Abstract
Background
Currently, statins are used to treat polycystic ovary syndrome (PCOS). This systematic review and meta-analysis aimed to investigate the effect of statins on serum or plasma levels of dehydroepiandrosterone (DHEA) in women with PCOS.
Material/Methods
Databases that were searched included PubMed, Embase, and the Cochrane Library from their inception to August of 2018. Published randomized controlled trials (RCTs) were identified that evaluated the impact of statins on plasma DHEA levels in women with PCOS. The Cochrane risk of bias tool was used to assess the quality of the included RCTs. A random-effects model was used to analyze the pooled results.
Results
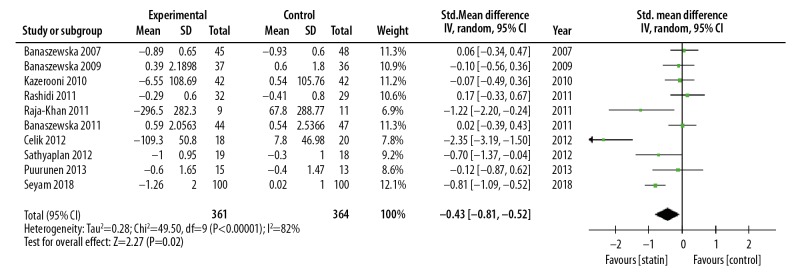
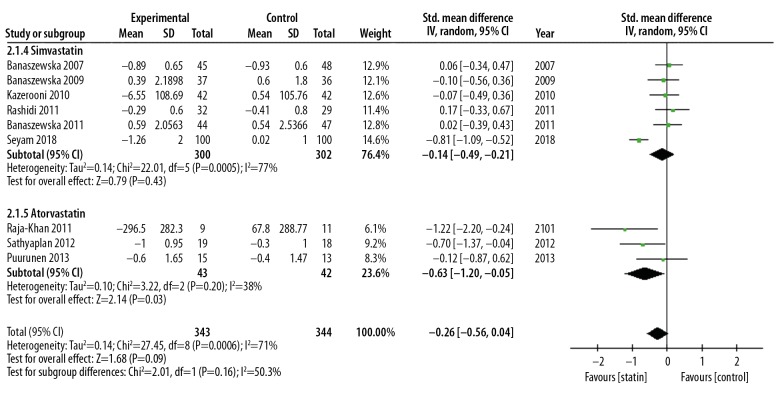
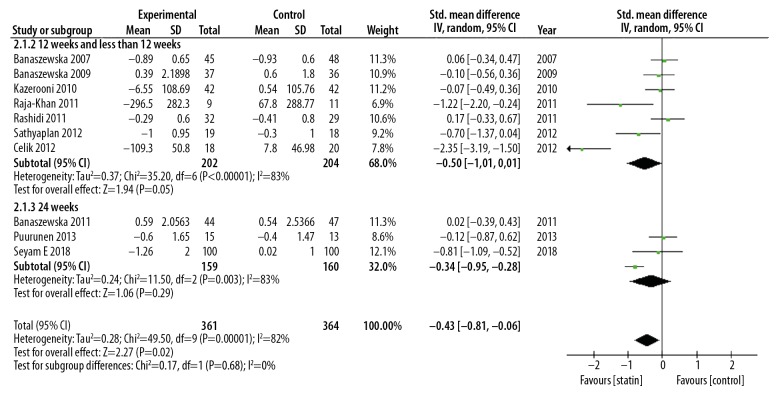
Meta-analysis was performed on data from ten published studies that included 735 patients and showed that statin treatment could significantly reduce plasma DHEA levels when compared with controls (SMD, −0.43; 95% CI, −0.81–0.06; p=0.02; I2=82%). Statins were significantly more effective than placebo in reducing the levels of DHEAs. Subgroup analysis based on statin type showed that atorvastatin significantly reduced DHEA levels (SMD, −0.63; 95% CI, −1.20 – −0.05; p=0.03; I2=38%) but simvastatin did not significantly reduce DHEA levels (SMD: −0.14; 95% CI, −0.49–0.28; p=0.43; I2=77%). Subgroup analysis based on duration of treatment showed no significant difference between 12 weeks of statin treatment (SMD, −0.61; 95% CI, −1.23–0.02; p=0.06; I2=85%) and 24 weeks (SMD, −0.34; 95% CI −0.95–0.28; p=0.29; I2=83%).
Conclusions
Meta-analysis showed that statins significantly reduced the levels of DHEA when compared with placebo in patients with PCOS.
MeSH Keywords: Dehydroepiandrosterone Sulfate; Diabetes Mellitus, Type 2; Hydroxymethylglutaryl-CoA Reductase Inhibitors; Meta-Analysis; Polycystic Ovary Syndrome
Background
Polycystic ovary syndrome (PCOS) is a common endocrine disorder that between 4–10% of women of childbearing age [1]. PCOS is characterized by hyperandrogenism, insulin resistance, dyslipidemia, polycystic ovaries, and chronic oligo-ovulation, or anovulation. Androgen excess is considered to be the main abnormality in women with PCOS and is one of the major diagnostic features included in current diagnostic criteria [2]. The main manifestations of hyperandrogenism are increased levels of testosterone, delta-androstenedione (delta-4-A), dehydroepiandrosterone (DHEA) and its sulfate, DHEAS.
Increased androgen production in PCOS originates from the ovaries and adrenal glands. Between 40–60% of patients with PCOS have excessive adrenal androgen production, which is characterized by increased serum or plasma levels of DHEA [3]. As the most abundant sex hormones in human plasma [4], DHEA and DHEAS are the precursors of androgen and estrogen. DHEA is produced from cholesterol, mainly in the zona reticularis of the adrenal cortex. In patients with PCOS, more than 95% of DHEA is derived from the adrenal gland and the rest is from the ovary [5]. Several previously published studies have shown that high levels of DHEA are associated with more favorable metabolic parameters, which affect insulin sensitivity and serum lipid profiles. DHEA levels have been negatively correlated with abdominal obesity, dyslipidemia, and insulin resistance in patients with PCOS [6–8].
Statins are hydroxy methyl glutaryl coenzyme A (HMG-CoA) reductase inhibitors. Statins are now widely used in the prevention and control of atherosclerotic cardiovascular disease (ASCVD) as they inhibit cholesterol synthesis. Several clinical trials have shown that statins can reduce the metabolic complications of PCOS and have beneficial effects on reproductive endocrine function [9–11]. In 2012, a meta-analysis showed that statin treatment in PCOS reduced the levels of total testosterone, triglyceride, and low-density lipoprotein cholesterol (LDL-C), which supported this treatment option for patients with PCOS [12]. However, the published data from clinical trials on the effect of statins on DHEA levels in patients with PCOS is inconsistent. Some clinical trials have shown that statin treatment can reduce serum or plasma levels of DHEA [13,14], whereas other studies have not supported these findings [15,16].
Therefore, this systematic review and meta-analysis aimed to investigate the effect of statins on levels of DHEA in women with PCOS.
Material and Methods
The systematic review and meta-analysis were conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [17].
Literature search
The Cochrane Library, PubMed, and Embase databases were systematically searched from inception to August of 2018. The following search terms were used: simvastatin, fluvastatin, rosuvastatin, atorvastatin, pravastatin, pitavastatin, lovastatin, cerivastatin, statins, dehydroepiandrosterone sulfate, DHEA, DHEA sulfate, prasterone sulfate, polycystic ovary syndrome, PCOS, and ovary polycystic disease.
Publication selection of randomized controlled trials (RCTs)
Publications that met the following criteria were considered to be eligible for meta-analysis: randomized placebo-controlled human clinical trials; studies that investigated the effects of statin treatment on serum or plasma levels of dehydroepiandrosterone (DHEA) and its sulfate (DHEAS) in women with polycystic ovary syndrome (PCOS); studies that used different types of statin at any dose that continued for at least two weeks; and studies that provided enough data for analysis. The full text of the published study was available for all studies included in the meta-analysis. The exclusion criteria included non-interventional studies without a placebo control group, studies that were conducted in animals, and the lack of adequate data on the baseline or post-intervention serum or plasma DHEA levels.
Data extraction
Data were extracted independently by two investigators and included first author, publication date, study site, study design, sample size, patient characteristics, study drop-out rates, and the reasons for withdrawal from the study, the type and dose of statin used, and the duration of patient follow-up. Data extracted from the publications also included the baseline and follow-up concentrations of DHEA.
Quality assessment
The Cochrane risk of bias tool for RCTs was used to assess the quality of the included studies [18]. The RCT bias was evaluated based on several methods that include random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome data, incomplete outcome data, and selective reporting. The impact of the quality of the studies on bias was described as low risk, high risk, and unknown risk.
Data synthesis and statistical analysis
The outcomes from each study were presented as mean values and standard deviation for each group. The standardized mean difference (SMD) and 95% confidence interval (CI) were expressed to show the degree of the effect. The change of DHEA was calculated by subtracting the measured values at the end of the follow-up from the baseline measurements. The variance was calculated using the 95% CI or statistical test values when studies did not directly report the standard deviation (SD) or variance.
The Q test and the I2 index were used to assess study heterogeneity. I2 values >50% and <25% represented large and small study heterogeneity, respectively. A random-effects model was used when there was study heterogeneity, and a fixed-effects model was used if there was no statistical heterogeneity. Subgroup analysis was based on the type and duration of statin therapy. Statistical analysis was performed using Review Manager 5.2 (RevMan 5.2) software. Sensitivity analysis was performed by sequentially removing one study at a time. Publication bias was evaluated by funnel plot analysis.
Results
Publications identified
The initial database search identified 127 randomized controlled trials (RCTs). After reading the titles and abstracts, 105 publications were excluded. Following the review of the remaining studies, 13 more publications were excluded due to the absence of details of the dehydroepiandrosterone (DHEA) assay (n=5), incomplete data (n=5), or duplicated data (n=3). Ten studies were included in the final meta-analysis. A flowchart of the publication selection is shown in Figure 1.
Figure 1.
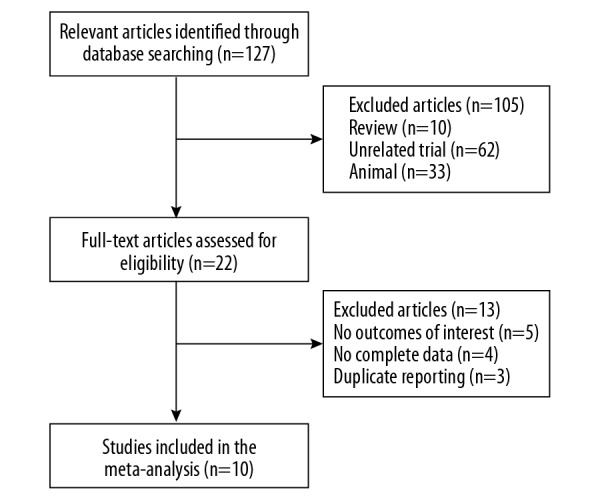
Flowchart of the publication selection for meta-analysis. There were initially 127 publications identified, of which 105 were excluded. Of the 22 full-text publications that were reviewed, a further 12 were excluded due to incomplete data, duplication of reported studies, and irrelevant outcome data. There were ten studies included in the final meta-analysis.
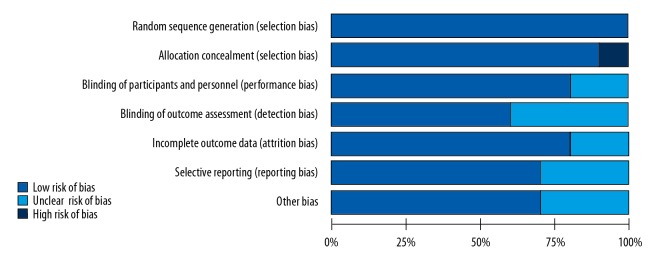
The ten RCTs included 735 patients with polycystic ovary syndrome (PCOS), 361 in the statin-treated group, and 374 patients in the non-treated control group. Six studies included statin treatment with simvastatin (20 mg/day) [15,16,19–21,24], two studies included treatment with atorvastatin (20 mg/day) [12,23], one study included atorvastatin (40 mg/day) [13], one study included treatment with rosuvastatin (10 mg/day) [22]. The duration of statin treatment in three studies was 24 weeks [21–24], and in the remaining studies, the duration of treatment was 12 weeks or less than 12 weeks. Three RCTs used intention-to-treat (ITT) analysis [13,14,16]. Three studies included Asian populations, six studies included European and American populations, and one study included an African population. The methods for detecting serum or plasma levels of DHEA included radioimmunoassay (RIA) (n=2), enzyme-linked immunoassay (ELISA) (n=1), electrochemiluminescence (n=3), chemiluminescence (n=3), and competitive immunoassay (n=1). The specific characteristics of all ten studies are summarized in Table 1. The quality of the included RCTs was assessed using the Cochrane risk of bias tool, and the findings of the quality of each study are shown in Figures 2 and 3.
Table 1.
Characteristics of the studies included in the meta-analysis.
| Publication author, year | Site | Statin, daily dose | Follow-up (weeks) | Sample size (case/control) | DHEA assessment method |
|---|---|---|---|---|---|
| Banaszewska, 2007 [15] | Poland | Simvastatin 20 mg | 12 weeks | 45/48 | Specific radioimmunoassays |
| Banaszewska, 2009 [19] | Poland | Simvastatin 20 mg | 12 weeks | 37/36 | Electrochemiluminescence assays |
| Kazerooni, 2010 [20] | Iran | Simvastatin 20 mg | 12 weeks | 42/42 | Radioimmunoassays |
| Banaszewska, 2011 [21] | Poland | Simvastatin 20 mg | 24 weeks | 44/47 | Electrochemiluminescence assays |
| Raja-Khan, 2011 [13] | US | Atorvastatin 40 mg | 6 weeks | 9/11 | Not given |
| Rashidi, 2011 [16] | Iran | Simvastatin 20 mg | 8 weeks | 32/39 | Chemiluminescence assays |
| Sathyapalan, 2012 [22] | UK | Atorvastatin 20 mg | 12weeks | 19/18 | Competitive immunoassay |
| Celik, 2012 [14] | Turkey | Rosuvastatin 10 mg | 12weeks | 18/20 | Enzyme-linked immunoassay (ELISA) |
| Puurunen, 2013 [23] | Finland | Atorvastatin 20 mg | 24 weeks | 15/13 | Chemiluminescence immunoassay |
| Seyam, 2018 [24] | Egypt | Simvastatin 20 mg | 24 weeks | 100/100 | Chemiluminescence |
Figure 2.
Risk of bias.
Figure 3.
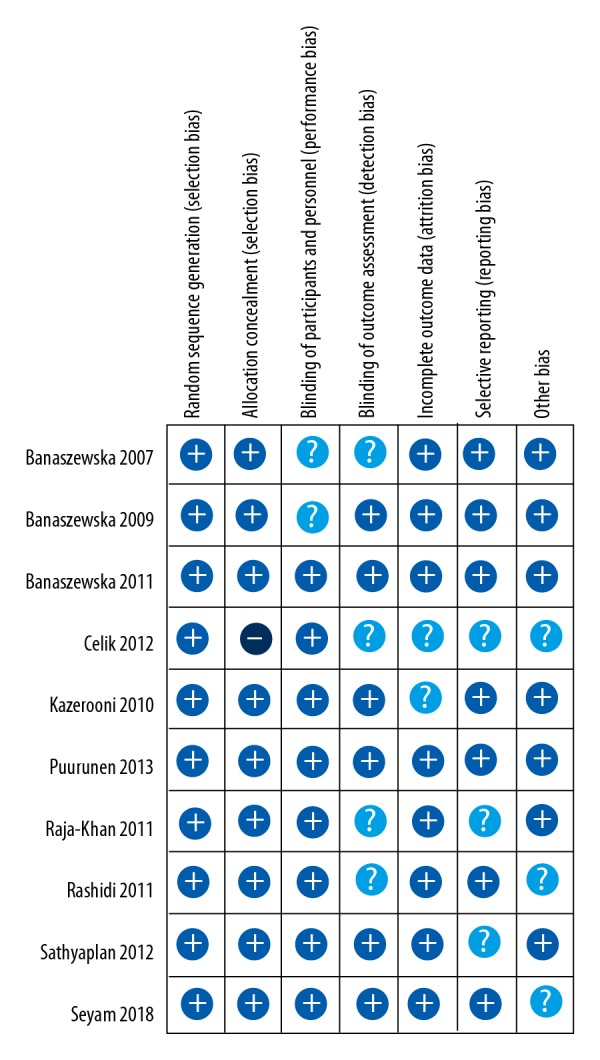
Summary of risk of bias.
Effect of statin therapy on serum or plasma DHEA levels
Meta-analysis showed that statin could reduce DHEA levels compared with the control group (SMD, −0.43; 95% CI, −0.81–0.06; p=0.02; I2=82%). Statin treatment was significantly more effective than placebo in reducing the levels of DHEA. The RCTs were heterogeneous (I2=82%), and so a random-effects model was used (Figure 4). Subgroup analysis based on statin type showed a significant difference in results. Subgroup analysis based on statin type showed that atorvastatin significantly reduced DHEA levels (SMD, −0.63; 95% CI, −1,20 – −0.05; p=0.03; I2=38%) but simvastatin did not significantly reduce DHEA levels (SMD: −0.14; 95% CI, −0.49–0.28; p=0.43; I2=77%) (Figure 5). Subgroup analysis based on duration of treatment showed no significant difference between 12 weeks of statin treatment (SMD, −0.61; 95% CI, −1.23–0.02; p=0.06; I2=85%) and 24 weeks of statin treatment (SMD, −0.34; 95% CI −0.95–0.28; p=0.29; I2=83%) (Figure 6). There was heterogeneity of pooled estimates from the included studies, which could be explained by heterogeneity of the baseline characteristics of the patients in the studies, and also because the DHEA detection methods used were different in the studies.
Figure 4.
Forest plot of the effect of statins on the levels of dehydroepiandrosterone (DHEA). 95% CI – 95% confidence interval; SD – standard deviation.
Figure 5.
Forest plot of the effect of statins on the levels of dehydroepiandrosterone (DHEA) using subgroup analysis of statin type. 95% CI – 95% confidence interval; SD – standard deviation.
Figure 6.
Forest plot of the effect of statins on the levels of dehydroepiandrosterone (DHEA) using subgroup analysis of statin treatment duration. 95% CI – 95% confidence interval; SD – standard deviation.
Sensitivity analysis and publication bias
Sensitivity analysis was conducted to evaluate the stability of the meta-analysis. The effect on the significance of the pooled standardized mean difference (SMD) of each study was evaluated. When any single study was removed, the overall statistical significance did not change, which indicated that the data in the meta-analysis was relatively stable. The study publication bias was assessed by a funnel plot, which showed no significant bias (Figure 7).
Figure 7.
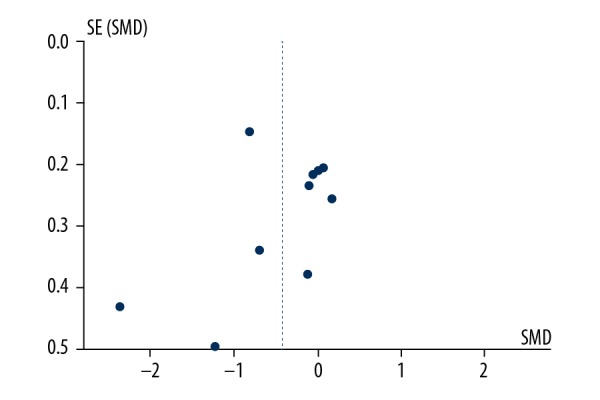
Funnel plot of the studies included in the meta-analysis.
Discussion
A systematic review of the literature and meta-analysis showed that in women with polycystic ovary syndrome (PCOS), statin treatment significantly reduced serum and plasma levels of dehydroepiandrosterone (DHEA) when compared with placebo controls. Subgroup analysis showed that atorvastatin reduced DHEA levels, but simvastatin did not. Subgroup analysis also showed that there was no significant correlation between the duration of statin use and the decrease in levels of DHEA.
Insulin-resistance with hyperinsulinemia is common in women with PCOS and approximately 40% of women with PCOS have glucose intolerance [25], and a significantly increased risk of impaired glucose tolerance and type 2 diabetes mellitus [26]. Previously published animal studies and epidemiological studies have shown the protective effect of DHEA on type 2 diabetes. The findings in a rat model of diabetes mellitus showed that treatment with DHEA sulfate (DHEAS) improved glucose tolerance, insulin sensitivity, and blood glucose control [27,28]. A recently published population-based prospective cohort study showed that serum DHEA levels were inversely related to the risk of type 2 diabetes and that reduced levels of DHEA increased the incidence of type 2 diabetes [29]. The mechanism of DHEA in type 2 diabetes remains unclear, but a possible mechanism includes a role for DHEA in increasing glucose uptake by adipocytes following stimulation of GLUT4 and GLUT1 translocation to the plasma membrane [30]. DHEA has been shown to inhibit oxidative stress and the formation of advanced glycation end products [31]. DHEA can also activate protein kinase C and phosphatidylinositol 3-kinase (PI3-kinase) to improve glucose uptake [32]. Finally, DHEA has been shown to improve endothelial function, which is affected in type 2 diabetes [33].
The mechanism by which statins reduce DHEAs is also unclear. Statins reduce cholesterol synthesis, and cholesterol is a substrate for the synthesis of DHEA. Also, statins directly inhibit the synthesis of adrenal and ovarian androgens [34]. The findings from a previously published meta-analysis that assessed statin treatment in PCOS did not conclude that statins reduced the levels of DHEA [35]. Although previously published studies have shown that statins can improve insulin resistance and increase insulin sensitivity in patients with PCOS, increasing evidence suggests that statins have an inhibitory role in glucose metabolism in patients with PCOS [13,23,36]. Meta-analysis data has shown that statin treatment can reduce insulin sensitivity and increase the risk of diabetes in women with PCOS [35] and women without PCOS [37]. The mechanisms of statin-induced changes in blood glucose disorders might include calcium signal-dependent insulin secretion by blocking calcium channels [38]. Statins increase blood-derived low-density lipoprotein cholesterol (LDL-C) intake, which affects insulin secretion [38]. Blood-derived LDL-C inhibits the activity of rate-limiting enzymes in the process of glucose metabolism in cells, thereby affecting calcium-dependent insulin secretion during glucose metabolism [38]. Statins can also inhibit insulin secretion by inhibiting the synthesis of the mevalonate metabolic pathway [38]. Statin treatment can also induce glucose tolerance by inhibiting the expression of glucose transporter 4 (GLUT-4) [39].
The findings of the present meta-analysis also showed that statin use in PCOS might increase the risk of diabetes by reducing the levels of DHEA, which suggests that the use of statins in patients with PCOS should be evaluated carefully. The effects of statins on blood glucose metabolism is time-dependent and dose-dependent [40]. The findings of the present study showed that atorvastatin significantly reduced the levels of DHEA when compared with simvastatin, which suggests that different types of statins may have different effects on DHEA. There have been few studies on the effects of different statins. However, it is possible that atorvastatin treatment in patients with PCOS might be associated with and increased risk of diabetes.
This systematic review of the literature and meta-analysis had several limitations. First, the ten studies that underwent meta-analysis showed significant study heterogeneity, and the baseline characteristics of the patients were heterogeneous in terms of age, ethnicity, type of statin treatment used, statin dose, and duration of treatment. Second, almost all the studies were not primarily designed to assess the effects of statins on DHEA levels. Third, the serum and plasma DHEA levels were measured by several different methods, which may mean that the results were not comparable. Subgroup analysis in our study did not find a relationship between the duration of statin use and the reduction in levels of DHEA, which might have been due to the small number of studies evaluated. Following the findings from this meta-analysis it is clear that further well-designed and large-scale RCTs are required that clarify the effects of different statins at different doses and for increasing duration in women with PCOS.
Conclusions
A systematic review of the literature and meta-analysis of ten published randomized controlled trials (RCTs) showed that treatment of women with polycystic ovary syndrome (PCOS) with statins reduced serum and plasma levels of DHEA when compared with placebo. Subgroup analysis showed that atorvastatin reduced the levels of DHEA, but might increase the risk of diabetes in women with PCOS, and should be used with caution in women with PCOS and an increased risk of diabetes. Further large scale, well-designed RCTs are required to determine the effects of statins on DHEA levels, the mechanism of action, and the relationship between statin treatment, the development of diabetes, and DHEA levels in women with PCOS.
Footnotes
Source of support: The National Natural Science Foundation of China (81471078, 81641030, and 81170764) and The Foundation of State Drug Administration of Traditional Chinese Medicine Clinical Research Base Construction Business Research Projects (JDZX2012007)
Conflict of interest
None.
References
- 1.Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223–36. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- 2.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 3.Vrbikova J, Hill M, Starka L, et al. The effects of long-term metformin treatment on adrenal and ovarian steroidogenesis in women with polycystic ovary syndrome. Eur J Endocrinol. 2001;144:619–28. doi: 10.1530/eje.0.1440619. [DOI] [PubMed] [Google Scholar]
- 4.Perrini S, Laviola L, Nataticchio A, et al. Associated hormone declines in aging: DHEAS. J Endocrinol Invest. 2005;28(3 Suppl):85–93. [PubMed] [Google Scholar]
- 5.Rosenfield RL, Mortensen M, Wroblewski K, et al. Determination of the source of androgen excess in functionally atypical polycystic ovary syndrome by a short dexamethasone androgen-suppression test and a low-dose ACTH test. Hum Reprod. 2011;26:3138–46. doi: 10.1093/humrep/der291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christodoulaki C, Trakakis E, Pergialiotis V, et al. Dehydroepiandrosterone sulfate, insulin resistance and ovarian volume estimation in patients with polycystic ovarian syndrome. J Family Reprod Health. 2017;11(1):24–29. [PMC free article] [PubMed] [Google Scholar]
- 7.Chen MJ, Chen CD, Yang JH, et al. High selxlm dehydroepiandrosterone sulfate is associated with phenotypic acne and a reduced risk of abdominal obesity in women with polycystic ovary syndrome. Hum Reprod. 2011;26(1):227–34. doi: 10.1093/humrep/deq308. [DOI] [PubMed] [Google Scholar]
- 8.Lerchbaum E, Schwetz V, Giuliani A, et al. Opposing effects of dehydroepiandrosterone sulfate and free testosterone on metabolic phenotype in women with polycystic ovary syndrome. Fertil Steril. 2012;98(5):1318–25. doi: 10.1016/j.fertnstert.2012.07.1057. [DOI] [PubMed] [Google Scholar]
- 9.Sokalska A, Piotrowski PC, Rzepczynska IJ, et al. Statins inhibit growth of human theca-interstitial cells in PCOS and non-PCOS tissues independently of cholesterol availability. J Clin Endocrinol Metab. 2010;95:5390–94. doi: 10.1210/jc.2010-0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raval AD, Hunter T, Stuckey B, et al. Statins for women with polycystic ovary syndrome not actively trying to conceive. Cochrane Database Syst Rev. 2011;10:CD008565. doi: 10.1002/14651858.CD008565.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Duleba AJ, Banaszewska B, Spaczynski RZ, et al. Simvastatin improves biochemical parameters in women with polycystic ovary syndrome: Results of a prospective, randomized trial. Fertil Steril. 2006;85:996–1001. doi: 10.1016/j.fertnstert.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 12.Gao L, Zhao F-L, Li S-C. Statin is a reasonable treatment option for patients with polycystic ovary syndrome: A meta-analysis of randomized controlled trials. Exp Clin Endocrinol Diabetes. 2012;120:367–75. doi: 10.1055/s-0032-1304619. [DOI] [PubMed] [Google Scholar]
- 13.Raja-Khan N, Kunselman AR, Hogeman CS, et al. Effects of atorvastatin on vascular function, inflammation, and androgens in women with polycystic ovary syndrome: A double-blind, randomized, placebo-controlled trial. Fertil Steril. 2011;95:1849–52. doi: 10.1016/j.fertnstert.2010.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Celik O, Acbay O. Effects of metformin plus rosuvastatin on hyperandrogenism in polycystic ovary syndrome patients with hyperlipidemia and impaired glucose tolerance. J Endocrinol Invest. 2012;35:905–10. doi: 10.3275/8371. [DOI] [PubMed] [Google Scholar]
- 15.Banaszewska B, Pawelczyk L, Spaczynski RZ, et al. Effects of simvastatin and oral contraceptive agent on polycystic ovary syndrome: Prospective, randomized, crossover trial. J Clin Endocrinol Metab. 2007;92:456–61. doi: 10.1210/jc.2006-1988. [DOI] [PubMed] [Google Scholar]
- 16.Rashidi B, Abediasl J, Tehraninejad E, et al. Simvastatin effects on androgens, inflammatory mediators, and endogenous pituitary gonadotropins among patients with PCOS undergoing IVF: results from a prospective, randomized, placebo-controlled clinical trial. J Investig Med. 2011;59:912–16. doi: 10.2310/JIM.0b013e31821bfd9c. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shuster JJ. Review: Cochrane handbook for systematic reviews for interventions, Version 5.1.0, published 3/2011. In: Higgins JPT, Green S, editors. Res Synth Methods. Vol. 2. 2011. pp. 126–30. [Google Scholar]
- 19.Banaszewska B, Pawelczyk L, Spaczynski RZ, et al. Comparison of simvastatin and metformin in treatment of polycystic ovary syndrome: Prospective randomized trial. J Clin Endocrinol Metab. 2009;94:4938–45. doi: 10.1210/jc.2009-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kazerooni T, Shojaei-Baghini A, Dehbashi S, et al. Effects of metformin plus simvastatin on polycystic ovary syndrome: A prospective, randomized, double-blind, placebo-controlled study. Fertil Steril. 2010;94:2208–13. doi: 10.1016/j.fertnstert.2009.11.045. [DOI] [PubMed] [Google Scholar]
- 21.Banaszewska B, Pawelczyk L, Spaczynski RZ, et al. Effects of simvastatin and metformin on polycystic ovary syndrome after six months of treatment. J Clin Endocrinol Metab. 2011;96(11):3493–501. doi: 10.1210/jc.2011-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sathyapalan T, Smith KA, Coady A-M, et al. Atorvastatin therapy decreases androstenedione and dehydroepiandrosterone sulphate concentrations in patients with polycystic ovary syndrome: randomized controlled study. Ann Clin Biochem. 2012;49:80–85. doi: 10.1258/acb.2011.011071. [DOI] [PubMed] [Google Scholar]
- 23.Puurunen J, Piltonen T, Puukka K, et al. Statin therapy worsens insulin sensitivity in women with polycystic ovary syndrome (PCOS): A prospective, randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2013;98:4798–807. doi: 10.1210/jc.2013-2674. [DOI] [PubMed] [Google Scholar]
- 24.Seyam E, Al Gelany S, Abd Al Ghaney A, et al. Evaluation of prolonged use of statins on the clinical and biochemical abnormalities and ovulation dysfunction in single young women with polycystic ovary syndrome. Gynecol Endocrinol. 2018;34(7):589–96. doi: 10.1080/09513590.2017.1418853. [DOI] [PubMed] [Google Scholar]
- 25.Pasquali R, Gambineri A. Glucose intolerance states in women with the polycystic ovary syndrome. J Endocrinol Invest. 2013;36(8):648–53. doi: 10.1007/BF03346757. [DOI] [PubMed] [Google Scholar]
- 26.Fauser BC, Tarlatzis BC, Rebar RW, et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): The Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97(1):28–38.e25. doi: 10.1016/j.fertnstert.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 27.Veras K, Almeida FN, Nachbar RT, et al. DHEA supplementation in ovariectomized rats reduces impaired glucose-stimulated insulin secretion induced by a high-fat diet. FEBS Open Bio. 2014;4:141–46. doi: 10.1016/j.fob.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Byrne JJ, Bradlow HL. DHEA-PC slows the progression of type 2 diabetes (non-insulin-dependent diabetes mellitus) in the ZDF/Gmi-fa/fa rat. Diabetes Technol Ther. 2001;3:211–19. doi: 10.1089/152091501300209570. [DOI] [PubMed] [Google Scholar]
- 29.Brahimaj A, Muka T, Kavousi M, et al. Serum dehydroepiandrosterone levels are associated with lower risk of type 2 diabetes: The Rotterdam Study. Diabetologia. 2017;60:98–106. doi: 10.1007/s00125-016-4136-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perrini S, Natalicchio A, Laviola L, et al. Dehydroepiandrosterone stimulates glucose uptake in human and murine adipocytes by inducing GLUT1 and GLUT4 translocation to the plasma membrane. Diabetes. 2004;53:41–52. doi: 10.2337/diabetes.53.1.41. [DOI] [PubMed] [Google Scholar]
- 31.Brignardello E, Runzo C, Aragno M, et al. Dehydroepiandrosterone administration counteracts oxidative imbalance and advanced glycation end product formation in type 2 diabetic patients. Diabetes Care. 2007;30:2922–27. doi: 10.2337/dc07-1110. [DOI] [PubMed] [Google Scholar]
- 32.Aoki K, Tajima K, Taguri M, et al. Effect of dehydroepiandrosterone (DHEA) on Akt and protein kinase C zeta (PKCζ) phosphorylation in different tissues of C57BL6, insulin receptor substrate (IRS)1(−/−), and IRS2(−/−) male mice fed a high-fat diet. J Steroid Biochem Mol Biol. 2016;159:110–20. doi: 10.1016/j.jsbmb.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 33.Kawano H, Yasue H, Kitagawa A, et al. Dehydroepiandrosterone supplementation improves endothelial function and insulin sensitivity in men. J Clin Endocrinol Metab. 2003;88:3190–95. doi: 10.1210/jc.2002-021603. [DOI] [PubMed] [Google Scholar]
- 34.Ortega I, Cress AB, Wong DH, et al. Simvastatin reduces steroidogenesis by inhibiting Cyp17a1 gene expression in rat ovarian theca interstitial cells. Biol Reprod. 2012;86(1):1–9. doi: 10.1095/biolreprod.111.094714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun J, Yuan Y, Cai R, et al. An investigation into the therapeutic effects of statins with metformin on polycystic ovary syndrome: A meta-analysis of randomised controlled trials. BMJ Open. 2015;5:e007280. doi: 10.1136/bmjopen-2014-007280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghazeeri G, Abbas HA, Skaff B, et al. Inadequacy of initiating rosuvastatin then metformin on biochemical profile of polycystic ovarian syndrome patients. J Endocrinol Invest. 2015;38:643–51. doi: 10.1007/s40618-015-0237-3. [DOI] [PubMed] [Google Scholar]
- 37.Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: A collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–42. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 38.Agouridis AP, Kostapanos MS, Elisaf MS. Statins and their increased risk of inducing diabetes. Expert Opin Drug Saf. 2015;14(12):1835–44. doi: 10.1517/14740338.2015.1096343. [DOI] [PubMed] [Google Scholar]
- 39.Ganesan S, Ito MK. Coenzyme Q10 ameliorates the reduction in GLUT4 transporter expression induced by simvastatin in 3T3-L1 adipocytes. Metab Syndr Relat Disord. 2013;11(4):251–60. doi: 10.1089/met.2012.0177. [DOI] [PubMed] [Google Scholar]
- 40.Park ZH, Juska A, Dyakov D, Patel RV. Statin-associated incident diabetes: A literature review. Consult Pharm. 2014;29(5):317–34. doi: 10.4140/TCP.n.2014.317. [DOI] [PubMed] [Google Scholar]