Abstract
Human rhinovirus (RV), the major cause of the common cold, triggers the majority of acute airway exacerbations in patients with asthma and chronic obstructive pulmonary disease. Nitric oxide, and the related metabolite S-nitrosoglutathione, are produced in the airway epithelium via nitric oxide synthase (NOS) 2 and have been shown to function in host defense against RV infection. We hypothesized that inhibitors of the S-nitrosoglutathione–metabolizing enzyme, S-nitrosoglutathione reductase (GSNOR), might potentiate the antiviral properties of airway-derived NOS2. Using in vitro models of RV-A serotype 16 (RV-A16) and mNeonGreen-H1N1pr8 infection of human airway epithelial cells, we found that treatment with a previously characterized GSNOR inhibitor (4-[[2-[[(3-cyanophenyl)methyl]thio]-4-oxothieno-[3,2-d]pyrimidin-3(4H)-yl]methyl]-benzoic acid; referred to as C3m) decreased RV-A16 replication and expression of downstream proinflammatory and antiviral mediators (e.g., RANTES [regulated upon activation, normal T cell expressed and secreted], CXCL10, and Mx1), and increased Nrf2 (nuclear factor erythroid 2-related factor 2)-dependent genes (e.g., SQSTM1 and TrxR1). In contrast, C3m had no effect on influenza virus H1N1pr8 replication. Moreover, a structurally dissimilar GSNOR inhibitor (N6022) did not alter RV replication, suggesting that the properties of C3m may be specific to rhinovirus owing to an off-target effect. Consistent with this, C3m antiviral effects were not blocked by either NOS inhibition or GSNOR knockdown but appeared to be mediated by reduced intercellular adhesion molecule 1 transcription and increased shedding of soluble intercellular adhesion molecule 1 protein. Collectively these data show that C3m has novel antirhinoviral properties that may synergize with, but are unrelated to, its GSNOR inhibitor activity.
Keywords: S-nitrosoglutathione reductase, inflammation, bronchial epithelial cells, rhinovirus
Rhinovirus (RV) infection is the major cause of the common cold. Owing to a large number of different serotypes of RV, no antirhinoviral drugs have reached the market. RVs are members of the Picornaviridae family of small, positive-stranded nonenveloped RNA viruses that account for the majority of acute wheezing illnesses in infants and children and virus-induced exacerbations in adults presenting with asthma and chronic obstructive pulmonary disease (1–5). The morbidity and mortality with rhinoviral infections are substantial and linked to RV-induced airway inflammation. The chemokines CXCL10 and RANTES (regulated upon activation, normal T cell expressed and secreted) are secreted by virus-infected airway epithelial cells (AECs) in response to viral infection and function as potent chemoattractants for lymphocytes, natural killer cells, monocytes, T cells, basophils and eosinophils. Both of these chemokines are suggested to contribute to the pathogenesis of RV-induced airway inflammation.
As the first line of defense against RV infection, the airway epithelium plays a critical role in regulating the inflammatory response to RV infection (6). Increased nitric oxide (NO) production is believed to be part of the host response to RV infection and has been postulated to aid in viral clearance (7). S-nitrosoglutathione (GSNO), a physiological NO molecule, has also been reported to have antiviral properties (8). Previously we showed that deletion or inhibition of S-nitrosoglutathione reductase (GSNOR) reduces metabolism of GSNO and protects against methacholine induced airway hyperresponsiveness (9). The effects of GSNOR inhibition often require nitric oxide synthase (NOS)2, the inducible NOS isoform, which is expressed after injury and inflammation by lung epithelia and macrophages. Here, we hypothesized that administration of a GSNOR inhibitor to RV-A serotype 16 (RV-A16)-infected human AECs would ameliorate RV-induced inflammation.
To address this hypothesis, we investigated the effects of two structurally distinct GSNOR inhibitors, 4-[[2-[[(3-cyanophenyl)methyl]thio]-4-oxothieno-[3,2-d]pyrimidin-3(4H)-yl]methyl]-benzoic acid (C3m) (10, 11) and N6022 (12), on RV-A16 replication and subsequent chemokine responses in human AECs. We found that C3m, but not N6022, inhibited RV-A16 replication in AECs, and the effects appeared to be independent of NOS and GSNOR activity.
Methods
Inhibitors and Viruses
C3m (13) and N6022 were synthesized by the Duke Small Molecule Facility. Lentivirus expressing nontargeting control or ADH5 shRNA (Sigma Mission TRC1 clone TRCN0000026476) was produced in HEK-293T cells by cotransfection with vesicular stomatitis virus G and delta-NRF plasmids as previously described (14). Viral production was confirmed by p24 ELISA (Cell Biolabs). Purified RV-A16 was provided by the Gern and Voelker laboratories. mNeonGreen-H1N1pr8 virus was a kind gift of Nicholas Heaton, Ph.D. (Duke University). (For further details, see the data supplement.)
Epithelial Cell Culture and Challenge
BEAS-2B cells were cultured in DMEM + 10% FBS + 1% penicillin/streptomycin on PureCol (Advanced BioMatrix)-coated plates. To generate cells stably expressing control or ADH5 shRNA, BEAS-2B cells in 6-well plates containing 2 ml of medium and 8 μg/ml hexadimethrine bromide were treated with 200 μl of lentivirus-containing medium per well. After 48 hours, stable cells were selected using 2 μg/ml puromycin.
Sections of trachea and large airway were obtained from unused segments of human donor lungs that were acquired for transplant using a study protocol approved by the institutional review board of Duke University Medical Center (protocol Pro00006669). Primary AECs were obtained using protease digestion as previously described (15). Unless noted, passage 1 AECs were plated on Transwell membranes and, at confluency, were differentiated at an air–liquid interface in a synthetic medium as described previously (16, 17). Before infection with RV-A16, cells were washed with PBS and cultured overnight in serum-free bronchial epithelial growth medium. Hydrocortisone-free medium was used for all mRNA and protein experiments (18).
Primary AECs were infected with RV-A16 (multiplicity of infection [MOI], 2) or stimulated with 100 μg/ml polyinosinic-polycytidylic acid [poly(I:C)] or vehicle (0.1% DMSO) (19, 20). GSNOR inhibitors were administered 1 hour before challenge as a pretreatment or 4 hours post-challenge as a therapeutic treatment. To test the effect of C3m on RV-A16 binding and replication, near-confluent BEAB-2B cells were pretreated in HEPES-buffered serum-free medium containing 50 μM C3m or 0.1% DMSO for 1 hour followed by RV-A16 (MOI, 3) infection for 1 hour and 4 hours or overnight, respectively. Cells were washed three times with PBS before RNA extraction. Infection with mNeonGreen-H1N1 pr8 (21) was performed on BEAS-2B cells seeded on a PureCol-coated 12-well dish. At confluency, cells were washed twice with PBS and infected with mNeonGreen-H1N1 pr8 (MOI, 2) in 200 μl of PBS + Ca2+ + Mg2+ + 1% BSA for 1 hour. Cells were washed two more times with PBS, then incubated in culture medium containing 50 μM C3m or 0.1% DMSO for 24 hours. All viral infections were performed at 34°C. (For further details of cell culture and reverse transcription RT-PCR and protein expression methods, see the data supplement.)
GSNOR Activity Assay
BEAS-2B cells expressing either random shRNA or GSNOR-knockdown ADH5 shRNA were lysed in PBS + 0.1% Nonidet P-40. After centrifugation, supernatants were adjusted to a protein concentration of 0.5 mg/ml. As a control, GSNOR was overexpressed in A549 cells using an adenovirus. These cells were lysed in the same buffer but adjusted to protein concentration at 0.1 mg/ml. GSNOR activity was measured with 200 μl of buffer alone or cell lysate in the presence of GSNO and nicotinamide adenine dinucleotide reduced, as described previously (22).
Quantitative Proteomics
Global changes in protein expression in AECs were quantified by gel-free, label-free, liquid chromatography–tandem mass spectrometry analysis of tryptic digests (see Supplemental Methods in the data supplement). A total of 18,728 peptides belonging to 3,133 proteins were quantified across all samples (see Tables E3 and E4 in the data supplement). The mass spectrometry data have been deposited in the Chorus database (https://chorusproject.org) under the project RV16/GSNORi.
Statistics
Data was expressed as mean ± SEM. Experiments were performed in triplicate. Comparisons between groups were tested with Student’s t test or one-way ANOVA using Prism version 5.1 software (GraphPad Software). P < 0.05 was considered statistically significant.
Results
C3m Inhibits Replication of RV in Primary AECs
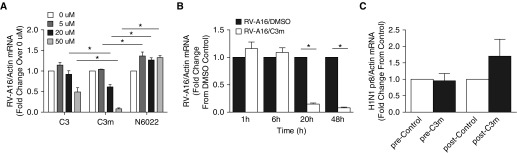
To investigate whether GSNOR activity alters RV infection, we tested the effects of two structurally distinct GSNOR inhibitors, C3m and N6022, on RV-A16 replication in infected BEAS-2B cells, an immortalized respiratory epithelial cell line. Submerged BEAS-2B cells were pretreated with increasing concentrations of C3m and N6022 (vs. vehicle control) for 1 hour, followed by 20-hour infection with RV-A16 (MOI, 3). RT-PCR analysis of total RNA 20 hours after infection demonstrated a marked decrease in RV RNA copy number in C3m–treated cells at 10 μM and 50 μM concentrations that was dose dependent, whereas N6022 treatment had no effect (Figure 1A).
Figure 1.
S-nitrosoglutathione reductase (GSNOR) inhibitor 4-[[2-[[(3-cyanophenyl)methyl]thio]-4-oxothieno-[3,2-d]pyrimidin-3(4H)-yl]methyl]-benzoic acid (C3m), but not N6022, decreases rhinovirus RV-A serotype 16 (RV-A16) replication in BEAS-2B cells and in primary human airway epithelial cells in a (A) dose-dependent and (B) time-dependent manner. (A) Viral RNA expression 20 hours postinfection (multiplicity of infection [MOI], 2) of BEAS-2B cells pretreated (1 h) with increasing concentrations of C3m (1–50 μM). (B) Time course of RV-A16 infection of primary airway epithelial cells pretreated (1 h) with C3m (50 μM). n = 3–6/group; experiments were performed in duplicate. *P < 0.05 by one-way ANOVA or Student’s t test. (C) C3m had no inhibitory effect on replication of influenza virus H1N1 pr8 in BEAS-2B cells pretreated with C3m (50 μM) for 1 hour before infection (pre) or 1 hour postinfection (post) (MOI, 2). n = 4–6/group. Experiments were performed in duplicate. Results were analyzed using Student’s t test.
To better recapitulate the pseudostratified mucociliary phenotype of the in vivo respiratory epithelium, we infected well-differentiated primary AECs grown at an air–liquid interface with RV-A16 (MOI, 2) for 1, 6, 20, or 48 hours in the presence of either DMSO (control) or C3m (50 μM). RT-PCR analysis of infected AECs showed a time-dependent decrease in RV-A16 RNA expression in the presence of C3m (Figure 1B). These data demonstrate inhibition of RV-A16 replication by C3m in AECs in a dose- and time-dependent manner.
We then sought to determine if the effects of C3m on rhinoviral replication are specific to RV or have a global effect on viral replication. BEAS-2B cells were infected with mNeonGreen-H1N1 pr8 strain of influenza A virus (MOI, 2). In contrast to RV, cellular tropism of influenza virus occurs through its preference for different conformers of sialic acid on host cell glycans, which mediates virus attachment and facilitates infection (23). As in the RV model, the cells underwent pretreatment with C3m for 1 hour or were treated at 1 hour postinfection. No inhibitory effect of C3m on mNeonGreen-H1N1 pr8 viral RNA expression was seen when administered pre- or postinfection, supporting an RV-specific effect (Figure 1C).
C3m Inhibits Downstream RV-induced Chemokine Production in AECs
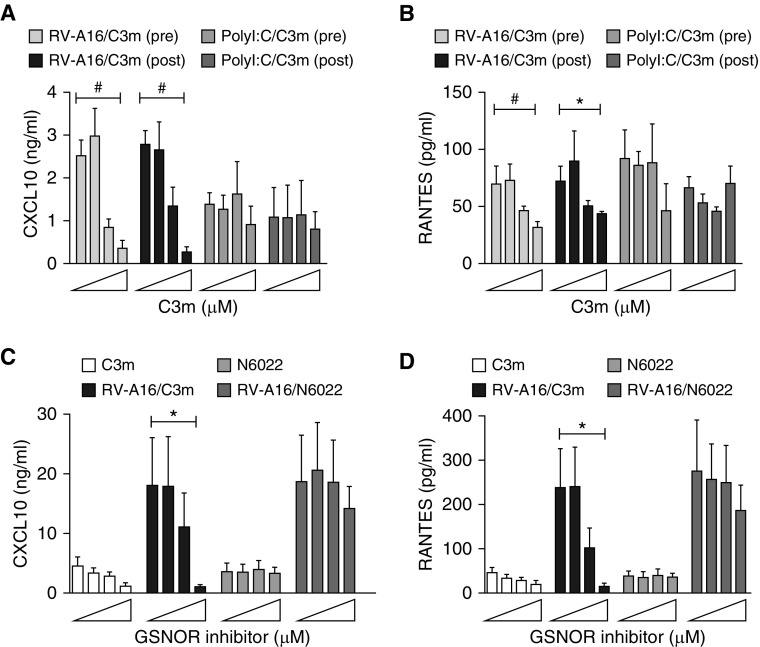
Chemokines CXCL10 and CCL5 (RANTES) are among the most highly induced cytokines upregulated after RV infection of AECs, where they function to recruit activated T lymphocytes, natural killer T cells, and macrophages to the sites of infection (18, 24–26). These chemokines are produced by virus-infected epithelial cells and are found in the respiratory secretions of patients with asthma (27–29). To determine the downstream effects of reduced RV-A16 replication on cellular antiviral and inflammatory responses, we measured CXCL10 and CCL5/RANTES protein and mRNA expression in RV-A16–infected AECs treated with increasing concentrations of C3m (0, 1, 10, and 50 μM). C3m administered either before or after infection significantly reduced protein (and mRNA; data not shown) expression of RANTES and CXCL10 in a dose-dependent manner (Figures 2A and 2B).
Figure 2.
C3m blocks RV-A16–induced RANTES (regulated upon activation, normal T cell expressed and secreted) and CXCL10 protein expression by inhibiting viral replication. (A) CXCL10 and (B) RANTES concentrations were measured in apical medium by ELISA 48 hours after RV-A16 infection or polyinosinic-polycytidylic acid (PolyI:C) stimulation. C3m (0, 1, 10, and 50 μM) and N6022 (0, 1, 10, and 50 μM) were administered 1 hour before infection (pre) or 4 hours after infection (post). (C) CXCL10 and (D) RANTES were measured as in A and B after pretreatment (1 h) with C3m or N6022. #P < 0.05; *P < 0.001 by Student’s t test. n = 5–6/group. The error bars reflect replicates of different airway epithelial cells from multiple donors. Experiments were performed in triplicate.
To mimic intracellular double-stranded RNA (dsRNA) generated normally during viral replication and to independently assess whether C3m might act directly on Toll-like receptor 3 (TLR3)-dependent signaling, a similar comparison was made using the TLR3 agonist poly(I:C). We found a dose–response effect of CXCL10 and RANTES to C3m only in RV-A16–infected AECs and not in poly(I:C)-infected AECs (Figures 2A and 2B), suggesting that C3m directly affects viral replication. Finally, we found that the inhibition of RANTES and CXCL10 expression was specific to C3m and not to N6022 (Figures 2C and 2D). Collectively, these results suggest that C3m has a modest effect on TLR3-dependent signaling and that the changes in antiviral proteins and related cytokines mostly reflect the inhibition of viral replication by C3m.
C3m Alters Global and RV-A16–inducible Protein Expression in AECs
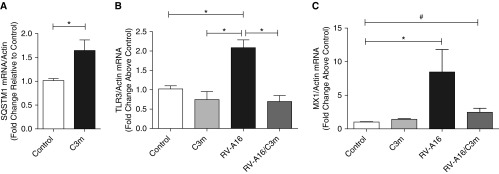
To confirm translation of this observation to human cells, we used a proteomic approach to examine the potential mechanisms, and downstream consequences, of C3m on RV-infected human AECs. Cell lysates were isolated from AECs obtained from three individual human donors after treatment with and without RV-A16 and with and without C3m (4 h postinfection; four conditions per donor); proteins were digested with trypsin; and more than 18,000 peptides, belonging to more than 3,000 proteins, were identified and quantified by liquid chromatography–tandem mass spectrometry (see Methods). We analyzed the data to identify proteins that were increased or decreased in expression by C3m treatment (independent of RV-A16 infection) and proteins that had RV-A16–inducible expression blocked by C3m treatment. The AEC proteins that were significantly upregulated by C3m treatment included numerous putative targets of the transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2), including sequestosome 1 (SQSTM1), aldo-keto reductases, glutamate cysteine ligase subunits, and thioredoxin reductase 1 (Table 1). Quantification of SQSTM1 mRNA expression after treatment with C3m alone by RT-qPCR confirmed the increase in SQSTM1 protein expression, as shown in Table 1 (see also Figure 3A).
Table 1.
Proteins with High Expression in C3m–treated versus Control*
| Primary Protein Name | Protein Description† | Protein Teller Probability | Number of Quantified Peptides | %CV QC Pool | Average Fold Change, C3m vs. Control | P Value (One-Way ANOVA) |
|---|---|---|---|---|---|---|
| GDF15_HUMAN | Growth/differentiation factor 15† | 1 | 5 | 3.24 | 5.89 | 5.76E-08 |
| SQSTM_HUMAN | Sequestosome-1† | 1 | 5 | 4.94 | 4.02 | 8.34E-06 |
| SRXN1_HUMAN | Sulfiredoxin-1† | 0.97 | 2 | 15.57 | 3.61 | 0.013 |
| TRXR1_HUMAN | Thioredoxin reductase 1, cytoplasmic† | 1 | 6 | 0.58 | 2.02 | 1.08E-05 |
| GFPT1_HUMAN | Glucosamine–fructose-6-phosphate aminotransferase [isomerizing] 1 | 1 | 14 | 2.54 | 1.88 | 6.85E-04 |
| AK1C1_HUMAN | Aldo-keto reductase family 1 member C1† | 1 | 27 | 1.21 | 1.88 | 1.11E-06 |
| NQO1_HUMAN | NAD(P)H dehydrogenase [quinone] 1† | 1 | 7 | 1.26 | 1.86 | 0.031 |
| OAT_HUMAN | Ornithine aminotransferase, mitochondrial | 1 | 11 | 2.16 | 1.82 | 0.013 |
| RISC_HUMAN | Retinoid-inducible serine carboxypeptidase | 1 | 7 | 2.82 | 1.79 | 0.017 |
| AK1C2_HUMAN | Aldo-keto reductase family 1 member C2† | 1 | 5 | 1.47 | 1.78 | 5.97E-04 |
| FKBP4_HUMAN | FK506-binding protein 4 | 1 | 13 | 1.78 | 1.75 | 0.02 |
| AK1C3_HUMAN | Aldo-keto reductase family 1 member C3† | 1 | 8 | 2.33 | 1.70 | 3.36E-06 |
| NFU1_HUMAN | NFU1 iron-sulfur cluster scaffold homolog, mitochondrial | 0.98 | 2 | 8.27 | 1.69 | 0.02 |
| AAAT_HUMAN | Neutral amino acid transporter B(0) | 1 | 6 | 2.51 | 1.66 | 0.038 |
| SYAC_HUMAN | Alanyl-tRNA synthetase, cytoplasmic | 1 | 13 | 3.40 | 1.66 | 9.25E-05 |
| ACSL4_HUMAN | Long-chain fatty-acid–CoA ligase 4 | 1 | 4 | 11.03 | 1.66 | 6.70E-10 |
| CYBP_HUMAN | Calcyclin-binding protein | 1 | 5 | 4.14 | 1.64 | 0.024 |
| XPP3_HUMAN | Probable Xaa-Pro aminopeptidase 3 | 1 | 4 | 3.68 | 1.60 | 8.78E-06 |
| UFL1_HUMAN | E3 UFM1-protein ligase 1 | 1 | 5 | 2.50 | 1.56 | 0.002 |
| MAOX_HUMAN | NADP-dependent malic enzyme | 1 | 11 | 5.31 | 1.56 | 7.36E-05 |
| GSH0_HUMAN | Glutamate–cysteine ligase regulatory subunit† | 1 | 4 | 7.16 | 1.54 | 2.64E-06 |
| HS105_HUMAN | Heat shock protein 105 kD | 1 | 18 | 1.20 | 1.54 | 2.08E-05 |
| HS90A_HUMAN | Heat shock protein HSP 90-alpha | 1 | 51 | 1.43 | 1.52 | 3.08E-13 |
| RETST_HUMAN | All-trans-retinol 13,14-reductase | 1 | 6 | 4.84 | 1.52 | 2.15E-06 |
| HTAI2_HUMAN | Oxidoreductase HTATIP2 | 1 | 6 | 0.50 | 1.51 | 1.50E-08 |
| GSH1_HUMAN | Glutamate–cysteine ligase catalytic subunit† | 1 | 20 | 1.99 | 1.49 | 4.84E-04 |
Definition of abbreviations: C3m = 4-[[2-[[(3-cyanophenyl)methyl]thio]-4-oxothieno-[3,2-d]pyrimidin-3(4H)-yl]methyl]-benzoic acid; CV = coefficient of variation; QC = quality control.
Data were filtered to include protein quantified by two or more peptides, average fold change approximately 1.5 or more between C3m and control groups. P < 0.05 (one-way ANOVA across four treatment groups).
Putative Nrf2-dependent proteins.
Figure 3.
Validation of quantitative proteomic analysis by RT-qPCR. Differentiated primary airway epithelial cells from three donors were infected (+/−) with RV-A16, followed by treatment with and without C3m 1 hour postinfection. mRNA quantification of (A) RV-A16–inducible sequestosome 1 (SQSTM1) and C3m–repressed (B) Toll-like receptor 3 (TLR3) and (C) Mx1 gene expression 48 hours postinfection. Data shown are mean ± SEM of n = 3–6/group; n = 3 replicates. *P < 0.05 by (A) Student’s t test and (B and C) one-way ANOVA. #P < 0.01.
IFN-inducible proteins, including the ubiquitin-like protein ISG15, uteroglobin (club cell secretory protein), and the IFN-induced GTP-binding protein Mx1, were among the proteins that were increased by RV-A16 infection but downregulated after C3m treatment (Table E5). These IFN-inducible genes are part of a signaling cascade that involves engagement of the pattern recognition receptor TLR3 and upregulation of IFN-inducible transcription factors, including IFN regulatory factor 1 (30, 31). We used RT-qPCR and Western blotting (data not shown) to determine the effect of C3m on TLR3 signaling in AECs. TLR3 and MX1 mRNA was significantly reduced in RV-A16–infected cells after C3m treatment (Figures 3B and 3C). These effects are consistent with a decrease in antiviral signaling and may be a consequence of reduced viral replication.
C3m Does Not Increase Cell Death of RV-infected Cells
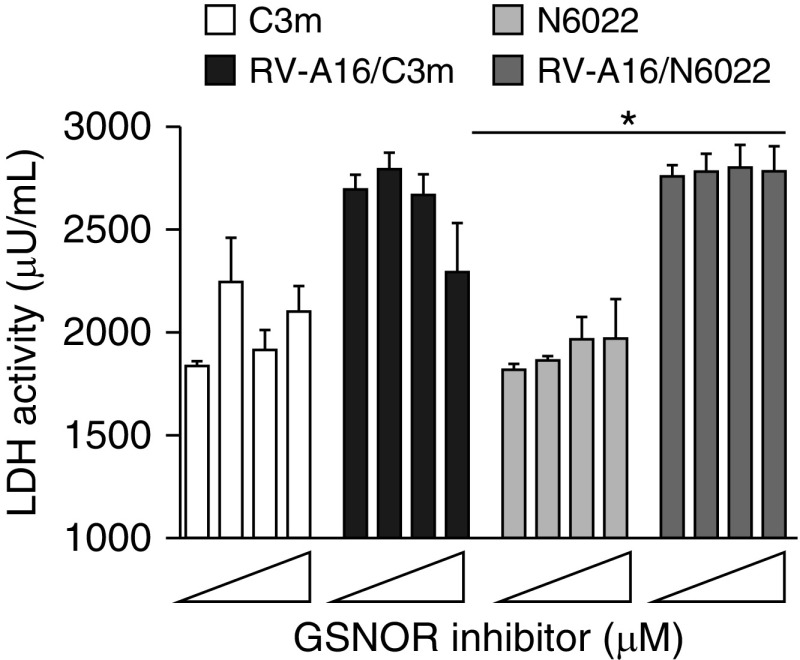
The potent inhibition of RV-A16 replication and downstream signaling in BEAS-2B cells and primary AECs, as well as the differential activity of two well-characterized in vitro inhibitors of GSNOR, led us to examine potential mechanisms. We first tested whether inhibition of RV-A16 replication might be explained by C3m–induced cytotoxicity. Compromise of cell integrity was monitored by measuring lactate dehydrogenase (LDH) activity in the apical supernatants of AECs treated with increasing concentrations of C3m (or N6022) for 1 hour followed by infection with RV-A16 (as described above). LDH activity, assessed at 48 hours postinfection, was modestly increased in RV-A16–infected cells, consistent with virus-induced cytotoxicity (Figure 4). Low doses of C3m (0, 1, and 10 μM) had no effect on LDH, with the exception of the highest C3m dose (50 μM), which parallels the decrease in viral replication observed in Figure 1. In contrast, addition of equivalent doses of N6022 had no effect on RV-induced LDH activity. Collectively, these data suggest that the inhibition of viral replication by C3m is associated with a modest reduction in RV-induced toxicity.
Figure 4.
C3m reduces RV-A16–induced cytotoxicity in human airway epithelial cells. Lactate dehydrogenase (LDH) activity measured 48 hours after RV-A16 infection (MOI, 1) of human airway epithelial cells pretreated with GSNOR inhibitors C3m and N6022 (0, 1, 10, 50 μM). Data shown are mean ± SEM of n = 3–6/group; n = 3 replicates. *P < 0.005 by one-way ANOVA.
Inhibitory Effect of C3m on Viral Replication Is Independent of NOS or GSNOR Activity
Despite being potent in vitro inhibitors of GSNOR, the cellular effects of C3m and N6022 are not well defined. We have previously shown, in mouse macrophages, a dependence of NOS activity for the biological effects of C3m, which is consistent with the production of GSNO via NOS activity (13). We wanted to determine if the inhibitory effect of C3m on viral replication in AECs also required NOS2. Because we had disparate results with the two different GSNOR inhibitors, we used both GSNOR inhibitors to test if NOS2 activity is required for C3m inhibition of viral replication.
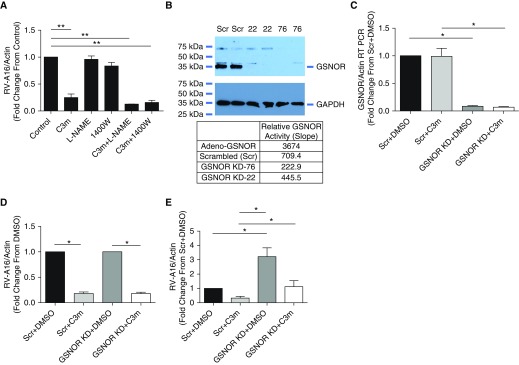
Although respiratory epithelial cells express multiple NOS isoforms, the inducible isoform (NOS2) is the major source of airway-derived NO and related metabolites in vivo. To determine whether NOS activity was required for the antiviral effects of C3m, BEAS-2B cells were exposed to the nonspecific NOS inhibitor l-nitroarginine methyl ester (l-NAME) or to the specific NOS2 inhibitor 1400W with or without C3m before addition of RV-A16. Neither l-NAME nor 1400W alone significantly altered RV-A16 RNA replication (Figure 5A).
Figure 5.
Inhibition of RV-A16 replication in BEAS-2B cells induced by C3m is independent of nitric oxide synthase (NOS) and GSNOR. (A) Neither l-nitroarginine methyl ester (l-NAME) (1 mM), a nonspecific NOS inhibitor, nor 1400W (100 μM), a specific NOS2 inhibitor, has a significant effect on RV-A16 RNA expression. n = 8/group; n = 3 replicates. **P < 0.001 by one-way ANOVA. (B) GSNOR protein expression (Western blot, upper panel) and activity (table, lower panel) in two different BEAS-2B clones (22 and 76) after GSNOR knockdown (GSNOR KD) or lentiviral transduction with scrambled RNA control (Scr). RT-qPCR quantification of (C) GSNOR and (D) RV-A16 RNA in BEAS-2B cells transduced with scrambled (Scr) or GSNOR-specific shRNA (KD) normalized to DMSO control (D) and scrambled (Scr) DMSO control (E). n = 6/group; n = 2 replicates. *P < 0.0001 by paired Student’s t test.
Finally, to determine whether the reduction of viral replication with administration of C3m is dependent on GSNOR activity, we used lentiviral transduction to generate BEAS-2B cells stably expressing either scrambled shRNA or GSNOR shRNA. GSNOR knockdown (KD) significantly decreased GSNOR protein expression and activity (Figure 5B) as well as mRNA level (Figure 5C). However, we observed no differences in RV-A16 replication in control (Scr + DMSO) versus KD cells (GSNOR KD + DMSO) (Figure 5C); moreover, the KD cells (Scr + C3m vs. GSNOR KD + C3m) had no effect on the antiviral properties of C3m. Collectively, these data show that the inhibition of viral replication by C3m does not require NOS activity and is insensitive to concentrations of GSNOR.
Decreased Viral Replication by C3m Is Secondary to Decreased Transcription of Intercellular Adhesion Molecule 1 and Increased Soluble Intercellular Adhesion Molecule 1 Shedding but Not Due to RV 3C Protease Inhibition
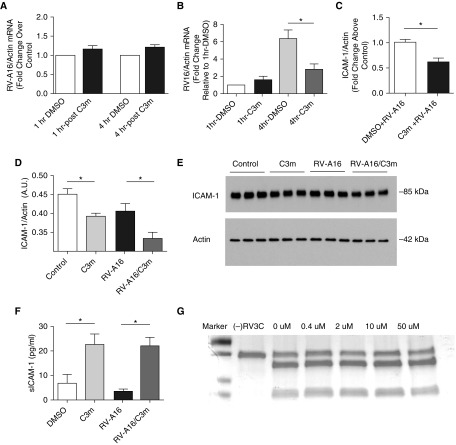
Initiation of viral replication is a complex process that involves multiple steps, including attachment, penetration, uncoating, replication, assembly, and release. To begin investigating a molecular basis for the inhibitory effects of C3m on viral replication, we first focused on two key steps of this process: rhinoviral attachment and replication. The initial interaction of the virus with the host AECs involves receptor binding and entry. Although we did not measure binding directly, RT-PCR has previously been used to assess viral attachment to host cells with high accuracy and reproducibility (32). Therefore, to determine the effect of C3m on RV-A16 binding, we measured RV-A16 RNA expression in BEAS-2B cells pretreated with 50 μM C3m for 1 hour before infection with RV-A16 for either 1 hour or 4 hours to capture the time by which peak binding has been shown to occur (33). No change in cell-associated RV-A16 RNA copy number was detected at 1 hour or 4 hours. On this basis, we concluded that 1-hour pretreatment with C3m does not affect initial viral binding to cells (Figure 6A). To determine the effect of prolonged treatment with C3m on viral attachment, we pretreated cells with either C3m (50 μM) or DMSO for 20 hours before infection with RV-A16 (MOI, 0.5) for either 1 hour or 4 hours. Viral RNA copy number significantly decreased at 4 hours postinfection (i.e., when full viral binding and entry have occurred and before efficient viral RNA replication starts) in BEAS-2B cells pretreated with C3m, suggesting that C3m inhibits viral binding and/or entry (Figure 6B).
Figure 6.
C3m decreases membrane-associated intercellular adhesion molecule (ICAM)-1 and increases soluble ICAM-1 (sICAM-1). (A) C3m does not affect viral binding after short treatment. BEAS-2B cells were pretreated with C3m (50 μM) for 1 hour before infection with RV-A16 (MOI, 3) for either 1 or 4 hours. n = 6/group; n = 3 replicates. (B) C3m reduces viral binding after prolonged treatment. BEAS-2B cells were pretreated with C3m (50 μM) for 20 hours before infection with RV-A16 (MOI, 0.5) for either 1 or 4 hours. n = 4–6/group; n = 3 replicates. *P < 0.05 by nonparametric Mann-Whitney U test. C3m reduces ICAM-1 (C) mRNA and (D) protein expression. (E) Western blot of whole-cell lysates of BEAS-2B cells pretreated with C3m (50 μM) for 1 hour, followed by infection with RV-A16 (MOI, 3) for 20 hours. n = 3–6/group; *P < 0.05 by Student’s t test. (F) C3m increases sICAM-1 protein in supernatants of BEAS-2B cells pretreated with C3m (50 μM) for 1 hour before infection with RV-A16 for 4 hours. n = 6–12/group; n = 3 replicates. *P < 0.005 by one-way ANOVA. (G) C3m does not affect RV 3C protease activity. Incubation of 1 μg of substrate with 1 μl of RV 3C protease (RV 3Cpro) and increasing concentrations of C3m for 20 minutes does not block protease activity (Pierce HRV 3C Protease; Pierce Biotechnology). SDS-PAGE gel was used. C = cleavable protein substrate without any protease treatment or C3m added. Readers may view the uncut gels for G in the data supplement.
We next sought to determine if the effect on viral replication is due to altered intercellular adhesion molecule (ICAM)-1 expression. ICAM-1 is a member of the immunoglobulin superfamily and is an adhesion protein that serves as the receptor for 90% of rhinoviruses A and B, including RV-A16 (33, 34). We measured ICAM-1 mRNA in primary AECs grown at an air–liquid interface (data not shown) and in submerged BEAS-2B cells pretreated with C3m at 50 μM for 1 hour followed by infection with RV-A16 (MOI, 3) for 20 hours. ICAM-1 mRNA (Figure 6C) and protein (Figures 6D and 6E) decreased significantly, suggesting that C3m downregulates ICAM-1 at the level of gene transcription. These data were in agreement with the C3m–induced inhibitory effects found at 20–48 hours postinfection with RV-A16 in the time-course experiments (Figure 1B), suggesting that virus progeny spread rather than initial virus binding was affected.
In addition to membranous ICAM-1, a soluble form of ICAM-1 (sICAM-1) has been described (35), which is believed to result from shedding of the extracellular domain of ICAM-1 (36, 37). We measured expression of sICAM-1 protein in cell supernatants at 1 hour and 4 hours after RV-A16 infection of BEAS-2B cells pretreated with 50 μM C3m for 1 hour. At 1 hour, sICAM-1 was below the detection limit of the assay, but it increased at 4 hours postinfection in the supernatant of both C3m alone and C3m–treated RV-A16–infected cells (Figure 6F), suggesting that the effects of C3m on shedding of sICAM-1 are independent of RV. The increase in sICAM-1 is consistent with decreased membranous ICAM-1 concentrations (Figure 6D). These findings are relevant because sICAM-1 has been demonstrated previously to inhibit RV replication in cell culture and exert an antiviral effect (38, 39).
Last, we investigated whether C3m alters RV replication via inhibition of RV 3C protease (3Cpro), a nonstructural protein that is essential for cleavage of viral polyprotein during viral replication (40). Because RV 3Cpro has a catalytic Cys residue, it (and related family members) can be sensitive to thiol-reactive compounds (41). Thus, we sought to address whether C3m might inhibit the protease via a similar mechanism. In an in vitro model of RV-A16 infection, incubation of RV 3Cpro with increasing doses of C3m did not affect protease activity even at the highest C3m concentration (Figure 6G).
Collectively, these data suggest that C3m does not directly affect viral replication but that it appears to inhibit initiation of the second RV-A16 replication cycle (short pretreatment) or initial virus binding (long pretreatment) by decreasing ICAM-1 expression at the level of gene transcription and by increasing sICAM-1 concentrations. Furthermore, RV 3Cpro protease does not appear to be a target.
Discussion
The “off-target” effects of drugs can often lead to unintended side effects and unwanted toxicity that may halt late-stage drug development. In contrast, drug repurposing or repositioning, based on the discovery of novel activities, has become an important tool in pharmacological discovery. Drugs are frequently found to have unexpected activities. These activities can be beneficial or injurious, resulting from the drug binding to an unknown target or by the drug altering a biochemical pathway. Such findings are often serendipitous, and the underlying mechanisms by which the effect is produced are often not identified.
Inhibition of GSNOR has emerged as a novel mechanism to regulate NO bioactivity. The GSNOR inhibitor, C3, an isomer of C3m, excludes GSNO from the binding site of GSNOR, which results in intracellular accumulation of S-nitrosothiols (11). Inhibition of GSNOR protects mice from the development of experimental allergic asthma (42) and induces vasorelaxation of preconstricted murine aorta (11). In this study, we identified a novel off-target effect of C3m (11) on RV-induced epithelial cell inflammation. Treatment of RV-infected AECs with C3m reduced rhinoviral replication and downstream RV-induced inflammation.
We were interested in studying the potential therapeutic use of GSNOR inhibitors in the treatment of rhinoviral infection, because NO has several reported antiviral effects on a variety of DNA and RNA viruses (43–47). Work by Sanders and colleagues (48) and Koetzler and collaborators (49) supports a role for NO in decreasing RV- and dsRNA-induced epithelial cell production of CXCL10 by NO donor, PAPA NONOate (3-[2-hydroxy-2-nitroso-1-propylhydrazino]-1-propanamine). S-nitrosothiols also have antiviral effects (43). The protective effects of S-nitrosothiols have been attributed to S-nitrosylation of cysteine residues of viral proteins needed for replication (41).
In the present study, we show that the GSNOR inhibitor, C3m, independent of NOS and GSNOR, inhibits RV replication and RV-induced epithelial cell production of CXCL10 and RANTES. C3m also decreased dsRNA-induced production of epithelial cell chemokines if administered before challenge with poly(I:C). These findings were unexpected because previously we had shown that inhibition of cytokine-stimulated inflammation in RAW264.7 cells by C3m was dependent on NOS2 (13), and Koetzler and colleagues found that the NO donor, PAPA NONOate, inhibited RV-induced transcriptional activation of CXCL10 in AECs through a cyclic guanosine monophosphate–independent pathway (48). In the present study, NOS inhibitors l-NAME and 1400W did not reverse RV- ;and dsRNA-induced epithelial cell production of CXCL10. The inhibition of rhinoviral replication by C3m in epithelial cells occurs early in the infectious cycle and coincides with a decrease of ICAM-1 mRNA transcription and an increase in shedding of sICAM-1 protein (38). An increase in sICAM-1 shedding is seen 4 hours after incubation with C3m, whereas a decrease in mRNA and protein expression of ICAM-1 is seen 20 hours after C3m and RV-A16 infection. It is well known that sICAM-1 tested in human embryonic lung fibroblasts has antirhinoviral activity against 88 of 90 human RVs belonging to the major receptor group, including RV-A16. sICAM-1 has also been found to be effective in inhibiting replication of at least five human RV serotypes in explants of human respiratory epithelial cells (39) and may be conferring protection against RV-A16 infection in bronchial epithelial cells.
An isomer of C3m, C3o, also appears to decrease viral inflammation by decreasing ICAM-1 expression and NF-κB activation (11). Work by Sanghani and colleagues showed that C3o decreases TNF-α (11) in RAW 264.9 cells and A549 cells. Similarly, we showed that C3m decreased RV-A16 induced NF-κB activity in primary AECs grown at an air–liquid interface. Taken together, our data suggest that C3m protects against RV-induced inflammation during the early steps of the RV replication cycle.
In addition to serving as the host cells for RV infection, AECs also initiate the local antiviral response to RV in part through production of chemokines, CXCL10, and RANTES, which are synthesized shortly after viral infection. Secretion of these chemokines by AECs is a key step in the host innate and acquired immune response to viral infection. We show that C3m blocks RV-A16–induced production of CXCL10 and RANTES by epithelial cells. On one hand, these findings are not surprising for CXCL10, because generation of CXCL10 has been shown to be dependent on viral replication (26). On the other hand, IFN-γ can enhance RV-A16–induced RANTES secretion by increasing viral binding and through a second receptor-independent pathway (50), and this mechanism may have been regulating RANTES in our study because our proteomic data show that C3m reduces IFN-inducible proteins and suggests that by blocking IFN signaling, C3m inhibits RANTES secretions and can function as an important regulator of the early immune response to RV infection (50).
This study has limitations. First, studies were restricted to in vitro cell culture and not in vivo animal exposures, owing to difficulty in infecting rodents with the major group RV serotype (51). Second, studies were performed in primary cells obtained from healthy lung transplant donors and not from subjects with asthma. Nonetheless, our findings are significant because they demonstrate protection against rhinoviral infection in human AECs by an unexpected activity of C3m. These improvements appear to be mediated by C3m inhibition of ICAM-1 mRNA transcription and increased shedding of sICAM-1, resulting in reduced viral binding and progeny spread and decreased rhinoviral replication and RV-associated inflammation. C3m was not effective against H1N1 pr8 influenza A virus, which binds to glycosylated oligosaccharides that terminate in a sialic acid (52), suggesting that the C3m effect is specific to ICAM-1–mediated effects on viral replication. Whether C3m will be effective in whole animals or human trials is unknown and will be the focus of future work. Because intransally delivered C3 has been shown to markedly reduce methacholine-induced airway hyperresponsiveness and airway inflammation in a murine model of experimental allergic asthma (53), it is plausible that C3m will play a future role in the treatment and prevention of airway inflammation and subsequent exacerbations by rhinoviral infection.
Supplementary Material
Footnotes
Supported by National Institutes of Health grants R01-HL107590-04 (L.G.Q. and M.W.F.) and U19 AI 25357 (D.R.V.).
Author Contributions: Conception and design: Z.Y., Y.A.B., M.W.F., and L.G.Q.; acquisition, analysis, or interpretation: Z.Y., Y.A.B., D.R.V., M.W.F., and L.G.Q.; drafting and revising of the work: Z.Y., Y.A.B., D.R.V., M.W.F., and L.G.Q.; final approval of the manuscript version to be published: Z.Y., Y.A.B., D.R.V., M.W.F., and L.G.Q.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2018-0058OC on August 29, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L, et al. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993;307:982–986. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corne JM, Marshall C, Smith S, Schreiber J, Sanderson G, Holgate ST, et al. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet. 2002;359:831–834. doi: 10.1016/S0140-6736(02)07953-9. [DOI] [PubMed] [Google Scholar]
- 4.Grissell TV, Powell H, Shafren DR, Boyle MJ, Hensley MJ, Jones PD, et al. Interleukin-10 gene expression in acute virus-induced asthma. Am J Respir Crit Care Med. 2005;172:433–439. doi: 10.1164/rccm.200412-1621OC. [DOI] [PubMed] [Google Scholar]
- 5.Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, Caramori G, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173:1114–1121. doi: 10.1164/rccm.200506-859OC. [DOI] [PubMed] [Google Scholar]
- 6.Bardin PG, Johnston SL, Sanderson G, Robinson BS, Pickett MA, Fraenkel DJ, et al. Detection of rhinovirus infection of the nasal mucosa by oligonucleotide in situ hybridization. Am J Respir Cell Mol Biol. 1994;10:207–213. doi: 10.1165/ajrcmb.10.2.8110476. [DOI] [PubMed] [Google Scholar]
- 7.Sanders SP, Proud D, Permutt S, Siekierski ES, Yachechko R, Liu MC. Role of nasal nitric oxide in the resolution of experimental rhinovirus infection. J Allergy Clin Immunol. 2004;113:697–702. doi: 10.1016/j.jaci.2004.01.755. [DOI] [PubMed] [Google Scholar]
- 8.Akaike T, Maeda H. Nitric oxide and virus infection. Immunology. 2000;101:300–308. doi: 10.1046/j.1365-2567.2000.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Que LG, Liu L, Yan Y, Whitehead GS, Gavett SH, Schwartz DA, et al. Protection from experimental asthma by an endogenous bronchodilator. Science. 2005;308:1618–1621. doi: 10.1126/science.1108228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster MW, Gwinn WM, Kelly FL, Brass DM, Valente AM, Moseley MA, et al. Proteomic analysis of primary human airway epithelial cells exposed to the respiratory toxicant diacetyl. J Proteome Res. 2017;16:538–549. doi: 10.1021/acs.jproteome.6b00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanghani PC, Davis WI, Fears SL, Green SL, Zhai L, Tang Y, et al. Kinetic and cellular characterization of novel inhibitors of S-nitrosoglutathione reductase. J Biol Chem. 2009;284:24354–24362. doi: 10.1074/jbc.M109.019919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green LS, Chun LE, Patton AK, Sun X, Rosenthal GJ, Richards JP. Mechanism of inhibition for N6022, a first-in-class drug targeting S-nitrosoglutathione reductase. Biochemistry. 2012;51:2157–2168. doi: 10.1021/bi201785u. [DOI] [PubMed] [Google Scholar]
- 13.Foster MW, Yang Z, Gooden DM, Thompson JW, Ball CH, Turner ME, et al. Proteomic characterization of the cellular response to nitrosative stress mediated by s-nitrosoglutathione reductase inhibition. J Proteome Res. 2012;11:2480–2491. doi: 10.1021/pr201180m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cockrell AS, Kafri T. Gene delivery by lentivirus vectors. Mol Biotechnol. 2007;36:184–204. doi: 10.1007/s12033-007-0010-8. [DOI] [PubMed] [Google Scholar]
- 15.Zheng S, Byrd AS, Fischer BM, Grover AR, Ghio AJ, Voynow JA. Regulation of MUC5AC expression by NAD(P)H:quinone oxidoreductase 1. Free Radic Biol Med. 2007;42:1398–1408. doi: 10.1016/j.freeradbiomed.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krunkosky TM, Fischer BM, Martin LD, Jones N, Akley NJ, Adler KB. Effects of TNF-α on expression of ICAM-1 in human airway epithelial cells in vitro: signaling pathways controlling surface and gene expression. Am J Respir Cell Mol Biol. 2000;22:685–692. doi: 10.1165/ajrcmb.22.6.3925. [DOI] [PubMed] [Google Scholar]
- 17.Krunkosky TM, Martin LD, Fischer BM, Voynow JA, Adler KB. Effects of TNFα on expression of ICAM-1 in human airway epithelial cells in vitro: oxidant-mediated pathways and transcription factors. Free Radic Biol Med. 2003;35:1158–1167. doi: 10.1016/s0891-5849(03)00498-2. [DOI] [PubMed] [Google Scholar]
- 18.Schroth MK, Grimm E, Frindt P, Galagan DM, Konno SI, Love R, et al. Rhinovirus replication causes RANTES production in primary bronchial epithelial cells. Am J Respir Cell Mol Biol. 1999;20:1220–1228. doi: 10.1165/ajrcmb.20.6.3261. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki T, Yamaya M, Kamanaka M, Jia YX, Nakayama K, Hosoda M, et al. Type 2 rhinovirus infection of cultured human tracheal epithelial cells: role of LDL receptor. Am J Physiol Lung Cell Mol Physiol. 2001;280:L409–L420. doi: 10.1152/ajplung.2001.280.3.L409. [DOI] [PubMed] [Google Scholar]
- 20.Yamaya M, Nishimura H, Hatachi Y, Yoshida M, Fujiwara H, Asada M, et al. Procaterol inhibits rhinovirus infection in primary cultures of human tracheal epithelial cells. Eur J Pharmacol. 2011;650:431–444. doi: 10.1016/j.ejphar.2010.09.056. [DOI] [PubMed] [Google Scholar]
- 21.Harding AT, Heaton BE, Dumm RE, Heaton NS. Rationally designed influenza virus vaccines that are antigenically stable during growth in eggs. MBio. 2017;8:e00669-17. doi: 10.1128/mBio.00669-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Que LG, Yang Z, Stamler JS, Lugogo NL, Kraft M. S-nitrosoglutathione reductase: an important regulator in human asthma. Am J Respir Crit Care Med. 2009;180:226–231. doi: 10.1164/rccm.200901-0158OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levinson B, Pepper D, Belyavin G. Substiuted sialic acid prosthetic groups as determinants of viral hemagglutination. J Virol. 1969;3:477–483. doi: 10.1128/jvi.3.5.477-483.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bai J, Smock SL, Jackson GR, Jr, MacIsaac KD, Huang Y, Mankus C, et al. Phenotypic responses of differentiated asthmatic human airway epithelial cultures to rhinovirus. PLoS One. 2015;10:e0118286. doi: 10.1371/journal.pone.0118286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kallal LE, Lukacs NW. The role of chemokines in virus-associated asthma exacerbations. Curr Allergy Asthma Rep. 2008;8:443–450. doi: 10.1007/s11882-008-0084-9. [DOI] [PubMed] [Google Scholar]
- 26.Spurrell JC, Wiehler S, Zaheer RS, Sanders SP, Proud D. Human airway epithelial cells produce IP-10 (CXCL10) in vitro and in vivo upon rhinovirus infection. Am J Physiol Lung Cell Mol Physiol. 2005;289:L85–L95. doi: 10.1152/ajplung.00397.2004. [DOI] [PubMed] [Google Scholar]
- 27.Teran LM, Seminario MC, Shute JK, Papi A, Compton SJ, Low JL, et al. RANTES, macrophage-inhibitory protein 1α, and the eosinophil product major basic protein are released into upper respiratory secretions during virus-induced asthma exacerbations in children. J Infect Dis. 1999;179:677–681. doi: 10.1086/314618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shelfoon C, Shariff S, Traves SL, Kooi C, Leigh R, Proud D. Chemokine release from human rhinovirus-infected airway epithelial cells promotes fibroblast migration. J Allergy Clin Immunol. 2016;138:114–122.e4. doi: 10.1016/j.jaci.2015.12.1308. [DOI] [PubMed] [Google Scholar]
- 29.Bochkov YA, Busse WW, Brockman-Schneider RA, Evans MD, Jarjour NN, McCrae C, et al. Budesonide and formoterol effects on rhinovirus replication and epithelial cell cytokine responses. Respir Res. 2013;14:98. doi: 10.1186/1465-9921-14-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bachmaier K, Neu N, Pummerer C, Duncan GS, Mak TW, Matsuyama T, et al. iNOS expression and nitrotyrosine formation in the myocardium in response to inflammation is controlled by the interferon regulatory transcription factor 1. Circulation. 1997;96:585–591. [PubMed] [Google Scholar]
- 31.Pitha PM, Au WC, Lowther W, Juang YT, Schafer SL, Burysek L, et al. Role of the interferon regulatory factors (IRFs) in virus-mediated signaling and regulation of cell growth. Biochimie. 1998;80:651–658. doi: 10.1016/s0300-9084(99)80018-2. [DOI] [PubMed] [Google Scholar]
- 32.Herrel NR, Johnson NL, Cameron JE, Leigh J, Hagensee ME. Development and validation of a HPV-32 specific PCR assay. Virol J. 2009;6:90. doi: 10.1186/1743-422X-6-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papi A, Johnston SL. Respiratory epithelial cell expression of vascular cell adhesion molecule-1 and its up-regulation by rhinovirus infection via NF-κB and GATA transcription factors. J Biol Chem. 1999;274:30041–30051. doi: 10.1074/jbc.274.42.30041. [DOI] [PubMed] [Google Scholar]
- 34.Papi A, Johnston SL. Rhinovirus infection induces expression of its own receptor intercellular adhesion molecule 1 (ICAM-1) via increased NF-κB-mediated transcription. J Biol Chem. 1999;274:9707–9720. doi: 10.1074/jbc.274.14.9707. [DOI] [PubMed] [Google Scholar]
- 35.Rothlein R, Mainolfi EA, Czajkowski M, Marlin SD. A form of circulating ICAM-1 in human serum. J Immunol. 1991;147:3788–3793. [PubMed] [Google Scholar]
- 36.Hashimoto S, Imai K, Kobayashi T, Amemiya E, Takahashi Y, Tomita Y, et al. Elevated levels of soluble ICAM-1 in sera from patients with bronchial asthma. Allergy. 1993;48:370–372. doi: 10.1111/j.1398-9995.1993.tb02408.x. [DOI] [PubMed] [Google Scholar]
- 37.Hollander C, Sitkauskiene B, Sakalauskas R, Westin U, Janciauskiene SM. Serum and bronchial lavage fluid concentrations of IL-8, SLPI, sCD14 and sICAM-1 in patients with COPD and asthma. Respir Med. 2007;101:1947–1953. doi: 10.1016/j.rmed.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 38.Arruda E, Crump CE, Marlin SD, Merluzzi VJ, Hayden FG. In vitro studies of the antirhinovirus activity of soluble intercellular adhesion molecule-1. Antimicrob Agents Chemother. 1992;36:1186–1191. doi: 10.1128/aac.36.6.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crump CE, Arruda E, Hayden FG. In vitro inhibitory activity of soluble ICAM-1 for the numbered serotypes of human rhinovirus. Antivir Chem Chemother. 1993;4:323–327. [Google Scholar]
- 40.Amineva SP, Aminev AG, Palmenberg AC, Gern JE. Rhinovirus 3C protease precursors 3CD and 3CD′ localize to the nuclei of infected cells. J Gen Virol. 2004;85:2969–2979. doi: 10.1099/vir.0.80164-0. [DOI] [PubMed] [Google Scholar]
- 41.Saura M, Zaragoza C, McMillan A, Quick RA, Hohenadl C, Lowenstein JM, et al. An antiviral mechanism of nitric oxide: inhibition of a viral protease. Immunity. 1999;10:21–28. doi: 10.1016/S1074-7613(00)80003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blonder JP, Mutka SC, Sun X, Qiu J, Green LH, Mehra NK, et al. Pharmacologic inhibition of S-nitrosoglutathione reductase protects against experimental asthma in BALB/c mice through attenuation of both bronchoconstriction and inflammation. BMC Pulm Med. 2014;14:3. doi: 10.1186/1471-2466-14-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rimmelzwaan GF, Baars MM, de Lijster P, Fouchier RA, Osterhaus AD. Inhibition of influenza virus replication by nitric oxide. J Virol. 1999;73:8880–8883. doi: 10.1128/jvi.73.10.8880-8883.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Croen KD. Evidence for antiviral effect of nitric oxide: inhibition of herpes simplex virus type 1 replication. J Clin Invest. 1993;91:2446–2452. doi: 10.1172/JCI116479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akarid K, Sinet M, Desforges B, Gougerot-Pocidalo MA. Inhibitory effect of nitric oxide on the replication of a murine retrovirus in vitro and in vivo. J Virol. 1995;69:7001–7005. doi: 10.1128/jvi.69.11.7001-7005.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bi Z, Reiss CS. Inhibition of vesicular stomatitis virus infection by nitric oxide. J Virol. 1995;69:2208–2213. doi: 10.1128/jvi.69.4.2208-2213.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin YL, Huang YL, Ma SH, Yeh CT, Chiou SY, Chen LK, et al. Inhibition of Japanese encephalitis virus infection by nitric oxide: antiviral effect of nitric oxide on RNA virus replication. J Virol. 1997;71:5227–5235. doi: 10.1128/jvi.71.7.5227-5235.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanders SP, Siekierski ES, Porter JD, Richards SM, Proud D. Nitric oxide inhibits rhinovirus-induced cytokine production and viral replication in a human respiratory epithelial cell line. J Virol. 1998;72:934–942. doi: 10.1128/jvi.72.2.934-942.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koetzler R, Zaheer RS, Wiehler S, Holden NS, Giembycz MA, Proud D. Nitric oxide inhibits human rhinovirus-induced transcriptional activation of CXCL10 in airway epithelial cells. J Allergy Clin Immunol. 2009;123:201–208.e9. doi: 10.1016/j.jaci.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 50.Konno S, Grindle KA, Lee WM, Schroth MK, Mosser AG, Brockman-Schneider RA, et al. Interferon-γ enhances rhinovirus-induced RANTES secretion by airway epithelial cells. Am J Respir Cell Mol Biol. 2002;26:594–601. doi: 10.1165/ajrcmb.26.5.4438. [DOI] [PubMed] [Google Scholar]
- 51.Vandal OH, Pierini LM, Schnappinger D, Nathan CF, Ehrt S. A membrane protein preserves intrabacterial pH in intraphagosomal Mycobacterium tuberculosis. Nat Med. 2008;14:849–854. doi: 10.1038/nmXXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ibricevic A, Pekosz A, Walter MJ, Newby C, Battaile JT, Brown EG, et al. Influenza virus receptor specificity and cell tropism in mouse and human airway epithelial cells. J Virol. 2006;80:7469–7480. doi: 10.1128/JVI.02677-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferrini ME, Simons BJ, Bassett DJ, Bradley MO, Roberts K, Jaffar Z. S-nitrosoglutathione reductase inhibition regulates allergen-induced lung inflammation and airway hyperreactivity. PLoS One. 2013;8:e70351. doi: 10.1371/journal.pone.0070351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.