Abstract
Upregulation of human epididymis protein 4 (HE4) is often observed in different types of cancers, including gastric cancer (GC), but the association of elevated HE4 level with radiation resistance in GC remains unclear. The expression of HE4 and hypoxia-inducible factor 1α subunit (HIF1α) was assessed in GC patient samples and cell lines. Chromatin immunoprecipitation (ChIP) and luciferase reporter assays were performed to reveal the regulation between HE4 and HIF1α. Stable HE4 knockdown and HIF1α overexpression were introduced into GC cell lines to study the role of HE4 in the resistance of GC to radiation therapy. Colony formation assay and the xenograft mouse model were used to investigate the effects of radiation on GC cells. HE4 and HIF1α were upregulated in both GC patient tissues and GC cells. Hypoxia and HIF1α upregulated HE4 by directly targeting the hypoxia response element in its promoter region. Stable HE4 knockdown significantly sensitized GC cells and xenograft tumors to radiation. HIF1α overexpression markedly elevated the radiation resistance of GC cells, which was almost completely abolished by HE4 knockdown. Hypoxia-induced upregulation of HE4 is responsible for resistance to radiation therapy of GC, suggesting that HE4 knockdown or inhibition, combined with radiation therapy, holds great potential in the clinical treatment of GC.
Keywords: hypoxia, human epididymis protein 4, WFDC2, radiation therapy, gastric cancer, hypoxia-inducible factor 1 α subunit, HIF1α, resistance
Introduction
Gastric cancer (GC) is one of the most frequently encountered illnesses worldwide1 and one of the top causes of cancer-related mortality in China.2 Despite the fact that the incidence of GC is declining, its 5-year survival rate between 2011 and 2017 was ∼27%.3 In addition, many drugs for chemotherapy do not have ideal curative effects but cause a number of adverse reactions. Hence, there is an urgent need to better understand the molecular mechanisms responsible for the development and progression of GC to explore specific and sensitive markers for accurate early diagnosis. There has been a long history of utilizing radiation therapy to shrink tumors in GC.4 However, due to resistance to radiation therapy, GC patients who are diagnosed at late stages still present a poor prognosis, as well as a low 5-year survival rate, even with surgical resection combined with adjuvant radiotherapy.5 Therefore, identifying new factors associated with radiotherapy resistance could potentially enhance the treatment efficacy and improve patient prognosis for GC patients.
Human epididymis protein 4 (HE4), encoded by the WFDC2 gene, is a member of the WFDC domain family, which features the characteristic WAP motif consisting of eight cysteine-formed disulfide bonds.6 Under physiological conditions, HE4 is secreted into the blood to act as a protease inhibitor and is reportedly involved in the maturation of sperm cells.7 Several recent studies have shown the upregulation of HE4 in various types of human cancers, including GC. In patients with GC, HE4 was detected in 74% of intestinal and over 90% of diffuse cancers.8 HE4 has also been reported to promote GC progression, suggesting its role as a promising prognostic factor for GC diagnosis.9 Recently, HE4 was suggested to be a useful addition to the current panel of immunocytochemistry markers for the diagnosis of gastrointestinal adenocarcinomas of Müllerian origin.10 However, the relevance of high-level HE4 to radiation resistance in GC still remains a question, which has prompted us to experimentally examine such possibility.
It has been widely suggested that hypoxia and hypoxia-inducible factor 1, α subunit (HIF1α), are linked to GC tumorigenesis. Hypoxia facilitates the growth, migration, and invasion of GC cells through the downregulation of RASSF8 and induction of microRNA-224.11 Hypoxia also boosts the resistance of GC cells to cisplatin via activation of interleukin-1α.12 In GC cells, the elevation of HIF1α expression induced by the activation of phosphatidylinositol 3-kinase (PI3K)-mammalian target of rapamycin (mTOR) acts as a mediator between cell-cycle arrest signaling and survival.13 Clinically, HIF1α is a known indicator of poor prognosis in GC patients, as evidenced by significant correlation between HIF1α expression and epithelial-mesenchymal transition markers in GC tissues, as well as the infiltration of tumor-associated macrophages.14 Further, prior investigations demonstrated that the radiosensitivity of GC cells was reduced by hypoxia,15 whereas reoxygenation was able to increase the radiosensitivity of hypoxic GC cells.16
In this study, we aimed to investigate the relationship between HE4 expression and hypoxia in GC patient tissues and to reveal the underlying molecular regulatory mechanism between these two important diagnostic factors in GC. In particular, we would like to understand the interplay between HE4 and hypoxia in the radiation sensitivity of GC.
Results
Correlative Upregulation of WFDC2, HE4, and HIF1α in GC Patient Tissues
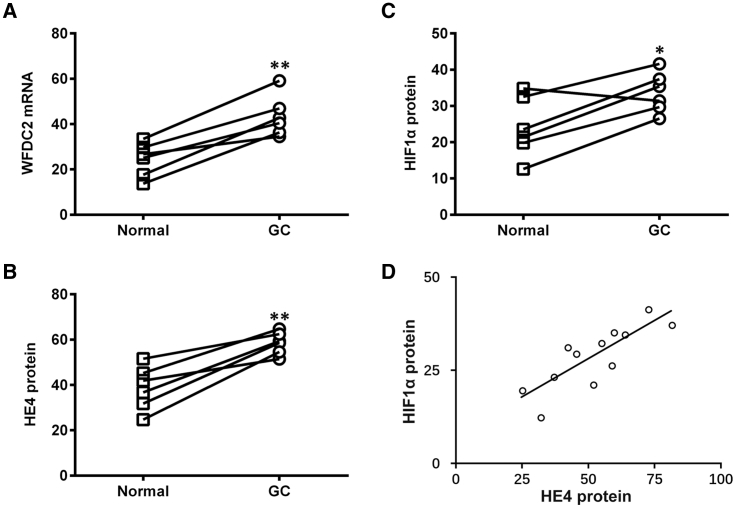
We first aimed to assess the expression levels of both WFDC2 mRNA and HE4 protein (encoded by the WFDC2 gene) in GC patient tumor and paired adjacent normal tissues. Our data indicated that the WFDC2 mRNA level was markedly elevated in samples from GC patients (Figure 1A), and so was the HE4 protein level (Figure 1B). Consistent with previous studies, we also observed significantly elevated HIF1α protein levels in GC tumor tissues compared to those of normal tissues (Figure 1C). Importantly, Pearson’s correlation coefficient test was performed between protein levels of HE4 and HIF1α, which yielded a strong lineal correlation between these protein factors in all tissue samples collected (Figure 1D).
Figure 1.
Expression Profiles of WFDC2 mRNA, HE4 Protein, and HIF1α Protein in GC Patient Tumor and Paired Adjacent Normal Tissues
(A–C) Expressions of WFDC2 mRNA (A), HE4 protein (B), and HIF1α protein (C) were assessed in GC patient tumor tissues (n = 6) and paired adjacent normal tissues (n = 6). (D) Lineal correlation between the expressions of HE4 protein and HIF1α protein was analyzed in all patient tissues (n = 12). Data are presented as mean ± SD. *p < 0.05; **p < 0.01, normal versus GC tissues.
WFDC2 Is Under Hypoxia Regulation Directly via the HRE in Its Promoter
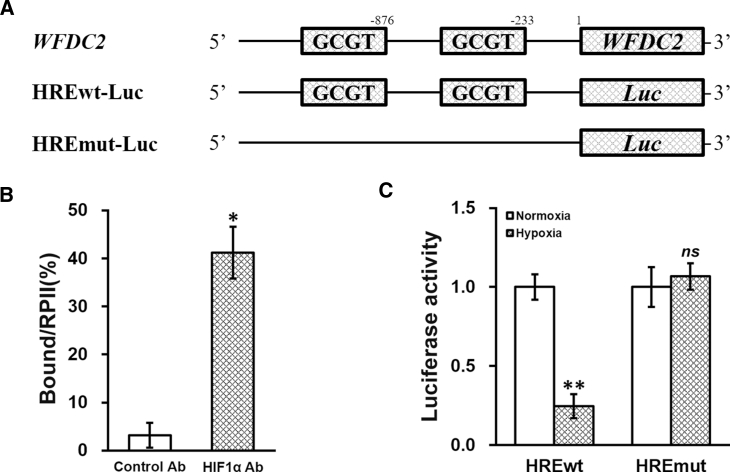
The aforementioned observed correlative upregulation of WFDC2, HE4, and HIF1α in GC patient tissues raised our interest regarding their regulatory relationship, using GC cells as the in vitro model. We examined the sequence of the WFDC2 promoter region and identified sequences constituting two putative hypoxia response elements (HREs) (Figure 2A). HIF1α could act as a transcriptional factor directly binding to the HREs in the promoter region of a variety of genes.17 Therefore, we conducted a chromatin immunoprecipitation (ChIP) assay with HIF1α antibody, using immunoglobulin G (IgG) as the control. As expected, we observed a strong direct binding of HIF1α to the HREs in the WFDC2 promoter region (Figure 2B). We next cloned the wild-type (HREwt-Luc) and mutated (HREmut-Luc) sequences of the WFDC2 promoter region into the 5′ of luciferase reporter open reading frame (Figure 2A), which were then separately transfected into GC cells under normoxia (0 μM CoCl2) or hypoxia (100 μM CoCl2) conditions (Figure 2C). The activity of luciferase under the control of wild-type HREwt-Luc was notably suppressed by hypoxia, whereas the luciferase activity of HREmut-Luc was not affected, suggesting that the WFDC2 promoter was under the induction of hypoxic conditions.
Figure 2.
WFDC2 Is Under Hypoxia Regulation Directly through the HRE in Its Promoter
(A) Consensus sequence for the hypoxia response element (HRE) was identified in the promoter region of WFDC2. Wild-type (HREwt-Luc) and mutated (HREmut-Luc) sequences from the WFDC2 promoter were respectively cloned at the 5′ of luciferase reporter open reading frame (Luc). (B) Binding of HIF1α to the HRE in the WFDC2 promoter was analyzed by ChIP assay, using control antibody (Ab) and HIF1α Ab, respectively. (C) Luciferase activities of HREwt-Luc and HREmut-Luc constructs were measured in GC cells incubated in media containing 0 μM (normoxia) or 100 μM (hypoxia) CoCl2, respectively. Data are presented as mean ± SD. *p < 0.05, control versus HIF1α Ab; **p < 0.01; ns, not significant, normoxia versus hypoxia.
WFDC2 mRNA and HE4 Protein Are Upregulated in GC Cell Lines
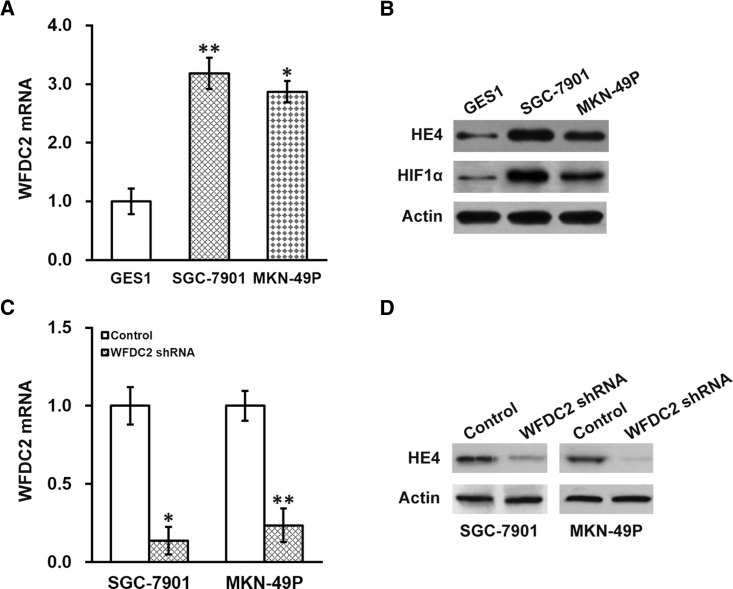
We then examined the relative expression levels of WFDC2 mRNA and HE4 protein in GC cell lines. Indeed, we observed higher expressions of WFDC2 mRNA (Figure 3A) and HE4 protein (Figure 3B) in the GC cell lines SGC-7901 and MKN-49P than those in the normal human gastric epithelial cell line GES1. Noteworthily, HIF1α protein expression was also higher in both GC cell lines than in the GES1 normal cell control (Figure 3B). As the in vitro observations correlated well with our in vivo findings (Figure 1), these two cell lines were used for further investigations into the role of WFDC2, HE4, and HIF1α in the radiotherapy resistance of GC cells.
Figure 3.
Expression Profiles of WFDC2 mRNA and HE4 Protein in GC Cell Lines
(A) Expressions of WFDC2 mRNA were assessed in GC cell lines SGC-7901 and MKN-49P, with the human gastric epithelial cell line GES1 used as normal control. (B) Expressions of HE4 and HIF1α proteins in GC cell lines SGC-7901 and MKN-49P, and human gastric epithelial cell line GES1. (C and D) Effect of WFDC2 shRNA on the expression of (C) WFDC2 mRNA and (D) HE4 protein GC cells. Data are presented as mean ± SD. *p < 0.05, and **p < 0.01, versus (A) GES1 or (C) control.
WFDC2 and HE4 Is Required for Resistance of GC to Radiation In Vitro and In Vivo
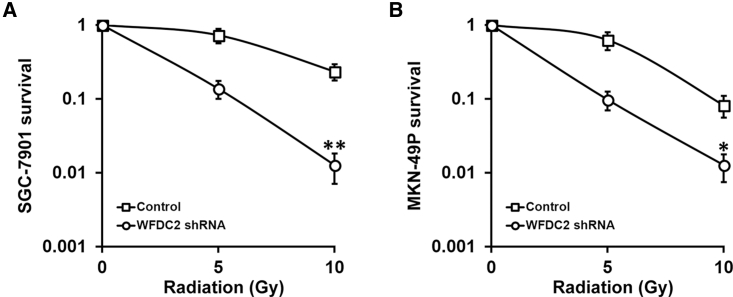
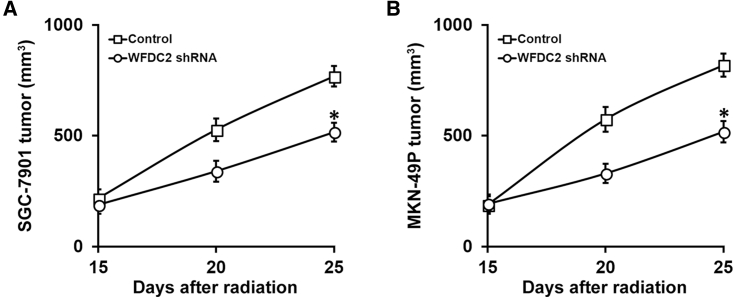
We next sought to determine whether WFDC2 and HE4 contributed to the radiation resistance of GC cells. We first established stable WFDC2 short hairpin RNA (shRNA) knockdown in both the SGC-7901 and MKN-49P GC cell lines, which was confirmed by both real-time PCR and immunoblotting assays showing that both WFDC2 mRNA (Figure 3C) and HE4 protein (Figure 3D) were repressed. Next, survival of these GC cells with stable WFDC2 knockdown after radiation treatments was evaluated using the clonogenic assay. We found that the survival fraction was greatly reduced in the stable WFDC2 knockdown SGC-7901 (Figure 4A) and MKN-49P (Figure 4B) cells compared with control. Further, we examined this effect in vivo, utilizing the xenograft mouse model. Consistent with our results obtained from the cell lines, WFDC2 knockdown markedly inhibited xenograft growth following radiation therapy (Figures 5A and 5B). Taken together, our results demonstrated that WFDC2 and HE4 was required for resistance of GC to radiation both in vitro and in vivo.
Figure 4.
WFDC2 and HE4 Is Required for Resistance of GC Cells to Radiation In Vitro
(A and B) Viability of GC cell lines SGC-7901 (A) and MKN-49P (B) stably expressing control or WFDC2 shRNA, after treatment with radiation dose as indicated, was assessed by clonogenic assay. Data are presented as mean ± SD. *p < 0.05, and **p < 0.01, control versus WFDC2 shRNA.
Figure 5.
WFDC2 and HE4 Is Required for Resistance of GC Xenograft Tumors to Radiation Therapy In Vivo
Mice (n = 6 each group) bearing SGC-7901 (A) and MKN-49P (B) xenograft tumors were subjected to 10 Gy of radiation on days 15 and 20 after inoculation, and sizes of xenograft tumors were measured on the indicated days. Data are presented as mean ± SD. *p < 0.05, control versus WFDC2 shRNA.
Hypoxia-Induced Upregulation of WFDC2 and HE4 Is Required for Resistance of GC to Radiation Therapy Both In Vitro and In Vivo
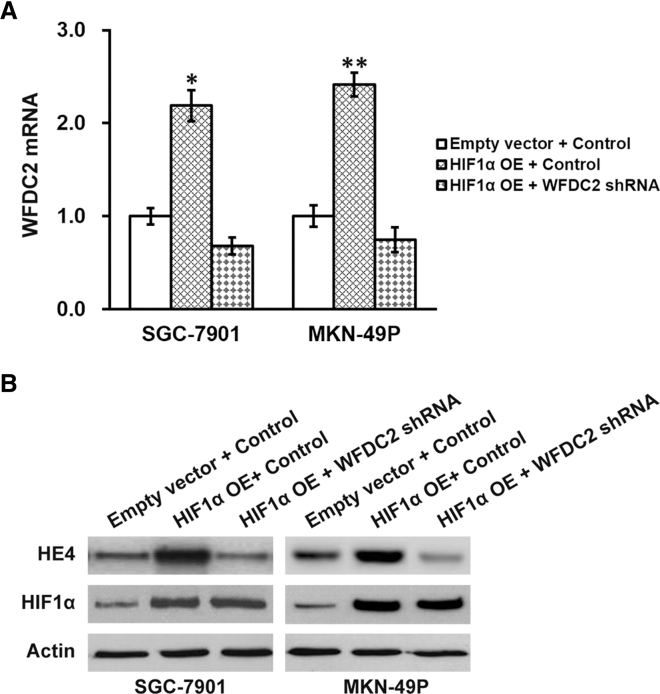
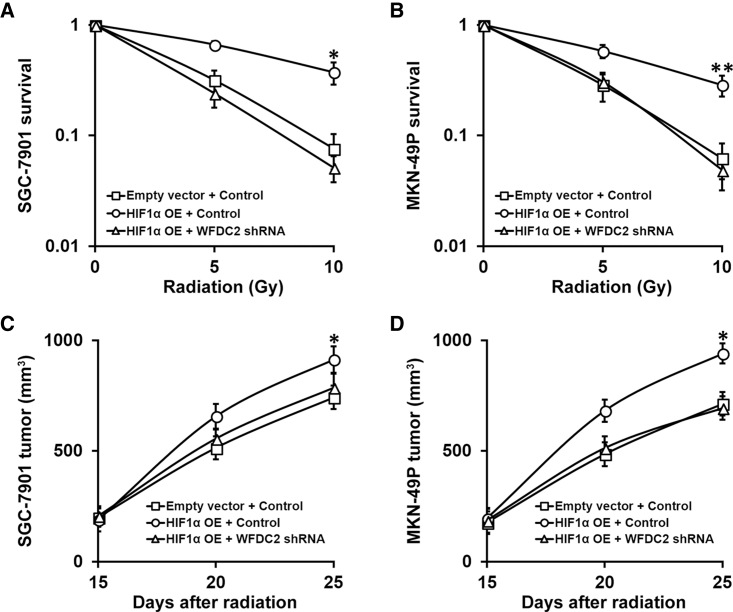
Next, we aimed to determine whether WFDC2 and HE4 expression was induced by HIF1α. Both GC cell lines with stable expression of HIF1α were evaluated with regard to WFDC2 mRNA and HE4 protein levels. As expected, HIF1α overexpression induced strong upregulation of both WFDC2 mRNA and HE4 protein (Figures 6A and 6B, first two lanes). Importantly, WFDC2 knockdown was able to completely abolish the HIF1α-induced upregulation of WFDC2 mRNA and protein (Figures 6A and 6B, last two lanes). These cell lines, combining HIF1α overexpression and WFDC2 knockdown, would then allow us to investigate whether this HIF1α-WFDC2-HE4 regulatory cascade was involved in the resistance of GC cells to radiation therapy. Indeed, overexpressing HIF1α in both GC cells induced stronger resistance to radiation than control cells, which could be restored by subsequent knockdown of WFDC2 (Figures 7A and 7B). Moreover, using the xenograft mouse model, we found that, following radiation therapy, tumor sizes from GC cells overexpressing HIF1α were significantly bigger than that from control, while WFDC2 knockdown was able to abolish this resistance (Figures 7C and 7D).
Figure 6.
Effect of HIF1α Overexpression on HE4 Level in GC Cells
(A) Expressions of WFDC2 mRNA were assessed in GC cell lines SGC-7901 and MKN-49P stably expressing control or WFDC2 shRNA, bearing either empty vector or HIF1α overexpression (OE), respectively. (B) Expressions of HE4 and HIF1α proteins were assessed in GC cell lines SGC-7901 and MKN-49P stably expressing control or WFDC2 shRNA, bearing either empty vector or HIF1α OE, respectively. Data are presented as mean ± SD. *p < 0.05, and **p < 0.01, HIF1α OE + control versus empty vector + control and HIF1α OE + WFDC2 shRNA.
Figure 7.
Hypoxia-Induced Upregulation of HE4 Is Required for Resistance of GC to Radiation Therapy Both In Vitro and In Vivo
(A and B) Viability of GC cell lines SGC-7901 (A) and MKN-49P (B) stably expressing control or WFDC2 shRNA, bearing either empty vector or HIF1α overexpression (OE), respectively, after treatment with radiation dose as indicated, was assessed by clonogenic assay. (C and D) Mice (n = 6 each group) bearing SGC-7901 (C) and MKN-49P (D) xenograft tumors stably expressing control or WFDC2 shRNA, bearing either empty vector or HIF1α OE, respectively, were subjected to 10 Gy of radiation on days 15 and 20 after inoculation, and sizes of xenograft tumors were measured on indicated days. Data are presented as mean ± SD. *p < 0.05, and **p < 0.01, HIF1α OE + control versus empty vector + control and HIF1α OE + WFDC2 shRNA.
Discussion
In the present study, we first validated previous reports on the upregulation of WFDC2 and HE4 at both mRNA and protein levels, as well as elevated hypoxic status, in GC tissues and cells. We further experimentally established that hypoxia and HIF1α upregulated HE4 expression via directly targeting the HRE in its promoter region. Stable HE4 knockdown significantly sensitized GC cells and xenograft tumors to radiotherapy. Similarly, HIF1α overexpression markedly elevated radiation resistance of GC cells, which was almost completely abolished by HE4 knockdown. Our findings clearly reveal that the hypoxia-HIF1α-WFDC2 cascade plays a critical role in the resistance to radiation in GC. Therefore, knocking down WFDC2 or inhibiting HE4 expression, combined with radiation therapy, holds great potential in the clinical treatment of GC, necessitating further laboratory and clinical studies.
HE4 is encoded by the WFDC2 gene and belongs to a family of secreted protease inhibitors containing 2 to 4 WAP domains.6 Their precise functions are not fully understood, but their putative roles as extracellular protease inhibitors7 imply that they potentially contribute to cell migration, invasion, and the regulation of the extracellular matrix. There has been an increasing recognition of HE4 as a protease inhibitor in cancer biology, and HE4 has been implicated in the therapeutic resistance of cancer. For instance, Lee et al.18 revealed that, in epithelial ovarian cancer, HE4 contributed to poor prognosis and chemoresistance. Ribeiro et al.19 observed that, in ovarian cancer, HE4 enhanced collateral resistance to paclitaxel and cisplatin. However, the involvement of HE4 in GC resistance to radiation therapy has not been previously addressed. Of particular relevance to our present study, HE4 was detected in 74% of intestinal and over 90% of diffuse cancers in GC patients8 and promoted GC progression.9 Data from these previous studies were in agreement with ours, in that we also observed significantly upregulated expression of HE4 protein in GC patient tissues, as compared to corresponding adjacent normal tissues. The defining novelty of our present study lies in that our results have uncovered hypoxia and HIF1α as the upstream regulator of WFDC2 gene and HE4 protein for the first time in GC. Importantly, apart from its known promotional role in GC progression, we have identified that HE4, under direct induction of hypoxia and HIF1α, is also responsible for radiotherapy resistance of GC tumors.
HIF1α is an essential transcription factor in the hypoxia-induced adaptive responses. The activity of HIF1 is primarily dictated by the modification of HIF1α.20 Under normoxic conditions, HIF1α is hydroxylated and ubiquitinated, which leads to its rapid degradation;21, 22 whereas during hypoxia, HIF1α degradation is inhibited, thereby allowing the formation of a stable HIF1 complex. HIF1α activity is also prominently elevated in many different types of cancer, presumably as a result of hypoxia within the tumor tissues plus genetic alterations. The radiation or reoxygenation of tumor tissues following radiotherapy reportedly stimulated the production of reactive oxygen species, generating a stable HIF1 complex.23 It is widely reported that hypoxia and HIF1α likely contribute to the tumorigenesis of GC. In line with our study, previous investigations reported that hypoxia reduced the sensitivity of GC cells to radiation.15 However, the exact downstream effector of hypoxia in GC resistance to radiation remains unknown. Our present data not only provide yet another instance implicating hypoxia and HIF1α as an active player in the radiation resistance of GC tumors but also reveal HE4 as the direct downstream target of HIF1α. HIF1α and HE4 are involved in conferring radioresistance of GC, suggesting their potential as diagnostic markers and therapeutic targets in clinical GC cases.
To summarize, our experimental results have shown that hypoxia and HIF1α induces upregulation of HE4 expression through directly binding to the HRE in the WFDC2 promoter region. Stable knockdown of HE4 significantly increases the sensitivity of GC cells and xenograft tumors to radiation therapy. Consistently, overexpression of HIF1α significantly promotes the resistance of GC cells to radiation, an effect that could be nearly completely prevented via HE4 knockdown. Findings presented in this study provide unambiguous evidence that the hypoxia-HIF1α-WFDC2 signal cascade plays a critical role in the resistance to radiation of GC cells and could potentially serve as diagnostic markers and/or therapeutic targets in clinical treatments against GC.
Materials and Methods
Patient Samples
Six GC tumor samples, including adjacent benign tissues, were harvested from the First Affiliated Hospital of Anhui Medical University. The protocols for human research were approved by the Ethics Committee of the First Affiliated Hospital of Anhui Medical University. All the patients involved in the donation provided written informed consents.
Cell Culture
GC cell lines MKN-49P and SGC-7901 were purchased from the America Type Culture Collection (ATCC, Manassas, VA, USA). The human gastric epithelial cell line GES1 was used as the control in the present study. Cells were cultured in PRMI-1640 medium supplemented with 1% PSG (penicillin-streptomycin-glutamine; GIBCO, Grand Island, NY, USA) and 10% fetal bovine serum (FBS; Hyclone, USA) and maintained in an incubator with 5% CO2 at 37°C.
Real-Time qPCR
The relative mRNA expression levels were assessed through qPCR with the use of the PowerUp SYBR Green Master Mix (Thermo Fisher Scientific, Waltham, MA, USA) according to the provided manual. In brief, the total RNA from the cell samples was extracted using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA). The quantity and quality of RNA samples were verified using BioAnalyzer 2100 (Agilent, Santa Clara, CA, USA). Next, total RNA (1 μg) underwent reverse transcription using the SuperScript III First-Strand Synthesis SuperMix (Thermo Fisher Scientific, Waltham, MA, USA). Then, real-time PCR was conducted using the PowerUp SYBR Green Master Mix (Thermo Fisher Scientific, Waltham, MA, USA). The relative levels of expression were calculated using the 2−ΔΔCt method with GAPDH as the internal control. The primers used in the present study were as follows: WFDC2, sense: 5′-GCT GGC CTC CTA CTA GGG TT-3′, anti-sense: 5′-AAC ACA CAG TCC GTA ATT GGT-3′; HIF1α, sense: 5′-CTC AAA GTC GGA CAG CCT CA-3′, anti-sense: 5′-CCC TGC AGT AGG TTT CTG CT-3′; GAPDH, sense: 5′-GGA GCG AGA TCC CTC CAA AAT-3′, anti-sense: 5′-GGC TGT TGT CAT ACT TCT CAT GG-3′.
Western Blot
The cell samples were lysed using RIPA lysis buffer, and concentrations of proteins were assessed using the BCA Protein System Kit. Protein samples of equal amounts were resolved on an SDS-PAGE gel and then transferred onto polyvinylidene fluoride (PVDF) membranes on ice. The membranes were blocked with 5% skim milk in Tris-buffered saline, 0.1% Tween 20 (TBST) buffer briefly and subsequently incubated with the primary antibody (anti-HE4, Abcam, ab24480, 1:1,000; anti-β-actin, Abcam, ab8226, 1:1,000) at 4°C overnight. After six washes with TBST (5 min each), the PVDF membrane was subjected to incubation with the appropriate secondary antibody (anti-mouse, Abcam, ab6789, 1:5,000; anti-rabbit, Abcam, ab6721, 1:5,000) for 1 hr at room temperature. The final bands were visualized with an enhanced chemiluminescence (ECL) kit (Millipore, Billerica, MA, USA) following the instructions of the manufacturer.
ChIP Assay
Formaldehyde (1%) was used to cross-link the cells, which were then subjected to immunoprecipitation using the antibody against HIF1α (Cell Signaling Technology, Danvers, MA, USA). The WFDC2 promoter regions containing both HREs were amplified using specific primers. IgG was used as a negative control. The amplification efficiency of the experimental samples was normalized to that of IgG and presented as fold increase.
Luciferase Reporter Assay
The WFDC2 promoter region, either wild-type or mutated, was cloned into pGL-3 luciferase reporter plasmid (Promega, Madison, WI, USA) using the double-digestion method. Cells were transfected with the resultant plasmid and cultured for 48 hr. Relative activities of luciferase were determined using the Bright-Glo Luciferase Assay System (Promega, Madison, WI, USA) according to the manufacturer’s guides.
shRNA Knockdown and Overexpression of WFDC2 mRNA
Cells with exponential growth were seeded into six-well plates for infection with lentivirus packaged with either WFDC2 shRNA or control and then were transferred to 100-mm Petri dishes for resistance selection after 24 hr. The efficiency of overexpression or knockdown was confirmed using both real-time PCR and western blot assay.
Colony Formation Assay
MKN-49P and SGC-7901 cells were digested into single cells, suspended in culture medium at the density of 200 cells per milliliter using a trypsin-EDTA solution (Sigma, St. Louis, MO, USA), and then added to six-well plates at 2 mL per well, two wells for each group. After 2 washes with PBS, cells were fixed using 4% paraformaldehyde and stained using hematoxylin solution. Colonies containing 50 or more cells were counted under a microscope. The area and diameter of individual colonies were normalized to those of the control group.
Xenograft Mouse Model
The immunodeficient NPG mice (4–6 weeks old) were housed in the pathogen-free environment and allowed to acclimatize for at least 1 week. All the procedures involving animals were conducted in conformity with protocols approved by the Animal Care and Use Committee of First Affiliated Hospital of Anhui Medical University. Single-cell suspensions (106 cells per 100 μL) were mixed with equal volumes of Basement Membrane Extracts (R&D Systems, Minneapolis, MN, USA) on ice and then inoculated subcutaneously into the lower flanks on both sides of the animal to establish xenograft tumors. Diameters of the tumors were measured using digital calipers, and the volumes of the tumors were calculated using the following formula: volume (mm3) = (width)2 × length/2.
Radiation Therapy
Cells were added to 60-mm Petri dishes to attach for 4 hr and then were radiated with different dosages (0, 5, and 10 Gy) using a 210-kV X-ray source at 2.16 Gy/min (RS-2000 biological irradiator; Rad Source Technologies, Buford, GA, USA). Afterward, cells were maintained in a 5% CO2 incubator at 37°C for 14 consecutive days; then, crystal violet staining was used to reveal the surviving colonies, and the survival fractions (SFs) were calculated. For in vivo experiments, GC cells were inoculated on day 0 (6 mice per group), followed by 10 Gy of irradiation treatment on days 15 and 20.
Statistical Analysis
All experiments were performed independently at least three times. Data were analyzed using SPSS 23.0 software and one-way ANOVA, followed by Tukey’s multiple comparisons test, which was utilized for comparisons between groups. p values less than 0.05 were regarded as statistically significant.
Author Contributions
C.P and C.Y designed the study; C.P., G.L., K.H., Q.Z., and Y.L. did the experiments; C.P, G.L., and K.H. analyzed the data; C.P. and C.Y. wrote the manuscript.
Conflicts of Interest
The authors declare no competing interests.
References
- 1.Bignotti E., Ragnoli M., Zanotti L., Calza S., Falchetti M., Lonardi S., Bergamelli S., Bandiera E., Tassi R.A., Romani C. Diagnostic and prognostic impact of serum HE4 detection in endometrial carcinoma patients. Br. J. Cancer. 2011;104:1418–1425. doi: 10.1038/bjc.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong B.C., Lam S.K., Wong W.M., Chen J.S., Zheng T.T., Feng R.E., Lai K.C., Hu W.H., Yuen S.T., Leung S.Y., China Gastric Cancer Study Group Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187–194. doi: 10.1001/jama.291.2.187. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R.L., Miller K.D., Jemal A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 4.Ng J., Lee P. The role of radiotherapy in localized esophageal and gastric cancer. Hematol. Oncol. Clin. North Am. 2017;31:453–468. doi: 10.1016/j.hoc.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Aurello P., D’Angelo F., Rossi S., Bellagamba R., Cicchini C., Nigri G., Ercolani G., De Angelis R., Ramacciato G. Classification of lymph node metastases from gastric cancer: comparison between N-site and N-number systems. Our experience and review of the literature. Am. Surg. 2007;73:359–366. [PubMed] [Google Scholar]
- 6.Chhikara N., Saraswat M., Tomar A.K., Dey S., Singh S., Yadav S. Human epididymis protein-4 (HE-4): a novel cross-class protease inhibitor. PLoS ONE. 2012;7:e47672. doi: 10.1371/journal.pone.0047672. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Kirchhoff C., Habben I., Ivell R., Krull N. A major human epididymis-specific cDNA encodes a protein with sequence homology to extracellular proteinase inhibitors. Biol. Reprod. 1991;45:350–357. doi: 10.1095/biolreprod45.2.350. [DOI] [PubMed] [Google Scholar]
- 8.O’Neal R.L., Nam K.T., LaFleur B.J., Barlow B., Nozaki K., Lee H.J., Kim W.H., Yang H.K., Shi C., Maitra A. Human epididymis protein 4 is up-regulated in gastric and pancreatic adenocarcinomas. Hum. Pathol. 2013;44:734–742. doi: 10.1016/j.humpath.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo Y.D., Wang J.H., Lu H., Li X.N., Song W.W., Zhang X.D., Zhang W.M. The human epididymis protein 4 acts as a prognostic factor and promotes progression of gastric cancer. Tumour Biol. 2015;36:2457–2464. doi: 10.1007/s13277-014-2858-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stiekema A., Van de Vijver K.K., Boot H., Broeks A., Korse C.M., van Driel W.J., Kenter G.G., Lok C.A. Human epididymis protein 4 immunostaining of malignant ascites differentiates cancer of Müllerian origin from gastrointestinal cancer. Cancer Cytopathol. 2017;125:197–204. doi: 10.1002/cncy.21811. [DOI] [PubMed] [Google Scholar]
- 11.He C., Wang L., Zhang J., Xu H. Hypoxia-inducible microRNA-224 promotes the cell growth, migration and invasion by directly targeting RASSF8 in gastric cancer. Mol. Cancer. 2017;16:35. doi: 10.1186/s12943-017-0603-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xuan Y., Wang Y.N. Hypoxia/IL-1α axis promotes gastric cancer progression and drug resistance. J. Dig. Dis. 2017;18:511–520. doi: 10.1111/1751-2980.12496. [DOI] [PubMed] [Google Scholar]
- 13.Canales J., Valenzuela M., Bravo J., Cerda-Opazo P., Jorquera C., Toledo H., Bravo D., Quest A.F. Helicobacter pylori induced phosphatidylinositol-3-OH kinase/mTOR activation increases hypoxia inducible factor-1α to promote loss of cyclin D1 and G0/G1 cell cycle arrest in human gastric cells. Front. Cell. Infect. Microbiol. 2017;7:92. doi: 10.3389/fcimb.2017.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang W.J., Chen C., Zhou Z.H., Gao S.T., Tee T.J., Yang L.Q., Xu Y.Y., Pang T.H., Xu X.Y., Sun Q. Hypoxia-inducible factor-1 alpha correlates with tumor-associated macrophages infiltration, influences survival of gastric cancer patients. J. Cancer. 2017;8:1818–1825. doi: 10.7150/jca.19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J.G., Yi J.M., Park S.J., Kim J.S., Son T.G., Yang K., Yoo M.A., Heo K. Histone demethylase JMJD2B-mediated cell proliferation regulated by hypoxia and radiation in gastric cancer cell. Biochim. Biophys. Acta. 2012;1819:1200–1207. doi: 10.1016/j.bbagrm.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Kato Y., Yashiro M., Fuyuhiro Y., Kashiwagi S., Matsuoka J., Hirakawa T., Noda S., Aomatsu N., Hasegawa T., Matsuzaki T. Effects of acute and chronic hypoxia on the radiosensitivity of gastric and esophageal cancer cells. Anticancer Res. 2011;31:3369–3375. [PubMed] [Google Scholar]
- 17.Wenger R.H., Gassmann M. Oxygen(es) and the hypoxia-inducible factor-1. Biol. Chem. 1997;378:609–616. [PubMed] [Google Scholar]
- 18.Lee S., Choi S., Lee Y., Chung D., Hong S., Park N. Role of human epididymis protein 4 in chemoresistance and prognosis of epithelial ovarian cancer. J. Obstet. Gynaecol. Res. 2017;43:220–227. doi: 10.1111/jog.13181. [DOI] [PubMed] [Google Scholar]
- 19.Ribeiro J.R., Schorl C., Yano N., Romano N., Kim K.K., Singh R.K., Moore R.G. HE4 promotes collateral resistance to cisplatin and paclitaxel in ovarian cancer cells. J. Ovarian Res. 2016;9:28. doi: 10.1186/s13048-016-0240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang G.L., Jiang B.-H., Rue E.A., Semenza G.L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berra E., Roux D., Richard D.E., Pouysségur J. Hypoxia-inducible factor-1 α (HIF-1 α) escapes O2-driven proteasomal degradation irrespective of its subcellular localization: nucleus or cytoplasm. EMBO Rep. 2001;2:615–620. doi: 10.1093/embo-reports/kve130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaakkola P., Mole D.R., Tian Y.-M., Wilson M.I., Gielbert J., Gaskell S.J., von Kriegsheim A., Hebestreit H.F., Mukherji M., Schofield C.J. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 23.Dewhirst M.W., Cao Y., Moeller B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat. Rev. Cancer. 2008;8:425–437. doi: 10.1038/nrc2397. [DOI] [PMC free article] [PubMed] [Google Scholar]