Abstract
Angiogenesis, the formation of new blood capillaries, plays a key role in organ development and regeneration. Inhibition of lung angiogenesis through the blockade of angiogenic signaling pathways impairs compensatory and regenerative lung growth after unilateral pneumonectomy (PNX). The Hippo signaling transducer, Yes-associated protein (YAP) 1 binds to TEA domain transcription factor (TEAD) and controls organ size and regeneration. However, the role of endothelial YAP1 in lung vascular and alveolar morphogenesis remains unclear. In this report, we demonstrate that knockdown of YAP1 in endothelial cells (ECs) decreases angiogenic factor receptor Tie2 expression, and inhibits EC sprouting and epithelial cell budding in vitro and vascular and alveolar morphogenesis in the gel implanted on the mouse lung. The expression levels of YAP1, TEAD1, and Tie2 increase in ECs isolated from the remaining mouse lungs after unilateral PNX and vascular formation is stimulated in the post-PNX mouse lungs. Knockdown of endothelial YAP1 inhibits compensatory lung growth and vascular and alveolar morphogenesis after unilateral PNX. These findings suggest that endothelial YAP1 is required for lung vascular and alveolar regeneration and modulation of YAP1 in ECs may be novel interventions for the improvement of lung regeneration.
Keywords: angiogenesis, lung regeneration, YAP1, TEAD1, Tie2
Clinical Relevance
We have demonstrated that endothelial YAP1 stimulates endothelial cell sprouting and epithelial morphogenesis through Tie2 signaling in vitro and in the gel implanted on the mouse lungs. Endothelial YAP1 is required for compensatory and regenerative lung growth after unilateral pneumonectomy. Modulation of endothelial YAP1 may improve the way for lung regeneration and potentially lead to the development of new therapeutic strategies for chronic lung diseases.
Lung transplantation is one of the options to save patients with end-stage lung diseases. However, because of the shortage of donor lungs and low 5-year survival rate, lung transplantation is not an optimal approach (1). Recent significant advances in stem cell research and bioengineering techniques allow us to use biomaterials as medical devices to repair defects and restore functions in simple orthopedic and periodontal tissues (e.g., cartilage, bone, skin) (2); however, these approaches have not been highly successful in restoring the complex structures and functions of vital organs, such as lungs. Compensatory and regenerative growth takes place in a number of human organs (e.g., heart, liver, lungs) after the organs are damaged or partially removed (3–5). We and other groups have reported that compensatory lung growth is induced in the remaining lung tissues after unilateral pneumonectomy (PNX) in humans and other species (e.g., rats, mice, dogs, cats, rabbits, and ferrets) (4, 6–9). Thus, stimulating the organs’ intrinsic regenerative ability could be one of the therapeutic strategies to restore structures and functions after resection of damaged organs.
Although epithelial signaling has been the major focus of regeneration research, endothelial cells (ECs) also play key roles in organ morphogenesis (10). The formation of the vascular system is one of the earliest and most important events in organ development and regeneration (11, 12). Newly formed vasculatures closely interact with alveolar epithelial cells and deliver oxygen, nutrients, and various cell components required for lung organ formation (10, 13–15). During lung development, the vascular plexus sprouts in parallel with alveolar budding (16) and provides instructive signals to surrounding nonvascular cells (e.g., epithelium, smooth muscle cells) (10, 15). Angiogenic signaling also plays a key role in regenerative alveolarization in adult lung tissues (9, 17–20). Thus, angiogenesis constitutes an essential part of the regenerative program in the lung.
A Hippo signaling transducer, Yes-associated protein (YAP) 1, binds to a TEA domain transcription factor (TEAD) (21) and controls organ size and regeneration (e.g., liver, heart, intestine, muscle, lung) (22, 23). It has been reported that YAP1-TEAD1 signaling controls angiogenesis and vascular function in ECs through various signaling pathways (e.g., angiopoietin [Ang] 2, matrix metalloproteinase 2, VE-cadherin, CDC42, peroxisome proliferator–activated receptor γ coactivator 1-α [PGC1α]) (24–28). For example, YAP1 controls retinal angiogenesis through Ang2-Tie2 signaling (24). YAP/transcriptional coactivator with PDZ-binding motif (TAZ) also controls EC morphology and junctional integrity by changing actin cytoskeleton structures (25, 27). In addition, YAP1 maintains vascular stability in response to flow in a zebrafish model (26). In the lung, YAP1 plays a key role in the differentiation of adult airway progenitors (29) and stimulates lung alveolar development (30). YAP activation in alveolar stem cells also promotes post-PNX alveolar regeneration (6). However, it remains unclear the role of endothelial YAP1 in regenerative growth of the lung.
Here, we found that knockdown of YAP1 in ECs decreases Tie2 expression, and inhibits angiogenesis and epithelial budding formation in vitro and compensatory lung growth and vascular formation after PNX in the mouse lung. Activation of endothelial YAP1 could be an efficient strategy for lung regeneration.
Methods
Molecular Biological and Biochemical Methods
qRT-PCR was performed as previously described (9, 14, 28, 31). β2 microglobulin and cyclophilin controlled for overall cDNA content. Details are described in the data supplement.
In Vitro Fibrin Gel Angiogenesis Assay
Fibrin gel angiogenesis assays were performed as previously described (9, 28, 32). Details are described in the data supplement.
Coculture of Human Bronchial Epithelial Cell 3-KT and HUVE Cells
Coculture of human bronchial epithelial cell (HBEC) 3-KT and human umbilical vein endothelial (HUVE) cells was performed as previously described with slight modifications (9, 33). Details are described in the data supplement.
Mouse Lung EC Isolation
Yap1fl/fl mice were obtained from Dr. Fernando Camargo (Harvard Medical School) (34) and crossed with Cdh5(PAC)-CreERT2 mice (obtained from Dr. Ralf Adams, Max Planck Institute) (35), an inducible cre deleter under the control of VE-cadherin promoter, to create VE-cadherin–specific Yap1 conditional knockout (Yap1fl/fl-Cdh5(PAC)-CreERT2) mice, in which cre recombination is induced in ECs by administration of tamoxifen. Mouse lung ECs were isolated from Yap1fl/fl and Yap1fl/fl-Cdh5(PAC)-CreERT2 mouse lungs using anti-CD31–conjugated magnetic beads as previously described (31).
Fibrin Gel Implantation on the Mouse Lung In Vivo
The in vivo animal study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the NIH. The protocol was reviewed and approved by the Animal Care and Use Committee of the Medical College of Wisconsin. Fibrin gel implantation was performed as described previously (31, 32). Details are described in the data supplement. This system is important to the study of vascular morphogenesis in the lung, because blood vessel structures in the gel are significantly different in the gel implanted on the mouse lung compared with those in the gel implanted under the skin; vascular density was 67% lower and vessel diameter was 4.1-times larger in the subcutaneously implanted gel, suggesting that blood vessels are formed in the gel in an organ-specific manner (see Figure E4A in the data supplement).
Unilateral PNX
Unilateral PNX was performed as described previously (9). Details are described in the data supplement. Vascular structure was characterized using the microfil vascular casting system (36). After heparinization, mice were killed and the cardiac apex was cut. Microfil (0.5–1.0 ml; Flow Tech) was injected into the pulmonary arteries through right ventricle. After solidification of Microfil, the lungs were fixed with 4% paraformaldehyde, dehydrated with ethanol, cleared with methyl salicylate, and imaged. Quantification of vasculatures was performed using the AngioTool software (NCI/NIH).
Statistical Analysis
All phenotypic analyses, including image selection and computational image analysis for EC sprouting assay, epithelial budding assay, mouse lung gel implantation assay, and lung tissues after PNX were performed by masked observers unaware of the identity of experimental groups. Error terms (SEM) and P values were determined from the results of three or more independent experiments. The F test (for two samples) or the Levene test (for more than two samples) was performed to confirm that the variances are homogeneous. Student’s t test was used for statistical significance for two groups. For more than two groups, one-way ANOVA with a post hoc analysis using the Bonferroni test was conducted.
Results
YAP1 Stimulates Angiogenesis through Ang1-Tie2 Signaling In Vitro
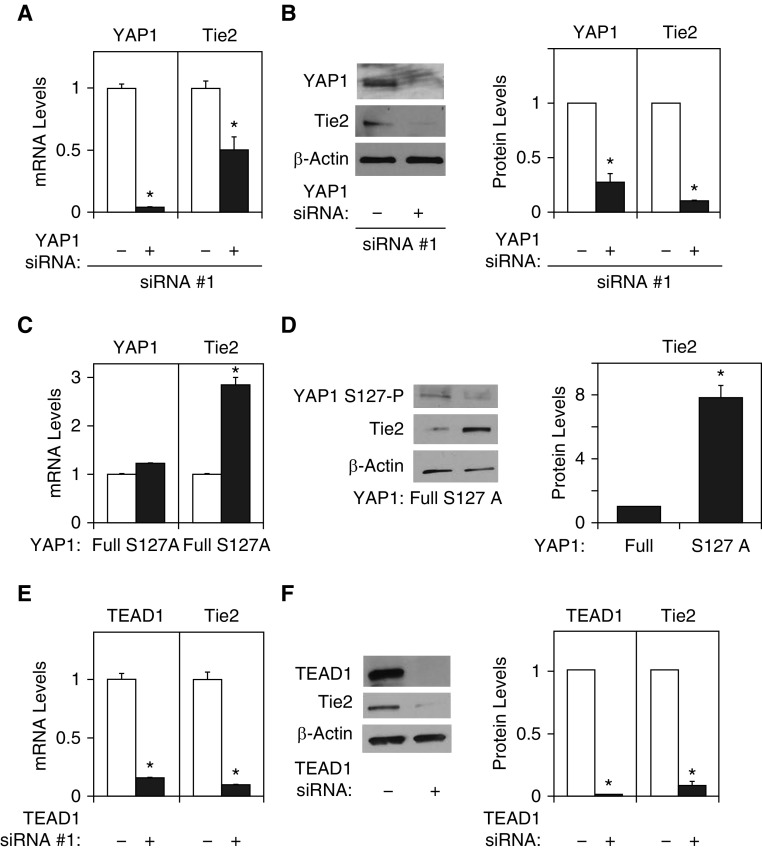
YAP-TEAD signaling controls angiogenesis and vascular function through various signaling pathways (24–28). We have demonstrated that angiogenic Ang1-Tie2 signaling is involved in regenerative lung growth in adult mice (9). Therefore, we examined whether YAP1 stimulates angiogenesis through Ang1-Tie2 signaling. When we knocked down YAP1 expression using siRNA transfection, YAP1 siRNA (#1) decreased mRNA levels of YAP1 and Tie2 by 95% and 52%, respectively, in human lung microvascular endothelial (L-HMVE) cells (Figure 1A). YAP1 siRNA (#1) also decreased protein levels of YAP1 and Tie2 by 78% and 91%, respectively, in L-HMVE cells (Figure 1B). We confirmed the results using the second siRNA targeting YAP1 (YAP1 siRNA #2), which revealed similar results (Figures E1A and E1B). Therefore, we used YAP1 siRNA #1 for the rest of the study. Because YAP1 Ser127 phosphorylation has a potent role in suppressing YAP1 activity (21–23), we next examined whether YAP1 Ser127 phosphorylation is involved in Tie2 expression. As expected, Tie2 mRNA and protein expression increased by 2.8 times and 7.8 times, respectively, in L-HMVE cells overexpressing YAP1 Ser127 mutant construct (YAP1S127A: active form), in which YAP1 S127 residue is mutated to alanine, compared with those treated with nonmutated, full-length YAP1 (Figures 1C and 1D).
Figure 1.
Yes-associated protein (YAP) 1 controls Tie2 expression in human lung microvascular endothelial (L-HMVE) cells. (A) Graph showing YAP1 and Tie2 mRNA levels in L-HMVE cells treated with YAP1 siRNA #1 or control siRNA with irrelevant sequences (n = 3, *P < 0.05). (B) Immunoblots showing YAP1, Tie2, and β-actin protein levels in L-HMVE cells treated with YAP1 siRNA #1 or control siRNA with irrelevant sequences (left). Graph showing YAP1 and Tie2 protein levels in L-HMVE cells treated with YAP1 siRNA #1 or control siRNA with irrelevant sequences (right, n = 3, *P < 0.05). (C) Graph showing YAP1 and Tie2 mRNA levels in L-HMVE cells treated with lentivirus encoding full-length YAP1 or YAP1S127A mutant construct (n = 3, *P < 0.05). (D) Immunoblots showing YAP1S127 phosphorylation and Tie2 and β-actin protein levels in L-HMVE cells treated with lentivirus encoding full-length YAP1 or YAP1S127A mutant construct (left). Graph showing Tie2 protein levels in L-HMVE cells treated with lentivirus encoding full-length YAP1 or YAP1S127A mutant construct (right, n = 3, *P < 0.05). (E) Graph showing TEA domain transcription factor (TEAD) 1 and Tie2 mRNA levels in L-HMVE cells treated with TEAD1 siRNA #1 or control siRNA with irrelevant sequences (n = 3, *P < 0.05). (F) Immunoblots showing TEAD1, Tie2, and β-actin protein levels in L-HMVE cells treated with TEAD1 siRNA #1 or control siRNA with irrelevant sequences (left). Graph showing the quantification of immunoblots (right, n = 3, *P < 0.05). Error bars represent SEM.
YAP1 binds to TEAD1 transcription factor and exerts its coactivator function (21). Thus, we also examined whether TEAD1 controls Tie2 expression. The Tie2 mRNA and protein expression levels were lower by 92% and 94%, respectively, in L-HMVE cells treated with TEAD1 siRNA (#1) compared with those treated with control siRNA with irrelevant sequences (Figures 1E and 1F). We confirmed the results using the second siRNA targeting TEAD1, which revealed similar results (Figure E1C), and therefore we used TEAD1 siRNA #1 for the rest of the study.
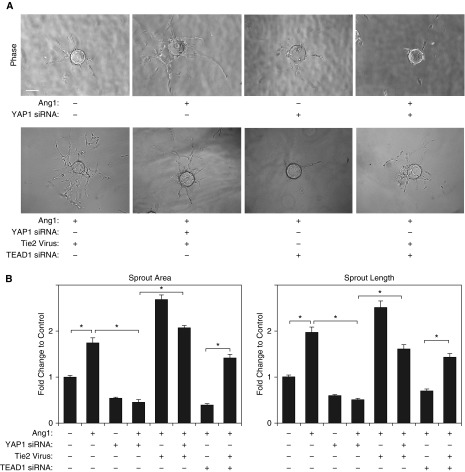
To examine whether YAP1 modulates blood vessel formation through the Ang1-Tie2 pathway in vitro, we performed a three-dimensional (3D) EC sprouting assay in the fibrin gel (9, 28, 32). After culturing beads coated with L-HMVE cells in the fibrin gel for 5 days, sprouting from the beads was quantified (Figure 2). Ang1 increased the area of the EC sprouts and sprout length by 1.8- and 1.9-fold, respectively, whereas YAP1 knockdown using siRNA transfection inhibited sprout formation induced by Ang1 (Figures 2A and 2B). Overexpression of YAP1S127A, which increases Tie2 expression, stimulated sprout formation; sprout area and sprout length increased by 1.2- and 1.3-fold, respectively, in L-HMVE cells compared with those treated with full-length, nonmutated YAP1 (Figures E2B and E2C). Importantly, Tie2 knockdown using siRNA transfection (Figure E2A) decreased sprout area and length induced by YAP1S127A (Figures E2B and E2C), whereas Tie2 overexpression using lentiviral transduction (Figure E2A) restored EC sprouting inhibited by YAP1 siRNA (Figures 2A and 2B), suggesting that YAP1 stimulates EC sprouting through Tie2 signaling.
Figure 2.
YAP1 is required for endothelial cell (EC) sprouting in vitro. (A) Phase–contrast images showing EC sprouting from each bead in L-HMVE cells treated with angiopoietin (Ang) 1 or in combination with YAP1 siRNA, TEAD1 siRNA, Tie2 lentivirus, control siRNA with irrelevant sequences, or control virus (vector alone). Scale bar: 150 μm. (B) Graphs showing changes in sprout area (left) and length of the sprout (right) in L-HMVE cells treated with Ang1 or in combination with YAP1 siRNA, TEAD1 siRNA, Tie2 lentivirus, control siRNA with irrelevant sequences, or control virus (vector alone) (n = 3, *P < 0.05). Error bars represent SEM.
We also examined whether TEAD1 controls EC sprouting. TEAD1 knockdown inhibited EC sprouting area and length induced by Ang1 by 82% and 69%, respectively, compared with those treated with control siRNA with irrelevant sequences, whereas Tie2 overexpression restored the EC sprouting (Figures 2A and 2B). These results suggest that YAP1-TEAD1-Tie2 signaling is necessary for angiogenesis in vitro.
Endothelial YAP1 Is Required for Epithelial Morphogenesis In Vitro and in Fibrin Gel Implanted on the Mouse Lung
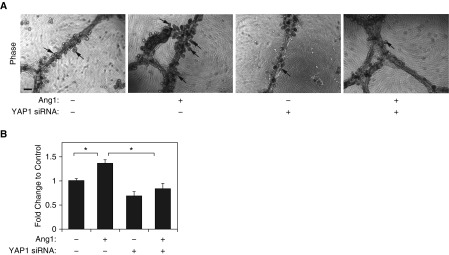
It has been recognized that ECs stimulate alveolar morphogenesis by instructing lung epithelial cells (9, 17, 19). We have reported that ECs stimulate alveolar morphogenesis in immortalized HBEC3-KT cells when these cells are cocultured together on growth factor–reduced Matrigel that is overlaid on top of a monolayer of human lung fibroblasts (9). Therefore, we used this system and examined whether endothelial YAP1 is required for alveolar morphogenesis. When HBEC3-KT cells were cocultured with HUVE cells on the growth factor–reduced Matrigel, the cells self-assembled into tubule-like structures (Figure 3A) similar to those observed in organotypic cultures with ECs (14) or HBEC3-KT cells alone (9, 33), and the small bud-like structures that have lumens and express alveolar epithelial markers (data not shown) (9) emerged from the tubules (Figure 3A). The number of alveolar buds increased by 1.3 times when treated with Ang1 compared with untreated cultures (Figures 3A and 3B). When we knocked down YAP1 expression in HUVE cells using siRNA transfection and cocultured with HBEC3-KT cells, epithelial budding induced by Ang1 was inhibited (Figures 3A and 3B), suggesting that endothelial YAP1 is necessary for alveolar bud formation induced by Ang1. YAP1S127A mutant construct, which increases Tie2 expression in ECs, stimulated EC sprouting (Figures 1C and 1D, Figures E2B and E2C). Thus, we next examined whether ECs overexpressing YAP1S127A mutant construct stimulate epithelial budding. YAP1S127A overexpression in ECs treated with Ang1 (20, 100 ng/ml) stimulated epithelial budding by 1.3 and 1.4 times, respectively, compared with those overexpressing nonmutated, full-length YAP1, whereas Tie2 knockdown in ECs attenuated the effects (Figure E3), suggesting that endothelial YAP1-Tie2 signaling is necessary for epithelial cell budding in vitro.
Figure 3.
Knockdown of endothelial YAP1 inhibits epithelial morphogenesis. (A) Phase–contrast images showing epithelial cell budding (scale bar: 50 μm) in coculture of human bronchial epithelial cell (HBEC) 3-KT and human umbilical vein endothelial (HUVE) cells treated with Ang1 or in combination with YAP1 siRNA or control siRNA with irrelevant sequences for 5 days. Arrows indicate the epithelial bud formation. (B) Graph showing the changes in the number of buds in coculture of HBEC3-KT and HUVE cells treated with Ang1 or in combination with YAP1 siRNA or control siRNA with irrelevant sequences for 5 days (n = 3, *P < 0.05). Error bars represent SEM.
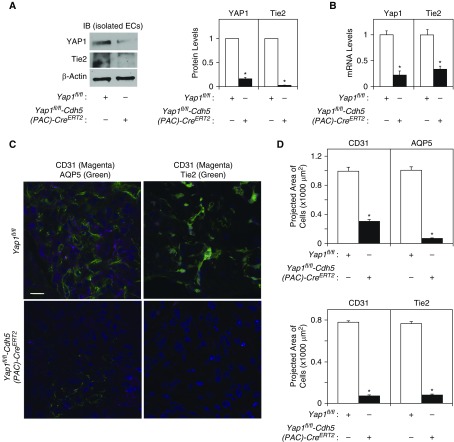
To characterize the effects of endothelial YAP1 on angiogenesis and epithelial morphogenesis in the mouse lung, we created tamoxifen-inducible Yap1fl/fl-Cdh5(PAC)-CreERT2 mice. The protein and mRNA levels of YAP1 in ECs isolated from Yap1fl/fl-Cdh5(PAC)-CreERT2 mouse lungs 48 hours after 4-hydroxytamoxifen treatment were lower by 82% and 79%, respectively, compared with those isolated from control Yap1fl/fl mouse lungs, confirming that the YAP1 expression is knocked down in ECs in Yap1fl/fl-Cdh5(PAC)-CreERT2 mouse lungs (Figures 4A and 4B). Consistent with the in vitro results with YAP1 siRNA–treated ECs, Tie2 expression was also significantly lower in ECs isolated from Yap1fl/fl-Cdh5(PAC)-CreERT2 mouse lungs (Figures 4A and 4B). We then implanted fibrin gel on the Yap1fl/fl-Cdh5(PAC)-CreERT2 or control Yap1fl/fl mouse lung surface (8–10 wk old) for 7 days (14, 31, 32). CD31- and Tie2-positive ECs and AQP5-positive type I alveolar epithelial cells were recruited from the host lung and made network formation in the gel implanted on the control Yap1fl/fl mouse lungs, whereas the recruitment of ECs and alveolar epithelial cells, as well as Tie2 expression, were attenuated when the gel was implanted on the Yap1fl/fl-Cdh5(PAC)-CreERT2 mouse lungs (Figures 4C and 4D), indicating that endothelial YAP1 is necessary for angiogenesis and epithelial cell morphogenesis in the gel implanted on the mouse lungs.
Figure 4.
Knockdown of endothelial YAP1 inhibits vascular and epithelial morphogenesis. (A) Immunoblots (IB) showing YAP1, Tie2, and β-actin protein levels in ECs isolated from Yap1fl/fl-Cdh5(PAC)-CreERT2 or control Yap1fl/fl mouse lungs treated with 4-hydroxytamoxifen (4-OHT) for 48 hours (left). Graph showing the quantification of IB (right, n = 4, *P < 0.05). Readers may view the uncut gels for Figure 4A in the data supplement. (B) Graph showing Yap1 and Tie2 mRNA levels (n = 4, *P < 0.05) in ECs isolated from Yap1fl/fl-Cdh5(PAC)-CreERT2 or Yap1fl/fl mouse lungs treated with 4-OHT for 48 hours. (C) Immunofluorescence micrographs showing CD31-positive blood vessels and AQP5-positive alveolar type I epithelial cells (left) and CD31-positive blood vessels and Tie2 expression (right) in the fibrin gel implanted on Yap1fl/fl-Cdh5(PAC)-CreERT2 or control Yap1fl/fl mouse lungs for 7 days (scale bar: 50 μm). (D) Graphs showing quantification of CD31-, AQP5-, or Tie2-positive cells in the gel implanted on the Yap1fl/fl-Cdh5(PAC)-CreERT2 or Yap1fl/fl mouse lungs for 7 days (n = 7, mean ± SEM, *P < 0.05).
Endothelial YAP1 Is Required for Compensatory Lung Growth after PNX
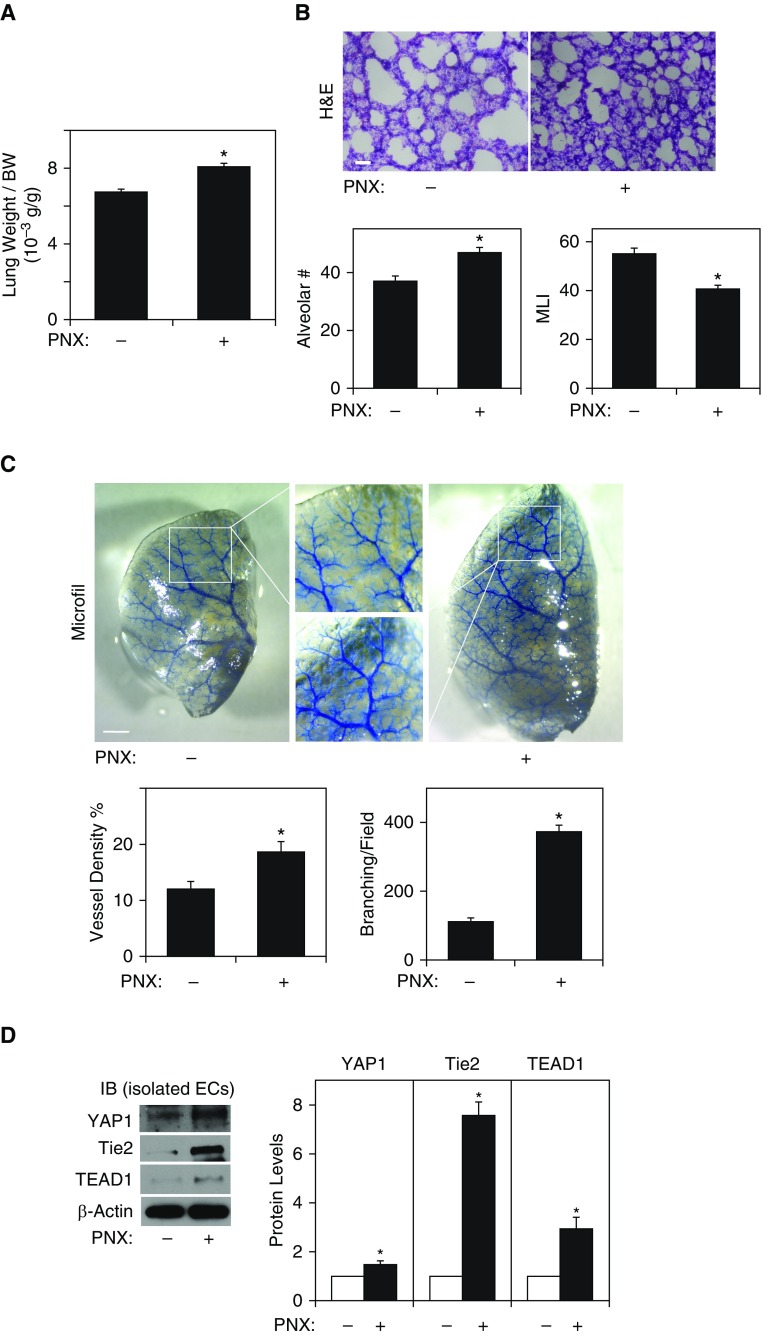
We have reported that lung vascular and alveolar regeneration after unilateral PNX is mediated by the Ang1-Tie2 pathway (9). Because YAP1 knockdown in ECs inhibits Tie2 expression (Figures 1 and 4, Figures E1A and E1B) and attenuates EC sprouting and alveolar morphogenesis in vitro (Figures 2 and 3) and in the gel implanted on the mouse lungs (Figure 4), we next examined whether endothelial YAP1 is required for vascular and alveolar regeneration using a mouse unilateral PNX model. Consistent with previous reports (9, 18, 20), there was a significant increase in the ratio of the weight of right cardiac lobe to mouse body weight 7 days after left unilateral PNX; the lung weight:body weight ratio was 6.3 × 10−3 (g/g) in the sham-operated control mice, whereas the ratio increased by 1.3-fold in the lungs 7 days after PNX (Figure 5A). Morphometric analysis of hematoxylin and eosin–stained mouse lungs also revealed that the size of the alveolar space measured by mean linear intercept decreased by 30%. The number of alveoli also increased by 1.3 times in the remaining lung lobe after left PNX compared with control sham-operated mouse lungs (Figure 5B). We also analyzed the effects of PNX on blood vessel formation using the microfil casting system. Vessel density and the number of branching points were 1.6- and 3.4-times higher in the mouse lungs after PNX compared with those in the sham-operated control mouse lungs (Figure 5C). The protein levels of YAP1, Tie2, and TEAD1 were significantly higher in ECs isolated from the mouse lungs 7 days after left PNX compared with those in the sham-operated control mouse lungs (Figure 5D).
Figure 5.
Angiogenesis is stimulated in the mouse lung after pneumonectomy (PNX). (A) Graph showing the ratio of the weight of right lung cardiac lobe to mouse body weight (BW) 7 days after PNX (n = 7, mean ± SEM, *P < 0.05). (B) Hematoxylin and eosin (H&E)–stained remaining right cardiac lobe 7 days after left PNX (top). Scale bar: 25 μm. Graphs showing quantification of alveolar number (left bottom) and alveolar size (mean linear intercept [MLI], right bottom) in the remaining mouse lung right cardiac lobe 7 days after PNX (n = 7, mean ± SEM, *P < 0.05). (C) Micrographs showing blood vessel structures in the mouse right lung lobe 7 days after left PNX analyzed using the Microfil casting system (top). Scale bar: 1 mm. Graphs showing the quantification of blood vessel density (left bottom) and the number of branching points (right bottom, n = 7, mean ± SEM, *P < 0.05). (D) IB showing the protein levels of YAP1, Tie2, TEAD1, and β-actin in ECs isolated from mouse lungs 7 days after PNX (left). Graph showing the quantification of YAP1, Tie2, and TEAD1 protein levels in ECs isolated from mouse lungs 7 days after PNX (right, n = 4, mean ± SEM, *P < 0.05).
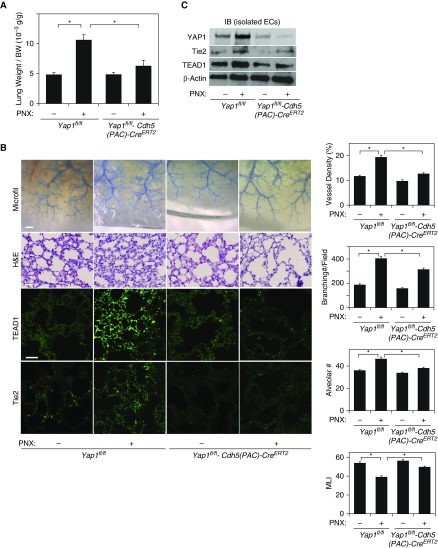
To further study whether endothelial YAP1 is necessary for regenerative lung growth, we performed unilateral PNX on Yap1fl/fl-Cdh5(PAC)-CreERT2 or control Yap1fl/fl mouse lungs. Although there was a significant increase in the weight of the remaining right cardiac lobe of Yap1fl/fl mice 7 days after left unilateral PNX, lung weight did not increase in Yap1fl/fl-Cdh5(PAC)-CreERT2 mouse lungs after PNX (Figure 6A). When we analyzed blood vessel density and branching using the microfil casting system, vessel density and the number of branching points were 1.6- and 2.1-times higher in the control Yap1fl/fl mouse lung after PNX, whereas these effects were attenuated in Yap1fl/fl-Cdh5(PAC)-CreERT2 mouse lungs after PNX (Figure 6B). Morphometric analysis of hematoxylin and eosin–stained mouse lungs also revealed that Yap1fl/fl mouse lungs after PNX exhibited thickened alveolar septa, a decrease in the size of the alveolar space measured by mean linear intercept, and an increase in the number of alveoli compared with control sham-operated lung, whereas these changes were attenuated in Yap1fl/fl-Cdh5(PAC)-CreERT2 mouse lungs after PNX (Figure 6B). The protein levels of Tie2 and TEAD1 in the lungs and in ECs isolated from the mouse lungs after PNX were also attenuated in Yap1fl/fl-Cdh5(PAC)-CreERT2 mouse lungs compared with those in Yap1fl/fl mouse lungs (Figures 6B and 6C, Figures E4B and E4C). These findings suggest that endothelial YAP1 is necessary for lung vascular and alveolar regeneration after PNX.
Figure 6.
Endothelial YAP1 is required for post-PNX compensatory lung growth and angiogenesis. (A) Graph showing the ratio of the weight of right lung cardiac lobe to mouse BW 7 days after left PNX in Yap1fl/fl-Cdh5(PAC)-CreERT2 or control Yap1fl/fl mice (n = 7, mean ± SEM, *P < 0.05). (B) Micrographs showing blood vessel structures in the mouse right lung lobe 7 days after left PNX analyzed using the Microfil casting system (top row; scale bar: 1 mm). H&E–stained cardiac lobe of Yap1fl/fl-Cdh5(PAC)-CreERT2 or Yap1fl/fl mice 7 days after PNX (second row; scale bar: 25 μm). Immunofluorescence micrographs showing TEAD1 (third row) and Tie2 expression (bottom row) in the Yap1fl/fl-Cdh5(PAC)-CreERT2 or control Yap1fl/fl mouse lungs 7 days after PNX (scale bar: 25 μm). Graphs showing the quantification of blood vessel density (right first) and the number of branching points (right second) in the microfil-casted lungs, and alveolar number (right third) and alveolar size (MLI, right fourth) in the H&E-stained lungs (n = 7, mean ± SEM, *P < 0.05). (C) IB showing YAP1, Tie2, TEAD1, and β-actin protein levels in ECs isolated from Yap1fl/fl-Cdh5(PAC)-CreERT2 or control Yap1fl/fl mouse lungs 7 days after PNX.
Discussion
Here, we have demonstrated that endothelial YAP1 controls angiogenesis and lung alveolar regeneration after unilateral PNX through Tie2 signaling. Knockdown of YAP1 in ECs decreases Tie2 expression, and inhibits EC sprouting and epithelial cell budding in vitro and vascular and alveolar morphogenesis in the gel implanted on the mouse lung. Knockdown of endothelial YAP1 also inhibits compensatory lung growth and vascular and alveolar morphogenesis after unilateral PNX. These findings suggest that endothelial YAP1 is required for lung vascular and alveolar regeneration and modulation of endothelial YAP1 could be novel interventions to restore structures and functions of the lungs after resection of damaged lungs.
We have demonstrated that Wnt coreceptor, LRP5, controls Tie2 expression in ECs and modulates lung development and regenerative lung growth (9, 14). It has been reported that YAP1 activity is controlled by Wnt signaling and vice versa (37, 38); when Wnt ligands bind to LRP5/6, YAP1 is released from the destruction complex, translocates to nucleus (37), and stimulates cell proliferation (39, 40), organ growth, and regeneration (6, 22, 29, 30, 37). LRP5 also regulates expression/activity of other angiogenic genes, including PDGFR (41). Wnt3a promotes definitive endoderm differentiation (42) and Wnt7b signals through LRP5 and regulates lung airway and vascular development (43). Thus, Wnt and the related signaling pathways may contribute to YAP1-induced angiogenesis during lung regeneration as well. Other Hippo signaling molecules, including TAZ, which is known to have distinct biological activities from YAP1 (22, 23), may also be involved. YAP1-TEAD1 signaling also controls angiogenesis and vascular integrity in ECs through various other angiogenic pathways (e.g., Ang2, matrix metalloproteinase 2, VE-cadherin, PGC1α) (24–28). Thus, YAP1 may stimulate regenerative lung growth after PNX through cooperation of multiple angiogenic signaling. We found that endothelial YAP1S127A significantly stimulates alveolar budding formation compared with nonmutated, full-length YAP1 (Figure E3). However, Ang1 did not significantly enhance the YAP1S127A response (Figure E3). This may be because YAP1S127A stimulates expression of another Tie2 ligand, Ang2 in ECs (24), which induces angiogenesis and epithelial morphogenesis in a distinct manner (24, 44, 45). YAP1 may stimulate epithelial budding through Ang1/Ang2-Tie2 signaling. Because the establishment of stable and functional blood vessel networks requires cooperation of multiple signaling pathways (11, 12), manipulation of YAP1-TEAD1 signaling, which interacts with the multiple angiogenic pathways, could be an optimal strategy for modulating lung vascular and alveolar morphogenesis.
In addition to signals from the chemical factors, the micromechanical environment controls vascular morphogenesis and function (46). It has been demonstrated that mechanical tension changes during lung regeneration after PNX (6, 7, 20), and that EC density and expression of angiogenic factors are differentially changed after PNX (20). YAP1 activity is controlled by various mechanical stimuli (e.g., cell density [21, 24], ECM stiffness [47], mechanical tension [6], flow [26]). Thus, YAP1 activity and the responses of ECs to YAP1 after PNX may be mechanosensitive and altered in a spatiotemporal manner.
We used coculture of HBEC3-KT and HUVE cells to study the role of endothelial YAP1 in epithelial morphogenesis. Although HBEC3-KT cells are p63 and keratin-14 positive, and potent to differentiate into alveolar epithelial cells (33), these cells are immortalized, and cell characteristics may be different from other epithelial stem and progenitor cells in the lung, such as basal cells, bronchioalveolar stem cells, and alveolar type II cells (19, 48). Usage of more physiologically relevant alveolar epithelial stem and progenitor cells (19, 48), in combination with the 3D organoid system (49), would help to further characterize the mechanism by which endothelial YAP1 controls alveolar morphogenesis in physiological and pathological conditions in vitro.
Characterization of angiogenesis in the lung is challenging, due to the high density of blood vessels in the lung and the difficulty in identifying newly formed blood vessels in histological sections. To study the process of angiogenesis in the lung, we developed a unique system to implant fibrin gel on the mouse lung (31, 32). This system is important to the study of lung angiogenesis, because the morphology of blood vessels in the gel seems to be organ specific; the blood vessel structure in the subcutaneously implanted gel is significantly different compared with that in the gel implanted on the lung (Figure E4A). These blood vessels are mainly derived from pulmonary artery, but not from pleural artery (bronchial artery), and connect to pulmonary circulation; when pulmonary artery is ligated, the blood vessel recruitment into the gel is significantly attenuated (32). Using the lung gel implantation model and the transgenic mouse model, we found that knockdown of endothelial YAP1 attenuates alveolar epithelial morphogenesis in the mouse lung (Figure 4). Other lung cells (e.g., epithelial cells, smooth muscle cells, immune cells) (50) may interact with ECs and change EC behaviors in the lung or in the implanted gel. These other cells also secrete angiogenic and other chemical factors, which, in turn, change the gradients of angiogenic and growth factors, and indirectly control vascular formation after PNX in a spatiotemporal manner. In fact, YAP1 expresses in alveolar epithelial cells and contributes to lung alveolar regeneration (6). Thus, changes in YAP1 expression/activity in other lung cells after PNX may affect angiogenesis during lung regeneration. Manipulation of gene expression in other lung cells will further elucidate the mechanism by which YAP1 controls lung vascular and alveolar regeneration.
In summary, we have demonstrated that endothelial YAP1 stimulates EC sprouting and epithelial morphogenesis through Tie2 signaling in vitro and in the gel implanted on the mouse lungs. Endothelial YAP1 is required for compensatory and regenerative lung growth after unilateral PNX. Modulation of endothelial YAP1 may improve the strategy for lung regeneration and potentially lead to the development of new therapeutic strategies for chronic lung diseases.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank F. Camargo for providing Yap1fl/fl mouse and R. Adams for providing Cdh5(PAC)-CreERT2 mouse.
Footnotes
This work was supported by funds from National Institutes of Health grant R21AG054830 (T.M. and A.M.), a Medical College of Wisconsin Research Affair Committee New Investigator Award (A.M.), and Medical College of Wisconsin Faculty Start-up funds (T.M. and A.M.).
Author Contributions: conceived and designed the experiments—T.M. and A.M.; performed the experiments—T.M., M.M., and A.M.; analyzed the data—T.M. and A.M.; contributed reagents/materials/analysis tools—T.M., M.M., and A.M.; wrote the paper—T.M. and A.M.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2018-0105OC on August 29, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Orens JB, Garrity ER., Jr General overview of lung transplantation and review of organ allocation. Proc Am Thorac Soc. 2009;6:13–19. doi: 10.1513/pats.200807-072GO. [DOI] [PubMed] [Google Scholar]
- 2.Chen FM, Zhang J, Zhang M, An Y, Chen F, Wu ZF. A review on endogenous regenerative technology in periodontal regenerative medicine. Biomaterials. 2010;31:7892–7927. doi: 10.1016/j.biomaterials.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 3.Liu M, Chen P. Proliferationinhibiting pathways in liver regeneration. Mol Med Rep. 2017;16:23–35. doi: 10.3892/mmr.2017.6613. . (Review) [DOI] [PubMed] [Google Scholar]
- 4.Butler JP, Loring SH, Patz S, Tsuda A, Yablonskiy DA, Mentzer SJ. Evidence for adult lung growth in humans. N Engl J Med. 2012;367:244–247. doi: 10.1056/NEJMoa1203983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cahill TJ, Choudhury RP, Riley PR. Heart regeneration and repair after myocardial infarction: translational opportunities for novel therapeutics. Nat Rev Drug Discov. 2017;16:699–717. doi: 10.1038/nrd.2017.106. [DOI] [PubMed] [Google Scholar]
- 6.Liu Z, Wu H, Jiang K, Wang Y, Zhang W, Chu Q, et al. MAPK-mediated YAP activation controls mechanical-tension–induced pulmonary alveolar regeneration. Cell Reports. 2016;16:1810–1819. doi: 10.1016/j.celrep.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 7.Thane K, Ingenito EP, Hoffman AM. Lung regeneration and translational implications of the postpneumonectomy model. Transl Res. 2014;163:363–376. doi: 10.1016/j.trsl.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Hsia CC, Herazo LF, Fryder-Doffey F, Weibel ER. Compensatory lung growth occurs in adult dogs after right pneumonectomy. J Clin Invest. 1994;94:405–412. doi: 10.1172/JCI117337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mammoto T, Chen Z, Jiang A, Jiang E, Ingber DE, Mammoto A. Acceleration of lung regeneration by platelet-rich plasma extract through the low-density lipoprotein receptor–related protein 5–Tie2 pathway. Am J Respir Cell Mol Biol. 2016;54:103–113. doi: 10.1165/rcmb.2015-0045OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crivellato E, Nico B, Ribatti D. Contribution of endothelial cells to organogenesis: a modern reappraisal of an old aristotelian concept. J Anat. 2007;211:415–427. doi: 10.1111/j.1469-7580.2007.00790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung AS, Ferrara N. Developmental and pathological angiogenesis. Annu Rev Cell Dev Biol. 2011;27:563–584. doi: 10.1146/annurev-cellbio-092910-154002. [DOI] [PubMed] [Google Scholar]
- 13.Jakkula M, Le Cras TD, Gebb S, Hirth KP, Tuder RM, Voelkel NF, et al. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am J Physiol Lung Cell Mol Physiol. 2000;279:L600–L607. doi: 10.1152/ajplung.2000.279.3.L600. [DOI] [PubMed] [Google Scholar]
- 14.Mammoto T, Chen J, Jiang E, Jiang A, Smith LE, Ingber DE, et al. LRP5 regulates development of lung microvessels and alveoli through the angiopoietin–Tie2 pathway. PLoS One. 2012;7:e41596. doi: 10.1371/journal.pone.0041596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thebaud B. Angiogenesis in lung development, injury and repair: implications for chronic lung disease of prematurity. Neonatology. 2007;91:291–297. doi: 10.1159/000101344. [DOI] [PubMed] [Google Scholar]
- 16.Metzger RJ, Klein OD, Martin GR, Krasnow MA. The branching programme of mouse lung development. Nature. 2008;453:745–750. doi: 10.1038/nature07005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding BS, Nolan DJ, Guo P, Babazadeh AO, Cao Z, Rosenwaks Z, et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell. 2011;147:539–553. doi: 10.1016/j.cell.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakurai MK, Lee S, Arsenault DA, Nose V, Wilson JM, Heymach JV, et al. Vascular endothelial growth factor accelerates compensatory lung growth after unilateral pneumonectomy. Am J Physiol Lung Cell Mol Physiol. 2007;292:L742–L747. doi: 10.1152/ajplung.00064.2006. [DOI] [PubMed] [Google Scholar]
- 19.Lee JH, Bhang DH, Beede A, Huang TL, Stripp BR, Bloch KD, et al. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4–NFATc1–thrombospondin-1 axis. Cell. 2014;156:440–455. doi: 10.1016/j.cell.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konerding MA, Gibney BC, Houdek JP, Chamoto K, Ackermann M, Lee GS, et al. Spatial dependence of alveolar angiogenesis in post-pneumonectomy lung growth. Angiogenesis. 2012;15:23–32. doi: 10.1007/s10456-011-9236-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ota M, Sasaki H. Mammalian tead proteins regulate cell proliferation and contact inhibition as transcriptional mediators of Hippo signaling. Development. 2008;135:4059–4069. doi: 10.1242/dev.027151. [DOI] [PubMed] [Google Scholar]
- 22.Piccolo S, Cordenonsi M, Dupont S. Molecular pathways: YAP and TAZ take center stage in organ growth and tumorigenesis. Clin Cancer Res. 2013;19:4925–4930. doi: 10.1158/1078-0432.CCR-12-3172. [DOI] [PubMed] [Google Scholar]
- 23.Panciera T, Azzolin L, Cordenonsi M, Piccolo S. Mechanobiology of YAP and TAZ in physiology and disease. Nat Rev Mol Cell Biol. 2017;18:758–770. doi: 10.1038/nrm.2017.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi HJ, Zhang H, Park H, Choi KS, Lee HW, Agrawal V, et al. Yes-associated protein regulates endothelial cell contact–mediated expression of angiopoietin-2. Nat Commun. 2015;6:6943. doi: 10.1038/ncomms7943. [DOI] [PubMed] [Google Scholar]
- 25.Kim J, Kim YH, Park DY, Bae H, Lee DH, Kim KH, et al. YAP/TAZ regulates sprouting angiogenesis and vascular barrier maturation. J Clin Invest. 2017;127:3441–3461. doi: 10.1172/JCI93825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakajima H, Yamamoto K, Agarwala S, Terai K, Fukui H, Fukuhara S, et al. Flow-dependent endothelial yap regulation contributes to vessel maintenance. Dev Cell. 2017;40:523–536.e526. doi: 10.1016/j.devcel.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 27.Sakabe M, Fan J, Odaka Y, Liu N, Hassan A, Duan X, et al. YAP/TAZ-CDC42 signaling regulates vascular tip cell migration. Proc Natl Acad Sci USA. 2017;114:10918–10923. doi: 10.1073/pnas.1704030114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mammoto A, Muyleart M, Kadlec M, Gutterman D, Mammoto T. YAP1-TEAD1 signaling controls angiogenesis and mitochondrial biogenesis through PGC1α. Microvasc Res. 2018;119:73–83. doi: 10.1016/j.mvr.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahoney JE, Mori M, Szymaniak AD, Varelas X, Cardoso WV. The Hippo pathway effector yap controls patterning and differentiation of airway epithelial progenitors. Dev Cell. 2014;30:137–150. doi: 10.1016/j.devcel.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin C, Yao E, Chuang PT. A conserved MST1/2-YAP axis mediates Hippo signaling during lung growth. Dev Biol. 2015;403:101–113. doi: 10.1016/j.ydbio.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mammoto T, Muyleart M, Konduri GG, Mammoto A. Twist1 in hypoxia-induced pulmonary hypertension through transforming growth factor-β–Smad signaling. Am J Respir Cell Mol Biol. 2018;58:194–207. doi: 10.1165/rcmb.2016-0323OC. [DOI] [PubMed] [Google Scholar]
- 32.Mammoto T, Jiang A, Jiang E, Mammoto A. The role of Twist1 phosphorylation in angiogenesis and pulmonary fibrosis. Am J Respir Cell Mol Biol. 2016;55:633–644. doi: 10.1165/rcmb.2016-0012OC. [DOI] [PubMed] [Google Scholar]
- 33.Kaisani A, Delgado O, Fasciani G, Kim SB, Wright WE, Minna JD, et al. Branching morphogenesis of immortalized human bronchial epithelial cells in three-dimensional culture. Differentiation. 2014;87:119–126. doi: 10.1016/j.diff.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, et al. YAP1 acts downstream of alpha-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature. 2010;465:483–486. doi: 10.1038/nature09002. [DOI] [PubMed] [Google Scholar]
- 36.Walker EJ, Shen F, Young WL, Su H. Cerebrovascular casting of the adult mouse for 3D imaging and morphological analysis. J Vis Exp. 2011;(57):e2958. doi: 10.3791/2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, et al. YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the wnt response. Cell. 2014;158:157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 38.Park HW, Kim YC, Yu B, Moroishi T, Mo JS, Plouffe SW, et al. Alternative Wnt signaling activates YAP/TAZ. Cell. 2015;162:780–794. doi: 10.1016/j.cell.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramos A, Camargo FD. The Hippo signaling pathway and stem cell biology. Trends Cell Biol. 2012;22:339–346. doi: 10.1016/j.tcb.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: Hippo signaling and beyond. Physiol Rev. 2014;94:1287–1312. doi: 10.1152/physrev.00005.2014. [DOI] [PubMed] [Google Scholar]
- 41.Caverzasio J, Biver E, Thouverey C. Predominant role of PDGF receptor transactivation in Wnt3a-induced osteoblastic cell proliferation. J Bone Miner Res. 2013;28:260–270. doi: 10.1002/jbmr.1748. [DOI] [PubMed] [Google Scholar]
- 42.Roszell B, Mondrinos MJ, Seaton A, Simons DM, Koutzaki SH, Fong GH, et al. Efficient derivation of alveolar type ii cells from embryonic stem cells for in vivo application. Tissue Eng Part A. 2009;15:3351–3365. doi: 10.1089/ten.tea.2008.0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Z, Shu W, Lu MM, Morrisey EE. Wnt7b activates canonical signaling in epithelial and vascular smooth muscle cells through interactions with Fzd1, Fzd10, and LRP5. Mol Cell Biol. 2005;25:5022–5030. doi: 10.1128/MCB.25.12.5022-5030.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Felcht M, Luck R, Schering A, Seidel P, Srivastava K, Hu J, et al. Angiopoietin-2 differentially regulates angiogenesis through TIE2 and integrin signaling. J Clin Invest. 2012;122:1991–2005. doi: 10.1172/JCI58832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu J, Srivastava K, Wieland M, Runge A, Mogler C, Besemfelder E, et al. Endothelial cell–derived angiopoietin-2 controls liver regeneration as a spatiotemporal rheostat. Science. 2014;343:416–419. doi: 10.1126/science.1244880. [DOI] [PubMed] [Google Scholar]
- 46.Mammoto A, Mammoto T, Ingber DE. Mechanosensitive mechanisms in transcriptional regulation. J Cell Sci. 2012;125:3061–3073. doi: 10.1242/jcs.093005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bertero T, Cottrill KA, Lu Y, Haeger CM, Dieffenbach P, Annis S, et al. Matrix remodeling promotes pulmonary hypertension through feedback mechanoactivation of the YAP/TAZ–MIR-130/301 circuit. Cell Reports. 2015;13:1016–1032. doi: 10.1016/j.celrep.2015.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hogan BL, Barkauskas CE, Chapman HA, Epstein JA, Jain R, Hsia CC, et al. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell. 2014;15:123–138. doi: 10.1016/j.stem.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barkauskas CE, Chung MI, Fioret B, Gao X, Katsura H, Hogan BL. Lung organoids: current uses and future promise. Development. 2017;144:986–997. doi: 10.1242/dev.140103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stevens T, Phan S, Frid MG, Alvarez D, Herzog E, Stenmark KR. Lung vascular cell heterogeneity: Endothelium, smooth muscle, and fibroblasts. Proc Am Thorac Soc. 2008;5:783–791. doi: 10.1513/pats.200803-027HR. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.