Abstract
We previously identified a novel selective androgen receptor modulator, S42, that does not stimulate prostate growth but has a beneficial effect on lipid metabolism. S42 also increased muscle weight of the levator ani in orchiectomized Sprague–Dawley rats. These findings prompted us to investigate whether S42 has a direct effect on cultured C2C12 myotubes. S42 significantly lowered expression levels of the skeletal muscle ubiquitin ligase (muscle atrophy-related gene), atrogin1 and Muscle RING-Finger Protein 1(MuRF1) in C2C12 myotubes, as determined by real time PCR. Phosphorylation of p70 S6 kinase (p70S6K), an essential factor for promoting protein synthesis in skeletal muscle, was significantly increased by S42 to almost the same extent as by insulin, but this was significantly prevented by treatment with rapamycin, an inhibitor of mechanistic target of rapamycin complex 1 (mTORC1). However, phosphorylation of Akt, upstream regulator of mTORC1, was not changed by S42. S42 did not increase insulin-like growth factor 1 (Igf1) mRNA levels in C2C12 myotubes. These results suggest that S42 may have an anabolic effect through activation of mTORC1–p70S6K signaling, independent of IGF-1-Akt signaling and may exert an anti-catabolic effect through inhibition of the degradation pathway in cultured C2C12 myotubes.
Keywords: SARM, C2C12, Myotube, Muscle
Highlights
-
•
A SARM, S42 lowered expression levels of atrogin1 and MuRF1 mRNA in C2C12 myotubes.
-
•
S42 increased phosphorylation of p70S6K through activation of mTORC1 in C2C12 myotubes.
-
•
S42 may have anti-catabolic and anabolic effect in vitro.
1. Introduction
Muscle satellite cells play a major role in muscle regeneration [1]. However, muscle mass mainly depends on protein turnover. The well-known effect of the anabolic hormones, insulin/insulin-like growth factor 1 (IGF-1), on muscle is mainly mediated by Akt signaling and its downstream target, mechanistic target of rapamycin complex 1 (mTORC1) [2]. Muscle atrophy is mainly controlled by two specific genes encoding muscle-specific ubiquitin ligases, atrogin1 (also known as MAFbx) and MuRF1 [3]. The mechanisms controlling muscle mass have attracted increasing attention because various clinical situations are associated with muscle mass reduction, including aging and many diseases such as diabetes, lung diseases and cancer cachexia [4]. Sarcopenia is one such medical problem and is defined as an age-related, involuntary loss of skeletal muscle mass and strength, causing functional decline and loss of independence in older adults [5]. Thus, innovative drugs that prevent muscle mass reduction with aging or in various pathological situations have been long awaited.
Testosterone deficiency or disruption of androgen receptor (AR) signaling is associated with increased risks of cardiovascular disease, metabolic syndrome and sarcopenia. [6], [7]. Consequently, testosterone-replacement therapy has been recommended for low-testosterone disorders such as late-onset hypogonadism [5], but this has the potential to accelerate the progression of overt or latent prostate cancer (PC). To mitigate this risk, a class of novel compounds termed selective androgen receptor modulators (SARMs) has been developed; these compounds have no significant androgen-like effects in the prostate, but act as agonists in selected androgen-responsive tissues [8], [9]. For example, it was reported that a ligand compound LGD2226 shows anabolic activity as androgen on the levator muscle as well as bone mass and strength, while having little effect on prostate size in a rodent model [8].
In an effort to develop a SARM with beneficial activity in energy homeostasis, we previously screened 119 steroid analogs for the ability to induce uncoupling protein-1 mRNA without increasing prostate-specific antigen promoter activity in the human PC cell line, LNCaP [10]. As a result, we identified a novel SARM, S42, which is a structural analog of testosterone [10]. In LNCaP cells, S42 does not induce AR transactivation, but antagonizes 5α-dihydrotestosterone (DHT)-induced AR activation [10]. We have recently demonstrated that S42 inhibits PC cell proliferation in vitro and in vivo [11]. Furthermore, administration of S42 to orchiectomized Sprague–Dawley rats for 3 weeks was as effective as DHT in increasing the weight of the levator ani muscle, but did not elevate the prostate weight at any dose, in contrast to the effect of DHT. These results suggest that, while sparing the prostate from potentially harmful effects, S42 had an anabolic action in orchiectomized rats [10].
In the present study, we investigated the direct effect of S42 on the muscle cell line, C2C12. We found that S42 has both anabolic and anti-catabolic effects on the myotubes formed by differentiating C2C12 cells in vitro.
2. Materials and methods
2.1. Chemicals
S42 (Patent No. 5789874, Japan) was synthesized by Aska Pharmaceutical Co., Ltd. (Tokyo, Japan). DHT was purchased from Sigma Chemical Co. (St. Louis, MO, USA). Insulin was purchased from Thermo Fisher Scientific (Waltham, MA, USA). S42, DHT and rapamycin were dissolved in dimethyl sulfoxide (DMSO) (Nacalai Tesque, Kyoto, Japan) and insulin was dissolved in a zinc solution.
2.2. Cell culture
Mouse myoblast C2C12 cells were purchased from the American Type Culture Collection (Manassas, VA, USA). The C2C12 cells were seeded into 6-well plates at 2 × 105 cells/well and maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum. When the cultured cells became 80–90% confluent, the medium was changed to DMEM supplemented with 2% horse serum (HS) and the cells were further cultured for 4 days to induce differentiation into myotubes. Next, the medium was changed to DMEM containing 2% dextran-coated-charcoal–stripped HS from one day before treatment with various drugs: 1 or 10 μM S42, 100 nM DHT, 2 μM insulin and 1 nM rapamycin were tested.
2.3. Reverse transcription and quantitative real-time polymerase chain reaction (qPCR)
Total RNA was isolated from C2C12 cells using Sepasol RNA I Super G (Nacalai Tesque, Kyoto, Japan) and reverse-transcribed to cDNA. qPCR reactions were performed using a LightCycler 2.0 system (Roche, Basel, Switzerland) and SYBR Premix Ex Taq II (Takara, Otsu, Japan). Each sample was analyzed in triplicate and mRNA levels were normalized against Gapdh mRNA levels. The primer sequences were as follows:
Gapdh, 5′-TGTGTCCGTCGTGGATCTCTGA-3′ (forward), 5′-TTGCTGGTTGAAGTCGCAGGA-3′ (reverse); Ar 5′-ATGTGGTCAAGTGGGCCAG-3′ (forward), 5′-ACCATCAGTCCCATCCAGGAA-3′ (reverse); atrogin1, 5′-TCTAGGCTTGTCCGGGCTTC-3′ (forward), 5′-AAGCCTCAGTTCTTGGGCTCTC-3′ (reverse); MuRF1, 5′-GTGCCAAGCAGCTCATCAAG-3′ (forward), 5′-CCAAAGTCAATGGCCCTCAA-3′ (reverse); Igf1, 5′-TCACTGCCCAATTGAAATACGA-3′ (forward), 5′-TTAGGCCCAGACAGTTTAAACAAAG-3′ (reverse).
2.4. Western blotting
Western blot analysis was performed essentially as previously described [11]. C2C12 cells were lysed with RIPA buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, Protease Inhibitor Cocktail, 0.1% sodium dodecyl sulfate). Samples of 20 µg total protein were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. The blots were then incubated with the following primary antibodies: AR (N-20) (#sc-816, Santa Cruz), phospho-p70S6K (Thr389) (#9234S, Cell Signaling Technology (CST) Japan, Tokyo), p70S6K (#9202S, CST Japan), phospho-Akt (Ser473) (#4058S, CST Japan), Akt (#9272S, CST Japan), phospho-p44/42 MAPK (p-Erk1/2) (#9101S, CST Japan), p44/42 MAPK (Erk1/2) (#9102S, CST Japan), GAPDH (#NB300–320, Novus Biological, Ontario). The secondary antibody was from CST Japan. Each sample was analyzed in triplicate and protein expression was normalized against Gapdh protein expression.
2.5. Statistical analysis
All results are expressed as a mean ± standard error (SE). Statistical analyses were performed using one-way analysis of variance (ANOVA) followed by Dunnett analysis, as appropriate. P values < 0.05 were considered to be statistically significant.
3. Results
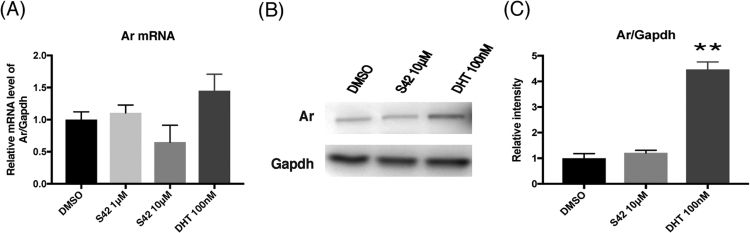
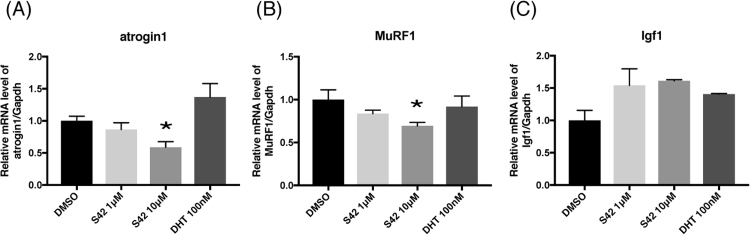
First, the effects of DHT or S42 on the expression levels of Ar in C2C12 myotubes were examined by qPCR and Western blot analysis. Administration of 100 nM DHT caused a 1.45 fold increase in the mRNA level but it was not significant (Fig. 1A). However, protein level of Ar was significantly increased to 4.5 fold by 100 nM DHT (Fig. 1B and C). No significant change in Ar was induced by 1–10 μM S42 at either the mRNA or the protein level (Fig. 1A-C). Next, the effects of DHT or S42 on the expression levels of atrogin1 and MuRF1, which are skeletal muscle ubiquitin ligases (muscle atrophy-related genes), was analyzed by qPCR (Fig. 2A and B). No effect of DHT on the expression of atrogin1 or MuRF1 was observed. However, S42 significantly lowered the expression levels of atrogin1 (P = 0.03) and MuRF1 (P = 0.046) compared with those by treatment with control (DMSO) in C2C12 myotubes (Fig. 2A and B). These results suggest that S42 exerts an inhibitory action against muscle protein degradation.
Fig. 1.
Effects of S42 or DHT on Ar expression on C2C12 myotubes. C2C12 myotubes were incubated with 1–10 µM of S42 or 100 nM of DHT or appropriate vehicle (DMSO) for 24 h. (A) Comparison of mRNA expression levels of Ar relative to those of Gapdh by qPCR. Data are expressed as mean ± SE of triplicate samples. (B)Western blot analysis showing Ar and Gapdh. (C) Statistical comparison of the expression levels of Ar relative to Gapdh by Western blot analysis. Data are expressed as mean ± SE of triplicate samples. In statistical comparisons in (A) and (C), the data of treated groups with DHT or S42 were compared with that of untreated group. **P < 0.01 vs DMSO by one-way ANOVA.
Fig. 2.
Effects of S42 or DHT on atrogin1, MuRF1, Igf1 expression on C2C12 myotubes. C2C12 myotubes were incubated with 1–10 µM of S42 or 100 nM of DHT or appropriate vehicle (DMSO) for 24 h. (A), (B), (C) Comparison of mRNA expression levels of atrogin1, MuRF1 and Igf1 relative to those of Gapdh, respectively are shown. Data are expressed as mean ± SE of triplicate samples. In statistical comparisons, the data of treated groups with DHT or S42 were compared with that of untreated group. *P < 0.05 vs DMSO by one-way ANOVA.
The effect of DHT or S42 on Igf1 mRNA was then examined by qPCR in C2C12 myotubes. However, no significant increase of Igf1 mRNAwas observed by treatment with DHT or S42 (Fig. 2C).
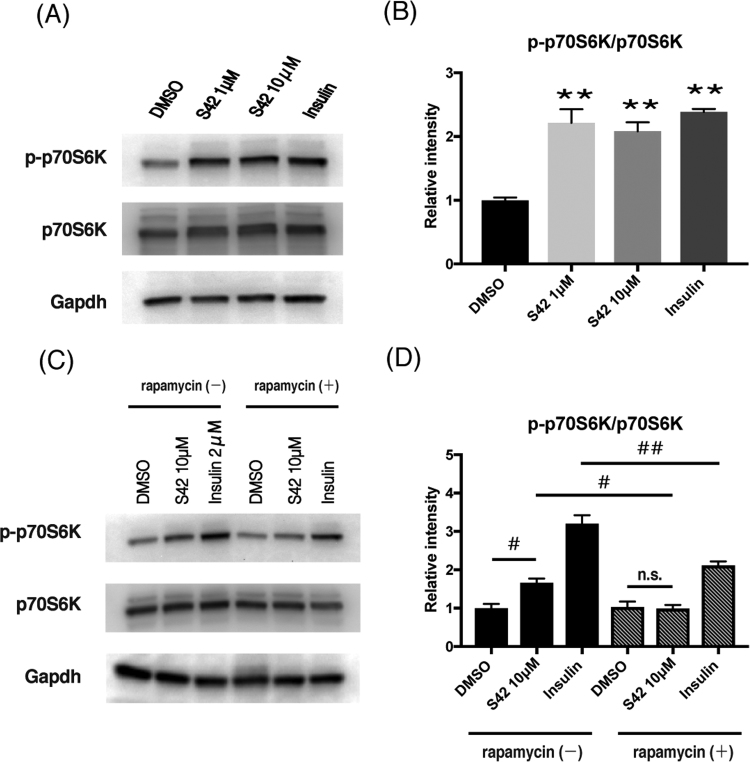
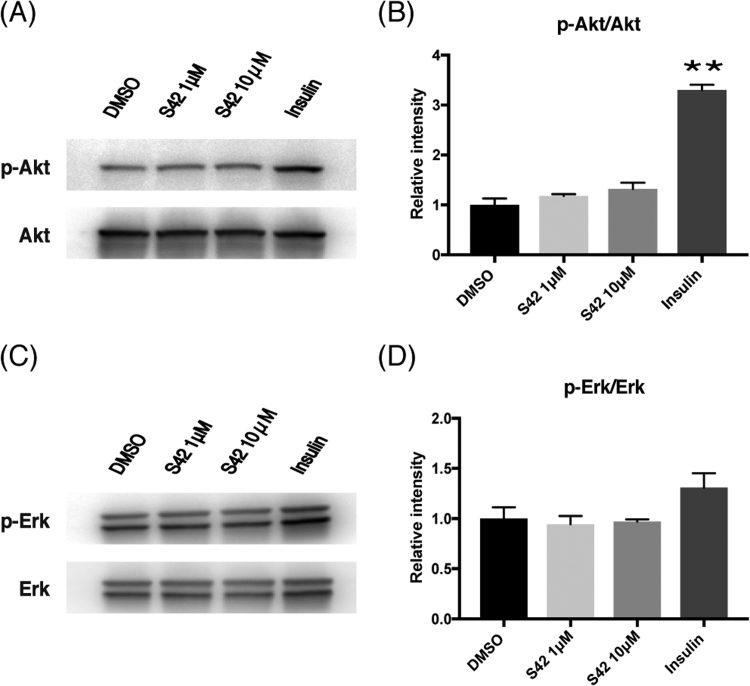
Phosphorylation of the mTORC1-p70S6K signaling pathway is an important factor for promoting protein synthesis in skeletal muscle. We therefore examined the phosphorylation of p70S6K by western blotting (Fig. 3A-D). 100 nM DHT did not show any effect on p70S6K phosphorylation (data not shown; 2 µM insulin treatment was used as a positive control). However, 1 µM and 10 µM S42 significantly increased p70S6K phosphorylation, to almost the same extent as that observed following treatment with the 2 µM insulin (Fig. 3A and B). Importantly, the effect was significantly canceled by treatment with 1 nM rapamycin, an inhibitor of mTORC1 (Fig. 3C and D). Next, the effect of S42 was examined on signaling upstream of mTORC1, namely on the phosphorylation of Akt or Erk (Fig. 4A and B). The phosphorylation of Akt and Erk was not changed by administration of 1 µM or 10 µM S42 while 2 µM of insulin significantly stimulated the phosphorylation of Akt (P < 0.01).
Fig. 3.
S42 increases phosphorylation of p70S6K (Thr389) in C2C12 cells. Effects of S42 or insulin on phosphorylation of p70S6K (p-p70S6K) on C2C12 myotubes by Western blot analysis (A) and their statistical evaluations (B). C2C12 myotubes were incubated with 1–10 μM of S42 or 2 μM of insulin or appropriate vehicle for 24 h. Effect of S42 or insulin on phosphorylation of p70S6K (p-p70S6K) on C2C12 myotubes in the presence or absence of rapamycin by Western blotting (C) and their statistical evaluations (D). C2C12 myotubes were incubated with 1–10 μM of S42 or 2 μM of insulin or appropriate vehicle in the presence or absence of 1 nM of rapamycin for 24 h. In statistical comparisons, expressions of p-p70S6K protein relative to p70S6K protein were determined and the data of treated groups with S42 or insulin were compared with that of untreated group. Data are expressed as mean ± SE of triplicate samples. *P < 0.05, **P < 0.01 vs DMSO, # P < 0.05, ##P < 0.01 in indicated comparisons by one-way ANOVA. ns, not significant.
Fig. 4.
Effects of S42 on phosphorylation of Akt (Ser473) or Erk on C2C12 myotubes by Western blot analysis (A, B) and their statistical evaluations (C, D). C2C12 myotubes were incubated with 1–10 μM of S42 or 2 μM of insulin or appropriate vehicle for 24 h. In statistical comparisons, expressions of p-Akt relative to Akt (B) or p-Erk relative to Erk (D) were determined. Data of treated groups with S42 or insulin were compared with those of untreated group. Data are expressed as mean ± SE of triplicate samples. **P < 0.01 vs DMSO by one-way ANOVA.
4. Discussion
In the present study, we demonstrated that a novel SARM, S42 has both anabolic and anti-catabolic effects on cultured C2C12 myotubes, as evidenced by the findings that S42 significantly lowered the expression levels of the skeletal muscle ubiquitin ligase, atrogin1 and MuRF1, and increased the phosphorylation of p70S6K, an important factor for promoting protein synthesis in skeletal muscle. Phosphorylation of p70S6K is implicated in androgen-induced skeletal muscle hypertrophy in vivo [12]. These results suggest that S42 may have a beneficial effect on the maintenance of muscle mass.
In orchiectomized rats, increased expression of atrogin1 and MuRF1 in atrophied skeletal muscle (the levator ani muscle) has been observed, but these phenomena have been shown to be fully reversed by SARM compounds [13]. Similarly, reduced androgen levels following the treatment of aged rats with gonadotropin-releasing hormone caused a systemic increase in muscle protein degradation and enhanced apoptosis owing to the decreased Akt-mediated muscle hypertrophy. This phenomenon was also reversed by testosterone administration [14]. Thus, it is clear that endogenous testosterone has an anabolic and anti-catabolic effect on muscle. In C2C12 myotubes, Ar protein expression was significantly increased by DHT treatment as previously noted in prostate cancer cells, LNCaP cells [10], [15]. This is probably due to stabilizing AR mRNA (15) since Ar mRNA level was not increased in C2C12 myotubes. However, in our study, DHT did not show any effect on the levels of atrogin1/MuRF1 in C2C12 myotubes. Although the reason for the lack of the effect of DHT in C2C12 myotubes remains unclear, little response to DHT in C2C12 cells have been reported previously [16] and relatively lower expression of Ar in C2C12 cells has been speculated to be the reason [16]. In this sense, the clear effect of S42 in the prevention of muscle protein degradation in C2C12 myotubes may be unique.
IGF-1, a circulating growth factor, is also produced locally by many tissues, including skeletal muscle [17]. IGF-1 and/or insulin signaling can suppress protein breakdown while promoting muscle growth [2]. Muscle-specific overexpression of a locally acting Igf-1 isoform in mice showed that localized Igf-1 expression sustains muscle growth and regeneration [18]. The IGF-1-Akt-mTORC1 signaling pathway has been thought to be essential for the regulation of skeletal muscles’ protein synthesis and degradation [19] although this pathway is still controversial, from the findings that the correlation between IGF-1 levels and Akt-mTORC1 was not consistent in several reports [20], [21]. In this study, Igf1 mRNA was unchanged following treatment of C2C12 myotubes with S42, suggesting little impact of S42 on the most upstream regulator of muscle cell, Igf1.
Phosphorylation of Akt and Erk, which are upstream regulators of mTORC1 [17], [22], was not changed by administration of S42. In the canonical IGF-1-Akt-mTORC1 pathway, Akt-mTOR activation and sequential phosphorylation of p70S6K is supposed to induce protein synthesis [23]. p70S6K is a serine/threonine kinase and its phosphorylation of S6 ribosomal protein induces protein synthesis at the ribosome. In our study, S42 significantly increased the phosphorylation of p70S6K, a downstream target of mTORC1 signaling, and importantly, this phosphorylation was prevented by treatment with rapamycin, an inhibitor of mTORC1. However, phosphorylation of Akt was not changed by the administration of S42. Because extracellular-signal-regulated kinase (ERK) pathway has been suggested as downstream signaling of IGF-1 [24] and because testosterone has been also suggested to affect ERK pathway [25], we also tested a possibility of involvement of ERK pathway in the effect of S42. However, this possibility was unlikely since phosphorylation of ERK was not increased by S42 treatment. These results suggest that S42 may stimulate mTORC1-p70S6K signaling, independent of canonical IGF-1-Akt signaling or ERK pathway. Further investigations are needed for the clarification of more detailed mechanism of S42 on muscle protein turnover.
Testosterone is known to increase AR expression in the muscle stem cells, satellite cells, and to activate the satellite cells, thus leading to muscle hyperplasia [26]. In this study, we did not examine the effect of S42 on muscle satellite cells.
Collectively, our results suggest that S42 may have an anabolic effect through the activation of mTORC1-p70S6K signaling and an anti-catabolic effect through the inhibition of the degradation pathway (atrogin 1 and MuRF1) in cultured C2C12 myotubes. This selective action on muscle by a SARM could encourage muscle growth while at the same time minimizing some of the unwanted aspects of testosterone, such as prostate growth in men. Currently, there are several SARMs in clinical trials [27]. One example is ostarine, a SARM developed to help with muscle wasting. In a double-blind clinical study of 120 healthy elderly people aged 60 years or older who took part in a 2-week treatment, the administration of 3 mg ostarine showed a significant improvement in lean body mass and physical ability (determined by stair climbing), compared with the placebo treatment group [28]. Our study is the initial step for the application of S42 in muscle–related diseases, and further studies are needed to clarify the effect of S42 on muscle in humans.
Acknowledgements
We thank Nicholas Rufaut, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Acknowledgments
Funding sources
This work was supported in part by a Grant-in-Aid for Scientific Research (B) Japan Society for the Promotion of Science (ID: 15H04854).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2019.01.006.
Appendix A. Transparency document
Supplementary material
References
- 1.McCarthy J.J., Mula J., Miyazaki M., Erfani R., Garrison K., Farooqui A.B., Srikuea R., Lawson B.A., Grimes B., Keller C., Van Zant G., Campbell K.S., Esser K.A., Dupont-Versteegden E.E., Peterson C.A. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development. 2011;138:3657–3666. doi: 10.1242/dev.068858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bodine S.C., Stitt T.N., Gonzalez M., Kline W.O., Stover G.L., Bauerlein R., Zlotchenko E., Scrimgeour A., Lawrence J.C., Glass D.J., Yancopoulos G.D. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 2001;3:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 3.Gomes M.D., Lecker S.H., Jagoe R.T., Navon A., Goldberg A.L. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc. Natl. Acad. Sci. USA. 2001;98:14440–14445. doi: 10.1073/pnas.251541198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fielding R.A., Vellas B., Evans W.J., Bhasin S., Morley J.E., Newman A.B., Abellan van Kan G., Andrieu S., Bauer J., Breuille D., Cederholm T., Chandler J., De Meynard C., Donini L., Harris T., Kannt A., Keime Guibert F., Onder G., Papanicolaou D., Rolland Y., Rooks D., Sieber C., Souhami E., Verlaan S., Zamboni M. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. J. Am. Med. Dir. Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Metter E.J., Conwit R., Tobin J., Fozard J.L. Age-associated loss of power and strength in the upper extremities in women and men. J. Gerontol. A Biol. Sci. Med. Sci. 1997;52:B267–B276. doi: 10.1093/gerona/52a.5.b267. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman J.M., Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr. Rev. 2005;26:833–876. doi: 10.1210/er.2004-0013. [DOI] [PubMed] [Google Scholar]
- 7.Liu P.Y., Swerdloff R.S., Veldhuis J.D. Clinical review 171: the rationale, efficacy and safety of androgen therapy in older men: future research and current practice recommendations. J. Clin. Endocrinol. Metab. 2004;89:4789–4796. doi: 10.1210/jc.2004-0807. [DOI] [PubMed] [Google Scholar]
- 8.Bhasin S., Jasuja R. Selective androgen receptor modulators as function promoting therapies. Curr. Opin. Clin. Nutr. Metab. Care. 2009;12:232–240. doi: 10.1097/MCO.0b013e32832a3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Negro-Vilar A. Selective androgen receptor modulators (SARMs): a novel approach to androgen therapy for the new millennium. J. Clin. Endocrinol. Metab. 1999;84:3459–3462. doi: 10.1210/jcem.84.10.6122. [DOI] [PubMed] [Google Scholar]
- 10.Min L., Yanase T., Tanaka T., Fan W., Nomura M., Kawate H., Okabe T., Takayanagi R., Nawata H. A novel synthetic androgen receptor ligand, S42, works as a selective androgen receptor modulator and possesses metabolic effects with little impact on the prostate. Endocrinology. 2009;150:5606–5616. doi: 10.1210/en.2009-0405. [DOI] [PubMed] [Google Scholar]
- 11.Kawanami T., Tanaka T., Hamaguchi Y., Nomiyama T., Nawata H., Yanase T. Selective androgen receptor modulator S42 suppresses prostate cancer cell proliferation. Endocrinology. 2018;159:1774–1792. doi: 10.1210/en.2018-00099. [DOI] [PubMed] [Google Scholar]
- 12.Xu T., Shen Y., Pink H., Triantafillou J., Stimpson S.A., Turnbull P., Han B. Phosphorylation of p70s6 kinase is implicated in androgen-induced levator ani muscle anabolism in castrated rats. J. Steroid Biochem. Mol. Biol. 2004;92:447–454. doi: 10.1016/j.jsbmb.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Jones A., Hwang D.-J., Narayanan R., Miller D.D., Dalton J.T. Effects of a novel selective androgen receptor modulator on dexamethasone-induced and hypogonadism-induced muscle atrophy. Endocrinology. 2010;151:3706–3719. doi: 10.1210/en.2010-0150. [DOI] [PubMed] [Google Scholar]
- 14.Kovacheva E.L., Hikim A.P., Shen R., Sinha I., Sinha-Hikim I. Testosterone supplementation reverses sarcopenia in aging through regulation of myostatin, c-Jun NH2-terminal kinase, Notch, and Akt signaling pathways. Endocrinology. 2010;151:628–638. doi: 10.1210/en.2009-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeap B.B., Krueger R.G., Leedman P.J. Differential posttranscriptional regulation of androgen receptor gene expression by androgen in prostate and breast cancer cells. Endocrinology. 1999;140:3282–3291. doi: 10.1210/endo.140.7.6769. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y., Lee N.K., Zajac J.D., MacLean H.E. Generation and analysis of an androgen-responsive myoblast cell line indicates that androgens regulate myotube protein accretion. J. Endocrinol. Investig. 2008;31:910–918. doi: 10.1007/BF03346441. [DOI] [PubMed] [Google Scholar]
- 17.Schiaffino S., Mammucari C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights from genetic models. Skelet. Muscle. 2011;1:4. doi: 10.1186/2044-5040-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Musaro A., McCullagh K., Paul A., Houghton L., Dobrowolny G., Molinaro M., Barton E.R., Sweeney H.L., Rosenthal N. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat. Genet. 2001;27:195–200. doi: 10.1038/84839. [DOI] [PubMed] [Google Scholar]
- 19.Glass D.J. Molecular mechanisms modulating muscle mass. Trends Mol. Med. 2003;9:344–350. doi: 10.1016/s1471-4914(03)00138-2. [DOI] [PubMed] [Google Scholar]
- 20.Markofski M.M., Dickinson J.M., Drummond M.J., Fry C.S., Fujita S., Gundermann D.M., Glynn E.L., Jennings K., Paddon-Jones D., Reidy P.T., Sheffield-Moore M., Timmerman K.L., Rasmussen B.B., Volpi E. Effect of age on basal muscle protein synthesis and mTORC1 signaling in a large cohort of young and older men and women. Exp. Gerontol. 2015;65:1–7. doi: 10.1016/j.exger.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandri M., Barberi L., Bijlsma A.Y., Blaauw B., Dyar K.A., Milan G., Mammucari C., Meskers C.G., Pallafacchina G., Paoli A., Pion D., Roceri M., Romanello V., Serrano A.L., Toniolo L., Larsson L., Maier A.B., Munoz-Canoves P., Musaro A., Pende M., Reggiani C., Rizzuto R., Schiaffino S. Signalling pathways regulating muscle mass in ageing skeletal muscle: the role of the IGF1-Akt-mTOR-FoxO pathway. Biogerontology. 2013;14:303–323. doi: 10.1007/s10522-013-9432-9. [DOI] [PubMed] [Google Scholar]
- 22.Frost R.A., Lang C.H. Protein kinase B/Akt: a nexus of growth factor and cytokine signaling in determining muscle mass. J. Appl. Physiol. 2007;103(1985):378–387. doi: 10.1152/japplphysiol.00089.2007. [DOI] [PubMed] [Google Scholar]
- 23.Ma X.M., Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 24.Perrini S., Laviola L., Carreira M.C., Cignarelli A., Natalicchio A., Giorgino F. The GH/IGF1 axis and signaling pathways in the muscle and bone: mechanisms underlying age-related skeletal muscle wasting and osteoporosis. J. Endocrinol. 2010;205:201–210. doi: 10.1677/JOE-09-0431. [DOI] [PubMed] [Google Scholar]
- 25.Wu Y., Bauman W.A., Blitzer R.D., Cardozo C. Testosterone-induced hypertrophy of L6 myoblasts is dependent upon Erk and mTOR. Biochem. Biophys. Res. Commun. 2010;400:679–683. doi: 10.1016/j.bbrc.2010.08.127. [DOI] [PubMed] [Google Scholar]
- 26.Sinha-Hikim I., Taylor W.E., Gonzalez-Cadavid N.F., Zheng W., Bhasin S. Androgen receptor in human skeletal muscle and cultured muscle satellite cells: up-regulation by androgen treatment. J. Clin. Endocrinol. Metab. 2004;89:5245–5255. doi: 10.1210/jc.2004-0084. [DOI] [PubMed] [Google Scholar]
- 27.Sakkiah S., Ng H.W., Tong W., Hong H. Structures of androgen receptor bound with ligands: advancing understanding of biological functions and drug discovery. Exp. Opin. Ther. Targets. 2016;20:1267–1282. doi: 10.1080/14728222.2016.1192131. [DOI] [PubMed] [Google Scholar]
- 28.Dalton J.T., Barnette K.G., Bohl C.E., Hancock M.L., Rodriguez D., Dodson S.T., Morton R.A., Steiner M.S. The selective androgen receptor modulator GTx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: results of a double-blind, placebo-controlled phase II trial. J. Cachex., Sarcopenia Muscle. 2011;2:153–161. doi: 10.1007/s13539-011-0034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material