Abstract
Background
Cancer, one of the leading causes of death worldwide, develops when the normal balance between mitosis and apoptosis is disrupted. The subsequently increased proliferation rate or decreased apoptosis rate of cells leads to uncontrolled cellular growth. Thus, the current aim of cancer research is to increase the apoptosis rate in tumor cells—while limiting the concurrent death of healthy cells—and to induce controlled apoptosis in abnormal cells. Staurosporine is a very potent inducer of apoptosis because it inhibits many different kinases. So far, many different kinase pathways of staurosporine-induced apoptosis have been discussed for various tumor entities.
Aims
To identify the effect of staurosporine in pancreatic and colorectal carcinoma cells and its apoptosis-inducing signaling pathway.
Methods
The apoptosis rate in pancreatic and colorectal carcinoma cells was analyzed by annexin V staining after staurosporine administration. Staurosporine stimulation and its effects on the expression of Bcl2, BAX, Bad, caspase-8, and caspase-9 were investigated with immunoblot.
Results
Staurosporine significantly increased apoptosis in pancreatic carcinoma cells. Western blot analysis showed activation of caspase-9 in PaTu 8988t and Panc-1 cells with 1 µM staurosporine. In addition, expression of Bcl2 and Bad was decreased in PaTu 8988t cells. In colorectal carcinoma cells SW 480, staurosporine stimulation did not induce apoptosis.
Conclusion
Modern therapeutic strategies for tumor diseases target the efficient modulation of specific signaling and transcription pathways. In this respect, the therapeutic potential of protein kinase inhibitors has been repeatedly discussed. Our study showed that staurosporine induces apoptosis in pancreatic carcinoma cells via the intrinsic signaling pathway. Thus, staurosporine is a suitable positive control for in vitro apoptosis tests for the pancreatic cancer cell lines PaTu 8988t and Panc-1. Further clinical studies should analyze the impact of this finding on cancer treatment.
Keywords: Staurosporine, Apoptosis, Pancreatic carcinoma, Colorectal carcinoma, Cancer
Background
Malignant tumors are one of the main scourges of humanity. In 2012 alone, about 8.2 million people died of carcinoma worldwide, and—according to the World Health Organization—[1] this figure is expected to rise to 13 million over the next 20 years.
From the molecular biology point of view, cancer is defined as any type of malignant neoplasm, independent of the organ or the tissue from which the tumor originates [2]. Cancer develops when the normal balance between mitosis and apoptosis is disrupted [3]. The subsequent increase in the proliferation rate or the decrease in the apoptosis rate of cells results in uncontrolled cellular growth [4, 5]. Thus, the current aim of cancer research is to increase the apoptosis rate in tumor cells—while simultaneously limiting concurrent death of healthy cells—and to induce controlled apoptosis in abnormal cells [6].
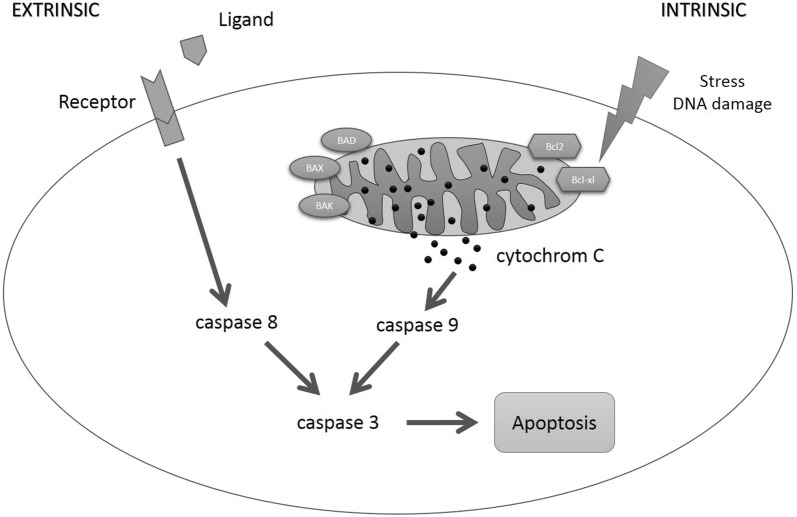
Therefore, comprehensive knowledge of the mechanisms of apoptosis in the different types of cells is vital for developing potential cancer therapies [7]. The term apoptosis refers to programmed cell death, which constitutes an important mechanism for maintaining tissue homeostasis [8]. The characteristic feature in this respect is that apoptosis is actively induced by the respective cell itself; thus, the cell is part of the metabolism. Activation of proteolytic enzymes termed caspases results in cellular shrinkage, condensation, and fragmentation of the cell nucleus, loss of cellular adhesion, and finally in apoptosis [9]. Apoptosis is induced by activation of important signal-transduction cascades [10]. Here, extrinsic and intrinsic signaling pathways have to be differentiated [11]. After the binding of a ligand to the death receptor of the tumor necrosis factor receptor family CD 95 (APO-1/Fas) or TRAIL receptors, caspase-8 is activated via the extrinsic signaling pathway [12, 13]. In turn, caspase-8 activates caspase-3 (effector caspase) [14], thus inducing apoptosis. The intrinsic signaling pathway is the cellular response to stress [15]. Changes in the mitochondrial membrane result in the collapse of its potential and thus in the release of cytochrome C, which subsequently irreversibly instigates the entire caspase cascade by activating caspase-9 [16] (Fig. 1).
Fig. 1.
Apoptotic signaling pathway. Caspase-8 is activated via the extrinsic signaling pathway by the binding of a ligand to the death receptor. The intrinsic signaling pathway is activated by changes in the mitochondrial membrane potential and the subsequent release of cytochrome C, which influences the pro-apoptotic factors BAK, Bad, and BAX as well as the anti-apoptotic factors Bcl2 and Bcl-xl and thus triggers caspase-9. Both pathways merge into a common pathway, in which effector caspase-3 finally induces apoptosis
A highly potent inductor of apoptosis is staurosporine, an alkaloid that inhibits many different kinases [17]. Several new derivatives of this substance, which was originally isolated from Streptomyces staurosporeus, have been synthesized in clinical trials to be used in anticancer therapies [18]. So far, many different kinase pathways of staurosporine-induced apoptosis have been discussed for various tumor entities [19–21].
The aim of this study was to identify the effect of staurosporine in pancreatic and colorectal carcinoma cells and its apoptosis-inducing signaling pathway.
Methods
Cell lines
The human pancreatic adenocarcinoma cell lines PaTu 8988t and Panc-1 were obtained from Professor Ellenrieder (Philipps University of Marburg, Germany). The colorectal carcinoma cell line SW 480 was purchased from the German Collection of Microorganism and Cell Culture (DSMZ). PaTu 8988t and Panc-1 cells were maintained in Dulbecco’s modified Eagle’s medium (Sigma-Aldrich, Steinsheim, Germany) or RPMI 1640 (Pan Biotech, Aidenbach, Germany), which was supplemented with 10% fetal calf serum (FCS) (Sigma-Aldrich) and 5% Myco Zap (Lonza Verviers SPRL, Verviers, Belgium) or 5% penicillin plus streptomycin (Sigma-Aldrich) for SW 480. Cells were cultured at 37 °C in humidified CO2 atmosphere (5%) and maintained in monolayer culture. Experiments were done with cells at ~ 70–80% confluence.
Antibodies and reagents
Staurosporine was purchased from Sigma-Aldrich. Final concentrations were achieved by diluting drugs in standard growth media. All solutions were prepared freshly prior to use. For immunoblotting, membranes were probed with antibodies against Bcl2, BAX, Bad, caspase-8, caspase-9 (cell signaling), and ß-actin (Sigma-Aldrich).
Subcellular fractionation and immunoblotting
Cells were washed twice with cold DPBS and collected by centrifugation at 4000 rpm at 4 °C for 10 min. Lysates were then resuspended in RIPAE-buffer (5 mL Triton X-100, 190 mg EDTA, 0.5 g SDS, 2.5 g deoxycholic acid, 500 mL DPBS, protease inhibitors) for 15 min and centrifuged at 13,000 rpm for 30 min. The supernatants were transferred to new cups and incubated on ice. 30 µg of the total lysates were analyzed by SDS-PAGE and blotted onto nitrocellulose. After protein extraction and gel transfer, the membranes were washed in TBS washing buffer and incubated with peroxidase-conjugated secondary antibodies. Immunoreactive proteins were visualized by means of an enhanced chemiluminescence detection system (Western Blotting Detection Reagent, GE Healthcare).
Apoptosis analysis
Apoptosis assays by annexin V staining were conducted according to the manufacturer’s instructions (BD Pharming). In brief, PaTu 8988t, Panc-1, and SW 480 cells were incubated with 1 µM of staurosporine. Standard growth medium was used for negative control. After 0 h, 3 h, 6 h, 9 h, 12 h, 16 h, or 24 h incubation time, the supernatant was decanted from the cells to preserve floating cells. Adherent cells were rinsed with warm PBS (Sigma-Aldrich) and harvested by standard trypsinization. Afterward, harvested and floating cells were mixed, washed, and resuspended in binding buffer at a final concentration of 106 cells/ml. 100 µL of cell suspension containing 105 cells was resuspended in 5 µL of FITC Annexin plus 5 µL of propidium iodide, followed by 15 min incubation at room temperature protected from light. The cells were washed and resuspended with 400 µL of binding buffer. Finally, the cells were analyzed by flow cytometry using FACS Calibur (BD Bioscience) and CellQuest Pro software (BD Bioscience). All tests were done in duplicate and the process was repeated twice.
Statistical analysis
Data are presented as mean ± SD. The non-parametric Mann–Whitney U test was used for statistical evaluation of the data. P values < 0.05 were considered significant. IBM SPSS Statistics (Vs. 20; IBM New York, US) and Excel Vs. 2010 (Microsoft, Redmond, USA) packages were employed for statistical analysis.
Results
Analysis of apoptosis and necrosis
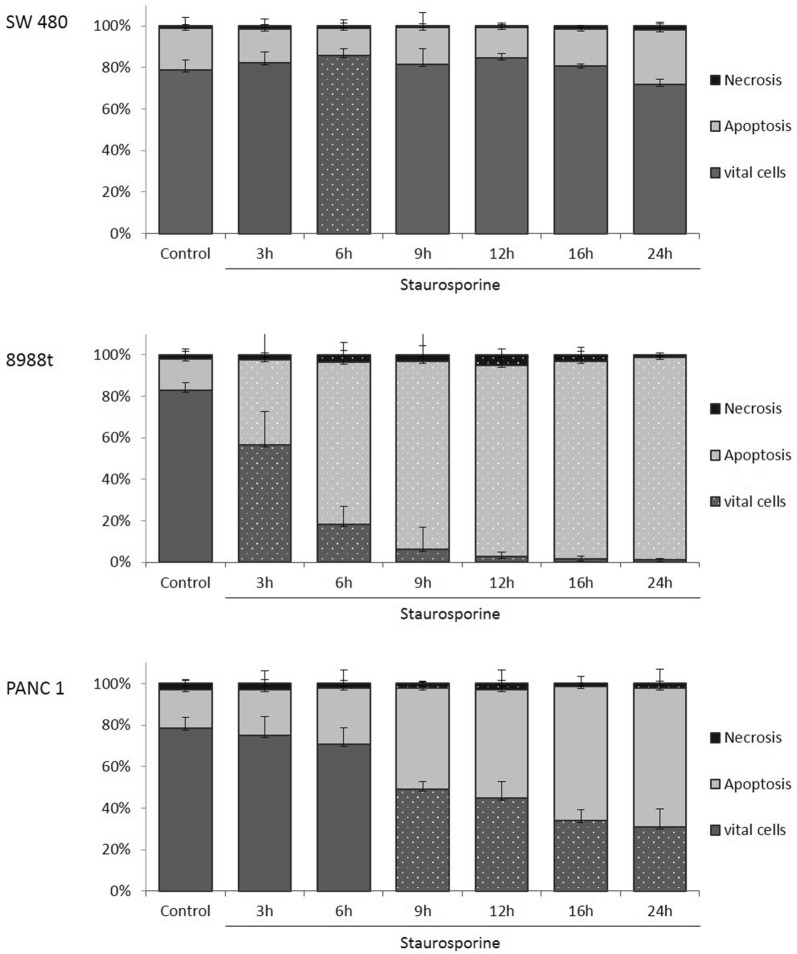
The annexin V staining apoptosis assay was used to determine whether incubation with staurosporine induced apoptosis or necrosis. Incubation with staurosporine for 6 h (Fig. 2a) increased the vital cell fraction phase of colorectal carcinoma cells SW 480 to 84.75% ± 3.57% compared to the untreated samples. No other significant changes in apoptosis rate or cell death behavior were observed during any of the other time frames.
Fig. 2.
The effects of staurosporine on apoptosis in in vitro SW 480 colorectal carcinoma (a) and PaTu 8988t (b) and Panc-1 (c) pancreatic carcinoma cell lines after time-dependent incubation. For apoptosis analysis, cancer cells were stained with annexin V. (*) indicates statistical significance at p < 0.05 compared to untreated control
In contrast to the untreated control samples in the pancreatic cancer cell line PaTu 8988t, incubation with staurosporine between 3 h and 24 h significantly increased the rate of apoptosis (Fig. 2b) and significantly reduced the number of vital cells. The necrosis rate was increased after 6 h, 12 h, and 16 h incubation. In Panc-1, stimulation with staurosporine (Fig. 2c) significantly increased apoptosis and significantly reduced the number of vital cells after 9 h, 12 h, 16 h, and 24 h.
Endogenic expression of Bcl2, Bad, BAX, caspase-8, and caspase-9 in pancreatic and colorectal carcinoma cells
The first aim was to obtain evidence for the actual expression of Bcl2, Bad, BAX, caspase-8, and caspase-9 in pancreatic and colorectal carcinoma cells (Fig. 3). The pancreatic cancer cell line PaTu 8988t (column 2) showed strong expression of each of the proteins investigated, whereas the cell lines SW 480 and Panc-1 showed only expression of BAX, caspase-8, and caspase-9. The proteins Bcl2 and Bad could not be detected at all. The endogenous expression of ß-actin serving as loading control can be seen in the lower blot (column 6).
Fig. 3.

Immunblotting and proof of endogenic expression of Bcl2, BAX, Bad, caspase-8, caspase-9, and ß-actin in colorectal cancer cells (SW 480) and pancreatic cancer cells (PaTu 8988t and Panc-1)
Western blot analysis after time-dependent incubation with 1 µM staurosporine and endogenic expression of Bcl2, BAX, Bad, caspase-8, and caspase-9 in pancreatic and colorectal carcinoma cells
The colorectal cancer cell line SW 480 did not show any time-dependent changes in the expression of the proteins BAX, caspase-8, and caspase-9 (Fig. 4a). The pancreatic cancer cell line PaTu 8988t (Fig. 4b) showed a time-dependent decrease in the signal strength of Bcl2 after incubation with staurosporine up to the complete absence of proteins after 24 h of incubation (column 1). In contrast, expression of BAX and caspase-8 was not influenced by staurosporine; here, only the band intensity was decreased after 24 h of incubation (column 2 and 4). Expression of Bad was considerably decreased after 3 h and 6 h of incubation in the reagent in contrast to untreated cells only incubated in the medium. After 9 h of incubation, protein was no longer detectable (column 3).
Fig. 4.
Time-dependent immunoblotting and proof of endogenic expression of Bcl2, BAX, Bad, caspase-8, caspase-9, and ß-actin in colorectal carcinoma cells (SW 480) and pancreatic cancer cells (PaTu 8988t and Panc-1) after stimulation with staurosporine
Use of the antibody caspase-9 enabled detection of full length caspase-9 as well as cleaved caspase-9. The control samples, which had only been incubated in the medium, showed considerable signal strength for full length caspase-9 that was markedly decreased after 3 h and 6 h of incubation with staurosporine. At the later time points (9 h to 24 h of incubation), protein was no longer detectable. In contrast, expression of cleaved caspase-9 first increased after incubation with staurosporine, showing the maximum signal strength after 6 h of incubation, but steadily decreased over the later time points (column 5). Column 6 depicts the endogenous expression of ß-actin that served as loading control.
In the pancreatic cancer cell line Panc-1 (Fig. 4c), BAX expression was not influenced by incubation with staurosporine (column 1), whereas the signal strength of caspase-8 steadily increased in a time-dependent manner (column 2). Expression of full length caspase-9 decreased, but expression of cleaved caspase-9 increased after stimulation with the reagent. After 24 h of incubation, the signal strength of both bands was decreased. Column 4 depicts the endogenous expression of ß-actin that served as loading control.
Discussion
Apoptosis is a key regulator of physiological growth control and tissue homeostasis. One of the most important findings in cancer research of the past few years is that apoptosis has a high impact on the development of tumors as well as on their response to chemotherapy [22, 23]. Bcl2 proteins residing on the mitochondrial membrane serve a regulatory function in the intrinsic apoptotic signaling pathway. They strictly control this pathway by inducing mitochondrial outer membrane permeabilization (MOMP). All proteins of the family show similar structural domains, called “Bcl2 homology (BH) domains” (named BH1, BH2, BH3, and BH4). They can be divided into three subgroups: the prosurvival members (Bcl2, Bcl-xl, Bcl-w, MCL-1, and A1), the pro-apoptotic members, which include the MOMP effectors (mainly BAX and BAK), and the BH3-only proteins, so called because they have only the BH3 domain (Bad, BIM, BID, PUMA, NOXA) [24].
Pro-apoptotic factors such as BAX, BAK, and Bad and the anti-apoptotic factors Bcl2 and Bcl-xl are delicately balanced, and this balance is often lacking in tumor cells [25]. For instance, dysfunction of BAX may further the tumor genesis of cells; thus, many chemotherapies try to indirectly intervene in this process [26]. Derivatives such as oblimersen sodium, AT-101, ABT-263, and GX15-070 are currently under clinical investigation [27, 28]. Furthermore, kinase inhibitors are also constantly used in cancer treatment [29, 30].
A highly potent inductor of apoptosis is staurosporine, an alkaloid that inhibits many different kinases [17]. In 2001, Stepczynska et al. [18] showed that staurosporine—as a broadband kinase inhibitor—induced apoptosis in Jurkat cells resistant to chemotherapeutic agents. Chae et al. [31] reported on the apoptosis-inducing effect of staurosporine in osteoblasts and Xue et al. [32] on this effect in breast carcinoma cells. The inhibiting effect of staurosporine on cell adhesion, mobilization, and invasion could also be shown in lung carcinoma cells [33]. To different degrees, staurosporine also seems to affect the induction of apoptosis in pancreatic and colorectal carcinoma cells. However, the signaling pathway by which staurosporine induces apoptosis has yet remained unclear.
The current study shows that—in the pancreatic and colorectal cancer cell lines Panc-1, PaTu 8988t, and SW 480—staurosporine does not influence BAX, but influences the anti-apoptotic factor Bcl2. The Bcl2 protein is often overexpressed in several types of cancer, for instance in breast, lung, and ovarian cancer or in malignant melanoma. Therefore, evidence of Bcl2 in cells is often associated with unfavorable outcome [34].
Bcl2 is expressed in the pancreatic cancer cell line PaTu 8988t. If Bcl2 is produced, apoptosis may be induced by suppressive medication. For this reason, the effect of BH3-mimetica has been repeatedly investigated in clinical trials, because these drugs induce apoptosis by binding and inhibiting anti-apoptotic members of the Bcl2 protein family [35, 36].
However, Bcl2 expression decreased after the administration of staurosporine in PaTu 8988t carcinoma cells. But why does staurosporine, as a kinase inhibitor, cause activation of the intrinsic apoptotic pathway? A possible explanatory approach could be that the imbalance between BAX and Bcl2—thus between pro-apoptotic and anti-apoptotic factors—probably induces apoptosis. This hypothesis is supported by the activation of full length caspase-9, shifting the balance to cleaved caspase-9. Cell apoptosis is eventually induced by activation of the intrinsic signaling pathway via caspase-9.
Bad—a further important protein in the apoptosis process—stands for Bcl2 antagonist of cell death. This protein may develop pro-apoptotic effects due to the heterodimerization with anti-apoptotic factors, for instance Bcl2, by binding to and thus blocking Bcl2 [37]. After stimulation with staurosporine, expression of Bad cannot any longer be detected by Western blot analysis. A possible explanation may be heterodimerization of Bad and Bcl2 that further shifts the balance between pro-apoptotic and anti-apoptotic factors in favor of pro-apoptotic factors. But degradation pathways at the level of transcription, translation level, or protein modification/clearance would also be conceivable.
Yuste et al. investigated whether overexpression of Bcl2 can rescue cells from staurosporine-induced apoptosis. They found that overexpression of Bcl2 increased the resistance of cells to staurosporine up to 1 µM. At higher doses, cytochrome c release from mitochondria occurred, caspases were activated, and cells died by apoptosis.
They also examined whether caspase inhibitors could rescue the cells from apoptosis induced by staurosporine. For this question they used the noncompetitive inhibitor of caspases z-VAD.fmk. The addition of z-VAD.fmk delayed the staurosporine-induced cell death [38].
The Bcl2 protein family plays a key role in the process of the intrinsic apoptotic signaling pathway, because dysfunctional Bcl2 protein may result in the development of both tumor cells and resistance to chemotherapies [39]. As a kinase inhibitor, staurosporine seems to be able to particularly influence the process of the intrinsic apoptotic signaling pathway.
In summary, this study showed that staurosporine induces apoptosis in pancreatic carcinoma cells via the intrinsic signaling pathway. Staurosporine is therefore a suitable positive control for in vitro apoptosis tests for the pancreatic carcinoma cell lines PaTu 8988t and Panc-1. In the colorectal cancer cell line SW 480, stimulation with staurosporine did not induce apoptosis.
Conclusion
Modern therapeutic strategies for tumor diseases target the efficient modulation of specific signaling and transcription pathways (for instance, VEGF antibodies [40], tyrosine kinase inhibitors in the treatment of chronic lymphatic leukemia [41], or EGFR antibodies in the therapeutic management of advanced colorectal carcinoma [42]). In this respect, the therapeutic potential of protein kinase inhibitors has been repeatedly discussed. Pharmaceutical companies increasingly focus on the development of new chemotherapies [43]. The approval of chemotherapies (for instance, imatinib for treating chronic myeloid leukemia, trastuzumab for treating breast cancer, or gefitinib and cetuximab for treating lung and colorectal cancer) has opened up new possibilities of treating different types of cancer [44]. A large number of kinase inhibitors are currently undergoing clinical development in both clinical and preclinical trials to analyze the potential of these substances for medical treatment [45]. However, their side effects and toxicity should be closely monitored because kinase inhibitors may also modulate important signaling cascades in healthy cells.
Ultimately, the basis and prerequisite of such new therapeutic approaches is a comprehensive knowledge of the respective carcinogenesis. The only types of cancer that may benefit from therapeutic protein kinase inhibitors are diseases marked by upregulation of the specific signaling pathways and thus by disturbed natural balance between mitosis and apoptosis. Furthermore, a combination therapy of different target-specific therapeutics will probably be required to avoid an increase in the proliferation rate or a decrease in the apoptosis rate of cells and thus the development of uncontrolled cellular growth [44].
The present work provides insight into the complexity of the Bcl2 family and the apoptotic pathways. Many further trials will be required to identify the underlying molecular mechanisms. Identifying and characterizing cellular receptors and their signal-transduction cascades will eventually help establish new therapeutic approaches in the treatment of pancreatic carcinoma, one of the most aggressive types of all cancers.
Authors’ contributions
All authors have made substantial contributions to the conception, design, analysis, and the interpretation of this research article. They have been involved in the critical revision of the manuscript with regard to important intellectual content. All authors have given their final approval for the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors read and approved the final manuscript.
Acknowledgements
We thank Sigrid Bamberger, Regina Lindner, Gabriele Bollwein, Marion Schindler, and Ruth Spaeth for technical assistance. We thank Monika Schoell for linguistic support.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The dataset supporting the conclusions of this article is included in the article.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
Funding was provided by Regensburger Forschungsförderung in der Medizin (ReForM), Faculty of Medicine, University of Regensburg.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- Bad
Bcl2 antagonist of cell death
- BAX
Bcl2-associated X
- Bcl2
B cell lymphoma 2
- BH3
Bcl2 homology domain 3
- EGFR
epidermal growth factor receptor
- MOMP
mitochondrial outer membrane permeabilization
- VEGF
vascular epithelial growth factor
Contributor Information
Manuela Malsy, Phone: +49 941 944 7801, Email: Manuela.Malsy@ukr.de.
Diane Bitzinger, Email: Diane.Bitzinger@ukr.de.
Bernhard Graf, Email: Bernhard.Graf@ukr.de.
Anika Bundscherer, Email: Anika.Bundscherer@ukr.de.
References
- 1.WHO. Cancer fact sheet No 297; 2012.
- 2.Oberholzer MJ. Pathologie verstehen, Molekulare Grundlagen der allgemeinen Pathologie. 2001; p. 55–64.
- 3.Byrne H. The effect of time delay on the dynamics of avascular tumor growth. Math Biosci. 1997;144:83–117. doi: 10.1016/S0025-5564(97)00023-0. [DOI] [PubMed] [Google Scholar]
- 4.Cotter TG. Apoptosis and cancer: the genesis of a research field. Nat Rev Cancer. 2009;9:501–507. doi: 10.1038/nrc2663. [DOI] [PubMed] [Google Scholar]
- 5.Evan GI, Vousden KH. Proliferation cell cycle and apoptosis in cancer. Nature. 2001;411:342–348. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- 6.Hassan M, Watari H, AbuAlmaaty A, Ohba Y, Sakuragi N. Apoptosis and molecular targeting therapy in cancer. Biomed Res Int. 2014;2014:150845. doi: 10.1155/2014/150845. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Lowe SW, Lin AW. Apoptosis in cancer. Carcinogenesis. 2000;21:285–295. doi: 10.1093/carcin/21.3.485. [DOI] [PubMed] [Google Scholar]
- 8.De Felici M, Piacebtini M. Programmed cell death in development and tumors. Int J Dev Biol. 2015;59:1–3. doi: 10.1387/ijdb.150168md. [DOI] [PubMed] [Google Scholar]
- 9.Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT, Liu B, Bao JK. Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012;45:487–498. doi: 10.1111/j.1365-2184.2012.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Degterev A, Boyce M, Yuan J. A decade of caspases. Oncogene. 2003;22:8543–8567. doi: 10.1038/sj.onc.1207107. [DOI] [PubMed] [Google Scholar]
- 11.Hengartner M. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 12.Barnhart BC, Lee JC, Alappat EC, Peter ME. The death effector domain protein family. Oncogene. 2003;22:8634–8644. doi: 10.1038/sj.onc.1207103. [DOI] [PubMed] [Google Scholar]
- 13.Fulda S. Caspase-8 in cancer biology and therapy. Cancer Lett. 2009;281:128–133. doi: 10.1016/j.canlet.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 14.Ashkenazi A. Targeting the extrinsic apoptosis pathway in cancer. Cytokine Growth Factor Rev. 2008;19:325–331. doi: 10.1016/j.cytogfr.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Fulda S. Evasion of apoptosis as a cellular stress response in cancer. Int J Cell Biol. 2010;2010:1–6. doi: 10.1155/2010/370835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schafer ZT, Kornbluth S. The apoptosome: physiological, developmental and pathological modes of regulation. Dev Cell. 2006;10:549–561. doi: 10.1016/j.devcel.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Zhang XD, Gillespie SK, Hersey P. Staurosporine induced apoptosis of melanoma by both caspase-dependent and independent apoptotic pathways. Mol Cancer Ther. 2004;3:187–197. [PubMed] [Google Scholar]
- 18.Stepczynska A, Lauber K, Engels IH, Janssen O, Kabelitz D, Wesselborg S, Schulze- Osthoff K. Staurosporine and conventional anticancer drugs induce overlapping, yet distinct pathways of apoptosis and caspase activation. Oncogene. 2001;20:1193–1202. doi: 10.1038/sj.onc.1204221. [DOI] [PubMed] [Google Scholar]
- 19.Yadav SS, Prasad CB, Prasad SB, Pandey LK, Singh S, Pradhan S, Narayan G. Anti- tumor activity of staurosporine in the tumor microenvironment of cervical cancer: an in vitro study. Life Sci. 2015;133:21–28. doi: 10.1016/j.lfs.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 20.Zhao L, Lu G, Zhao Q, Zhang M, Chen M, Zhang J, Dai K. Staurosporine induces platelet apoptosis through p38 mitogen-activated protein kinase signaling pathway. Clin Lab. 2015;61:717–726. doi: 10.7754/clin.lab.2014.141103. [DOI] [PubMed] [Google Scholar]
- 21.Nardo T, Micalizzi G, Vicinanza R, De Iuliis F, Taglieri L, Scarpa S. Adhesion to type V collagen enhances staurosporine-induced apoptosis of adrenocortical cancer cells. Tumour Biol. 2014;35:9949–9955. doi: 10.1007/s13277-014-2281-6. [DOI] [PubMed] [Google Scholar]
- 22.Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapie. Oncogene. 2006;25:4798–4811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- 23.Hamacher R, Schmid R, Saur D, Schneider G. Apoptotic pathways in pancreatic ducal adenocarcinoma. Mol Cancer. 2008;7:1–10. doi: 10.1186/1476-4598-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Besbes S, Mirshahi M, Pocard M, Billard C. New dimension in therapeutic targeting of BCL2 family proteins. Oncotarget. 2015;6:12862–12871. doi: 10.18632/oncotarget.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bundscherer A, Malsy M, Bitzinger D, Graf BM. Interaction of anesthetics with tumor cells. Anaesthesist. 2014;63:313–325. doi: 10.1007/s00101-014-2310-2. [DOI] [PubMed] [Google Scholar]
- 26.Liu Z, Ding Y, Ye N, Wild C, Chen H, Zhou J. Direct activation of Bax protein for cancer therapy. Med Res Rev. 2016;36:313–341. doi: 10.1002/med.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang MH, Reynolds CP. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin Cancer Res. 2009;15:1126–1132. doi: 10.1158/1078-0432.CCR-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leber B, Geng F, Kale J, Andrewa DW. Drugs targeting Bcl-2 family members as an emerging strategy in cancer. Expert Rev Mol Med. 2010 doi: 10.1017/s1462399410001572. [DOI] [PubMed] [Google Scholar]
- 29.Zeidner JF, Karp JE. Clinical activity of alvocidib (flavopiridol) in acute myeloid leukemia. Leuk Res. 2015;39:1312–1318. doi: 10.1016/j.leukres.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 30.Ou SH, Soo RA. Dacomitinib in lung cancer: a “lost generation” EGFR tyrosine-kinase inhibitor from a bygone era? Drug Des Devel Ther. 2015;9:5641–5653. doi: 10.2147/DDDT.S52787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chae HJ, Kang JS, Byun JO, Han KS, Kim DU, Oh SM, Kim HM, Chae SW, Kim HR. Molecular mechanism of staurosporine-induced apoptosis in osteoblasts. Pharmacol Res. 2000;42:373–381. doi: 10.1006/phrs.2000.0700. [DOI] [PubMed] [Google Scholar]
- 32.Xue LY, Chiu SM, Oleinick NL. Staurosporine-induced death of MCF-7 human breast cancer cells: a distinction between caspase-3-dependent steps of apoptosis and the critical lethal lesions. Exp Cell Res. 2003;283:135–145. doi: 10.1016/S0014-4827(02)00032-0. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Yang H, Liu H, Huang J, Song X. Effect of staurosporine on the mobility and invasiveness of lung adenocarcinoma A549 cells: an in vitro study. BMC Cancer. 2009;9:174. doi: 10.1186/1471-2407-9-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Talaiezadeh A, Jalali F, Galehdari H, Khodadadi A. Time depended Bcl-2 inhibition might be useful for a targeted drug therapy. Cancer Cell Int. 2015;15:105. doi: 10.1186/s12935-015-0254-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delbridge AR, Strasser A. The BCL-2 protein family, BH3-mimetics and cancer therapy. Cell Death Differ. 2015;22:1071–1080. doi: 10.1038/cdd.2015.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vela L, Marzo I. Bcl-2 family of proteins as drug targets for cancer chemotherapy: the long way of BH3 mimetics from bench to bedside. Curr Opin Pharmacol. 2015;23:74–81. doi: 10.1016/j.coph.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 37.Yang E, Korsmeyer SJ. Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood. 1996;88:386–401. [PubMed] [Google Scholar]
- 38.Yuste VJ, Sanchez-Lopez I, Sole C, Encinas M, Bayascas JR, Boix J, Comella JX. The prevention of the staurosporine-induced apoptosis by Bcl-X(L), but not by Bcl-2 or caspase inhibitors, allows the extensive differentiation of human neuroblastoma cells. J Neurochem. 2002;80:126–139. doi: 10.1046/j.0022-3042.2001.00695.x. [DOI] [PubMed] [Google Scholar]
- 39.Adams JM, Huang DC, Strasser A, Willis S, Chen L, Wei A, van Delft M, Fletcher JI, Puthalakath H, Kuroda J, Michalak EM, Kelly PN, Bouillet P, Villunger A, O’Reilly L, Bath ML, Smith DP, Egle A, Harris AW, Hinds M, Colman P, Cory S. Subversion of the Bcl-2 life/death switch in cancer development and therapy. Cold Spring Harb Symp Quant Biol. 2005;70:469–477. doi: 10.1101/sqb.2005.70.009. [DOI] [PubMed] [Google Scholar]
- 40.Bidart M, Berger F, Pelletier L. Anti-angiogenetic therapies: from theory to practice. Ann Biol Clin. 2013;71:527–535. doi: 10.1684/abc.2013.0883. [DOI] [PubMed] [Google Scholar]
- 41.Burger J, Montserrat E. Coming full circle: 70 years of chronic lymphocytic leukemia cell redistribution, from glucocorticoids to inhibitors of B-cell receptor signalling. Blood. 2013;121:1501–1509. doi: 10.1182/blood-2012-08-452607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Modest D, Hiddemann W, Heinemann V. Chemotherapy of metastatic colorectal cancer. Internist. 2014;55:37–42. doi: 10.1007/s00108-013-3314-8. [DOI] [PubMed] [Google Scholar]
- 43.Chahrour O, Cairns D, Omran Z. Small molecule kinase inhibitors as anti-cancer therapeutics. Mini Rev Med Chem. 2012;12:399–411. doi: 10.2174/138955712800493915. [DOI] [PubMed] [Google Scholar]
- 44.Novak K. Conference report-protein kinase inhibitors in cancer treatment: mixing and matching? Highlights of the keystone symposium on protein kinases and cancer. MedGenMed. 2004;6:25. [PMC free article] [PubMed] [Google Scholar]
- 45.Dancey J, Sausville EA. Issues and progress with protein kinase inhibitors for cancer treatment. Nat Rev Drug Discov. 2003;2:296–313. doi: 10.1038/nrd1066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset supporting the conclusions of this article is included in the article.