Abstract
Adaptive immunity is essentially required to control acute infection with enteropathogenic Yersinia pseudotuberculosis (Yptb). We have recently demonstrated that Yptb can directly modulate naïve CD4+ T cell differentiation. However, whether fully differentiated forkhead box protein P3 (Foxp3+) regulatory T cells (Tregs), fundamental key players to maintain immune homeostasis, are targeted by Yptb remains elusive. Here, we demonstrate that within the CD4+ T cell compartment Yptb preferentially targets Tregs and injects Yersinia outer proteins (Yops) in a process that depends on the type III secretion system and invasins. Remarkably, Yop-translocation into ex vivo isolated Foxp3+ Tregs resulted in a substantial downregulation of Foxp3 expression and a decreased capacity to express the immunosuppressive cytokine interleukin-10 (IL-10). Together, these findings highlight that invasins are critically required to mediate Yptb attachment to Foxp3+ Tregs, which allows efficient Yop-translocation and finally enables the modulation of the Foxp3+ Tregs' suppressive phenotype.
Keywords: regulatory T cells, Yersinia pseudotuberculosis, Yersinia outer proteins, invasins, cytotoxic necrotizing factor y, Foxp3
Introduction
Regulatory T cells (Tregs) are central players to confer tolerance towards self and harmless foreign antigens. They are characterized by expression of the lineage-specification transcription factor forkhead box protein P3 (Foxp3), which not only is a reliable marker to identify Tregs, but also is a key transcriptional regulator for the suppressive phenotype of these immunoregulatory cells [1]. Foxp3+ Tregs are integral to mediate immune tolerance. Thus, manipulation of Foxp3 expression by either enhancing its stability in the context of autoimmune diseases or ameliorating its stability in the context of cancer or chronic infections poses a promising strategy to further improve therapeutic approaches targeting Foxp3+ Tregs [2, 3].
In general, most infections overcoming epithelial barrier functions result in an inflammatory environment in the underlying tissues and the draining lymph nodes (LN), driving the establishment of T helper cell 1 (Th1) and Th17 responses. Importantly, the infection-induced skewing towards effector T cell responses can result in limitation of interleukin-2 (IL-2), a cytokine critically required to maintain stability of Foxp3 expression in Tregs [4–8]. In the context of gastrointestinal infections, we could recently show that upon oral infection with Yersinia pseudotuberculosis (Yptb), naïve CD4+ T cells get directly targeted by the pathogen, which ultimately drives naïve CD4+ T cell differentiation towards Th17 cells rather than Tregs [9]. Yet, the extent to which the Treg population is targeted by Yptb is unknown.
Yptb can efficiently translocate effector molecules, so called Yersinia outer proteins (Yops), into target cells [10]. Yops activate and abrogate homeostatic localization of Ras homologous-GTPase (Rho-GTPase) proteins, mitigate phosphorylation patterns, and limit activity of different small G-proteins, resulting in the deregulation of actin cytoskeleton assembly and cell-type dependently altering molecular functionalities [11]. The cytotoxic necrotizing factor y (CNFy) is a Yptb-secreted protein that exhibits its immunomodulatory effect by activating the Rho-GTPase proteins Rho A, Rac1, and Cdc42 [12–14]. Rho-GTPase proteins are key components of T cell development, activation, differentiation, and migration, acting via modulation of the T cell cytoskeleton [15]. In particular, activation of Rap1, a Rho-GTPase protein, was shown to enhance both thymic and peripheral Treg development [16]. Importantly, effective translocation of Yops via the type III secretion system (T3SS) is only possible via direct cell–Yptb contact, established by different invasins (inv). Particularly, invasin A acts as a potent β1 integrin ligand (mainly via α4β1 integrin) [17–19], potentially allowing effective binding to β1 integrin-expressing cells, including epithelial cells and Foxp3+ Tregs [20].
Although it is known that Yptb can modulate naïve CD4+ T cell differentiation relying on the T3SS [9], direct modulation of Foxp3+ Treg stability by Yptb virulence factors has not been described, yet. Here, we demonstrated that Yptb preferentially targets Tregs across the T cell compartment in a T3SS-dependent manner and directly modulates the stability and functional properties of Tregs by decreasing Foxp3 and IL-10 expression, respectively.
Materials and Methods
Mouse Strains
Foxp3hCD2 reporter mice (C57BL/6 background) were used as organ donors for the isolation of T cells within the study. All animals were bred and housed under specific pathogen-free conditions at the Helmholtz Centre for Infection Research (Braunschweig, Germany).
Bacterial Strains and Recombinant Proteins
Y. pseudotuberculosis strains, namely Yptb-WT-Bla, Yptb-ΔT3SS-Bla, Yptb-Δinv and Yptb-ΔCNFy-Bla, were used throughout the study [13]. Overnight cultures of Yptb strains were grown at 25 °C in Lysogeny broth (LB) medium, and subsequently diluted at 1:50, followed by 2 h of culture at 25 °C and 3 h at 37 °C. Cultures were performed in the presence of 50 μg/mL kanamycin (Sigma Aldrich) for all strains except Yptb-ΔCNFy-Bla, which was cultured in the presence of 50 μg/mL chloramphenicol (Sigma Aldrich). Recombinant invasins A, D, and E as well as CNFy were produced as described before [13, 21, 22].
Antibodies and Flow Cytometry
Fluorochrome-conjugated anti-human CD2 (RPA-2.10), anti-CD4 (RM4-5), anti-CD8α (53-6.7), anti-CD11c (N418), anti-CD45R (RA3-6B2), anti-CD62L (MEL-14), anti-F4/80 (BM8), anti-Foxp3 (FJK-16S), anti-IFNγ (XMG1.2), anti-IL-10 (JES5-16E3), and anti-IL-17A (eBio17B7) antibodies were purchased from BioLegend and eBioscience. Prior to cytokine staining, cells were stimulated with phorbol 12-myristate 13-acetate (PMA, 10 ng/mL) and Ionomycin (0.5 μg/mL) for 4 h, with Brefeldin A (10 μg/mL) added for the final two h (all Sigma-Aldrich). Surface staining was performed for 15 min on ice in PBS (Gibco) containing 0.2 % bovine serum albumin (BSA, Sigma-Aldrich). Intracellular Foxp3 staining was performed using Foxp3 staining buffer set (eBioscience) according to the manufacturer's instructions. Absolute cell numbers were determined using Accurri C6 Cytometer (Becton Dickinson). Dead cell exclusion was carried out using LIVE/DEAD Fixable Blue Dead Cell Stain (Thermo Fisher Scientific). Cells were washed, resuspended in phosphate buffered saline/bovine serum albumin (PBS/BSA), and measured at LSR-II SORP with Diva software 6.1 (BD Biosciences). Data were analyzed using FlowJo software (Tree Star).
In Vitro Treg Stability Assay
Total CD4+ T cells were enriched from spleens and LNs of Foxp3hCD2 mice via magnetic sorting using autoMACS (Miltenyi Biotec). From these CD4-enriched cells, CD4+Foxp3hCD2+ Tregs or CD4+Foxp3hCD2– conventional T cells (Tconv) were sorted on Aria II SORP (BD Biosciences) or MoFlo XDP (Beckman Coulter) cell sorters. Tregs or Tconv were co-cultured with Yptb-WT-Bla, Yptb-ΔT3SS-Bla, Yptb-Δinv, or Yptb-ΔCNFy-Bla at the indicated multiplicity of infection (MOI) at 37 °C for 1 h and washed twice with Roswell Park Memorial Institute (RPMI) medium supplemented with 50 μg/mL gentamicin (Sigma-Aldrich) to eliminate bacteria. Cells were labeled with the proliferation dye CellTrace™ Violet (CTV, Thermo Fisher Scientific) and cultured in 96-well plates pre-coated with anti-CD3 (17A2, 1 μg/mL) and anti-CD28 (37.51, 1 μg/mL) antibodies (both BioLegend) in complete RPMI (cRPMI, 10% FCS, 50 U/mL penicillin, 50 U/mL streptomycin, 1 mM sodium pyruvate, 25 mM HEPES, and 50 μM β-mercaptoethanol, all Gibco) supplemented with 50 ng/mL IL-2 (R&D) and 50 μg/mL gentamicin (Sigma-Aldrich) for 3 days. For studies involving recombinant proteins (CNFy, invasin A, D, and E), Tregs were cultured as described above in the presence of the indicated proteins. At the end of the culture, cells were harvested and analyzed by flow cytometry.
β-Lactamase Reporter Assay
For in vitro analysis of Yop translocation, total CD4+ T cells were isolated from spleens and LNs of Foxp3hCD2 mice using anti-CD4-Microbeads and the autoMACS Pro separation system (Miltenyi Biotec). Subsequently, cells were co-cultured with Yptb-WT-Bla or Yptb-ΔT3SS-Bla at the indicated MOIs at 37 °C for 1 h and washed twice with RPMI medium supplemented with 50 μg/mL gentamicin to eliminate bacteria. Cells were stained for cell surface markers anti-CD4 and anti-hCD2 and labeled with CCF4-AM, using the LiveBLAzer-FRET B/G loading kit (Thermo Fisher Scientific) for 1 h at 24 °C in the presence of 1.5 mM probenecid (Sigma-Aldrich) and 50 μg/mL gentamicin. Finally, cells were harvested and analyzed by flow cytometry.
Yptb Binding to Tregs
Tregs were isolated as described above and co-cultured with Yptb-WT-Bla or Yptb-Δinv at a MOI of 50 for 1 h at 37 °C. Subsequently, cell-Yptb single cell solutions were repeatedly washed by centrifugation at 400 g to sediment eukaryotic cells, limiting the sedimentation of prokaryotic Yptb. Cell-Yptb single cell solutions were resuspended in LB medium, and serial dilutions were plated on LB plates. These were kept at 37 °C for 36 h, and colony forming units (CFU) per Treg were calculated.
Statistical Analysis
For all figures, each data point represents the mean of at least 3 technical replicates per independent experiment, if not stated otherwise. For comparison of unmatched groups, two-tailed Mann-Whitney statistical test was applied. The comparison of more than 2 groups was performed by Kruskall-Wallis test followed by Dunn's Multiple Comparison test. All data are presented as mean or mean ± SD, and p-values < 0.05 are considered as significant (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001). Prism software (GraphPad) was applied for all statistical analyses and graphs.
Ethics
Animals were handled with appropriate care and welfare in accordance with good animal practice as defined by FELASA and the national animal welfare body GV-SOLAS under supervision of the institutional animal welfare officer, and all efforts were made to minimize suffering.
Results
Yptb Preferentially Targets Tregs Within the CD4+ T Cell Compartment
We have recently shown that Yptb can directly interact with CD4+ T cells and efficiently translocate Yops into both Tconv and Foxp3+ Tregs in vivo [9]. Interestingly, this translocation allowed a direct modulation of naïve CD4+ T cell differentiation and resulted in an enhanced Th17 differentiation and decreased induction of Foxp3+ Tregs in vitro [9]. Here, we further dissected the efficiency of Yptb to target Tconv and Foxp3+ Tregs in vitro. To this end, we used the β-lactamase reporter assay [13], which relies on the chromosomal integration of a YopE-β-lactamase fusion protein (Yptb-WT-Bla) and allows the identification of cells into which Yops were successfully translocated by Yptb. Total CD4+ T cells, obtained from Foxp3hCD2 reporter mice, were co-cultured with Yptb-WT-Bla at a MOI of 35, followed by labeling with the dye CCF4-AM (green fluorescence), which turns into blue fluorescence upon conversion by β-lactamase. Flow cytometric analysis of Foxp3hCD2– Tconv and Foxp3hCD2+ Tregs revealed a significantly higher frequency of blue cells among Tregs when compared to Tconv (Figure 1). These in vitro data, which are in line with our previous observations in vivo [9], demonstrate that Yptb preferentially targets Tregs within the CD4+ T cell compartment.
Figure 1.
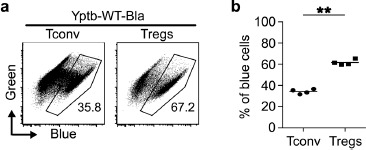
Tregs are efficiently targeted by Yptb for translocation of effector proteins. CD4+ T cells isolated from Foxp3hCD2 reporter mice were co-cultured with Yptb-WT-Bla at 37 °C for 1 h at MOI 35, labelled with CCF4-AM and subsequently analyzed by flow cytometry. Alive unmodulated cells are labelled in “Green”, and cells that were modulated by Yptb show “Blue” fluorescence. (a) Representative dot plots show frequency of translocation in the indicated T-cell subsets. Numbers indicate the frequency of modulated “Blue” cells in the corresponding gates. (b) Scatter-plot summarizes the frequency of modulated “Blue” cells among the indicated T-cell subsets. Data were pooled from 4 independent experiments (**p < 0.01; Bla, β-lactamase; MOI, multiplicity of infection)
Yptb Suppresses Foxp3 and IL-10 Expression in Tregs in a T3SS-Dependent Manner
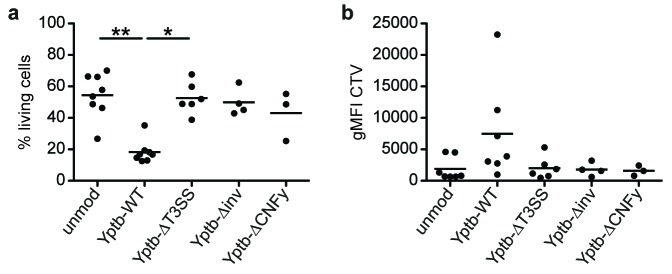
Based on the preferential targeting of Foxp3+ Tregs and our previous observation that Yptb can directly modulate naïve CD4+ T cell differentiation [9], we next asked if Yptb can also modulate the phenotype of already differentiated Foxp3+ Tregs. Thus, sorted Foxp3hCD2+ Tregs were co-cultured with Yptb-WT for 1 h, and subsequently, Yptb function was abrogated by applying gentamicin to the culture. Co-cultures with Yptb-ΔT3SS served as a control to investigate the importance of Yop translocation [9]. Afterwards, the modulated Foxp3hCD2+ Tregs were cultured for 3 days in vitro on plate-bound anti-CD3/28 in the presence of high-dose IL-2. At the end of the culture, flow cytometric analysis revealed a significantly decreased Foxp3 expression in Tregs that were previously modulated by Yptb-WT, when compared to unmodulated Tregs, as indicated by both a reduced frequency of Foxp3+ cells, as well as a lower geometric mean fluorescence intensity (gMFI) (Figure 2a and b). Interestingly, this reduction of Foxp3 expression was fully dependent on Yop translocation as Tregs that were modulated by Yptb-ΔT3SS did show neither a reduced frequency of Foxp3+ cells nor a lowered gMFI. It has to be mentioned that Tregs modulated with Yptb-WT, but not with Yptb-ΔT3SS, showed an increased cell death and impaired proliferative response at day 3 of the culture when compared to unmodulated controls (Supplementary Figure 1), most likely due to the direct interference with T cell receptor downstream signaling as previously reported [9]. However, the abovementioned reduction of Foxp3 expression upon modulation with Yptb-WT cannot be due to a selective killing of Tregs and survival of Foxp3– cells as Foxp3hCD2+ Tregs sorted to highest purity were used in these in vitro cultures. In accordance with the reduced Foxp3 expression, we also observed a significantly decreased IL-10 expression when Tregs were modulated by Yptb-WT, while again no effect was observed upon modulation by Yptb-ΔT3SS (Figure 2c and d). Importantly, the reduced Foxp3 and IL-10 expression was not accompanied by an increase in IL-17A, IFNγ, T-bet and RORγt expression (data not shown), suggesting that the modulated Tregs do not acquire an inflammatory phenotype upon downregulation of Foxp3 expression.
Figure 2.
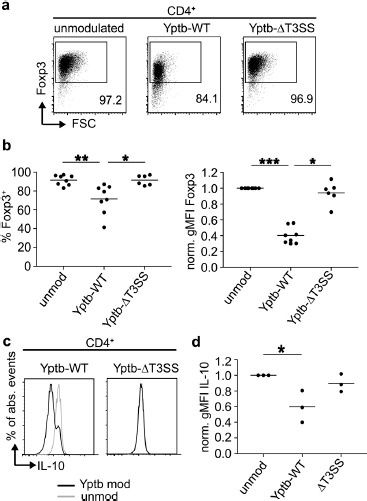
Foxp3 and IL-10 expression are suppressed in Tregs by Yptb in a T3SS-dependent manner. Foxp3+ Tregs isolated from Foxp3hCD2 reporter mice were co-cultured with Yptb-WT-Bla or Yptb-ΔT3SS-Bla at 37 °C for 1 h at MOI 50. Subsequently, bacteria were killed by antibiotics treatment, and Tregs were cultured on anti-CD3/CD28-coated plates in the presence of IL-2. At day 3 of culture, cells were analyzed by flow cytometry. (a) Representative dot plots show Foxp3 expression among alive CD4+ cells from indicated cultures. Numbers indicate the frequency of Foxp3+ cells in the corresponding gates. (b) Scatterplots summarize the frequencies of Foxp3+ cells (left) and gMFI of Foxp3 (normalized to unmodulated control, right) among alive CD4+ cells from the indicated cultures. (c) Representative histograms show IL-10 expression among alive CD4+ cells from the indicated cultures. (d) Scatterplot summarizes gMFI of IL-10 among alive CD4+ cells from indicated cultures. Each dot represents mean of 3 technical replicates. Data were pooled from 3 to 8 independent experiments (*p < 0.05; **p < 0.01; ***p < 0.001; gMFI, geometric mean fluorescence intensity; MOI, multiplicity of infection; mod, modulated; unmod, unmodulated; T3SS, Type III secretion system)
Viewed as a whole, our data demonstrate that Yptb can directly affect Foxp3 and IL-10 expression within Tregs, a process that fully relies on the T3SS, indicating that the injection of Yops functions as a key factor in modulating the stability and functional properties of Tregs.
Invasins and CNFy are Key Effector Virulence Factors for Effective Yop Translocation into Tregs
Translocation of Yops via the T3SS can be enhanced by invasins and CNFy [13, 18]. To determine whether these effector molecules are required for the reduction of Foxp3 expression, sorted Foxp3hCD2+ Tregs were co-cultured with Yptb either lacking all invasins (Yptb-Δinv) or CNFy (Yptb-ΔCNFy) for 1 h, followed by an elimination of all remaining Yptb by gentamicin treatment. Subsequently, the modulated Foxp3hCD2+ Tregs were cultured for 3 days in vitro on plate-bound anti-CD3/28 in the presence of high-dose IL-2. Remarkably, at the end of the culture, neither Tregs modulated by Yptb-Δinv nor Yptb-ΔCNFy showed any decrease in Foxp3 expression when compared to Tregs modulated by Yptb-WT (Figure 3a and b), suggesting that invasins and CNFy are both critically required for the reduction of Foxp3 expression. As abovementioned, Tregs modulated with Yptb-WT, but neither with Yptb-Δinv nor with Yptb-ΔCNFy, showed an increased cell death and impaired proliferative response at day 3 of the culture when compared to unmodulated controls (Supplementary Figure 1). Yet, in vitro culture of sorted Foxp3hCD2+ Tregs in the presence of recombinant CNFy, invasin A, invasin D, or invasion E did not result in any reduced Foxp3 expression (Figure 3c). Together, these findings implicate that CNFy and invasins cannot directly affect Foxp3 expression within Tregs on their own, yet they are both independently required for efficient translocation of Yops via the T3SS and thereby contribute to the modulation of the Tregs' phenotype.
Figure 3.
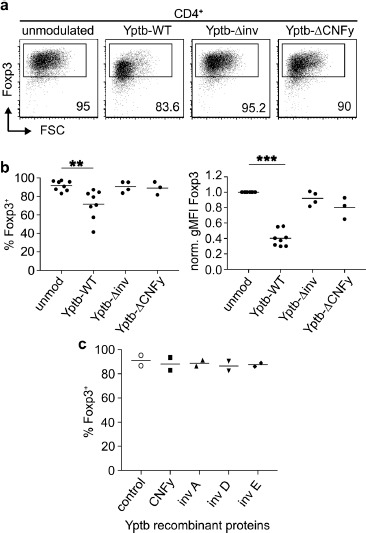
Invasins and CNFy are required for efficient Yop translocation into Tregs. Foxp3+ Tregs isolated from Foxp3hCD2 reporter mice were co-cultured with Yptb-WT, Yptb-Δinv or Yptb-ΔCNFy at 37 °C for 1 h at MOI 50. Subsequently, bacteria were killed by antibiotics treatment, and Tregs were cultured on anti-CD3/CD28-coated plates in the presence of IL-2. At day 3 of culture, cells were analyzed by flow cytometry. (a) Representative dot plots show Foxp3 expression among alive CD4+ cells from the indicated cultures. Numbers indicate the frequency of Foxp3+ cells in the corresponding gates. (b) Scatterplots summarize frequencies of Foxp3+ cells (left) and gMFI of Foxp3 (normalized to unmodulated control, right) among alive CD4+ cells from the indicated cultures. Unmodulated controls and Tregs modulated with Yptb-WT, same as in Figure 2b. (c) Foxp3+ Tregs isolated from Foxp3hCD2 reporter mice were cultured as described above in the presence of CNFy, invasin A, D, or E. At day 3 of culture, cells were analyzed by flow cytometry. Scatterplot summarizes the frequencies of Foxp3+ cells among alive CD4+ cells from the indicated cultures. Each dot represents mean of 3 technical replicates. Data were pooled from 2 to 8 independent experiments (**p < 0.01; ***p < 0.001. inv, invasin; CNFy, cytotoxic necrotizing factor y; unmod, unmodulated; MOI, multiplicity of infection)
Invasins are Required for Attachment of Yptb to Tregs
Having demonstrated that invasins are critically required for the reduction of Foxp3 expression in Tregs upon modulation by Yptb, we next aimed to determine the molecular mechanism involved and asked if invasins can foster the attachment of Yptb to Tregs. To this end, sorted Foxp3hCD2+ Tregs were co-cultured with Yptb-WT or Yptb-Δinv for 1 h. Subsequently, unbound bacteria were removed by repeated washing and centrifugation, and the remaining cell pellet was plated in serial dilutions to determine the number of bacteria bound to the cells. Strikingly, while Tregs co-cultured with Yptb-WT showed a high number of attached Yptb when compared to the negative control (Yptb-WT without Tregs), any specific binding was observed when Tregs were co-cultured with Yptb-Δinv (Figure 4). These findings indicate that invasins are crucially required to mediate Yptb attachment to Tregs, thereby enabling their efficient modulation through the translocation of Yops.
Figure 4.
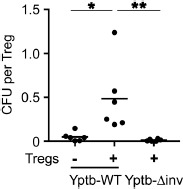
Attachment of Yptb to Tregs essentially requires invasins. Foxp3+ Tregs isolated from Foxp3hCD2 reporter mice were co-cultured with Yptb-WT or Yptb-Δinv at 37 °C for 1 h at MOI 50. Yptb-WT without Tregs served as control. After repeated washing and centrifugation to remove unbound bacteria, the remaining cell pellet was plated in serial dilutions, and CFU per Treg were determined. “+” indicates the presence of Tregs in the co-cultures. Each dot represents a biological unicate. Data were pooled from 6 independent experiments (*p < 0.05; **p < 0.01; inv, invasin; MOI, multiplicity of infection; CFU, colony forming unit)
Discussion
Within the intestinal tract, effector and regulatory immune cell subsets need to be tightly balanced to maintain immune homeostasis and, at the same time, combat infections effectively. We have recently shown that Yptb directly interacts with CD4+ T cells, which results in an efficient translocation of Yops and a skewing of naïve CD4+ T cell differentiation from immunosuppressive Tregs to inflammatory Th17 cells [9]. Here, we expanded our findings on fully differentiated Tregs and could demonstrate that Yptb directly impairs Treg stability by suppressing Foxp3 expression and limiting their capacity to produce IL-10.
During acute infections the inflammatory environment limits Treg expansion and their immunosuppressive functions to enable the establishment of effector and memory T cell responses and subsequently an efficient eradication of the invading pathogens [18, 23, 24]. Therefore, pathogens including Mycobacteria, Salmonella, Helicobacter pylori, and helminths have evolved mechanisms to utilize the suppressive function of Tregs in order to support initial infection or establish chronicity [25]. In contrast to these pathogens, we here could show that Yptb directly interacts with Tregs and impairs lineage stability by interfering with proper Foxp3 expression, in line with our previous observation of Yptb-induced, ameliorated de novo Treg induction [9]. Together, these findings indicate that Yptb does not rely on Treg-mediated suppression of the immune response for successful establishment within the host.
Several molecular mechanisms have been identified that allow pathogens to directly modulate the function of Tregs [26], including the induction of Tregs by secretory antigens of Heligmosomoides polygyrus or tolerogenic polarization of dendritic cells by Helicobacter pylori [23, 27]. Due to the effectiveness of pathogens to utilize Treg functionality, the molecular mechanism of pathogen-mediated modulation of Tregs is of high interest to develop novel therapeutic tools specifically targeting Tregs, e.g. in the context of tumor therapy [28]. Using various Yptb mutant strains lacking CNFy, invasins, or T3SS, we could demonstrate that Yop translocation via the T3SS is essential to inhibit Foxp3 expression. Importantly, CNFy-dependent inhibition of Foxp3 expression only took place in the presence of viable Yptb and not in the presence of recombinant CNFy, underlining the importance of the Yop translocation-enhancing properties of CNFy in this context [13]. Furthermore, Yptb requires close proximity to Tregs and utilizes invasin-mediated binding, presumably by interaction of invasin A with β1 integrin [17–19], which is expressed at high levels on Foxp3+ Tregs [20]. Both, CNFy and invasins, were independently required for efficient translocation of Yops via the T3SS and thereby contributed to the modulation of the Tregs' phenotype. The extent to which the different Yops alter Treg function and mitigate Foxp3 expression requires further studies. Potentially interesting candidates might include YopH, a tyrosine phosphatase that was reported to impair proximal T cell receptor signaling by interfering with tyrosine-phosphorylation of downstream adapter molecules LAT and SLP-76 upon T cell activation [29, 30]. Additionally, YopJ can act as a deubiquitinating enzyme, interacting with stimulator of interferon genes (STING) that in turn inhibits IRF3 signaling [31], which might influence T cell differentiation. In the context of utilizing Yop translocation by combining different Yop functionalities, including the capacity of YopM to efficiently self-translocate [32], Yptb-originated effector proteins could be used to effectively target Tregs in the context of therapeutic approaches encompassing engineered bacteria for tumor therapy. So far, bacterial agents have been predominantly studied in tumor therapies using attenuated bacterial strains ranging from Clostridium novyi to auxotrophic Salmonella conferring effective anti-tumor immune responses with promising efficiency [33, 34]. Thus, the capacity of Yptb to preferentially target Tregs in a T3SS-dependent manner and to directly modulate the stability and functional properties of Tregs by decreasing Foxp3 and IL-10 expression, respectively, could be utilized in the future to leverage the development of novel Treg targeting approaches to boost anti-tumor immune responses.
Acknowledgement
We thank Maria Ebel and Beate Pietzsch for technical assistance, Lothar Groebe and Maria Hoexter for cell sorting and Juhao Yang and Jana Niemz for valuable discussions.
Footnotes
Funding Sources
This work was supported by the Deutsche Forschungsgemeinschaft (SFB854 project B16 to JH). Work in the group of AS is partially funded by the Helmholtz Association (Young Investigator grant number VH-NG-727). PS gratefully acknowledges support by the President's Initiative and Networking Funds of the Helmholtz Association of German Research Centers (HGF) under contract number VH-GS-202.
Authors' Contribution
A.E., A.B., J.P., M.P., P.C., and P.S. performed experiments and interpreted data. N.E. and M.H. provided expertise and feedback. A.S. and P.D. provided reagents. A.E., A.B., J.P., M.P., P.D. and J.H. designed research, interpreted data, and wrote the manuscript.
Conflict of Interest
The authors declared no conflict of interest.
References
- 1.Huehn J, Beyer M. Epigenetic and transcriptional control of Foxp3 regulatory T cells. Semin Immunol. 2015;27:10–8. [DOI] [PubMed] [Google Scholar]
- 2.Miyara M, Ito Y, Sakaguchi S. T-cell therapies for autoimmune rheumatic diseases. Nat Rev Rheumatol. 2014. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27:109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oldenhove G, Bouladoux N, Wohlfert EA, Hall JA, Chou D, Dos Santos L, et al. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity. 2009;31:772–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakaguchi S, Vignali DA, Rudensky AY, Niec RE, Waldmann H. The plasticity and stability of regulatory T cells. Nat Rev Immunol. 2013;13:461–7. [DOI] [PubMed] [Google Scholar]
- 6.Bhela S, Varanasi SK, Jaggi U, Sloan SS, Rajasagi NK, Rouse BT. The Plasticity and Stability of Regulatory T Cells during Viral-Induced Inflammatory Lesions. J Immunol. 2017;199:1342–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Z, Zhang W, Guo J, Gu Q, Zhu X, Zhou X. Activation and Functional Specialization of Regulatory T Cells Lead to the Generation of Foxp3 Instability. J Immunol. 2017;198:2612–25. [DOI] [PubMed] [Google Scholar]
- 8.Toomer KH, Malek TR. Cytokine Signaling in the Development and Homeostasis of Regulatory T cells. Cold Spring Harb Perspect Biol. 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pasztoi M, Bonifacius A, Pezoldt J, Kulkarni D, Niemz J, Yang J, et al. Yersinia pseudotuberculosis supports Th17 differentiation and limits de novo regulatory T cell induction by directly interfering with T cell receptor signaling. Cell Mol Life Sci. 2017;74:2839–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viboud GI, Bliska JB. Yersinia outer proteins: role in modulation of host cell signaling responses and pathogenesis. Annu Rev Microbiol. 2005;59:69–89. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, Mei M, Yu C, Shen W, Ma L, He J, Yi L. The Functions of Effector Proteins in Yersinia Virulence. Pol J Microbiol. 2016;65:5–12. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann C, Pop M, Leemhuis J, Schirmer J, Aktories K, Schmidt G. The Yersinia pseudotuberculosis cytotoxic necrotizing factor (CNFY) selectively activates RhoA. J Biol Chem. 2004;279:16026–32. [DOI] [PubMed] [Google Scholar]
- 13.Schweer J, Kulkarni D, Kochut A, Pezoldt J, Pisano F, Pils MC, et al. The Cytotoxic Necrotizing Factor of Yersinia pseudotuberculosis (CNFY) Enhances Inflammation and Yop Delivery during Infection by Activation of Rho GTPases. PLoS Pathog. 2013;9:e1003746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolters M, Boyle EC, Lardong K, Trulzsch K, Steffen A, Rottner K, et al. Cytotoxic necrotizing factor-Y boosts Yersinia effector translocation by activating Rac protein. J Biol Chem. 2013;288:23543–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saoudi A, Kassem S, Dejean A, Gaud G. Rho-GTPases as key regulators of T lymphocyte biology. Small GTPases. 2014;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Kim J, Boussiotis VA. Rap1A regulates generation of T regulatory cells via LFA-1-dependent and LFA-1-independent mechanisms. Cell Immunol. 2010;266:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arencibia I, Suarez NC, Wolf-Watz H, Sundqvist KG. Yersinia invasin, a bacterial beta1-integrin ligand, is a potent inducer of lymphocyte motility and migration to collagen type IV and fibronectin. J Immunol. 1997;159:1853–9. [PubMed] [Google Scholar]
- 18.Maldonado-Arocho FJ, Green C, Fisher ML, Paczosa MK, Mecsas J. Adhesins and host serum factors drive Yop translocation by yersinia into professional phagocytes during animal infection. PLoS Pathog. 2013;9:e1003415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deuschle E, Keller B, Siegfried A, Manncke B, Spaeth T, Koberle M, et al. Role of beta1 integrins and bacterial adhesins for Yop injection into leukocytes in Yersinia enterocolitica systemic mouse infection. Int J Med Microbiol. 2016;306:77–88. [DOI] [PubMed] [Google Scholar]
- 20.Huehn J, Siegmund K, Lehmann JC, Siewert C, Haubold U, Feuerer M, et al. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J Exp Med. 2004;199, 303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sadana P, Monnich M, Unverzagt C, Scrima A. Structure of the Y. pseudotuberculosis adhesin InvasinE. Protein Sci. 2017;26:1182–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sadana P, Geyer R, Pezoldt J, Helmsing S, Huehn J, Hust M, et al. The invasin D protein from Yersinia pseudotuberculosis selectively binds the Fab region of host antibodies and affects colonization of the intestine. J Biol Chem. 2018;293:8672–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grainger JR, Smith KA, Hewitson JP, McSorley HJ, Harcus Y, Filbey KJ, et al. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-beta pathway. J Exp Med. 2010;207:2331–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shafiani S, Dinh C, Ertelt JM, Moguche AO, Siddiqui I, Smigiel KS, et al. Pathogen-specific Treg cells expand early during mycobacterium tuberculosis infection but are later eliminated in response to Interleukin-12. Immunity. 2013;38:1261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boer MC, Joosten SA, Ottenhoff TH. Regulatory T-Cells at the Interface between Human Host and Pathogens in Infectious Diseases and Vaccination. Front Immunol. 2015;6, 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garib FY, Rizopulu AP. T-Regulatory Cells as Part of Strategy of Immune Evasion by Pathogens. Biochemistry (Mosc). 2015;80:957–71. [DOI] [PubMed] [Google Scholar]
- 27.Kao JY, Zhang M, Miller MJ, Mills JC, Wang B, Liu M, et al. Helicobacter pylori immune escape is mediated by dendritic cell-induced Treg skewing and Th17 suppression in mice. Gastroenterology. 2010;138:1046–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klages K, Mayer CT, Lahl K, Loddenkemper C, Teng MW, Ngiow SF, et al. Selective depletion of Foxp3+ regulatory T cells improves effective therapeutic vaccination against established melanoma. Cancer Res. 2010;70, 7788–99. [DOI] [PubMed] [Google Scholar]
- 29.Yao T, Mecsas J, Healy JI, Falkow S, Chien Y. Suppression of T and B lymphocyte activation by a Yersinia pseudotuberculosis virulence factor, yopH. J Exp Med. 1999;190:1343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerke C, Falkow S, Chien YH. The adaptor molecules LAT and SLP-76 are specifically targeted by Yersinia to inhibit T cell activation. J Exp Med. 2005;201:361–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao Y, Guan K, He X, Wei C, Zheng Z, Zhang Y, et al. Yersinia YopJ negatively regulates IRF3-mediated antibacterial response through disruption of STING-mediated cytosolic DNA signaling. Biochim Biophys Acta. 2016;1863:3148–59. [DOI] [PubMed] [Google Scholar]
- 32.Ruter C, Buss C, Scharnert J, Heusipp G, Schmidt MA. A newly identified bacterial cell-penetrating peptide that reduces the transcription of pro-inflammatory cytokines. J Cell Sci. 2010;123:2190–8. [DOI] [PubMed] [Google Scholar]
- 33.Roberts NJ, Zhang L, Janku F, Collins A, Bai RY, Staedtke V, et al. Intratumoral injection of Clostridium novyi-NT spores induces antitumor responses. Sci Transl Med. 2014;6:249ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Felgner S, Kocijancic D, Frahm M, Heise U, Rohde M, Zimmermann K, et al. Engineered Salmonella enterica serovar Typhimurium overcomes limitations of anti-bacterial immunity in bacteria-mediated tumor therapy. Oncoimmunology. 2018;7:e1382791. [DOI] [PMC free article] [PubMed] [Google Scholar]