuller DD. Cervical spinal cord injury exacerbates ventilator-induced diaphragm dysfunction. J Appl Physiol 2016; 120:166–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.De Cunto A, Paviotti G, Bua J, Demarini S. Theophylline increases diaphragmatic contractility in mechanically ventilated newborns. J Crit Care 2017; 37:264–5 [DOI] [PubMed] [Google Scholar]
- 88.Aydin NB, Teke T, Toy H, Uzun K. The effect of theophylline on the prevention of mechanical ventilation-induced diaphragm atrophy in rats. Adv Clin Exp Med 2014; 23:33–8 [DOI] [PubMed] [Google Scholar]
- 89.Kim WY, Park SH, Kim WY, Huh JW, Hong SB, Koh Y, Lim CM. Effect of theophylline on ventilator-induced diaphragmatic dysfunction. J Crit Care 2016; 33:145–50 [DOI] [PubMed] [Google Scholar]
- 90.van Hees HW, Dekhuijzen PN, Heunks LM. Levosimendan enhances force generation of diaphragm muscle from patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2009; 179:41–7 [DOI] [PubMed] [Google Scholar]
- 91.Doorduin J, Sinderby CA, Beck J, Stegeman DF, van Hees HW, van der Hoeven JG, Heunks LM. The calcium sensitizer levosimendan improves human diaphragm function. Am J Respir Crit Care Med 2012; 185:90–5 [DOI] [PubMed] [Google Scholar]
- 92.Schellekens WJ, van Hees HW, Linkels M, Dekhuijzen PN, Scheffer GJ, van der Hoeven JG, Heunks LM. Levosimendan affects oxidative and inflammatory pathways in the diaphragm of ventilated endotoxemic mice. Crit Care 2015; 19:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cho HJ, Park J, Lee HW, Lee YS, Kim JB. Regulation of adipocyte differentiation and insulin action with rapamycin. Biochem Biophys Res Commun 2004; 321:942–8 [DOI] [PubMed] [Google Scholar]
- 94.Laufenberg LJ, Kazi AA, Lang CH. Salutary effect of aurintricarboxylic acid on endotoxin- and sepsis-induced changes in muscle protein synthesis and inflammation. Shock 2014; 41:420–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yu T, Wang M, Wen Y, Cao Y, Shen G, Jiang X, Wu J, Lu W, Jin X. Activation of mammalian target of rapamycin induces lipid accumulation in the diaphragm of ventilated rats and hypoxia-treated C2C12 cells. J Surg Res 2018; 225:82–9 [DOI] [PubMed] [Google Scholar]
- 96.Guillamat-Prats R, Camprubi-Rimblas M, Bringue J, Tantinya N, Artigas A. Cell therapy for the treatment of sepsis and acute respiratory distress syndrome. Ann Transl Med 2017; 5:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007; 131:861–72 [DOI] [PubMed] [Google Scholar]
- 98.Schellekens WJ, van Hees HW, Doorduin J, Roesthuis LH, Scheffer GJ, van der Hoeven JG, Heunks LM. Strategies to optimize respiratory muscle function in ICU patients. Crit Care 2016; 20:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Petrof BJ, Hussain SN. Ventilator-induced diaphragmatic dysfunction: what have we learned? Curr Opin Crit Care 2016; 22:67–72 [DOI] [PubMed] [Google Scholar]