Abstract
Although cellular senescence may be a protective mechanism in modulating proliferative capacity, fibroblast senescence is now recognized as a key pathogenic mechanism in idiopathic pulmonary fibrosis (IPF). In aged mice, abundance and persistence of apoptosis-resistant senescent fibroblasts play a central role in nonresolving lung fibrosis after bleomycin challenge. Therefore, we investigated whether quercetin can restore the susceptibility of senescent IPF fibroblasts to proapoptotic stimuli and mitigate bleomycin-induced pulmonary fibrosis in aged mice. Unlike senescent normal lung fibroblasts, IPF lung fibroblasts from patients with stable and rapidly progressing disease were highly resistant to Fas ligand (FasL)-induced and TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis. Senescent IPF fibroblasts exhibited decreased expression of FasL and TRAIL receptors and caveolin-1, as well as increased AKT activation, compared with senescent normal lung fibroblasts. Although quercetin alone was not proapoptotic, it abolished the resistance to FasL- or TRAIL-induced apoptosis in IPF fibroblasts. Mechanistically, quercetin upregulated FasL receptor and caveolin-1 expression and modulated AKT activation. In vivo quercetin reversed bleomycin-induced pulmonary fibrosis and attenuated lethality, weight loss, and the expression of pulmonary senescence markers p21 and p19-ARF and senescence-associated secretory phenotype in aged mice. Collectively, these data indicate that quercetin reverses the resistance to death ligand–induced apoptosis by promoting FasL receptor and caveolin-1 expression and inhibiting AKT activation, thus mitigating the progression of established pulmonary fibrosis in aged mice. Therefore, quercetin may be a viable therapeutic option for IPF and other age-related diseases that progress with the accumulation of senescent fibroblasts.
Keywords: flavonoid, cellular senescence, lung fibrosis, aging
Idiopathic pulmonary fibrosis (IPF) is the most common and lethal form of idiopathic interstitial pneumonia, despite the advent of therapeutic interventions. It is characterized histologically by the presence of usual interstitial pneumonia and fibroblastic foci, which are speculated to be the site of active tissue remodeling (1). The disease course of patients with IPF is highly variable, with a subset of patients exhibiting disease stability for prolonged periods of time (stable IPF), whereas other patients exhibit rapid disease progression and deterioration (rapid IPF) (2, 3). Although progression is a key feature of IPF, little is known about what dictates clinical progression of this disease.
Cellular senescence is a state of permanent growth arrest combined with stereotyped phenotypic changes that have important role(s) in maintaining physiological homeostasis (4). However, accumulating evidence indicates that senescent cells may also have causal and/or contributing roles in tissue remodeling and many age-related diseases (5), including IPF and chronic obstructive pulmonary disease (6–10). In fact, it was recently shown that fibroblastic foci are comprised predominantly of senescent myofibroblasts. Furthermore, senescent fibroblasts are detected in the lungs of aged mice, where their persistence prevented the resolution of bleomycin-induced pulmonary fibrosis (11).
Senescent cells secrete proinflammatory cytokines, chemokines, and extracellular matrix proteases, which collectively constitute the senescence-associated secretory phenotype (SASP) (6, 12). The SASP generated by senescent fibroblasts robustly stimulates a fibrotic phenotype in healthy human fibroblasts, which may explain the correlation between their buildup and progressive fibrosis (6). Consequently, therapeutic interventions that reduce the burden of senescent cells attenuate the progression of pulmonary fibrosis and other age-related diseases (6, 11, 13–16).
Zhu and colleagues showed that the flavonoid quercetin had direct “senolytic” effects on senescent adipocytes and human umbilical vein endothelial cells by increasing the sensitivity of these cells to apoptosis (14). More recently, studies have shown therapeutic potential for quercetin associated with dasatinib (an inhibitor of multiple tyrosine kinases commonly used to treat chronic myeloid leukemia [17]) in bleomycin-induced pulmonary fibrosis in mice (6, 13). The administration of the combination of dasatinib + quercetin (D + Q) during the fibrotic phase of this model decreased both senescence biomarkers and fibrosis burden in the lungs of mice (6). The beneficial effect of D + Q in eliminating senescent cells during lung fibrosis was also demonstrated by Lehmann and colleagues, who demonstrated that D + Q depleted senescent cells by inducing apoptosis and reduced SASP factors in primary fibrotic mouse alveolar epithelial type (AT)II cells derived from bleomycin-treated fibrotic lungs (13).
Although the combination of D + Q showed a potent capacity to clear senescent cells in vitro and in vivo, these previous studies were conducted either in mice or on normal primary cells (6, 13, 14), leading us in the present study to query whether quercetin had any effects on the apoptotic responses of senescent lung fibroblasts from patients with IPF. Moreover, the effectiveness of quercetin alone on senescent human lung fibroblasts or experimental lung fibrosis in senescent mice has not been investigated yet. Thus, considering the deleterious effects of apoptosis-resistant senescent cells in the progression of fibrosis and recent evidence for senescent cell apoptotic sensitization by quercetin, we assessed the effects of this flavonoid on apoptosis-resistant senescent IPF fibroblasts and in promoting resolution of pulmonary fibrosis in aged mice.
Methods
Additional details of the methods are provided in the data supplement.
Primary Pulmonary Fibroblasts
This study was approved by an institutional review board at Cedars-Sinai Medical Center (approval number Pro34067). Lung fibroblasts were derived from diagnostic biopsies from patients who exhibited slowly or rapidly progressing IPF (slow IPF and rapid IPF, respectively) over the first year after diagnosis (2). Primary normal lung (NL) fibroblasts were derived from nonfibrotic lung samples lacking any evidence of disease. Fibroblasts were cultured in complete media (DMEM; Lonza) containing 15% FBS (Cell Generation), 100 IU of penicillin and 100 μg/ml streptomycin (Lonza), 292 μg/ml l-glutamine (Lonza), and 100 μg/ml Primocin (InvivoGen) at 37°C and 10% CO2.
Generation of Senescent Fibroblasts
Proliferating NL and IPF lung fibroblasts were serially passaged in culture until the cells showed a senescent phenotype (flattened morphology, permanent growth arrest, and altered gene expression [upregulation of CDKN1A, CDKN2A, IL6, and IL8 genes]) and senescence-associated β-galactosidase activity (β-Galactosidase Staining Kit; BioVision).
Apoptosis Studies
Senescent lung fibroblasts (5 × 104 cells per well) were treated with quercetin (50 μM; Sigma-Aldrich), cross-linked recombinant human Fas ligand (rhFasL) (75 or 150 ng/ml; R&D Systems), cross-linked recombinant human TNF-related apoptosis-inducing ligand (rhTRAIL) (100 or 200 ng/ml; R&D Systems), and/or vehicle (0.05% DMSO in complete media) for 24 hours. rhFasL and rhTRAIL were cross-linked by mouse anti-polyHistidine monoclonal antibody (R&D Systems). Apoptosis was assessed by measuring caspase-3 activity, cell viability, and lactate dehydrogenase (LDH) release. Phase-contrast images of fibroblasts were collected using IncuCyte ZOOM Live-Cell Imaging (Essen BioScience).
Bleomycin-induced Lung Injury
Aged (≥12 mo) male and female C57BL/6 mice (Faculdade de Medicina de Ribeirão Preto- USP) received intratracheal bleomycin (1.25 U/kg) (Biossintéctica Farmacêutica, Ltda.) or saline as previously described (11, 18). Mice were treated every other day with quercetin (30 mg/kg) or vehicle (saline) intraperitoneally, starting at Day 7 after bleomycin instillation and up until the end of the experiments. Pulmonary fibrosis was quantified by hydroxyproline assay and visualized by Masson’s trichrome staining of lung sections. All animal experiments were approved by the animal use ethics committee of Universidade Estadual de Londrina (protocol number 19898.2016.55).
qPCR
Expression of genes was quantitated by real-time qPCR (ViiA 7 Real-Time PCR System; Life Technologies) using predesigned primers (Integrated DNA Technologies) and primers and probe sets (Life Technologies) (see Tables E1–E3 in the data supplement). Transcript levels were normalized to human 18S or mouse Gapdh mRNA, and the fold change in expression was calculated using DataAssist software (Life Technologies). A heat map was generated manually using the relative expression values between quercetin and vehicle (control)-treated cells.
Western Blot Analysis
Cells were lysed using lysis buffer (Cell Signaling Technology) containing protease inhibitor cocktail (Roche). An equal amount of protein was loaded into a Bolt 4–12% Bis-Tris Plus gel (Life Technologies). Samples were transferred onto a nitrocellulose membrane and blotted using specific antibodies.
Statistical Analysis
Analyses were performed using Prism software (GraphPad Software). Data were expressed as mean ± SEM and assessed for significance by one-way ANOVA followed by Tukey’s multiple comparisons test. P < 0.05 was considered significant.
Results
Senescent IPF Fibroblasts Are Resistant to Apoptosis
In the present study, cultured lung fibroblasts derived from stable and rapid IPF lung biopsies exhibited a slower growth rate than their normal counterparts at similar passages (i.e., passages 7 and 8) (Figure 1A). Furthermore, regardless of clinical progression, IPF fibroblasts senesced after significantly fewer serial passage rounds than primary NL fibroblasts (Figure E1A). Stable and rapid IPF fibroblasts expressed twofold or higher expression of CDKN1A and CDKN2A genes (Figure 1B), which encode cycle inhibitor proteins p21 and p16, respectively. Upregulated p21 and/or p16 proteins are widely used markers of cellular senescence (4). Together, these findings suggested that, unlike NL fibroblasts, IPF lung fibroblasts are much more prone to senesce in culture.
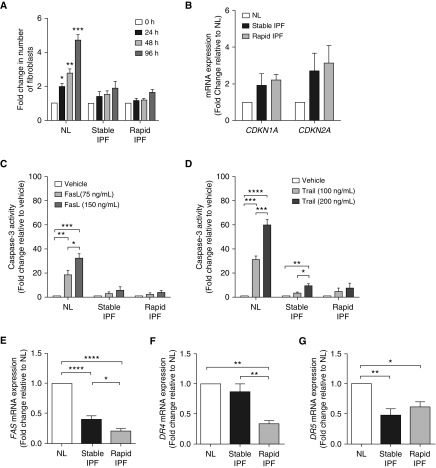
Figure 1.
Lung fibroblasts isolated from patients with idiopathic pulmonary fibrosis (IPF) exhibited a senescent phenotype and an apoptosis-resistant phenotype. Equal numbers of fibroblasts isolated from the lungs of normal patients (NL) or patients with stable or rapidly progressing IPF (stable IPF and rapid IPF, respectively) were plated simultaneously at time 0 hours. (A) The number of fibroblasts at 0, 24, 48, and 96 hours after plating were counted by flow cytometry. (B) CDKN1A and CDKN2A mRNA expression in fibroblasts at passages 7 and 8. For (C–G), replicative senescence was induced by serially passaging fibroblasts in culture until cells became senescent. Caspase-3 activity induced by (C) Fas ligand (FasL; 75 or 150 ng/ml) or (D) TNF-related apoptosis-inducing ligand (TRAIL; 100 or 200 ng/ml) and (E) FAS, (F) DR4, and (G) DR5 mRNA expression in senescent fibroblasts. Data are presented as mean ± SEM (n = 3 or 4 per group). *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; and ****P ≤ 0.0001 as indicated by the bars.
To undertake further study of senescent NL and IPF fibroblasts (n = 3 of each), we first serially passaged fibroblast lines until all cells in these cultures ceased to proliferate and acquired flattened morphology (Figures E1B). Replicative senescence was further confirmed in NL and IPF lines by upregulation of CDKN1A, CDKN2A, IL6, and IL8 expression (Figure E1C) and positive senescence-associated β-galactosidase staining in NL, stable IPF, and rapid IPF fibroblasts (data not shown). We next determined the susceptibility of senescent human fibroblasts to proapoptotic ligands. Senescent NL and IPF fibroblasts were exposed to FasL (75 or 150 ng/ml) or TRAIL (100 or 200 ng/ml) for 24 hours, and caspase-3 activity was measured. Senescent NL fibroblasts exhibited a dose-dependent increase in casapse-3 activity after exposure to FasL or TRAIL (Figures 1C and 1D). In contrast, neither stable nor rapid senescent IPF fibroblasts exhibited equivalent responsiveness to FasL- or TRAIL-induced apoptosis (i.e., caspase-3 activity) (Figures 1C and 1D). Thus, these findings demonstrate that senescent IPF fibroblasts are markedly less susceptible to FasL- and TRAIL-induced apoptosis.
Expression of Fas, DR4, and DR5 Is Decreased in Senescent IPF Fibroblasts
To determine why senescent IPF fibroblasts are resistant to FasL- and TRAIL-induced apoptosis, we next assessed the expression of Fas (i.e., FasL receptor) and death receptor (DR)4 and DR5 by qPCR. Both rapid and stable senescent IPF fibroblasts showed significantly decreased expression of FAS and DR5 compared with NL fibroblasts (Figures 1E and 1G). Senescent rapid IPF fibroblasts also showed decreased expression of DR4 compared with NL and stable IPF fibroblasts (Figure 1F). Interestingly, FAS and DR4 expression was approximately twofold lower in rapid IPF fibroblasts than in stable IPF fibroblasts. Decreased DR expression is implicated in apoptosis resistance (19–21); thus, the resistance of senescent IPF fibroblasts to apoptotic signals might be attributed to decreased expression of FasL and TRAIL receptors by these cells.
Quercetin Induces Fas Expression in Senescent IPF Lung Fibroblasts
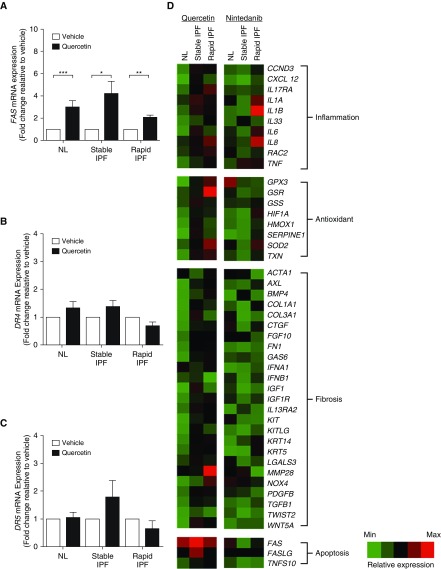
Previously, quercetin was shown to selectively kill senescent cells via induction of apoptosis (14). To determine whether quercetin modulates apoptosis in senescent IPF fibroblasts, cells were treated with quercetin (at 50 μM) or vehicle (i.e., 0.05% DMSO) for 24 hours, and FAS, DR4, and DR5 mRNA expression was quantified. Quercetin induced an approximately twofold increase in FAS mRNA expression in NL, stable IPF, and rapid IPF fibroblasts compared with vehicle-treated groups (Figure 2A). In contrast, no difference was observed in DR4 or DR5 mRNA expression in NL or IPF fibroblasts treated with quercetin compared with the vehicle groups (Figures 2B and 2C). A qPCR array revealed that quercetin had modest effects on fibrosis-associated transcripts but markedly upregulated FAS in all fibroblast groups (Figure 2D). In contrast, nintedanib (also referred to as BIBF 1120) markedly downregulated the expression of genes involved in fibrosis but did not alter FAS or FasL compared with vehicle-treated senescent fibroblasts (Figure 2D). These data demonstrate that quercetin upregulates the expression of Fas in senescent NL and IPF fibroblasts.
Figure 2.
Quercetin increased FasL receptor expression in senescent human lung fibroblasts. Effect of quercetin on (A) FAS, (B) DR4, and (C) DR5 mRNA expression in lungs of senescent normal patients (NL) and in patients with stable or rapidly progressing IPF (stable IPF and rapid IPF, respectively). (D) Heat map of the expression of inflammatory-, antioxidant-, fibrosis-, and apoptosis-related genes in lung fibroblasts from NL and stable IPF and rapid IPF treated with quercetin (50 μM, 24 h) or nintedanib (300 nM) relative to control (vehicle; DMSO 0.05%) (i.e., quercetin or nintedanib vs. vehicle). Each transcript was first normalized to the housekeeping gene 18S and is represented by a single row of colored bars. Red indicates upregulation of gene expression, and green denotes the downregulation of gene expression, compared with vehicle-treated fibroblasts. Data are presented as mean ± SEM (n = 3 or 4 per group). *P ≤ 0.05; **P ≤ 0.01; and ***P ≤ 0.001 as indicated by the bars.
Quercetin Increases the Susceptibility of Senescent Lung Fibroblasts to FasL- and TRAIL-induced Apoptosis
We next examined whether quercetin had a direct proapoptotic effect on senescent lung fibroblasts. NL and IPF fibroblasts were treated with quercetin (50 or 100 μM) or vehicle for 24 hours, and caspase-3 activity, cell viability, and LDH release were measured. Compared with the vehicle-treated groups, neither 50 μM nor 100 μM quercetin changed caspase-3 (Figure E2A), cell viability (Figure E2B), or LDH release (Figure E2C) in senescent NL or IPF fibroblasts, indicating that quercetin alone did not induce apoptosis in senescent lung fibroblasts.
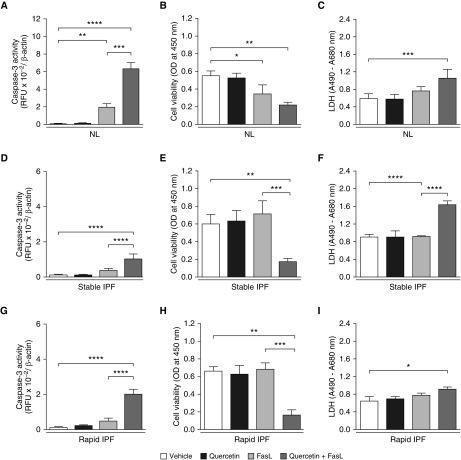
Given these findings, we next examined whether quercetin rendered senescent IPF fibroblasts susceptible to proapoptotic stimuli. NL and IPF fibroblasts were treated with FasL (75 ng/ml) for 24 hours with DMSO or quercetin (50 μM), and apoptosis was assessed using the three parameters indicated above. Neither quercetin nor FasL alone induced significant changes in caspase-3 (Figures 3A, 3D, and 3G), cell viability (Figures 3B, 3E, and 3H), or LDH release (Figures 3C, 3F, and 3I) in any of the senescent fibroblast groups. In contrast, the combination of quercetin + FasL significantly induced caspase-3 activity, reduced cell viability, and increased LDH release in NL and IPF senescent fibroblast cultures compared with fibroblasts treated with DMSO alone (Figures 3A–3I). Microscopically, a marked decrease in the number of viable cells was also observed in cultures of senescent fibroblasts treated with quercetin + FasL (Figures E3A–E3L), confirming that quercetin increased the susceptibility of both NL and IPF senescent fibroblasts to FasL-induced apoptosis.
Figure 3.
Quercetin increased the susceptibility to FasL-induced apoptosis in senescent human lung fibroblasts. Caspase-3 activity, cell viability, and lactate dehydrogenase (LDH) release by senescent lung fibroblasts from normal patients (NL) (A–C) and patients with stable (D–F) or rapidly progressing IPF (G–I) (stable IPF and rapid IPF, respectively) after 24-hour treatment with vehicle (DMSO 0.05%), quercetin (50 μM), FasL (75 ng/ml), or quercetin + FasL. Caspase-3 activity was expressed as relative fluorescence units (RFU) normalized to β-actin concentrations, cell viability as optical density (OD) values at 450 nm, and LDH release as the difference in the absorbance values at 490 and 680 nm. Data are presented as mean ± SEM (n = 3 or 4 per group). *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; and ****P ≤ 0.0001 as indicated by the bars.
Although we observed negligible effects of quercetin on DR4 and DR5 (Figures 2B and 2C), we next assessed whether quercetin altered the responsiveness of senescent IPF fibroblasts to TRAIL. Fibroblasts were treated with TRAIL (100 ng/ml) for 24 hours in the presence of DMSO or quercetin (50 μM), and changes in apoptosis parameters were assessed. Unlike monotreatment with quercetin or TRAIL, the addition of quercetin + TRAIL (100 ng/ml) significantly induced caspase-3 activity, reduced cell viability, and increased LDH release in senescent NL and IPF fibroblast cultures compared with DMSO vehicle–treated controls (Figure E4). A microscopically evident decrease in the number of cells was also observed in NL and IPF senescent fibroblast cultures treated with the combination of quercetin + TRAIL (Figures E5A–E5L). Collectively, these data demonstrate that quercetin renders senescent NL and IPF fibroblasts susceptible to FasL- and TRAIL-induced apoptosis.
Quercetin Upregulates Caveolin-1 Expression
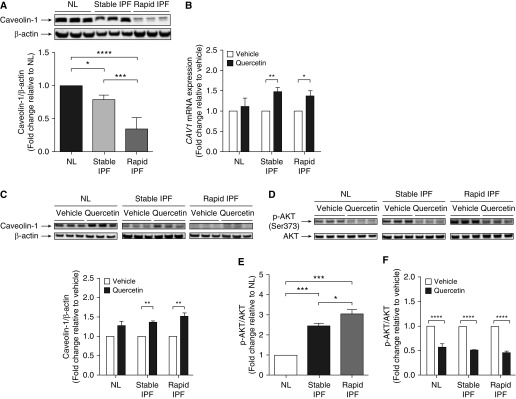
Low caveolin-1 expression in proliferating IPF fibroblasts appears to account, in part, for the reduced susceptibility of these cells to proapoptotic stimuli (22). In the present study, both stable and rapid senescent IPF fibroblasts exhibited markedly lower caveolin-1 protein expression than senescent NL fibroblasts (Figure 4A), suggesting that the association between loss of caveolin-1 and increased resistance to apoptosis is also present in senescent lung fibroblasts in IPF. We examined the effect of quercetin on caveolin-1 by measuring CAV1 mRNA by qPCR and protein expression by Western blotting. Quercetin induced an approximately 1.5-fold in CAV1 mRNA expression in stable and rapid IPF fibroblasts, but not in NL fibroblast cultures (Figure 4B). In line with this observation, caveolin-1 protein expression was significantly increased in stable and rapid IPF fibroblasts treated with quercetin (Figure 4C). It is noteworthy that although caveolin-1 protein expression was increased, quercetin did not completely restore caveolin-1 protein expression in stable or rapid IPF fibroblasts to concentrations observed in NL fibroblast cultures (Figure 4C). Thus, these findings suggest that the increased susceptibility of senescent IPF fibroblasts to proapoptotic ligands might be due, in part, to increased caveolin-1 concentrations in these cells.
Figure 4.
Quercetin modulated caveolin-1 expression and AKT activation in senescent human lung fibroblasts. (A–C) Caveolin-1 expression and (D–F) AKT activation in senescent lung fibroblasts isolated from lungs of normal patients (NL) and patients with stable or rapidly progressing IPF (stable IPF and rapid IPF, respectively). (A) Representative Western blot of basal caveolin-1/β-actin protein expression. Effect of quercetin (50 μM, 24 h) on (B) CAV1 mRNA expression and (C) caveolin-1/β-actin protein expression. (D) Representative Western blot of basal phosphorylated AKT (p-AKT)/AKT protein expression, (E) fold change in basal p-AKT/AKT protein expression, and (C) effect of quercetin (50 μM, 24 h) on p-AKT/AKT protein expression. Data are presented as mean ± SEM (n = 3 or 4 per group). *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; and ****P ≤ 0.0001 as indicated by the bars.
Increased Susceptibility to Apoptosis by Quercetin Is Independent of Cellular Redox Homeostasis
Stable and rapid IPF fibroblasts showed increased NOX4 mRNA expression (Figure E6A), hydrogen peroxide (H2O2) release (Figure E6B), and reactive oxygen species (ROS) (Figure E6C) concentrations compared with NL fibroblasts. Because previous studies have shown that increased ROS concentrations can induce the degradation of caveolin-1 and that quercetin can preserve caveolin-1 in stromal fibroblasts by reducing oxidative imbalance (23, 24), we hypothesized that quercetin might increase caveolin-1 expression in senescent IPF fibroblasts by modulating the oxidative imbalance. Although quercetin is well known for having antioxidant properties, it did not reduce NOX4 mRNA expression, H2O2 release, or ROS concentrations in NL or IPF fibroblasts. Furthermore, quercetin showed no modulatory effect on NRF2 (Figure E6D) or NRF2-responsive target gene HO1 (Figure E6E) mRNA expression, which suggests that quercetin increased caveolin-1 via a mechanism that is independent of restoring the oxidative balance in IPF fibroblasts.
Quercetin Modulates AKT Activation
AKT is a central component of the PI3K/AKT cell survival pathway (25), and AKT activation has been implicated in low caveolin-1 expression in IPF fibroblasts (26). To address whether quercetin modulates AKT activation in senescent IPF fibroblasts, we measured basal phosphorylated AKT (p-AKT) expression in NL and IPF fibroblasts (Figures 4D and 4E). In agreement with previous reports (22), p-AKT expression was significantly higher in stable and rapid IPF fibroblasts (Figure 4E). Treatment with quercetin resulted in an approximately twofold decrease in the expression of p-AKT in both NL and IPF fibroblasts (Figure 4F). Thus, one potential mechanism of quercetin-mediated increase in caveolin-1 expression and susceptibility to apoptosis is modulation of AKT activation in senescent NL and IPF fibroblasts.
Quercetin Reverses Bleomycin-induced Pulmonary Fibrosis
To test the in vivo efficacy of quercetin in resolving fibrosis in aged mice, a bleomycin-induced lung injury model in C57BL/6 mice aged 12 months or older was employed. Before testing the effect of quercetin, we assessed if supplementation with FasL and TRAIL would be necessary, because we had previously observed depletion of TRAIL concentrations in the lungs of young mice after bleomycin exposure (27). Both the depletion of TRAIL and the depletion of FasL are known to exacerbate pulmonary lung injury and fibrosis (28, 29). Aged mice received intratracheal instillation of bleomycin, and lung FASL and TRAIL mRNA expression was assessed. Bleomycin induced an approximately 1.5–3-fold increase in FASL mRNA expression between Days 7 and 28 (Figure E7A). In contrast, a twofold decrease in TRAIL mRNA expression was observed on Day 7 after bleomycin lung injury (Figure E7B). However, by Day 14, TRAIL mRNA expression was not significantly different between bleomycin- and saline-treated mice, suggesting that the supplementation with these apoptotic ligands was not necessary in this model.
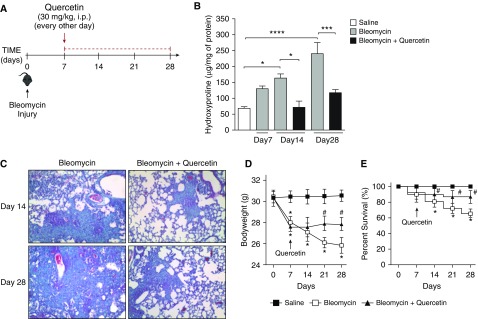
To assess the therapeutic efficacy of quercetin in this model, hydroxyproline concentrations, Masson’s trichrome staining of histological lung tissue sections, weight loss, and lethality were assessed (Figure 5). On Day 7 post-bleomycin instillation, an increase in collagen deposition in the lungs was noted, as shown by the significant increase in lung hydroxyproline concentrations (∼2-fold) (Figure 5B) and Masson’s trichrome blue staining for collagen (Figure E8A). Moreover, bleomycin-treated mice showed significant weight loss by Day 7 (Figure 5D). Given that the efficacy of therapeutics in this model is best determined after the inflammatory phase (7 d after bleomycin administration) (30), aged mice were treated with quercetin (30 mg/kg) 7 days after bleomycin administration and every other day thereafter until the end of the experimental time course (i.e., Day 14 or 28 after bleomycin administration) (Figure 5A). Compared with saline, bleomycin significantly increased hydroxyproline concentrations (Figure 5B) and collagenous Masson’s trichrome blue staining (Figure 5C) in the lungs of mice 14 and 28 days after administration. Bleomycin-induced weight loss and lethality were also observed after 14, 21, and 28 days (Figures 5D and 5E, respectively). Compared with vehicle, quercetin (30 mg/kg) significantly reduced all of the aforementioned parameters, indicating that therapeutic administration of quercetin was protective in aged mice with established pulmonary fibrosis induced by bleomycin.
Figure 5.
Quercetin reversed established bleomycin-induced pulmonary fibrosis. Aged C57BL/6 mice received intratracheal instillation of bleomycin (1.25 U/kg) or saline (equal volume). On Day 7 post–bleomycin-induced injury, treatment with quercetin (30 mg/kg i.p.) or vehicle (saline) was initiated. Mice were treated every other day over a period of 1 or 3 weeks (Day 14 or 28 endpoints, respectively). (A) Schematic diagram illustrating the time course of treatment with quercetin after bleomycin-induced lung injury. The effect of quercetin on pulmonary fibrosis was assessed by (B) quantitative lung hydroxyproline assay and confirmed by (C) Masson’s trichrome blue staining for collagen (magnification, 100×). (D) Body weight (g) and (E) survival rates (percent survival) are presented at 7-day intervals. Data are presented as mean ± SEM (n = 7–10 mice per group). *P ≤ 0.05; ***P ≤ 0.001; and ****P ≤ 0.0001 compared with saline group; #P ≤ 0.05 compared with bleomycin group or as indicated by the bars.
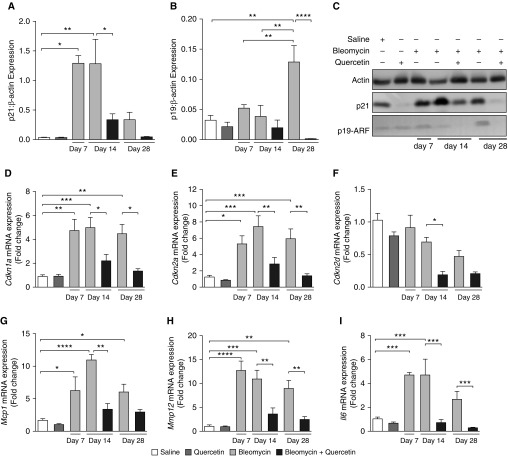
Quercetin Reduces the Expression of Senescence Markers p21 and p19-ARF and SASP in the Lungs of Aged Mice
To investigate whether the therapeutic efficacy of quercetin in this model was due to the reduction in the number of senescent cells in the lungs of mice, we investigated the effect of quercetin on the expression of p21 and p19-ARF (Figures 6A–6C). Intratracheal administration of bleomycin resulted in increased expression of p21 at Days 7, 14, and 28 postinstillation. Unlike p21, p19-ARF expression increased only at the latest time point. Treatment with quercetin (at 30 mg/kg) resulted in a significant reduction in bleomycin-induced p21 expression at Day 14 and a sevenfold decrease in this cell marker of senescence at Day 28 in the lungs of aged mice. The expression of p19-ARF was also reduced in the lungs of quercetin-treated mice at Day 28 after bleomycin. In line with these findings, Cdkn1a, Cdkn2a, and Cdkn2d mRNA expression was also reduced by treatment with quercetin. Moreover, the expression of Mcp1, Mmp12, and Il6 transcripts of the SASP were also mitigated by quercetin (Figures 6D–6I), suggesting that the therapeutic effect of quercetin in aged mice with bleomycin-induced pulmonary fibrosis was due to the reduction of the senescent cell burden in the lungs.
Figure 6.
Quercetin reduced the expression of senescence markers p21 and p19-ARF and senescence-associated secretory phenotype in the lungs of aged mice. Aged C57BL/6 mice received intratracheal instillation of bleomycin (1.25 U/kg) or saline (equal volume). On Day 7 after bleomycin-induced injury, treatment with quercetin (30 mg/kg i.p.) or vehicle (saline) was initiated. Mice were treated every other day over a period of 1 or 3 weeks (Day 14 or 28 endpoints, respectively). (A) p21, (B) p19-ARF, (C) representative Western blots of p21/β-actin and p21/β-actin, (D) Cdkn1a, (E) Cdkn2a, (F) Cdkn2d, (G) Mcp1, (H) Mmp12, and (I) Il6. Data are presented as mean ± SEM (n = 7–10 mice per group). *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; and ****P ≤ 0.0001 as indicated.
Discussion
Although IPF is a fibroproliferative disease, there is growing evidence of increased fibroblast senescence (6, 31), particularly within the fibroblastic foci of IPF lung biopsies (11). During bleomycin-induced pulmonary fibrosis in aged mice, an apoptosis-resistant phenotype favors the persistence of senescent myofibroblasts, and these cells contribute to a nonresolving, progressive lung fibrosis (11, 32). The secretome of senescent fibroblasts robustly stimulates a fibrotic phenotype in healthy human fibroblasts and promotes senescence of the surrounding cells (6, 33). In line with this concept, the clearance of senescent cells favors the resolution of fibrosis and improves pulmonary function in mice (6, 11, 13, 34). Therefore, targeting of these cells is recognized as a promising therapeutic approach for IPF (16, 35, 36).
In the present study, we provide evidence that primary lung fibroblasts derived from patients with IPF (regardless of the pace of disease progression) exhibited a senescent phenotype. Specifically, lung fibroblasts from patients with slow or rapid IPF exhibited 1) a limited replicative capacity in vitro, 2) upregulation of genes that encode for cell cycle inhibitor proteins, and 3) senescence-associated β-galactosidase staining (data not shown). More notably, we observed that, unlike senescent NL fibroblasts, senescent IPF fibroblasts were highly resistant to FasL- or TRAIL-induced apoptosis. This resistance to ligand-induced apoptosis was abolished with the coaddition of quercetin and either of these apoptosis-inducing ligands to cultures of senescent IPF fibroblasts. Quercetin appeared to target multiple mechanisms in senescent IPF fibroblasts that have been implicated in the resistance of proliferating IPF fibroblasts to apoptosis, including FasL receptor and caveolin-1 expression, and AKT activation. Thus, quercetin promoted responsiveness of senescent IPF fibroblasts to proapoptotic ligands. In vivo treatment with quercetin ameliorated the progression of pulmonary fibrosis, weight loss, and lethality in the bleomycin-challenged aged mice.
Decreased expression of proapoptotic DRs is a key component in the loss of susceptibility to apoptosis in many cell types (19, 20, 26, 37); thus, reduced expression of Fas, DR4, and DR5 might contribute to apoptosis resistance in senescent IPF fibroblasts. Numerous studies have reported lower Fas expression in fibroblasts from fibrotic lung diseases (21), including IPF (26, 37), than normal fibroblasts. However, before the present study, the expression levels of DR4 and DR5 in fibroblasts had not been reported in IPF, to our knowledge. Likewise, quercetin was known to sensitize leukemia and cancer cell lines to FasL, TRAIL, and other proapoptotic stimuli (38, 39), but these types of studies were lacking in the context of cells from patients with IPF. Although a recent study demonstrated a direct proapoptotic (or senolytic) effect of this compound on various senescent cell types (14), we did not observe a direct proapoptotic effect with similar or higher doses of quercetin (50–100 μM) on senescent IPF fibroblasts. A plausible explanation for this divergence might be cell, tissue, and/or disease specific (14). We observed that senescent IPF fibroblasts showed significantly lower expression of both FasL and agonist TRAIL receptors than senescent NL fibroblasts. Quercetin treatment enhanced FAS, but not DR4 or DR5, expression in senescent IPF fibroblasts, suggesting that the increased susceptibility of these cells to FasL-induced apoptosis could be attributed to enhanced Fas expression. In agreement with this, quercetin was found to increase the expression of genes in the Fas/apoptosis pathway in both senescent NL and IPF fibroblasts. Interestingly, this effect seemed to be specific to quercetin because two other flavonoids tested, namely naringenin and silymarin, did not show similar effects (data not shown). The enhanced responsiveness of quercetin-treated IPF fibroblasts to TRAIL-induced apoptosis appeared to be independent of changes in either DR4 or DR5, presumably indicating that quercetin might be modulating the expression/activation of DcR1 or DcR2, two receptors that antagonize the proapoptotic functions of TRAIL (40). Thus, although quercetin alone did not appear to have direct proapoptotic effects on senescent NL or IPF fibroblasts, quercetin can render senescent, apoptosis-resistant IPF fibroblasts susceptible to apoptosis induced by FasL and TRAIL.
Caveolin-1 is a main constituent of cellular membrane structures termed caveolae, and low caveolin-1 expression results in reduced Fas membrane expression (26, 41). In line with the previously established role for caveolin-1 deficiency in the apoptosis resistance observed in IPF fibroblasts (26, 42), we observed that caveolin-1 expression was markedly reduced in senescent stable and rapid IPF fibroblasts. Because it was previously shown that the sensitivity to proapoptotic stimuli is improved when caveolin-1 concentrations are increased in fibroblasts (26, 43), we speculated that quercetin might increase caveolin-1 expression in IPF fibroblasts, thereby increasing the susceptibility of these cells to proapoptotic stimuli. Indeed, treatment with quercetin increased both caveolin-1 mRNA and protein expression in senescent IPF cells. In contrast to previous data showing that quercetin preserves caveolin-1 concentrations in fibroblasts via the reduction of oxidative stress, quercetin did not alter ROS concentrations or the expression of Nrf2 or its downstream target heme oxygenase-1 in senescent IPF fibroblasts. It is therefore unlikely that quercetin preserves caveolin-1 by modulating the oxidative balance in these cells. These results were counterintuitive, owing to the remarkable antioxidant properties of quercetin in other systems (44). It is noteworthy that quercetin may modulate Fas expression via caveolin-1, which coincides with our finding that quercetin increased both caveolin-1 and Fas expression in senescent IPF fibroblasts. Thus, our findings support a role for quercetin in the upregulation of caveolin-1 transcript and protein expression rather than protecting loss of caveolin-1 concentrations owing to oxidative degradation in senescent IPF fibroblasts.
AKT is a central component of the PI3K/AKT prosurvival pathway, and aberrant AKT activation promotes apoptosis resistance (25, 26). In IPF, pathological activation of the PI3K/AKT pathway has been attributed to low caveolin-1 expression in fibroblasts. Low caveolin-1 at the plasma membrane creates a membrane microenvironment depleted of PTEN phosphatase activity, thereby favoring sustained PI3K/AKT activation (22). Supporting this concept, upregulating caveolin-1 expression results in decreased PI3K/AKT activation, which restores the susceptibility of fibroblasts to apoptosis (26, 43). In the present study, we confirmed that both stable and rapid senescent IPF fibroblasts exhibited increased AKT activation compared with senescent NL fibroblasts. Our present data suggest that quercetin might render senescent IPF fibroblasts susceptible to proapoptotic stimuli via the caveolin-1–dependent regulation of AKT activation. However, an inverse relationship between caveolin-1 and AKT activity cannot be excluded, because Nho and colleagues showed that low caveolin-1 expression is a result of increased activation of the PI3K/AKT pathway (26). Thus, although the mechanism whereby quercetin inhibits AKT activation requires further investigation, it is likely that the reduced activation of this antiapoptotic signaling pathway after quercetin treatment restores the susceptibility of senescent IPF fibroblasts to apoptosis.
Our in vivo results strongly support therapeutic potential for quercetin in IPF. First, quercetin mitigated fibrosis and promoted overall health benefit in established pulmonary fibrosis in mice. Treatment with quercetin was initiated 7 days after bleomycin-induced lung injury (i.e., when increased hydroxyproline concentrations and collagen deposition in the lung tissue were already observed). Second, quercetin alone reduced the expression of senescent cell markers p21 and p19-ARF (11, 13, 34) and cell cycle inhibitors Cdkn1a, Cdkn2a, and Cdkn3a transcripts and inhibited the progression of lung fibrosis in mice. Senescent cells express many of the signaling cascades that are also present in persistent fibroproliferation; thus, their clearance favors the resolution of fibrosis and recovery of lung function (6, 11, 34). Importantly, in the present study, quercetin also mitigated the expression of SASP-related transcripts (Mcp1, Mmp12, and Il6), further highlighting the therapeutic potential of quercetin. The secretome of senescent fibroblasts contains an array of factors with established roles in regulating fibrotic and inflammatory aspects of IPF (6).
Although previous studies had already shown that quercetin decreases the burden of senescent cells and fibrosis in experimental pulmonary fibrosis (6, 13), these effects were not produced by quercetin alone. Quercetin was associated with the drug dasatinib, which may have several off-target effects (17, 45). It is noteworthy that dasatinib was recently shown to increase the susceptibility to pulmonary hypertension in experimental models and in patients with chronic myeloid leukemia (46). Pulmonary hypertension is a well-recognized comorbidity in IPF (47); therefore, the use of dasatinib in patients with IPF could have potential aggravating effects in the long term.
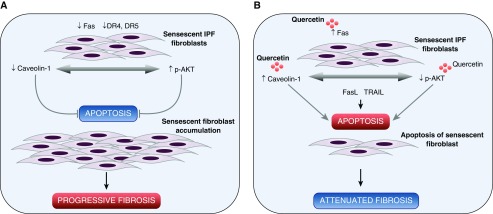
In conclusion, we demonstrate that the flavonoid quercetin renders senescent IPF fibroblasts susceptible to apoptosis induced by FasL and TRAIL, and we provide insight into the mechanisms that are associated with quercetin’s effect on senescent IPF fibroblasts. Specifically, quercetin regulates caveolin-1 and Fas expression and modulates AKT activation, thereby collectively accounting for the increased susceptibility of senescent fibroblasts to undergo apoptosis (Figure 7). In vivo quercetin also demonstrated promising therapeutic potential. Quercetin inhibited the progression of lung fibrosis, reduced the expression of senescent cell markers and SASP, and promoted overall health benefit in an experimental fibrosis model in aged mice. Last, we conclude that the data provided in our study are very promising and may add to current therapeutic strategies for IPF and other fibrotic disorders.
Figure 7.
Schematic illustration of the mechanism by which quercetin renders senescent lung IPF fibroblasts susceptible to apoptosis by FasL and TRAIL. (A) Senescent lung fibroblasts derived from patients with IPF (regardless of the pace of disease progression) exhibited a senescent state. Decreased expression of FasL and TRAIL receptors, Fas and death receptor (DR) 4 and DR5, respectively, and caveolin-1 proteins, in addition to increased AKT activation, confer senescent IPF lung fibroblasts with apoptosis resistance to ligands such as FasL and/or TRAIL. The apoptosis-resistant phenotype favors the accumulation of senescent IPF lung fibroblasts and most likely progressive lung fibrosis. (B) Treatment with quercetin produces notable increases in Fas and caveolin-1 expression and modulates AKT activation in senescent IPF lung fibroblasts, thereby restoring susceptibility of senescent fibroblasts to proapoptotic FasL and/or TRAIL and favoring the resolution of fibrosis.
Supplementary Material
Footnotes
Supported by A Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (150559/2017-8) scholarships (M.S.H.), a CNPq fellowship (308052/2013-7 [W.A.V.]), funding from Cedars Sinai Medical Center (C.M.H.), and an NIH R01 grant (HL123899 [C.M.H.]).
Author Contributions: Conception and design: M.S.H., D.M.H., and C.M.H.; acquisition of data: M.S.H. and A.L.C.; analysis and interpretation of data: M.S.H., D.M.H., and C.M.H.; and drafting the manuscript and intellectual content: M.S.H., D.M.H., W.A.V., and C.M.H.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2017-0289OC on August 15, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Habiel DM, Hogaboam C. Heterogeneity in fibroblast proliferation and survival in idiopathic pulmonary fibrosis. Front Pharmacol. 2014;5:2. doi: 10.3389/fphar.2014.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trujillo G, Meneghin A, Flaherty KR, Sholl LM, Myers JL, Kazerooni EA, et al. TLR9 differentiates rapidly from slowly progressing forms of idiopathic pulmonary fibrosis. Sci Transl Med. 2010;2:57ra82. doi: 10.1126/scitranslmed.3001510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinez FJ, Safrin S, Weycker D, Starko KM, Bradford WZ, King TE, Jr, et al. IPF Study Group. The clinical course of patients with idiopathic pulmonary fibrosis. Ann Intern Med. 2005;142:963–967. doi: 10.7326/0003-4819-142-12_part_1-200506210-00005. [DOI] [PubMed] [Google Scholar]
- 4.Tominaga K. The emerging role of senescent cells in tissue homeostasis and pathophysiology. Pathobiol Aging Age Relat Dis. 2015;5:27743. doi: 10.3402/pba.v5.27743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Childs BG, Durik M, Baker DJ, van Deursen JM. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med. 2015;21:1424–1435. doi: 10.1038/nm.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ, et al. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun. 2017;8:14532. doi: 10.1038/ncomms14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dagouassat M, Gagliolo JM, Chrusciel S, Bourin MC, Duprez C, Caramelle P, et al. The cyclooxygenase-2–prostaglandin E2 pathway maintains senescence of chronic obstructive pulmonary disease fibroblasts. Am J Respir Crit Care Med. 2013;187:703–714. doi: 10.1164/rccm.201208-1361OC. [DOI] [PubMed] [Google Scholar]
- 8.Minagawa S, Araya J, Numata T, Nojiri S, Hara H, Yumino Y, et al. Accelerated epithelial cell senescence in IPF and the inhibitory role of SIRT6 in TGF-β-induced senescence of human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2011;300:L391–L401. doi: 10.1152/ajplung.00097.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alder JK, Chen JJ, Lancaster L, Danoff S, Su SC, Cogan JD, et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci USA. 2008;105:13051–13056. doi: 10.1073/pnas.0804280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sundar IK, Rashid K, Gerloff J, Li D, Rahman I. Genetic ablation of p16INK4a does not protect against cellular senescence in mouse models of chronic obstructive pulmonary disease/emphysema. Am J Respir Cell Mol Biol. 2018;59:189–199. doi: 10.1165/rcmb.2017-0390OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hecker L, Logsdon NJ, Kurundkar D, Kurundkar A, Bernard K, Hock T, et al. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci Transl Med. 2014;6:231ra47. doi: 10.1126/scitranslmed.3008182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coppé JP, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehmann M, Korfei M, Mutze K, Klee S, Skronska-Wasek W, Alsafadi HN, et al. Senolytic drugs target alveolar epithelial cell function and attenuate experimental lung fibrosis ex vivo. Eur Respir J. 2017;50:1602367. doi: 10.1183/13993003.02367-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14:644–658. doi: 10.1111/acel.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ovadya Y, Krizhanovsky V. Strategies targeting cellular senescence. J Clin Invest. 2018;128:1247–1254. doi: 10.1172/JCI95149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pardo A, Selman M. Lung fibroblasts, aging, and idiopathic pulmonary fibrosis. Ann Am Thorac Soc. 2016;13(Suppl 5):S417–S421. doi: 10.1513/AnnalsATS.201605-341AW. [DOI] [PubMed] [Google Scholar]
- 17.Aguilera DG, Tsimberidou AM. Dasatinib in chronic myeloid leukemia: a review. Ther Clin Risk Manag. 2009;5:281–289. doi: 10.2147/tcrm.s3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helms MN, Torres-Gonzalez E, Goodson P, Rojas M. Direct tracheal instillation of solutes into mouse lung. J Vis Exp. 2010;(42):1941. doi: 10.3791/1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang XD, Nguyen T, Thomas WD, Sanders JE, Hersey P. Mechanisms of resistance of normal cells to TRAIL induced apoptosis vary between different cell types. FEBS Lett. 2000;482:193–199. doi: 10.1016/s0014-5793(00)02042-1. [DOI] [PubMed] [Google Scholar]
- 20.Huang SK, Scruggs AM, Donaghy J, Horowitz JC, Zaslona Z, Przybranowski S, et al. Histone modifications are responsible for decreased Fas expression and apoptosis resistance in fibrotic lung fibroblasts. Cell Death Dis. 2013;4:e621. doi: 10.1038/cddis.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bühling F, Wille A, Röcken C, Wiesner O, Baier A, Meinecke I, et al. Altered expression of membrane-bound and soluble CD95/Fas contributes to the resistance of fibrotic lung fibroblasts to FasL induced apoptosis. Respir Res. 2005;6:37. doi: 10.1186/1465-9921-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xia H, Khalil W, Kahm J, Jessurun J, Kleidon J, Henke CA. Pathologic caveolin-1 regulation of PTEN in idiopathic pulmonary fibrosis. Am J Pathol. 2010;176:2626–2637. doi: 10.2353/ajpath.2010.091117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mougeolle A, Poussard S, Decossas M, Lamaze C, Lambert O, Dargelos E. Oxidative stress induces caveolin 1 degradation and impairs caveolae functions in skeletal muscle cells. PLoS One. 2015;10:e0122654. doi: 10.1371/journal.pone.0122654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez-Outschoorn UE, Balliet RM, Rivadeneira DB, Chiavarina B, Pavlides S, Wang C, et al. Oxidative stress in cancer associated fibroblasts drives tumor-stroma co-evolution: a new paradigm for understanding tumor metabolism, the field effect and genomic instability in cancer cells. Cell Cycle. 2010;9:3256–3276. doi: 10.4161/cc.9.16.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Downward J. PI 3-kinase, Akt and cell survival. Semin Cell Dev Biol. 2004;15:177–182. doi: 10.1016/j.semcdb.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Nho RS, Peterson M, Hergert P, Henke CA. FoxO3a (Forkhead box O3a) deficiency protects idiopathic pulmonary fibrosis (IPF) fibroblasts from type I polymerized collagen matrix-induced apoptosis via caveolin-1 (cav-1) and Fas. PLoS One. 2013;8:e61017. doi: 10.1371/journal.pone.0061017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Habiel DM, Moreira AP, Ismailoglu UB, Dunleavy MP, Cavassani KA, van Rooijen N, et al. TRAIL-dependent resolution of pulmonary fibrosis Mediators Inflamm 2018. 20187934362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGrath EE, Lawrie A, Marriott HM, Mercer P, Cross SS, Arnold N, et al. Deficiency of tumour necrosis factor-related apoptosis-inducing ligand exacerbates lung injury and fibrosis. Thorax. 2012;67:796–803. doi: 10.1136/thoraxjnl-2011-200863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallach-Dayan SB, Elkayam L, Golan-Gerstl R, Konikov J, Zisman P, Dayan MR, et al. Cutting edge: FasL+ immune cells promote resolution of fibrosis. J Autoimmun. 2015;59:67–76. doi: 10.1016/j.jaut.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 30.Moeller A, Ask K, Warburton D, Gauldie J, Kolb M. The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int J Biochem Cell Biol. 2008;40:362–382. doi: 10.1016/j.biocel.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pechkovsky DV, Hogaboam CM, Yao E, Khalil N, Selman M, Knight DA. Fibroblast senescence in UIP/IPF: a contributing factor or consequence of the disease? [abstract] Am J Respir Crit Care Med. 2012;185:A5282. [Google Scholar]
- 32.Huang WT, Akhter H, Jiang C, MacEwen M, Ding Q, Antony V, et al. Plasminogen activator inhibitor 1, fibroblast apoptosis resistance, and aging-related susceptibility to lung fibrosis. Exp Gerontol. 2015;61:62–75. doi: 10.1016/j.exger.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adnot S, Amsellem V, Boyer L, Marcos E, Saker M, Houssaini A, et al. Telomere dysfunction and cell senescence in chronic lung diseases: therapeutic potential. Pharmacol Ther. 2015;153:125–134. doi: 10.1016/j.pharmthera.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 34.Hashimoto M, Asai A, Kawagishi H, Mikawa R, Iwashita Y, Kanayama K, et al. Elimination of p19ARF-expressing cells enhances pulmonary function in mice. JCI Insight. 2016;1:e87732. doi: 10.1172/jci.insight.87732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mailleux AA, Crestani B. Licence to kill senescent cells in idiopathic pulmonary fibrosis? Eur Respir J. 2017;50:1701360. doi: 10.1183/13993003.01360-2017. [DOI] [PubMed] [Google Scholar]
- 36.Thannickal VJ, Murthy M, Balch WE, Chandel NS, Meiners S, Eickelberg O, et al. Blue journal conference: aging and susceptibility to lung disease. Am J Respir Crit Care Med. 2015;191:261–269. doi: 10.1164/rccm.201410-1876PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wynes MW, Edelman BL, Kostyk AG, Edwards MG, Coldren C, Groshong SD, et al. Increased cell surface Fas expression is necessary and sufficient to sensitize lung fibroblasts to Fas ligation-induced apoptosis: implications for fibroblast accumulation in idiopathic pulmonary fibrosis. J Immunol. 2011;187:527–537. doi: 10.4049/jimmunol.1100447. [DOI] [PubMed] [Google Scholar]
- 38.Jacquemin G, Shirley S, Micheau O. Combining naturally occurring polyphenols with TNF-related apoptosis-inducing ligand: a promising approach to kill resistant cancer cells? Cell Mol Life Sci. 2010;67:3115–3130. doi: 10.1007/s00018-010-0407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Russo M, Palumbo R, Mupo A, Tosto M, Iacomino G, Scognamiglio A, et al. Flavonoid quercetin sensitizes a CD95-resistant cell line to apoptosis by activating protein kinase Cα. Oncogene. 2003;22:3330–3342. doi: 10.1038/sj.onc.1206493. [DOI] [PubMed] [Google Scholar]
- 40.LeBlanc HN, Ashkenazi A. Apo2L/TRAIL and its death and decoy receptors. Cell Death Differ. 2003;10:66–75. doi: 10.1038/sj.cdd.4401187. [DOI] [PubMed] [Google Scholar]
- 41.Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- 42.Sanders YY, Liu H, Scruggs AM, Duncan SR, Huang SK, Thannickal VJ. Epigenetic regulation of caveolin-1 gene expression in lung fibroblasts. Am J Respir Cell Mol Biol. 2017;56:50–61. doi: 10.1165/rcmb.2016-0034OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu J, Lee P, Galbiati F, Kitsis RN, Lisanti MP. Caveolin-1 expression sensitizes fibroblastic and epithelial cells to apoptotic stimulation. Am J Physiol Cell Physiol. 2001;280:C823–C835. doi: 10.1152/ajpcell.2001.280.4.C823. [DOI] [PubMed] [Google Scholar]
- 44.Boots AW, Haenen GR, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol. 2008;585:325–337. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 45.Steegmann JL, Cervantes F, le Coutre P, Porkka K, Saglio G. Off-target effects of BCR-ABL1 inhibitors and their potential long-term implications in patients with chronic myeloid leukemia. Leuk Lymphoma. 2012;53:2351–2361. doi: 10.3109/10428194.2012.695779. [DOI] [PubMed] [Google Scholar]
- 46.Guignabert C, Phan C, Seferian A, Huertas A, Tu L, Thuillet R, et al. Dasatinib induces lung vascular toxicity and predisposes to pulmonary hypertension. J Clin Invest. 2016;126:3207–3218. doi: 10.1172/JCI86249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harari S, Elia D, Humbert M. Pulmonary hypertension in parenchymal lung diseases: any future for new therapies? Chest. 2018;153:217–223. doi: 10.1016/j.chest.2017.06.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.