Graphical abstract
Keywords: Mushroom poisoning, Epidemiology, Phalloidin, Amanitin, Amanita virosa
Highlights
-
•
Globally, mushroom poisoning leads to a considerable number of deaths annually. However, no definite antidote has been introduced yet.
-
•
A mushroom-poisoning outbreak occurred in 2018 in Iran; this overview presents geographical distribution of Amanita virosa along with studies reporting A. virosa poisonings.
-
•
Also, main toxins of A. virosa, their toxicity mechanisms and pharmacological management of mushroom-poisoned individuals are presented.
Abstract
Mushrooms account for a part of human diet due to their exquisite taste and protein content as well as their promising health effects unveiled by scientific research. Toxic and non-toxic mushrooms frequently share considerable morphological similarities, which mislead the collectors/consumers, resulting in mycotoxicity. Numerous mushroom species are considered “poisonous” as they produce dangerous toxins. For instance, members of the genus Amanita, especially A. phalloides, A. virosa and A. verna, are responsible for severe and even life-threatening noxious consequences. Globally, mushroom poisoning is a crucial healthcare issue as it leads to a considerable number of deaths annually. However, no definite antidote has been introduced to treat this poisoning. The present article discusses the characteristics of A. virosa in terms of epidemiology, mechanisms of toxicity, poisoning features and management.
1. Introduction
Mushrooms are increasingly found in human diet due to their exquisite taste and protein content as well as their health-promoting effects revealed by numerous scientific studies [[1], [2], [3], [4], [5], [6]]. In this regard, several pharmacologically active compounds have been characterized in mushrooms [7]. Historically, it was noted that athletes in the 3rd century BC consumed mushrooms to enhance their performance [8]. In many countries, including Iran, collection and consumption of wild mushrooms found in forests and grasslands are traditional social activities [4,[9], [10], [11], [12]]. Different types of wild mushrooms are routinely picked and eaten by local inhabitants. Of more than 2000 mushrooms species, about 50 are toxic to humans [13]. Despite marked morphologic similarities, discrimination between toxic and non-toxic mushrooms is usually based on experience-related knowledge and observation. However, increasing interest in wild edible mushrooms has led to frequent collection and ingestion of poisonous species leading to poisonings with persistent issues in diagnosis and management [4,9,14].
Information on mushrooms poisoning in Iran has not been thoroughly recorded. It is therefore hard to retrieve informative data. However, recent reports showed that A. virosa is the most-prevalent Amanita species in Iran, consistent with reports from East Asian and European countries [15,16]. Additionally, in 2018, an outbreak of mushroom poisoning, later found to be caused by A. virosa, took lives of people in Western Iran [17]. Since our preliminary literature search showed no recent comprehensive review on A. virosa toxicological profile, we aimed to review the toxic effects of Amanita mushrooms, with a special focus on A. virosa.
2. Epidemiology of mushroom poisonings
Mushrooms poisoning is known as a major problem in Western countries [16,18] representing about 5.8% of the total poisonings in the US [11]. According to a report published by Litovitz et al. in 2002, 8996 mushroom poisoning cases were documented by the American Association of Poison Control Centers (AAPCC). Of these, 576 cases had mild poisoning, 56 had severe clinical conditions and six individuals died [19]. The Annual Report of the National Register of poisoning (U.S., 2009) reported 4083 (73.9%) children with mushroom poisoning with 3012 (54.5%) of them being <6 years old [20]. Later, in 2016, 6421 mushroom-poisoned cases were reported to AAPCC, including 39 severe and two fatal cases [21]. In Turkey, 143 mushroom-poisoned patients were admitted over a four-year period (between 1996 and 2000) to the central hospital of Osmangazi University, of which four patients died [4]. Another report from Turkey, reported 62 deaths in children aging 0–18 years, between 2009 and 2013 in Trabzon. It was found that 4 children (6.5%) were died due to mushroom poisoning; 3 of them were 0–3 years old [22].
In Switzerland, in a retrospective study conducted on 6307 patients with mushroom exposure (from 1995 to 2009), A. virosa was regarded as the cause of toxicity in one mild and 1 moderate cases. Generally, it was described that fatal poisonings were caused by amatoxin-containing species [23].
In Iran, Amanita species grow in many forested regions such as Mazandaran and Gilan, Northern Iran, as well as Azerbaijan (north-western Iran) and Western provinces [15,20,[22], [23], [24], [25], [26], [27]], but scarce information is available concerning mushrooms poisoning [24,25]. In 1993, three cases of A. virosa poisoning were reported from Hamadan, Western Iran, where one patient died [15]. The epidemiological pattern of mushroom poisoning among children aged 11–15 years admitted between 1988 and 1993, to Loghman Hakim Hospital, Tehran, Iran showed a mortality rate of 71% [24,28]. Another report from the same hospital indicated that from eight mushroom-poisoning cases, two patients (25%) died due to hepatic encephalopathy and gastrointestinal bleeding [29]. In a study conducted in 2006 in Iran, 72,421 suspected cases were examined and 37 patients (68% male and 32% female with an average age of 31 years old) were found to be intoxicated by poisonous mushrooms [24]. Another study reported 32 mushroom-poisoned patients (with an average age of 24.6 years old) referred to the Toxicology Center of Mashhad, Khorasan Razavi, Eastern Iran, from 2005 to 2011. Mushroom intoxication represented 0.1% of all intoxication cases admitted to the hospital [26].
In the most recent outbreak of mushroom poisoning in Iran, 1200 intoxicated individuals were referred to hospitals in 13 Western and Northwestern cities of Iran (over 90% of patients were from Kermanshah, Lorestan, Kordestam, and West Azerbaijan provinces) [25]. Of these patients, 8.9% were hospitalized and 1.5% died. Early signs and symptoms included abdominal pain, nausea, vomiting, and diarrhea. Though 50 toxic species of mushrooms grow in Iran, this recent report held Lepiota brunneioncarnata, Hypholoma fascicalare, and Coprinopsis atramentaria responsible for this outbreak [25]. Nevertheless, according to Food and Drug Administration, Health Ministry of Iran (available at https://www.tehrantimes.com/news/423947/Mushroom-poisoning-kills-18-in-Iran), A. virosa was the cause of poisoning in this scenario [17].
Variations in the mortality rate can usually be attributed to the type of mushroom species ingested, different levels of included toxins, and the vulnerability of poisoned subjects [26]. In most studies, spring and autumn were shown to have the highest incidence rates of mushroom poisoning [17,23,27,30,31].
3. Classification and toxicity of Amanita mushrooms
Numerous toxic mushrooms are found around the world, including those containing cyclopeptides, usually regarded as the most toxic species [32]. The genus Amanita belongs to the family Amanitaceae and includes the majority of mushrooms that are toxic to humans [18]. Amanita genus has about 900 to 1000 species, of which nine are known to produce poisonous amatoxins. Although the genus Lepiota has the largest number of amatoxin-producing species, the species from the Amanita genus are responsible for most of mushroom-poisoning deaths [[32], [33], [34], [35]]. The most well-known species of Amanita are A. phalloides, A. virosa, and A. verna; also, A. muscaria, A. smithiana, A. thiersii, A. ocreata, A. suballiacea, A. tenuifolia, A. nauseosa, A. virgineoides and A. bisporigera are other members of this genus. Among these different species, A. phalloides, A. verna, and A. virosa exert the highest toxicity, mainly involving the liver, kidneys and central nervous system [33,36,37]. More than 90% of the mushroom-related fatalities that are attributed to these Amanita mushrooms in Central Europe and North America, result from life-threatening acute hepatitis. Three other species, A. exitialis, A. fuliginea and A. subjunquillea, found in East Asia, contain cyclopeptides and have also been shown to cause liver failure and death [10,33,[37], [38], [39], [40], [41], [42], [43]]. The other Amanita mushrooms mainly cause nephrotoxicity [[44], [45], [46], [47]]. Interestingly, A. muscaria and A. pantherina contain ibotenic acid and muscimol, which produce hallucinogenic effects in addition to acute renal failure [37,44,48,49]. Among the rare edible species of Amanita mushrooms, A. lanei is frequently mistaken by species of the genus Agaricus [34]. A. virosa has a pure white appearance, like a veil of angels, and its roots are smoother compared to A. verna, but due to its deadly nature, it has been called "The destroying angel" (Fig. 1) [18]. Like other Amanita's mushrooms, it has a sweet smell and taste. The color of A. virosa cap is white and the color of the center becomes yellow or brown as it matures. A. virosa has white spores of 8–10 mm in diameter, with a length-to-width ratio <1.25 (Fig. 1) [18,34]. One of the most beautiful and widespread species of Amanita is the red and white A. muscaria also known as "fly agaric" [50].
Fig. 1.
Amanita virosa.
Since a long time, three types of mushrooms namely, A. virosa, Russula vesca and Russula persicina, have been identified in Iran [27]. Recent studies have shown that in Iran, A. virosa is more prevalent than A. phalloides [15,[24], [25], [26], [27]].
4. Toxins of Amanita mushrooms and mechanisms of toxicity
Major toxin classes found in the genus Amanita are amatoxins, phallotoxins and virotoxins, all classified as cyclopeptides with a sulfur-linked tryptophan and some unusual hydroxylated amino-acids [51]. Amatoxins are at least eight related toxic compounds of eight amino-acid residues arranged in a conserved pentacyclic structure. Phallotoxins are at least seven compounds, all of which are bicyclic heptapeptides. Virotoxins are monocyclic peptides formed by at least five different compounds. Their structure and biological activity are similar to those of phallotoxins, thus suggesting that they share common precursor pathways.
The two main toxins of A. phalloides are named phalloidin and amanitin. Phalloidin (MW of 900 Da) was first identified by Wieland in 1937 [52] in A. phalloides while phallotoxins were found in A. virosa for the first time in 1974 [53]. Amanitin, mainly alpha-amanitin with MW of around 900 Da [16], was discovered in A. virosa in 1966 [54], although its presence in this species remained controversial [55]. Interestingly, while amaninamide may be found in A. virosa, γ-amanitin is produced by A. phalloides and other Amanita species [37,56]. To date, the majority of the studies considered that A. virosa contains two major amatoxins namely alpha-amanitin and beta-amanitin, and two phallotoxins namely phalloidin and phallacidin [18,57,58]. However, in a study performed by Buku in 1980, amaninamide and alpha-amanitin but not beta- amanitin, were found in A. virosa. These results were supported by the findings reported by Yocum and Simons, following the chemical characterization of A. virosa mushrooms collected from different US regions [55,57,59]. Phalloidin and alpha-amanitin mechanisms of action are presented in Fig. 2, Fig. 3.
Fig. 2.
Phalloidin attacks the cell membranes causing leakage of calcium atoms, followed by loss of potassium ions. Reproduced based on a previously published report [60] with permission from the Estate of Bunji Tagawa.
Fig. 3.
Amanitin disintergrates hepatic cell nucleus. Reproduced based on a previously published report [60] with permission from the Estate of Bunji Tagawa.
Virotoxins are monocyclic peptides, and in terms of biological activity they are similar to phallotoxins [[61], [62], [63]]. Some articles indicated that virotoxins are only present in A. virosa, but other studies found virotoxins in A. subpallidorosea and A. virosa species. A. subpallidorosea and A. virosa are clustered according to phylogenetic analysis. Also, according to previous studies, the characteristics of toxin cyclopeptide are consistent with phylogenetic molecular relationships [56,57,64]. Virotoxins were mainly found in mushrooms collected from Europe and North-America. Toxovirin isolated from A. virosa has no mono- and diamino-oxidase activity but high oxidase activity for specific amino acids. Toxovirin-related effect on L-amino acids is double than that for DL-racemic mixtures. This toxin is chemically and structurally similar to toxophallin isolated from A. phalloides [65]. Viroidin cyclopeptide, a monocyclic peptide, was first detected in 2016 in A. virosa [37]. Like A. phalloides and A. virosa, other Amanita species like A. bisporigera and A. verna can also produce toxic peptides (amatoxin, phallotoxins, and virotoxin) [55,66,67]. Amatoxins have eight amino acids instead of seven. Again, sulfur atom joins two side chains; hydroxyl group (outlined) is essential for toxicity. Alpha-amanitin phalloidin structures (Fig. 4, Fig. 5) were worked out by Theodor Hermann Felix Wieland (1913–1995) at The Max Plack Institute for Medical Research in Heidleberg in 1974 [68].
Fig. 4.
Phalloidin as a phallotoxin, is a cyclic, or ring molecule made up of seven amino acids (outlined). A sulfur atom, connects the side chains of two amino acids on opposite sides of the ring. Reproduced based on a previously published report [60] with permission from the Estate of Bunji Tagawa.
Fig. 5.
Eight amino acids of amatoxin. Reproduced based on a previously published report [60] with permission from the Estate of Bunji Tagawa.
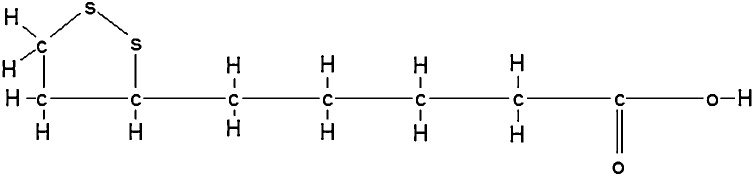
Thoitic acid (also known as alpha lipoic acid) is a possible antidote to amanita poisons. It contains a ring of carbons and sulfur atoms and a chain of carbon atoms (Fig. 6) [60].
Fig. 6.
Thiotic Acid chemical structure. Reproduced based on a previously published report [60] with permission from the Estate of Bunji Tagawa.
Though phallotoxins and virotoxins act faster, amatoxins are 10–20 times more toxic and are responsible for observed fatalities [37,50]. Amatoxins are potent and selective inhibitors of RNA polymerase II, a vital enzyme in the synthesis of mRNA, microRNA, and small nuclear RNA, leading to protein synthesis interruption and cell death. The lethal dose of orally-administered alpha-amanitin in humans is 0.1 mg/kg [26,69]. Amanitins including those produced by A. virosa, can damage the liver, kidneys and brain, eventually causing mortality [65,70,71]. Phallotoxins, although highly toxic to the liver and muscular cells, strongly bind actin [57], but mildly contribute to amanita-related toxicity since they are not absorbed by the gastrointestinal tract. Like phallotoxins, virotoxins have limited toxic effects after oral exposure. Their interaction with actin, which is even weaker than that of phallotoxins, stabilizes the bonds between actin monomers and prevents microfilaments depolymerization. A. phalloides and A. virosa also produce toxic lectins, differing in their carbohydrate moieties but leading to similar hemolysis [65,72]. Additionally, two amino-acids namely, 2,3-trans-3,4-dihydroxy-l-proline and 20-(methylsulphonyl)-l-tryptophan, were identified in A. virosa.
The physicochemical properties of the majority of these toxins make them heat-resistant and food processing methods like grilling, boiling, frying, and steaming cannot completely eliminate them [4,73,74]. Ingestion of toxin-containing Amanita mushrooms leads to challenging hepatotoxicity although additional disturbances like allergic gastroenteritis may also result [14,24,52,[75], [76], [77]]. Interestingly, nephrotoxic effects were also demonstrated in different studies [33,[78], [79], [80], [81]], which may have an important impact on the renal excretion of absorbed toxins. Therefore, the final observed clinical toxicity is related to the type and amount of ingested mushrooms, i.e. the content and specific toxicity of the involved cyclopeptides [82]. Different toxins found in A. virosa along with their mode(s) of action are presented in Table 1.
Table 1.
Features of Amanita virosa toxins.
| Type of toxin | Chemical structure | Target organ | Mechanism of action | Similar toxins | References |
|---|---|---|---|---|---|
| Virotoxin | Monocyclic heptapeptides (containing D-serine) | Liver and kidney | Disturbing Ca2+ homeostasis and reacting with actin | Ala-viroidin, Viroisin, Deoxoviroisin, Viroidin, Ala-desoxoviroidin and Phallotoxin | [57,64] |
| Amatoxin | Cyclic heptapeptides | Liver and kidney | Inhibition of DNA-dependent RNA polymerase II | α –amanitin, β -amanitin | [64] |
| Phallotoxin | Cyclic heptapeptides | Liver and kidney | Reacting with actin in the liver | Virotoxin | [64] |
| Amaninamide | Bicyclic peptides and (analogous to α –amanitin) | Liver | Inhibition of RNA polymerase II | Amatoxins | [59] |
| α -amanitin | Bicyclic peptides | Liver | Inhibition of RNA polymerase II | Amatoxin | [34] |
| β -amanitin | Bicyclic peptides | Liver | Inhibition of RNA polymerase III | Amatoxin | [34] |
| Viroisin | Cyclopeptide | liver | Reacting with actin in the liver | Virotoxin | [75] |
| Viroidin | Cyclopeptide | Liver | Reacting with actin in the liver | Virotoxin | [75] |
| Ala-viroidin | Cyclopeptide | Liver | Reacting with actin in the liver | Virotoxin | [75] |
| Deoxoviroisin | Cyclopeptide | Liver | Reacting with actin in the liver | Virotoxin | [75] |
| Ala-deoxoviroidin | Cyclopeptide | Liver | Reacting with actin in the liver | Virotoxin | [75] |
| Phalloidin | Cyclopeptide | Liver | Reacting with actin in the liver | Phallotoxin | [58,64] |
| Phallacidin | Cyclopeptide | Liver | Inhibition of RNA polymerase | Phallotoxin | [57,64] |
| Toxovirin | Cyclopeptide | Liver | Highly toxic against mammalian cells (its L-amino acid oxidase activity induces apoptosis in cancerous cells) | Toxophallin and lectin | [65] |
From a toxicokinetic point of view, the liver can excrete about 60% of the toxins in the bile [83] but returns to the liver through enterohepatic recirculation. Alpha-amanitin is quickly cleared from the serum by the kidneys [84]. A large amount of amatoxin is taken by the hepatocytes and undergoes extensive enterohepatic circulation [52,72]. The duration of action of these toxins is about 10–15 h in humans [50].
5. Toxic features and management
Most of Amanita poisonings are related to A. phalloides, A. virosa and A. verna, respectively. Nonetheless, since the incidence of A. virosa intoxication is increasing, more studies should be performed on this species [85].
Most mushroom intoxications are initially presented with gastrointestinal symptoms alone and usually resolve over time, mimicking viral gastroenteritis, but potentially lethal liver dysfunction may occur with Amanita. In A. virosa-poisoned patients, nausea and vomiting are the most common symptoms [17,23,26,27,30,31]; abdominal pain, diarrhea, irritability, vertigo and hepatitis may also occur. A. virosa poisoning develops in three clinical stages, starting 8–12, 12–48 and 72 h after the ingestion, respectively. The pancreas, testicles and blood are also affected by this intoxication. During the first stage the gastrointestinal tract is stimulated this effect is generally attributed to the phalloidin toxin and its active metabolites. The second stage of Amanita poisoning presents marked reduction of abdominal symptoms; however, hepatic and renal failure may occur. During the third phase, death happens because of coagulopathy (epistaxis, hematuria, melena and hematemesis), encephalopathy (muscular twitching, delirium, coma, seizures) and infrequently cardiomyopathy [86].
High Performance Liquid Chromatography (HPLC) has been the most common method used for the quantitative and qualitative analysis of Amanita mushroom toxins in biological specimens [[87], [88], [89], [90]]. Nevertheless, inconsistencies in methods of extraction of toxins and HPLC conditions do not allow drawing conclusions based on information reported by different laboratories. For instance, deadly cyclopeptidic toxins of A. fuligineoides and A. rimosa are yet to be discovered [37].
Mushrooms with incubation period <6 h contain muscarine, coprin, ibotenic acid and psilocybin toxins, cause mild clinical symptoms that disappear in a short time [26]. By contrast, this time delay was found to be a major and independent predictor of fatality in amatoxin poisoning [23,26,91,92].
Management of Amanita mushroom poisoning is mainly supportive in combination to gastrointestinal decontamination. Activated charcoal efficacy and/or gastric lavage is most useful if attempted within 1 h after the ingestion of a potentially life threatening poison. Of note, activated charcoal (20–40 g every 3–4 h) has also been administered routinely because it may also interrupt the enterohepatic circulation of amatoxins and potentially reduce their toxicity. On the other hand, gastric lavage is contraindicated in patients with loss of airway protective reflexes [93]. The main current goals of mushroom poisoning treatment are to reduce the serum concentrations of mushroom toxins in order to limit the extent of exposure and lessen the risks of organ damage [36,94,95]. Intravenous fluids should be given for forced diuresis and also to replenish fluids and electrolytes lost during the gastrointestinal phase [93]. The use of different extracorporeal techniques to enhance the toxin elimination was successfully reported including plasmapheresis, hemoperfusion, Molecular Absorbent Regenerating System (MARS®) dialysis and the fractionated plasma separation and adsorption system (Prometheus®). In the presence of acute liver failure caused by Amanita poisoning, indication of urgent liver transplantation should be considered, based on the standard King's College Criteria [96].
No specific life-saving approach exists. Various pharmacotherapies have been tested including intravenous penicillin G, thioctic acid, N-acetylcysteine, cimetidine, steroids, polymixin B, vitamin C, silymarin, and silibinin (Table 2) [32,34,36,84,[97], [98], [99], [100]]. Thioctic acid, an antioxidant used in cosmetics and anti-aging products due to its ability to scavenge free-radicals, was successfully used to treat amanitin poisoning cases [36], but has been abandoned, being considered inefficient. Silymarin and N-acetylcysteine have been found to play comparable protective properties mediated through the restoration of hepatic glutathione levels [98]. Recently, polymixin B which has a chemical structure similar to that of alpha-amanitin, has been found effective in preventing mushroom-induced hepatorenal damage [99]. In Iran, treatments such as penicillin G and silymarin, are routinely administered to most patients [100].
Table 2.
Characteristics of the different compounds used to treat Amanita virosa poisoning.
| Compound | Mode of action | Activity against | Dose | References |
|---|---|---|---|---|
| Silymarin (Silibinin) | Maintenance of hepatic glutathione level (reduces amatoxin uptake in the liver) | A. phalloides poisoning | 25-50 mg/kg/day | [26,32,34,96] |
| Penicillin G | Binding plasma proteins, prevention of toxin absorption in the liver and excretion of toxins through the kidneys | Amanitin | 1 million units/kg/day | [32,34] |
| Benzylpenicillin | Inhibition of transporter protein | alpha-amanitin | NS | [32,34] |
| Polymixin B | Binding RNA polymerase II | alpha-amanitin | NS | [34] |
| Thioctic acid | Acting as a coenzyme in the oxidative decarboxylation of pyruvate | Amanitin | 300 to 600 mg/kg with glucose | [53] |
| N-acetylcysteine | Hepatoprotective activity in acetaminophen poisoning but not in mushroom poisoning | NS | NS | [32,34] |
| Steroids (dexamethasone) | Controversial reprots | NS | 20–40 mg intravenously | [101] |
| Cimetidine | Suppression of amatoxins metabolism to toxic metabolites | Amatoxins | NS | [34,95] |
| Ethanol | Induction of toxin uptake by liver cells | Toxins | Solutions of 30–33% | [53] |
| Vitamin C | Antioxidant activity | Toxins | 3 g/day | [53] |
NS: not specified.
Toxic mushrooms cannot be easily identified based on their appearance. Mushroom-induced morbidities may result from consumer negligence and the delay for medical consultation after the ingestion of suspected mushrooms. Increasing public awareness seems an appropriate and effective approach to prevent mushroom poisoning. Education is the most effective way for preventing the toxicity. For training and educating the health professionals and public, full instructions provided by the International Chemical Safety Program (IPCS) team can be used [24].
6. Conclusions
Recent studies suggest that A. virosa is the most prevalent cause of mushroom poisoning in Iran, similar to some Asian and Eastern European countries. Like the well-known A. phalloides, A. virosa may be responsible for life-threatening toxic liver failure and even death. These two Amanita species share similar toxins, including amatoxins, phallotoxins and virotoxins. Poisoning management is supportive, although various specific therapies have been used with currently low-level evidence of usefulness to reduce the risks of morbidities and death. Prevention is thus essential and mainly based on information and education.
Conflicts of interest
None.
References
- 1.Cheung P.C.K. The nutritional and health benefits of mushrooms. Nutr. Bull. 2010;35:292–299. [Google Scholar]
- 2.Fischbein C.B., Mueller G.M., Leacock P.R., Wahl M.S., Aks S.E. Digital imaging: a promising tool for mushroom identification. Acad. Emerg. Med. 2003;10:808–811. doi: 10.1111/j.1553-2712.2003.tb00079.x. [DOI] [PubMed] [Google Scholar]
- 3.Felice S. Chemical constituents of some mushrooms. J. Sci. Food Agric. 1992;58:499–503. [Google Scholar]
- 4.Unluoglu I., Tayfur M. Mushroom poisoning: an analysis of the data between 1996 and 2000. Eur. J. Emerg. Med. 2003;10:23–26. doi: 10.1097/00063110-200303000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Grotto D., Gerenutti M., Souza V., Barbosa F., Jr Deficiency of macro-and micronutrients induced by Lentinula edodes. Toxicol. Rep. 2015;2:401–404. doi: 10.1016/j.toxrep.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin C.-C., Kumar K.S., Liao J.-W., Kuo Y.-H., Wang S.-Y. Genotoxic, teratotoxic and oral toxic assessments of Antrodia cinnamomea health food product (Leader Deluxe Antrodia cinnamomea®) Toxicol. Rep. 2015;2:1409–1417. doi: 10.1016/j.toxrep.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogbomida E.T., Omofonmwan K., Aganmwonyi I., Fasipe I.P., Enuneku A., Ezemonye L.I. Bioactive profiling and therapeutic potential of mushroom (Pleurotus tuberregium) extract on Wistar albino rats (Ratus norvegicus) exposed to arsenic and chromium toxicity. Toxicol. Rep. 2018;5:401–410. doi: 10.1016/j.toxrep.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsatsakis A., Vassilopoulou L., Kovatsi L., Tsitsimpikou C., Karamanou M., Leon G., Liesivuori J., Hayes A., Spandidos D. The dose response principle from philosophy to modern toxicology: the impact of ancient philosophy and medicine in modern toxicology science. Toxicol. Rep. 2018;5:1107–1113. doi: 10.1016/j.toxrep.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pilz D., Molina R. Commercial harvests of edible mushrooms from the forests of the Pacific Northwest United States: issues, management, and monitoring for sustainability. For. Ecol. Manage. 2002;155:3–16. [Google Scholar]
- 10.Wieland T. Poisonous principles of mushrooms of the genus amanita. Science. 1968;159:946–952. doi: 10.1126/science.159.3818.946. [DOI] [PubMed] [Google Scholar]
- 11.Trestrail J.H. Mushroom poisoning in the United States, an analysis of 1989 United States Poison Center data. Clin. Toxicol. 1991;29:459–465. doi: 10.3109/15563659109025741. [DOI] [PubMed] [Google Scholar]
- 12.Trim G.M., Lepp H., Hall M.J., McKeown R.V., McCaughan G.W., Duggin G.G., Le Couteur D.G. Poisoning by Amanita phalloides (‘deathcap’) mushroom in the Australian capital territory. Med. J. Aust. 1999;171:247–249. doi: 10.5694/j.1326-5377.1999.tb123631.x. [DOI] [PubMed] [Google Scholar]
- 13.Grossman C.M., Malbin B. Mushroom poisoning: a review of the literature and report of two cases caused by a previously undescribed species*. Ann. Intern. Med. 1954;40:249–259. doi: 10.7326/0003-4819-40-2-249. [DOI] [PubMed] [Google Scholar]
- 14.Paydas S., Kocak R., Erturk F., Erken E., Zaksu H.S., Gurcay A. Poisoning due to amatoxin-containing Lepiota species. Br. J. Clin. Pract. 1990;44:450–453. [PubMed] [Google Scholar]
- 15.Omidynia E., Rashidpourai R., Qaderi M.T., Ameri E. Mycetismus in Hamadan, of west Iran. Southeast Asian J. Trop. Med. Publ. Health. 1997;28:438–439. [PubMed] [Google Scholar]
- 16.Klein A.S., Hart J., Brems J.J., Goldstein L., Lewin K., Busuttil R.W. Amanita poisoning: treatment and the role of liver transplantation. Am. J. Med. 1989;86:187–193. doi: 10.1016/0002-9343(89)90267-2. [DOI] [PubMed] [Google Scholar]
- 17.Administration IFaD . Iran Food and Drug Administration; 2018. Mushroom Poisoning in Iran. [Google Scholar]
- 18.Bonnet M.S., Basson P.W. The toxicology of Amanita virosa: the destroying angel. Homeopathy. 2004;93:216–220. doi: 10.1016/j.homp.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Litovitz T.L., Klein-Schwartz W., Rodgers G.C., Jr., Cobaugh D.J., Youniss J., Omslaer J.C., May M.E., Woolf A.D., Benson B.E. 2001 Annual report of the American association of poison control centers toxic exposure surveillance system. Am. J. Emerg. Med. 2002;20:391–452. doi: 10.1053/ajem.2002.34955. [DOI] [PubMed] [Google Scholar]
- 20.Bronstein A.C., Spyker D.A., Cantilena L.R., Green J.L., Rumack B.H., Dart R.C. 2010 Annual report of the American association of poison control centers’ National Poison Data System (NPDS): 28th annual report. Clin. Toxicol. (Phila.) 2011;49:910–941. doi: 10.3109/15563650.2011.635149. [DOI] [PubMed] [Google Scholar]
- 21.Gummin D.D., Mowry J.B., Spyker D.A., Brooks D.E., Fraser M.O., Banner W. 2016 Annual report of the American association of poison control centers’ National Poison Data System (NPDS): 34th annual report. Clin. Toxicol. 2017;55:1072–1254. doi: 10.1080/15563650.2017.1388087. [DOI] [PubMed] [Google Scholar]
- 22.Karadeniz H., Birincioglu I., Turna O., Ketenci H.C., Beyhun N.E. Fatal poisoning of chilhood in the Eastern Black Sea region of Turkey (2009–2013) J. Forensic Leg. Med. 2015;34:109–112. doi: 10.1016/j.jflm.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Schenk-Jaeger K.M., Rauber-Lüthy C., Bodmer M., Kupferschmidt H., Kullak-Ublick G.A., Ceschi A. Mushroom poisoning: a study on circumstances of exposure and patterns of toxicity. Eur. J. Intern. Med. 2012;23:e85–e91. doi: 10.1016/j.ejim.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 24.Pajoumand A., Shadnia S., Efricheh H., Mandegary A., Hassanian-Moghadam H., Abdollahi M. A retrospective study of mushroom poisoning in Iran. Hum. Exp. Toxicol. 2005;24:609–613. doi: 10.1191/0960327105ht572oa. [DOI] [PubMed] [Google Scholar]
- 25.Soltaninejad K. Outbreak of mushroom poisoning in Iran: April–May, 2018. Int. J. Occup. Environ. Med. 2018;9:152–156. doi: 10.15171/ijoem.2018.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dadpour B., Tajoddini S., Rajabi M., Afshari R. Mushroom poisoning in the northeast of Iran; a retrospective 6-year epidemiologic study. Emerg. (Tehran) 2017;5:e23. [PMC free article] [PubMed] [Google Scholar]
- 27.Saadatm S., Azarbooyeh F. New records of fungi from Iran. Afr. J. Biotechnol. 2012;11:1900–1903. [Google Scholar]
- 28.Aji D.Y., Çalişkan S., Nayir A., Mat A., Can B., Yaşar Z., Özşahin H., Çullu F., Sever L. Haemoperfusion in Amanita phalloides poisoning. J. Trop. Pediatr. 1995;41:371–374. doi: 10.1093/tropej/41.6.371. [DOI] [PubMed] [Google Scholar]
- 29.Hady Sheikhol-Eslami S.H. Study of wild mushrooms poisoning between 1992/1994 in Loghmn-e-Hakim Hospital Poisoning Center. Forensic Med. J. 1995;4:20–25. [Google Scholar]
- 30.Durukan P., Yildiz M., Cevik Y., Ikizceli I., Kavalci C., Celebi S. Poisoning from wild mushrooms in Eastern Anatolia region: analyses of 5 years. Hum. Exp. Toxicol. 2007;26:579–582. doi: 10.1177/0960327106079545. [DOI] [PubMed] [Google Scholar]
- 31.Cevik A.A., Unluoglu I. Factors affecting mortality and complications in mushroom poisonings over a 20 year period: a report from Central Anatolia. Turk. J. Emerg. Med. 2014;14:104–110. doi: 10.5505/1304.7361.2014.36024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karlson-Stiber C., Persson H. Cytotoxic fungi—an overview. Toxicon. 2003;42:339–349. doi: 10.1016/s0041-0101(03)00238-1. [DOI] [PubMed] [Google Scholar]
- 33.Diaz J.H. Syndromic diagnosis and management of confirmed mushroom poisonings. Crit. Care Med. 2005;33:427–436. doi: 10.1097/01.ccm.0000153531.69448.49. [DOI] [PubMed] [Google Scholar]
- 34.Diaz J.H. Amatoxin-containing mushroom poisonings: species, toxidromes, treatments, and outcomes. Wilderness Environ. Med. 2018;29:111–118. doi: 10.1016/j.wem.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Ramirez P., Parrilla P., Bueno F.S., Robles R., Pons J.A., Bixquert V., Nicolas S., Nuñez R., Alegria M.S., Miras M., Rodriguez J.M. Fulminant hepatic failure after Lepiota mushroom poisoning. J. Hepatol. 1993;19:51–54. doi: 10.1016/s0168-8278(05)80175-8. [DOI] [PubMed] [Google Scholar]
- 36.Piering W.F., Bratanow N. Role of the clinical laboratory in guiding treatment of Amanita virosa mushroom poisoning: report of two cases. Clin. Chem. 1990;36:571–574. [PubMed] [Google Scholar]
- 37.Tang S., Zhou Q., He Z., Luo T., Zhang P., Cai Q., Yang Z., Chen J., Chen Z. Cyclopeptide toxins of lethal amanitas: compositions, distribution and phylogenetic implication. Toxicon. 2016;120:78–88. doi: 10.1016/j.toxicon.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 38.Alves A., Gouveia Ferreira M., Paulo J., França A., Carvalho Á. Mushroom poisoning with Amanita phalloides — a report of four cases. Eur. J. Intern. Med. 2001;12:64–66. doi: 10.1016/s0953-6205(00)00127-8. [DOI] [PubMed] [Google Scholar]
- 39.Bonnet M.S., Basson P.W. The toxicology of Amanita phalloides. Homeopathy. 2002;91:249–254. doi: 10.1054/homp.2002.0056. [DOI] [PubMed] [Google Scholar]
- 40.Vetter J. Toxins of Amanita phalloides. Toxicon. 1998;36:13–24. doi: 10.1016/s0041-0101(97)00074-3. [DOI] [PubMed] [Google Scholar]
- 41.Karlson-Stiber C., Persson H. Cytotoxic fungidan overview. Toxicon. 2003;42:339–349. doi: 10.1016/s0041-0101(03)00238-1. [DOI] [PubMed] [Google Scholar]
- 42.Berger K.J., Guss D.A. Mycotoxins revisited: part I. J. Emerg. Med. 2005;28:53–62. doi: 10.1016/j.jemermed.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 43.Chen Z., Zhang P., Zhang Z. Investigation and analysis of 102 mushroom poisoning cases in Southern China from 1994 to 2012. Fungal Divers. 2014;64:123–131. [Google Scholar]
- 44.Kirchmair M., Carrilho P., Pfab R., Haberl B., Felgueiras J., Carvalho F., Cardoso J., Melo I., Vinhas J., Neuhauser S. Amanita poisonings resulting in acute, reversible renal failure: new cases, new toxic Amanita mushrooms. Nephrol. Dial. Transplant. 2012;27:1380–1386. doi: 10.1093/ndt/gfr511. [DOI] [PubMed] [Google Scholar]
- 45.Warden C.R., Benjamin D.R. Acute renal failure associated with suspected Amanita smithiana mushroom ingestions: a case series. Acad. Emerg. Med. 1998;5:808–812. doi: 10.1111/j.1553-2712.1998.tb02508.x. [DOI] [PubMed] [Google Scholar]
- 46.West P.L., Lindgren J., Horowitz B.Z. Amanita smithiana mushroom ingestion: a case of delayed renal failure and literature review. J. Med. Toxicol. 2009;5:32–38. doi: 10.1007/BF03160979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang W.S., Lin C.H., Huang J.W., Fang C.C. Acute renal failure caused by mushroom poisoning. J. Formos. Med. Assoc. 2006;105:263–267. doi: 10.1016/S0929-6646(09)60317-X. [DOI] [PubMed] [Google Scholar]
- 48.Michelot D., Melendez-Howell L.M. Amanita muscaria: chemistry, biology, toxicology, and ethnomycology. Mycol. Res. 2003;107:131–146. doi: 10.1017/s0953756203007305. [DOI] [PubMed] [Google Scholar]
- 49.Saviuc P., Danel V. New syndromes in mushroom poisoning. Toxicol. Rev. 2006;25:199–209. doi: 10.2165/00139709-200625030-00004. [DOI] [PubMed] [Google Scholar]
- 50.Li C., Oberlies N.H. The most widely recognized mushroom: chemistry of the genus Amanita. Life Sci. 2005;78:532–538. doi: 10.1016/j.lfs.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 51.Wieland T., Goetzendoerfer C., Dabrowski J., Lipscomb W.N., Shoham G. Unexpected similarity of the structures of the weakly toxic amanitin (S)-sulfoxide and the highly toxic (R)-sulfoxide and sulfone as revealed by proton nuclear magnetic resonance and x-ray analysis. Biochemistry. 1983;22:1264–1271. doi: 10.1021/bi00274a043. [DOI] [PubMed] [Google Scholar]
- 52.Wieland O. Changes in liver metabolism induced by the poisons of amanita phalloides. Clin. Chem. 1965;11(Suppl):323–338. [PubMed] [Google Scholar]
- 53.Faulstich H., Georgopoulos D., Bloching M., Wieland T. Analysis of the toxins of amanitin-containing mushrooms. Z. Naturforschung C. 1974:86. [PubMed] [Google Scholar]
- 54.Tyler V.E., Benedict R.G., Brady L.R., Robbers J.E. Occurrence of amanita toxins in american collections of deadly amanitas. J. Pharm. Sci. 1966;55:590–593. doi: 10.1002/jps.2600550612. [DOI] [PubMed] [Google Scholar]
- 55.Yocum R.R., Simons D.M. Amatoxins and phallotoxins in Amanita species of the northeastern United States. Lloydia. 1977;40:178–190. [PubMed] [Google Scholar]
- 56.Wieland T. Springer; Berlin, Heidelberg: 1986. Molecular Pathology of the Amanita Peptides. Peptides of Poisonous Amanita Mushrooms; pp. 101–180. Berlin Heidelberg. [Google Scholar]
- 57.Faulstich H., Buku A., Bodenmuller H., Wieland T. Virotoxins: actin-binding cyclic peptides of Amanita virosa mushrooms. Biochemistry. 1980;19:3334–3343. doi: 10.1021/bi00555a036. [DOI] [PubMed] [Google Scholar]
- 58.Malak S.H. Occurence of phallotoxins in American collections of Amanita virosa. Planta Med. 1976;29:80–85. doi: 10.1055/s-0028-1097632. [DOI] [PubMed] [Google Scholar]
- 59.Buku A., Wieland T., Bodenmuller H., Faulstich H. Amaninamide, a new toxin of Amanita virosa mushrooms. Experientia. 1980;36:33–34. doi: 10.1007/BF02003953. [DOI] [PubMed] [Google Scholar]
- 60.Litten W. The most poisonous mushrooms. Sci. Am. 1975;232:90–101. doi: 10.1038/scientificamerican0375-90. [DOI] [PubMed] [Google Scholar]
- 61.Klán J. A review of mushrooms containing amanitins and phalloidines. Cas. Lek. Cesk. 1993;132:449–451. [PubMed] [Google Scholar]
- 62.Ko¨ppel C. Clinical symptomatology and management of mushroom poisoning. Toxicon. 1993;31:1513–1540. doi: 10.1016/0041-0101(93)90337-i. [DOI] [PubMed] [Google Scholar]
- 63.Turcotte A., Gicquaud C., Gendreau M., St-Pierre S. Separation of the virotoxins of the mushroom Amanita virosa and comparative study of their interaction on actin in vitro. Can. J. Biochem. Cell Biol. 1984;62:1327–1334. [PubMed] [Google Scholar]
- 64.Wei J., Wu J., Chen J., Wu B., He Z., Zhang P., Li H., Sun C., Liu C., Chen Z., Xie J. Determination of cyclopeptide toxins in Amanita subpallidorosea and Amanita virosa by high-performance liquid chromatography coupled with high-resolution mass spectrometry. Toxicon. 2017;133:26–32. doi: 10.1016/j.toxicon.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 65.Antonyuk V.O., Klyuchivska O.Y., Stoika R.S. Cytotoxic proteins of Amanita virosa Secr. mushroom: purification, characteristics and action towards mammalian cells. Toxicon. 2010;55:1297–1305. doi: 10.1016/j.toxicon.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 66.Preston J.F., Stark H.J., Kimbrough J.W. Quantitation of amanitins in Amanita verna with calf thymus RNA polymerase B. Lloydia. 1975;38:153–161. [PubMed] [Google Scholar]
- 67.Seeger R., Stijve T. Amanitin content and toxicity of Amanita verna bull. Z. Naturforschung C. 1979:330. doi: 10.1515/znc-1979-5-603. [DOI] [PubMed] [Google Scholar]
- 68.Witkop B., Eigen M. Theodor Hermann Felix Wieland (5 June 1913–24 November 1995) Proc. Am. Philos. Soc. 1998;142:317–319. [PubMed] [Google Scholar]
- 69.Bergis D., Friedrich-Rust M., Zeuzem S., Betz C., Sarrazin C., Bojunga J. Treatment of Amanita phalloides intoxication by fractionated plasma separation and adsorption (Prometheus) J. Gastrointestin. Liver Dis. 2012;21:171–176. [PubMed] [Google Scholar]
- 70.Kröncke K.D., Fricker G., Meier P.J., Gerok W., Wieland T., Kurz G. Alpha-Amanitin uptake into hepatocytes. Identification of hepatic membrane transport systems used by amatoxins. J. Biol. Chem. 1986;261:12562–12567. [PubMed] [Google Scholar]
- 71.Lutsik-Kordovsky M.D., Stasyk T.V., Stoika R.S. Analysis of cytotoxicity of lectin and non-lectin proteins from Amanita mushrooms. Exp. Oncol. 2001;23:43–45. [Google Scholar]
- 72.Antonyuk V.O. Study on carbohydrate specificity of hemolytic lectin from death-cap mushroom (Amanita phalloides (Vaill. Fr.) Secr) Biopolym. Cell. 2005;21:319–325. [Google Scholar]
- 73.Brandenburg W. Mushroom poisoning. Wilderness Environ. Med. 2017;28:368–369. [Google Scholar]
- 74.Nicholson F.B., Korman M.G. Death from Amanita poisoning. Aust. N. Z. J. Med. 1997;27:448–449. doi: 10.1111/j.1445-5994.1997.tb02212.x. [DOI] [PubMed] [Google Scholar]
- 75.MARY C.F. A puppy death and Amanita phalloides. Aust. Vet. J. 1993;70:271–272. doi: 10.1111/j.1751-0813.1993.tb08052.x. [DOI] [PubMed] [Google Scholar]
- 76.Parish R.C., Doering P.L. Treatment of Amanita mushroom poisoning: a review. Vet. Hum. Toxicol. 1986;28:318–322. [PubMed] [Google Scholar]
- 77.Loranger A., Tuchweber B., Gicquaud C., St-Pierre S., Cote M.G. Toxicity of peptides of Amanita virosa mushrooms in mice. Fundam. Appl. Toxicol. 1985;5:1144–1152. doi: 10.1016/0272-0590(85)90151-4. [DOI] [PubMed] [Google Scholar]
- 78.Nieminen L., Bjondahl K., Ojanen H., Mottonen M., Hirsimaki P., Hirsimaki Y. Sex differences in renal damage induced in the mouse by Amanita virosa. Exp. Pathol. (Jena) 1977;14:136–140. doi: 10.1016/s0014-4908(77)80060-4. [DOI] [PubMed] [Google Scholar]
- 79.Ergin M., Dundar Z.D., Kilinc I., Colak T., Oltulu P., Girisgin A.S. Alpha-Amanitin poisoning, nephrotoxicity and oxidative stress: an experimental mouse model. Iran. Red Crescent Med. J. 2015;17 doi: 10.5812/ircmj.28068. e28068-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Diaz J.H. Evolving global epidemiology, syndromic classification, general management, and prevention of unknown mushroom poisonings. Crit. Care Med. 2005;33:419–426. doi: 10.1097/01.ccm.0000153530.32162.b7. [DOI] [PubMed] [Google Scholar]
- 81.Faulstich H., Kirchner K., Derenzini M. Strongly enhanced toxicity of the mushroom toxin α-amanitin by an amatoxin-specific Fab or monoclonal antibody. Toxicon. 1988;26:491–499. doi: 10.1016/0041-0101(88)90188-2. [DOI] [PubMed] [Google Scholar]
- 82.Barbato M.P. Poisoning from accidental ingestion of mushrooms. Med. J. Aust. 1993;158:842–847. doi: 10.5694/j.1326-5377.1993.tb137674.x. [DOI] [PubMed] [Google Scholar]
- 83.Faulstich H. New aspects of Amanita poisoning. Klin. Wochenschr. 1979;57:1143–1152. doi: 10.1007/BF01491754. [DOI] [PubMed] [Google Scholar]
- 84.Becker C.E., Tong T.G., Roe R.L., Scott R.A.T., MacQuarrie M.B., Boerner U., Bartter F. Diagnosis and treatment of Amanita phalloides-type mushroom poisoning: use of thioctic acid. West. J. Med. 1976;125:100–109. [PMC free article] [PubMed] [Google Scholar]
- 85.Ye Y., Liu Z. Management of Amanita phalloides poisoning: a literature review and update. J. Crit. Care. 2018;46:17–22. doi: 10.1016/j.jcrc.2018.03.028. [DOI] [PubMed] [Google Scholar]
- 86.Das R., Parajuli S., Jayakumar J. “Last supper with mushroom soup”: a case report of amatoxin poisoning. McGill J. Med. 2007;10:93. [PMC free article] [PubMed] [Google Scholar]
- 87.Enjalbert F., Gallion C., Jehl F., Monteil H., Faulstich H. Simultaneous assay for amatoxins and phallotoxins in Amanita phalloides Fr. by high-performance liquid chromatography. J. Chromatogr. A. 1992;598:227–236. doi: 10.1016/0021-9673(92)85052-u. [DOI] [PubMed] [Google Scholar]
- 88.Defendenti C., Bonacina E., Mauroni M., Gelosa L. Validation of a high performance liquid chromatographic method for alpha amanitin determination in urine. Forensic Sci. Int. 1998;92:59–68. doi: 10.1016/s0379-0738(98)00006-1. [DOI] [PubMed] [Google Scholar]
- 89.Mcknight T.A., Mcknight K.B., Skeels M.C. Matoxin and phallotoxin concentration in Amanita bisporigera spores. Mycologia. 2010;102:763–765. doi: 10.3852/09-131. [DOI] [PubMed] [Google Scholar]
- 90.Jinsong H., Ping Z., Jun Z., Zuohong C. Determination of amatoxins in different tissues and development stages of Amanita exitialis. J. Sci. Food Agric. 2012;92:2664–2667. doi: 10.1002/jsfa.5685. [DOI] [PubMed] [Google Scholar]
- 91.Escudié L., Francoz C., Vinel J.-P., Moucari R., Cournot M., Paradis V., Sauvanet A., Belghiti J., Valla D., Bernuau J., Durand F. Amanita phalloides poisoning: reassessment of prognostic factors and indications for emergency liver transplantation. J. Hepatol. 2007;46:466–473. doi: 10.1016/j.jhep.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 92.Ganzert M., Felgenhauer N., Schuster T., Eyer F., Gourdin C., Zilker T. Amanita poisoning—comparison of silibinin with a combination of silibinin and penicillin. Dtsch. Med. Wochenschr. 2008;133:2261–2267. doi: 10.1055/s-0028-1091268. [DOI] [PubMed] [Google Scholar]
- 93.Garcia J., Costa V.M., Carvalho A., Baptista P., de Pinho P.G., de Lourdes Bastos M., Carvalho F. Amanita phalloides poisoning: mechanisms of toxicity and treatment. Food Chem. Toxicol. 2015;86:41–55. doi: 10.1016/j.fct.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 94.Healey K., Woo O.F., Olson K.R., Pond S.M., Seward J., Becker C.E. Amanita phalloides-type mushroom poisoning. West. J. Med. 1982;137:282–289. [PMC free article] [PubMed] [Google Scholar]
- 95.Bartoloni St Omer F., Giannini A., Botti P., Caramelli L., Ledda F., Peruzzi S., Zorn M. Amanita poisoning: a clinical-histopathological study of 64 cases of intoxication. Hepatogastroenterology. 1985;32:229–231. [PubMed] [Google Scholar]
- 96.Kieslichova E., Frankova S., Protus M., Merta D., Uchytilova E., Fronek J., Sperl J. Acute liver failure due to Amanita phalloides poisoning: therapeutic approach and outcome. Transplant. Proc. 2018;50:192–197. doi: 10.1016/j.transproceed.2017.11.032. [DOI] [PubMed] [Google Scholar]
- 97.Schneider S.M., Borochovitz D., Krenzelok E.P. Cimetidine protection against alpha-amanitin hepatotoxicity in mice: a potential model for the treatment of Amanita phalloides poisoning. Ann. Emerg. Med. 1987;16:1136–1140. doi: 10.1016/s0196-0644(87)80472-9. [DOI] [PubMed] [Google Scholar]
- 98.Saller R., Meier R., Brignoli R. The use of silymarin in the treatment of liver diseases. Drugs. 2001;61:2035–2063. doi: 10.2165/00003495-200161140-00003. [DOI] [PubMed] [Google Scholar]
- 99.Garcia J., Costa V.M., Carvalho A.T.P., Silvestre R., Duarte J.A., DFAR Dourado, Arbo M.D., Baltazar T., Dinis-Oliveira R.J., Baptista P., de Lourdes Bastos M., Carvalho F. A breakthrough on Amanita phalloides poisoning: an effective antidotal effect by polymyxin B. Arch. Toxicol. 2015;89:2305–2323. doi: 10.1007/s00204-015-1582-x. [DOI] [PubMed] [Google Scholar]
- 100.Chen W.C., Kassi M., Saeed U., Frenette C.T. A rare case of amatoxin poisoning in the state of Texas. Case Rep. Gastroenterol. 2012;6:350–357. doi: 10.1159/000339692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kroncke K.D., Fricker G., Meier P.J., Gerok W., Wieland T., Kurz G. α-Amanitin uptake into hepatocytes. Identification of hepatic membrane transport systems used by amatoxins. J. Biol. Chem. 1986;261:12562–12567. [PubMed] [Google Scholar]