Abstract
Krüppel-like factor 4 (KLF4) is a transcription factor with conserved zinc finger domains. As an essential regulator of vascular homeostasis, KLF4 exerts a protective effect in endothelial cells (ECs), including regulating vasodilation, inflammation, coagulation and oxidative stress. However, the underlying mechanisms modifying KLF4 activity in mediating vascular function remain poorly understood. Recently, essential roles for S-nitrosation have been implicated in many pathophysiologic processes of cardiovascular disease. Here, we demonstrated that KLF4 could undergo S-nitrosation in response to nitrosative stress in ECs, leading to the decreased nuclear localization with compromised transactivity. Mass-spectrometry and site-directed mutagenesis revealed that S-nitrosation modified KLF4 predominantly at Cys437. Functionally, KLF4 dependent vasodilatory response was impaired after S-nitrosoglutathione (GSNO) treatment. In ECs, endothelin-1 (ET-1) induced KLF4 S-nitrosation, which was inhibited by an endothelin receptor antagonist Bosentan. In hypoxia-induced rat model of pulmonary arterial hypertension (PAH), S-nitrosated KLF4 (SNO-KLF4) was significantly increased in lung tissues, along with decreased nuclear localization of KLF4. In summary, we demonstrated that S-nitrosation is a novel mechanism for the post-translational modification of KLF4 in ECs. Moreover, these findings suggested that KLF4 S-nitrosation may be implicated in the pathogenesis of vascular dysfunction and diseases such as PAH.
Keywords: Krüppel-like factor 4, S-nitrosation, Endothelium, Pulmonary arterial hypertension
Highlights
-
•
KLF4 undergoes S-nitrosation as a new post-translational modification.
-
•
The S-nitrosation at cysteine 437 impairs nuclear localization and transcriptional capacity of KLF4.
-
•
GSNO induced KLF4 nitrosation and impairs vasorelaxation of pulmonary arteries.
-
•
S-nitrosation of KLF4 is induced by endothelin-1 and inhibited by Bosentan.
-
•
S-nitrosation of KLF4 is increased in pulmonary arteries from rat model for pulmonary arterial hypertension.
1. Introduction
Krüppel-like factor 4 (KLF4), also known as gut-enriched Krüppel-like factor, is a member of the Krüppel-like transcription factor family with important biological functions in cell proliferation, differentiation, development and carcinogenesis [1], [2], [3]. In vasculature, KLF4 is constitutively expressed in endothelial cells (ECs) and smooth muscle cells (SMCs), playing critical roles in the regulation of inflammation, coagulation and phenotypic switching [4], [5]. KLF4 is implicated in the pathogeneses of a number of cardiovascular diseases including atherosclerosis and thrombosis [6], neointimal formation [5] and aortic aneurysm [7]. Recently, it has been suggested that endothelial KLF4 was critically involved in the development of pulmonary arterial hypertension (PAH) [8].
The KLF4 expression is regulated by diverse pathophysiological and pharmacological factors including blood flow, statins, sirolimus as well as pro-inflammatory cytokines such as TNFα, IL-1β, IFN-γ [9], [10], [11], [12]. Apart from the transcriptional regulation, KLF4 is also subjected to post-translational modification [13] via phosphorylation [14], [15], ubiquitination [16], [17], acetylation [18], sumoylation [19] and methylation [20]. These modifications fine-tune the function of KLF4 by changing its protein stability, DNA binding capacity and transcriptional activity. Protein S-nitrosation is a redox sensitive modification at cysteine residues by nitric oxide (NO) or NO-derived species to form an S-nitrosothiol (SNO). It was established as a significant route of NO mediated cellular function in addition to classical sGC/cGMP/Ca2+ signaling pathway. However, aberrant S-nitrosation was closely associated with various diseases such as atherosclerosis, diabetes, arrhythmia and PAH as well [21], [22], [23], [24], [25]. Thus, whether KLF4 could be modulated through S-nitrosation remains an important question.
Here, we demonstrated that S-nitrosation is a novel mechanism to post-translationally modify KLF4 activity. Such a modification can impair the vasoprotective function of KLF4 and may be implicated in the pathogenesis of PAH.
2. Materials and methods
2.1. Reagents and cells
Primary antibodies against KLF4 and S-nitroso-cysteine (SNO-Cys) were from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and Sigma-Aldrich (St. Louis, MO, USA). MG132, ascorbate, N-acetylcysteine (NAC), biotin-maleimide, streptavidin-agarose, glutathione (GSH), acetylcholine (ACh), phenylephrine (Phe), Nω-Nitro-L-arginine methyl ester hydrochloride (L-NAME), sodium nitroprusside (SNP) were from Sigma-Aldrich (St. Louis, MO, USA). S-nitrosoglutathione (GSNO) and S-nitrosocysteine (CSNO) were synthesized from glutathione using acidified nitrite. Endothelin-1 was from Tocris Bioscience (Bristol, UK) and Bosentan was from Selleck Chemicals (Houston, TX, USA).
Human umbilical vein endothelial cells (HUVECs) were cultured as previously described [26] with M199 medium containing heparin, acidic fibroblast growth factor and 20% (v/v) fetal bovine serum (FBS). HEK293 cells were grown in DMEM supplemented with 10% (v/v) FBS.
2.2. Animals and treatment
Animal care and experimental procedures were conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals with the approval by the Animal Research Committee of Peking University Health Science Center. For hypoxia-induced PAH, male Sprague-Dawley rats were maintained in hypoxic (10 ± 0.5% O2) or normoxic (21% O2) conditions 8 h per day for 3 weeks [27] and each group contains 9–10 rats. Rats were anesthetized with intraperitoneal injection of pentobarbital sodium (50 mg/kg) and right ventricle systolic pressure (RVSP) was obtained by invasive right heart catheterization. Right ventricular hypertrophy was assessed by calculating right ventricle vs. left ventricle plus septum (RV/LV+S) and right ventricle vs. body weight (RV/BW). KLF4 transgenic (Tg) mice on the FVB background were previously described [28]. Male KLF4-Tg mice and their wild-type (WT) littermates were used for isometric tension measurement and each group contains 5–8 mice. Animals were euthanized by intraperitoneal injection with an overdose of pentobarbital sodium.
2.3. Isometric tension measurement
Arterial tension was measured using myograph as we previously reported [29]. Pulmonary arteries from KLF4-Tg or WT mice were dissected in cold Krebs solution containing (in mmol/L): 119.0 NaCl, 4.7 KCl, 2.5 CaCl2, 1.0 MgCl2, 25.0 NaHCO3, 1.2 KH2PO4, and 11.0 D-glucose. The arteries were cut into ring segments of 2 mm long and cultured in M199 containing 20% (v/v) FBS with or without GSNO for 12 h. The isometric force was measured by myograph system (Danish Myo Technology, Denmark). Each ring was suspended between 2 tungsten wires (diameter, 25 µm) in the chamber under optimal resting tension (1.5 mN as previously determined for the pulmonary arteries) and left for 60-min equilibration. The ACh-induced endothelium-dependent relaxations were detected after the precontraction induced by Phe (10 μmol/L). SNP-induced endothelium-independent relaxations were measured in the presence of L-NAME (100 μmol/L).
2.4. Plasmids, adenoviruses and transfection
pcDNA3.1-KLF4 was subcloned from pMT3-KLF4 [10]. Mutagenesis of Cys402 or Cys437 to Alanine were performed by using QuikChange® Lightning Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, CA, USA) and confirmed by sequencing. GST-KLF4 was constructed through subcloning of the mouse KLF4 coding region from pMT3-KLF4 into pGEX-5X−1 vector. Plasmids were transfected using Lipofectamine™2000. Adenoviruses encoding mouse KLF4 (Ad-KLF4) and tetracycline (Tc)-responsive transactivator (tTA) were described previously [10]. In light of the previous report that adenoviral vectors containing E4 gene promoted endothelial survival and Akt signaling [30], we used the tet-off system to minimize potential confounding due to the adenoviral infection. In this system, both control and KLF4-exppressing groups of cells were co-infected by Ad-KLF4 and Ad-tTA. Tc (0.1 μg/mL) was used to turn-off the expression of KLF4.
2.5. Detection of S-nitrosation by irreversible biotinylation procedure (IBP)
S-nitrosation of KLF4 was detected in cells or tissues by using irreversible biotinylation procedure (IBP). IBP was performed as previously described [31]. Briefly, cells and lung tissues were homogenized in HEN buffer (250 mM HEPES pH 7.7, 0.1 mM EDTA, 10 mM neocuproine) with 1% (v/v) Nonidet P-40 (NP-40), protease inhibitor cocktail and 20 mM MMTS. After centrifugation, 2.5% (w/v) SDS was added to the supernatant and incubated at 50 °C for 30 min to block free thiols. After ice-cold acetone precipitation and centrifugation, the pellet was recovered in HENS buffer (HEN buffer containing 2.5% (w/v) SDS) with 0.2 mM biotin-maleimide and 10 mM ascorbate and incubated at 37 °C for 1 h. Then, excess biotin-maleimide was removed by ice-cold acetone precipitation. The protein pellet was resuspended and boiled in HENS buffer containing 200 mM DTT for 15 min to reduce potential intermolecular disulfide bonds. After neutralization with triple volumes of HEN buffer with 150 mM NaCl, the biotinylated protein was purified with streptavidin-agarose and eluted with HENS buffer. Samples were analyzed by western blotting to detect S-nitrosated KLF4.
2.6. Expression and purification of recombinant KLF4 protein
Recombinant KLF4 was made by transforming BL21 cells with GST-KLF4 and induced by IPTG (0.5 mM, 16 °C O/N). Cells were harvested in Tris-buffered saline (TBS) containing DTT, PMSF and EDTA, and the supernatant was sonicated and incubated with Glutathione-Sepharose beads. The fusion protein was eluted from the beads with TBS buffer containing glutathione (GSH, 20 mM). The purified protein was desalted with TBS buffer containing EDTA (1 mM) and DTT (25 μM) to remove GSH and maintain the activity of the protein.
2.7. Quantitative reverse-transcription PCR (qRT-PCR)
RNA sample isolated from cells and lung tissues were reverse transcribed. Quantitative PCR was performed with specific primers (primer sequences listed in Table S1) in a Stratagene Mx3000P qPCR System (Agilent Technologies). β-actin was used as an internal control.
2.8. Western blotting
Proteins in cytoplasmic fraction were extracted using hypotonic lysis buffer (10 mM Tris–HCl, pH 7.5, 1.5 mM MgCl2, 10 mM KCl, 0.5% (v/v) NP-40). Nuclear proteins were extracted using a high-salt buffer (20 mM Tris–HCl, pH 7.5, 1.5 mM MgCl2, 420 mM NaCl, 10% (v/v) glycerol, 0.2 mM EGTA). Protein samples were then denatured, separated by 10% sodium dodecyl sulfate-polyacrylamide gels (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes. Western blotting was performed with KLF4 antibody and a horseradish peroxidase (HRP)-conjugated secondary antibody, followed with chemiluminescence detection.
2.9. Immunofluorescence
Cells were fixed with 4% (w/v) PFA, blocked with 5% (v/v) donkey serum containing 0.3% (v/v) Triton X-100, then incubated with the primary antibodies for KLF4 or S-nitroso-cysteine (SNO-Cys) at 4 °C overnight. Negative controls were performed with the primary antibodies replaced by isotype matched IgG. After incubation with Alexa Fluor-conjugated secondary antibodies at 37 °C for 1 h, cells were counterstained with 4,6-diamidino-2-phenylindole (DAPI). Digital images were acquired using Leica TCS SP8 confocal laser scanning microscope.
2.10. Mass spectrometry determination of S-nitrosated sites
S-nitrosation of the recombinant KLF4 was detected using liquid chromatography with tandem mass spectrometry (LC-MS/MS) analysis. Purified GST-KLF4 protein (200 μg) was incubated with GSNO (0.5 mM) for 30 min at room temperature. The free thiols were blocked by MMTS and S-nitrosated proteins were labeled by biotin-maleimide as described in IBP. Biotinylated proteins were trypsinized (using a 1:50 ratio of protein: trypsin) and desalted by using a C18 ZipTip (Millipore, Billerica, MA, USA), followed by drying in a speed vac. For liquid chromatography with tandem mass spectrometry (LC-MS/MS) analysis, peptides were separated using a NanoLC 1D Plus system. Peptide cations ionization were performed with nanospray and analyzed at LTQ-Orbitrap XL mass spectrometer. Dynamic exclusion for selected precursor ions was set at 90 s. MS/MS spectra were searched against UniProt mouse complete proteome database (Release 2016_01) using Sequest with the following parameters: precursor mass tolerance 10 ppm, MS/MS mass tolerance 0.6 Da, two missed cleavage for trypsinized peptides, variable modifications oxidation (M, +15.9949 Da), carbamidomethyl (C, +57.021 Da), biotin-maleimide (B, +451.1889 Da) as previously described [32]. The results were filtered for a 1% FDR at the PSM level utilizing the percolator-based algorithm.
2.11. Statistical analysis
Quantitative results were expressed as the mean ± SEM. To determine the statistical significance between two groups, an unpaired Student's t-test was performed. One-way ANOVA with post-hoc tests was performed for multiple-group analysis. The vascular reactivity in pulmonary arteries was analyzed by repeated-measures two-way ANOVA with post-hoc tests. p < 0.05 was considered statistically significant. All data were analyzed by using Prism 6.0 (GraphPad Software).
3. Results
3.1. KLF4 was modulated by S-nitrosation
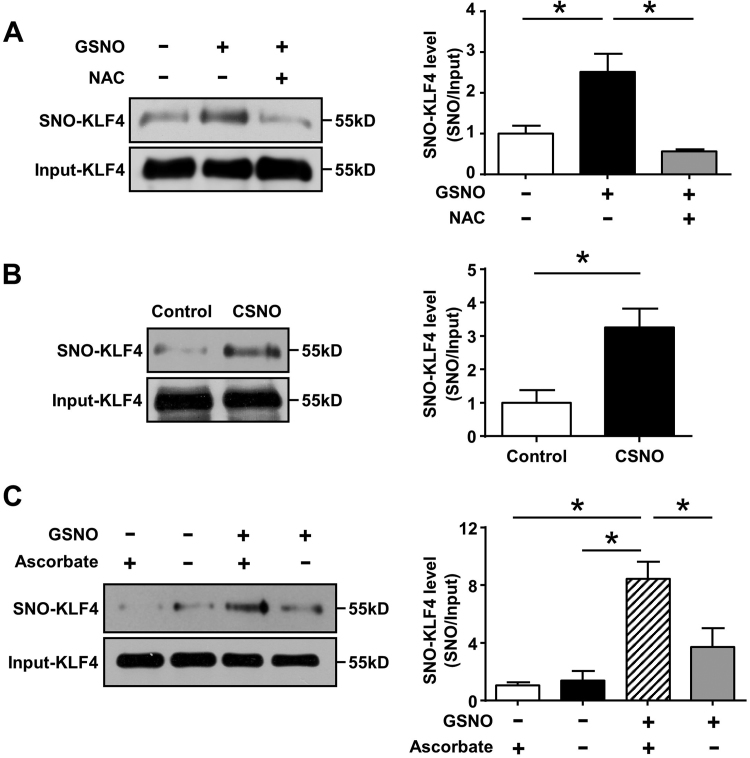
To examine whether KLF4 protein can be modified by S-nitrosation, human umbilical vein ECs (HUVECs) were exposed to S-nitrosoglutathione (GSNO, 0.5 mmol/L, 4 h). These samples were then subjected to the irreversible biotinylation procedure (IBP) detection, western blotting showed marked S-nitrosation of KLF4, which was abolished by pretreatment with a reductant N-acetylcysteine (NAC) (Fig. 1A). In addition, we confirmed the capacity of S-nitrosocysteine (CSNO) to induce the S-nitrosation of KLF4 (Fig. 1B). The specificity of detection of S-NO bond by the IBP was established in the presence of ascorbate which facilitated S-nitrosated cysteines to be biotinylated and detected (Fig. 1C). These results suggested that KLF4 could undergo S-nitrosation.
Fig. 1.
S-nitrosation of KLF4 in ECs. (A) After infection with Ad-KLF4 for 24 h, HUVECs were exposed to GSNO (0.5 mmol/L, 4 h) with or without NAC pretreatment (10 mmol/L, 3 h). Cell lysates were subjected to IBP. *p < 0.05, by one-way ANOVA. n = 5. (B) Similarly, cells were exposed to CSNO (0.5 mmol/L, 1 h) and subjected to IBP. *p < 0.05, by unpaired Student's t-test. n = 3. (C) S-nitrosated KLF4 was diminished in the absence of ascorbate. *p < 0.05, by one-way ANOVA. n = 3.
3.2. S-nitrosation mitigated KLF4 transcriptional activity
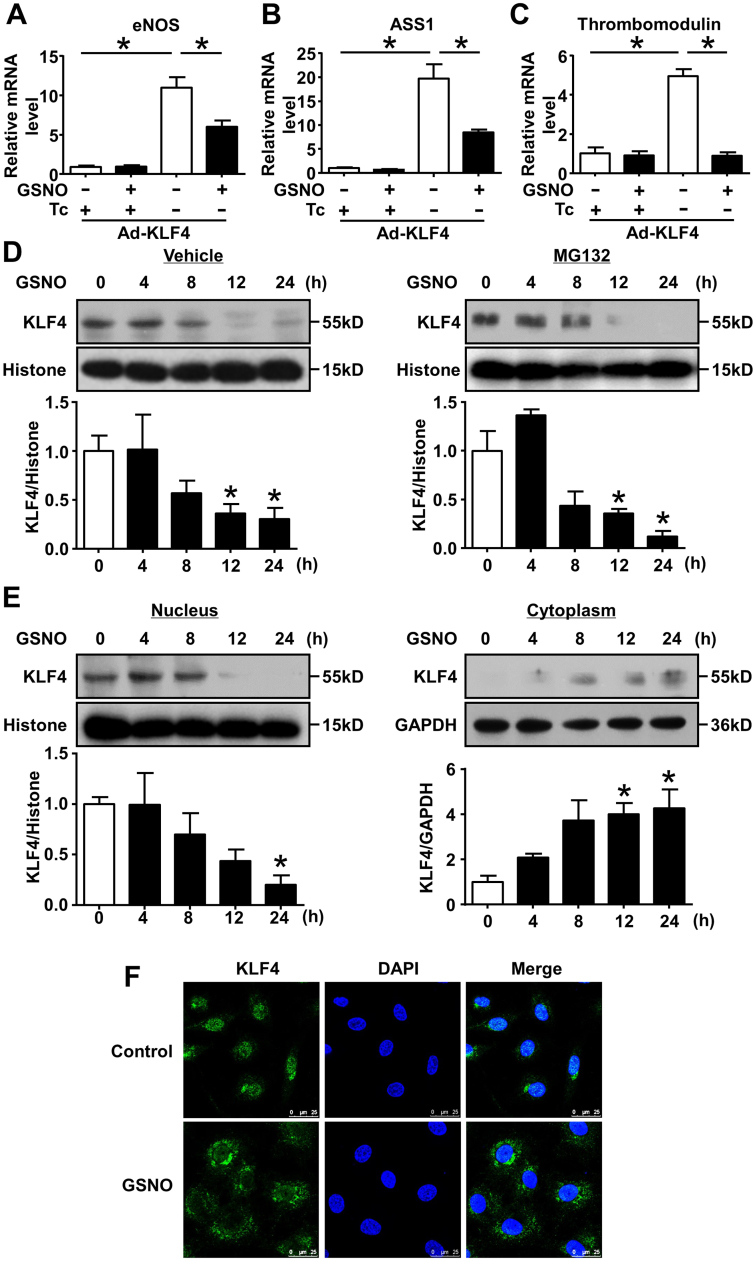
S-nitrosation of a transcription factor often affects its transcriptional activity [32], [33], [34]. To investigate the effect of S-nitrosation on KLF4, ECs were infected with adenovirus expressing KLF4 and exposed to GSNO. In Fig. 2A-C, qRT-PCR showed that overexpression of KLF4 induced the expression of its known target genes eNOS, argininosuccinate synthetase1 (ASS1) and thrombomodulin. However, the inductions of these genes were significantly decreased by GSNO treatment, suggesting that S-nitrosation mitigated the transcriptional activity of KLF4 on its target genes.
Fig. 2.
Suppression of KLF4 transcriptional activity and nuclear localization by GSNO. (A-C) ECs were infected with Ad-KLF4 in the presence or absence of tetracycline (Tc, 0.1 μg/mL) for 24 h and then exposed to GSNO. The mRNA levels of KLF4 target genes (eNOS, ASS1 and thrombomodulin) were analyzed by qRT-PCR. *p < 0.05, by one-way ANOVA. n = 4–6. (D) After pretreatment with or without MG132 (1 μmol/L, 1 h), HUVECs were exposed to GSNO for indicated times. Nuclear proteins were extracted and analyzed by western blotting. *p < 0.05 vs. 0 h, by one-way ANOVA. n = 3. (E) Immunoblots of nuclear and cytoplasmic fractions from HUVECs after treatment with GSNO (0.5 mmol/L) for indicated times. *p < 0.05 vs. 0 h, by one-way ANOVA. n = 3. (F) Immunofluorescence of HUVECs stimulated with GSNO (0.5 mmol/L, 24 h) depicting the subcellular distribution of KLF4. Images displayed KLF4 in green and DAPI in blue. Rabbit IgG was used as negative control. n = 3. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
3.3. S-nitrosation decreased nuclear localization of KLF4
To investigate the mechanism of decreased transcriptional activity of KLF4, we found that the protein of endogenous KLF4 in the nucleus was decreased under stimulation of GSNO (Fig. 2D), while the KLF4 mRNA level did not change significantly (Fig. S1). We then examined whether the decreased protein level of KLF4 was associated with the proteasome-mediated degradation [17]. However, inhibition of proteasome with MG132 did not prevent KLF4 from the GSNO-triggered decrease (Fig. 2D).
Next, we examined the subcellular localization of KLF4. Western blotting showed that, after the exposure to GSNO, KLF4 protein levels decreased in nuclear fractions with a simultaneous increase in the cytosol (Fig. 2E). Further, immunofluorescence staining indicated that a large portion of KLF4 was re-distributed in cytosol 24 h after GSNO treatment (Fig. 2F). The results shown in Fig. 2E strengthened the immunofluorescence finding that GSNO led to a cytosolic distribution of KLF4. Taken together, these findings suggested that S-nitrosation of KLF4 impaired its nuclear localization.
3.4. Identification of S-nitrosated cysteine residues of KLF4
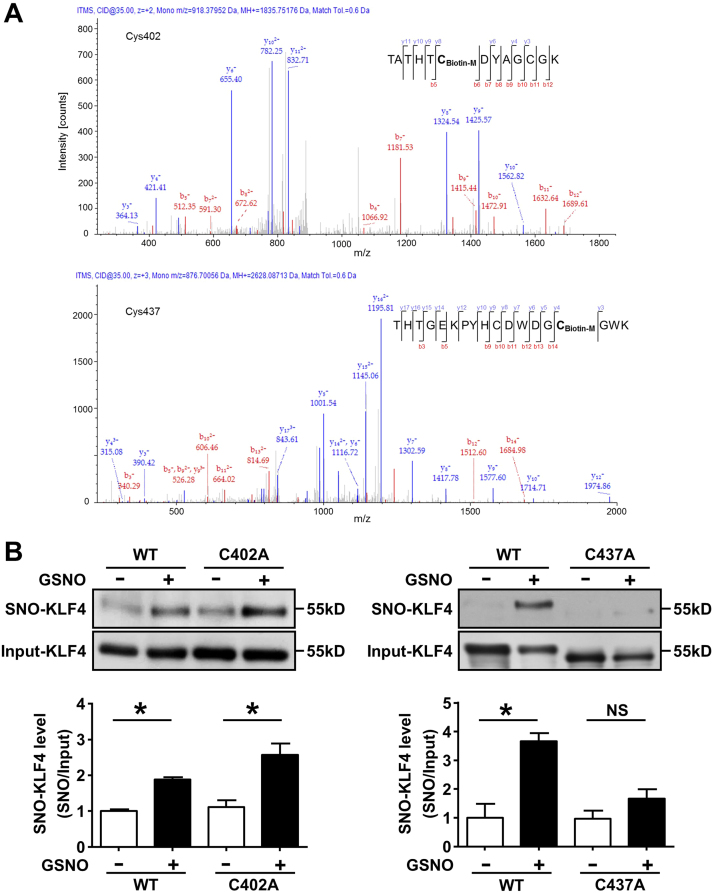
We performed LC-MS/MS to identify the potential cysteine residues undergoing S-nitrosation. Recombinant KLF4 protein was exposed to GSNO and the S-nitrosated cysteines were labeled with biotin-maleimide. As shown in Fig. 3A, cysteine residues at 402 and 437 were found to be S-nitrosated respectively. To further confirm the sites of S-nitrosation in vivo, we replaced the two corresponding cysteines in KLF4 with alanines (C402A, C437A) by using site-directed mutagenesis. The expression plasmids of the wildtype or mutated KLF4 were transfected into HEK293 cells and treated with GSNO. We found that KLF4 mutation at C437A but not at C402A abolished GSNO-induced S-nitrosation (Fig. 3B). It was suggested that S-nitrosation modified KLF4 predominantly at Cys437.
Fig. 3.
LC-MS/MS identification of S-nitrosated cysteine residues in KLF4. (A) S-nitrosation of GST-KLF4 was detected by using LC-MS/MS. Sequence-informative fragmentation ions were summarized on the peptide sequence and annotated in red (b-ions) and blue (y-ions). (B) HEK293 cells were transfected with the KLF4 mutants (C402A, C437A) and, 24 h later, treated with GSNO. S-nitrosated KLF4 was analyzed by using IBP followed by western blotting. *p < 0.05, by one-way ANOVA. n = 3. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
3.5. S-nitrosation of KLF4 impaired vasorelaxation
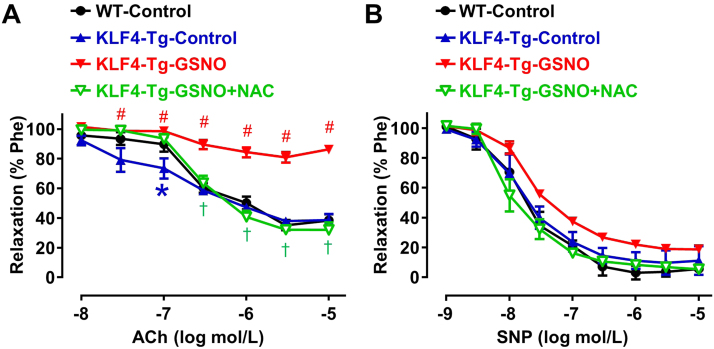
Emerging evidence show that KLF4 has protective roles in vascular functions and its dysregulation is potentially implicated in the pathogenesis of PAH [4], [8]. We interrogated whether S-nitrosation impaired the protective role of KLF4 in pulmonary arteries. Pulmonary arteries isolated from KLF4 transgenic (KLF4-Tg) and wild-type (WT) mice were used to measure the isometric tension. Pulmonary arteries from KLF4-Tg mice had improved relaxation response to acetylcholine (ACh). However, pretreatment of the arteries with GSNO nearly abrogated the relaxation. Importantly, such a detrimental effect of GSNO was largely absent in the NAC pre-incubated arteries (Fig. 4A). On the other hand, pulmonary arteries from wildtype and KLF4 Tg mice had similar responses in sodium nitroprusside (SNP)-induced relaxations. In addition, the SNP-induced endothelium-independent response was also barely affected by GSNO (Fig. 4B). The results indicated that endothelial KLF4-dependent vasodilation in pulmonary arteries was impaired under nitrosative stress.
Fig. 4.
Impairment of KLF4-mediated vasodilation by GSNO. Pulmonary artery rings were isolated from WT and KLF4-Tg mice, with or without NAC (10 mmol/L, 3 h) pretreatment, were exposed to GSNO (0.5 mmol/L, 12 h). (A) ACh induced vasodilatory responses; (B) SNP induced endothelium-independent dilation. *p < 0.05 vs. WT-Control group, #p < 0.05 vs. KLF4-Tg-Control group, †p < 0.05 vs. KLF4-Tg-GSNO group, by two-way repeated measures ANOVA followed by post-hoc tests. n = 5–8.
3.6. Endothelin-1 stimulated S-nitrosation of KLF4 in ECs
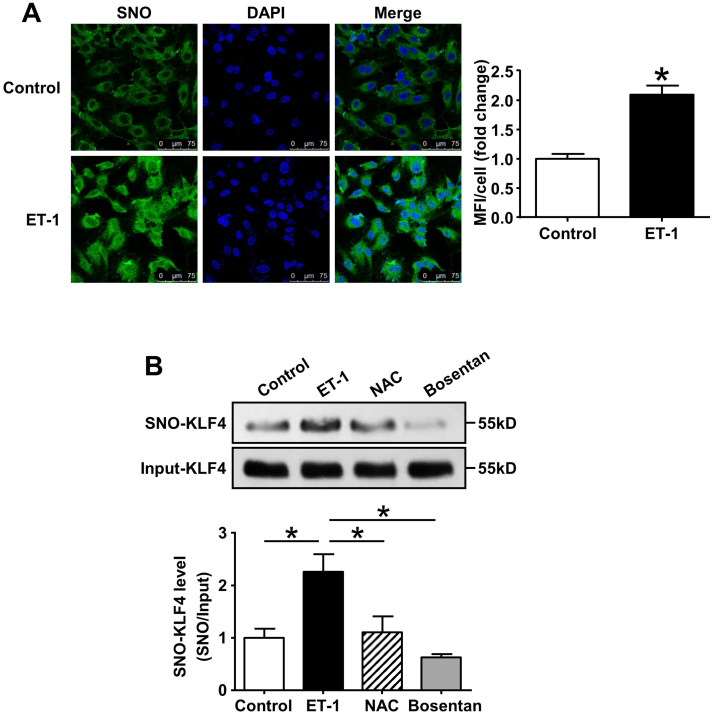
Endothelial dysfunction is the major player in the progression of PAH with increased production of vasoconstrictors. Endothelin-1 (ET-1), a key mediator in PAH pathogenesis, is elevated in plasma and lung tissues of the PAH patients, as well as animal models [35]. Immunofluorescence staining using an antibody against S-nitrosocysteine (SNO) showed that the exposure to ET-1 (0.1 μmol/L, 1 h) increased SNO-protein levels in ECs (Fig. 5A). Most importantly, S-nitrosation of KLF4 was significantly increased in ECs induced by ET-1 and diminished in the presence of endothelin receptor antagonist Bosentan, which is widely used in the treatment of PAH (Fig. 5B).
Fig. 5.
S-nitrosation of KLF4 in ET-1 stimulated ECs. (A) HUVECs were treated with ET-1 (0.1 μmol/L, 1 h). Immunofluorescence was done with antibody against S-nitrosocysteine (SNO). Nuclei were counterstained with DAPI. Rabbit IgG was used as negative control. *p < 0.05, by unpaired Student's t-test, n = 3. MFI, mean fluorescence intensity. (B) Cells were exposed to ET-1 (0.1 μmol/L, 1 h) with or without pretreatment with NAC (10 mmol/L, 3 h) or Bosentan (10 μmol/L, 1 h). Cell lysate was subjected to IBP to detect S-nitrosation of KLF4. *p < 0.05, by one-way ANOVA. n = 3.
3.7. KLF4 S-nitrosation was increased in rat model with PAH
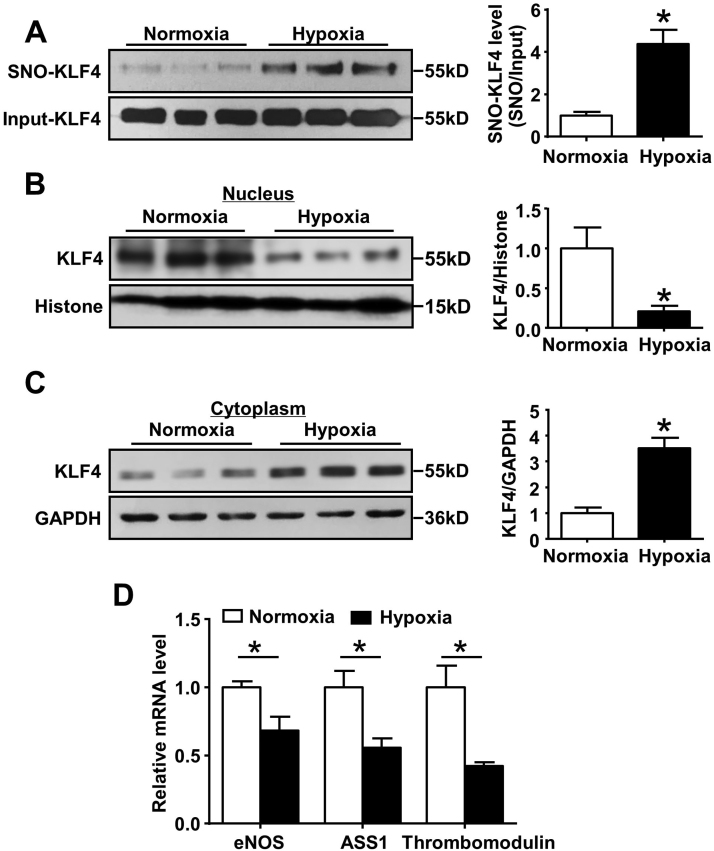
Recent studies indicated a pathological role of aberrant level of SNOs in the development of PAH [36]. To assess the level of KLF4 S-nitrosation in PAH, we used a hypoxia-induced rat model of PAH. Hypoxia significantly increased right ventricle systolic pressure (RVSP, 48.8 mmHg ± 1.77) compared with normoxia group (30.7 mmHg ± 0.70, n = 9, *p < 0.05) (Fig. S2A), and resulted in right ventricle (RV) hypertrophy, as illustrated by increased ratios of right ventricle vs. body weight (RV/BW) and right ventricle vs. left ventricle plus septum (RV/LV+S) (Fig. S2B). We observed a significant increase of SNO-KLF4 in lung tissues after hypoxia treatment (Fig. 6A). Moreover, we also found the reduced nuclear localization of KLF4 in lung tissues of PAH rats compared to normoxia group (Fig. 6B and C). Consistently, the mRNA levels of eNOS, ASS1 and thrombomodulin genes were also decreased in lung tissues of PAH rats (Fig. 6D).
Fig. 6.
S-nitrosation of KLF4 in lung tissues from PAH rats. (A) S-nitrosated KLF4 in the lung tissues from control and PAH rats. *p < 0.05, by unpaired Student's t-test. n = 6. (B and C) KLF4 protein levels in nuclear and cytoplasmic fractions from lung tissues of rats. *p < 0.05, by unpaired Student's t-test. n = 6. (D) Relative mRNA levels of KLF4 target genes in lung tissues of rats. *p < 0.05, by unpaired Student's t-test. n = 9–10.
4. Discussion
This study revealed a novel mechanism by which the KLF4 protein was post-translationally modified via S-nitrosation under nitrosative stress. Such a modification resulted in decreased nuclear location and transcriptional activity of KLF4 in ECs and impaired the vascular protective function of KLF4. Interestingly, as an endogenous pathological stimulus in PAH, ET-1 could induce KLF4 S-nitrosation in ECs, which was attenuated by Bosentan. We also indicated that KLF4 S-nitrosation was increased in the lung tissues of animal models, suggesting that this modification may be implicated in the development of vascular dysfunction and progression of PAH.
Protein S-nitrosation has been regarded as a redox-based modification of cysteine residues to covey biological influence of NO and its derivatives as reactive radicals. Several transcription factors including NF-κB, HIF-1α, c-Myb, PPARγ and ERα are known to be post-translationally regulated via S-nitrosation [32], [33], [34], [37], [38]. Here, we demonstrated that KLF4 was S-nitrosated under GSNO and CSNO condition, resulting in an inhibition of its transcriptional activity (Fig. 1 and Fig. 2). Recent studies indicated that KLF4 was a vascular protective factor. KLF4 attenuated NF-κB activity and inhibited the expression of vascular cell adhesion molecule-1 (VCAM-1) and plasminogen activator inhibitor-1 (PAI-1); KLF4 also induces the gene expression of eNOS and thrombomodulin to maintain endothelium under an anti-adhesive and anti-thrombotic state [4]. In contrast, endothelium-specific deficiency of KLF4 showed higher atherosclerotic burden with increased inflammatory cell infiltration [39]. Our previous work showed that the expression of KLF4 was decreased during vascular injury while overexpression of KLF4 prevented neointimal formation in the balloon-injured arteries [10]. Yoshida et al. indicated that loss of KLF4 in endothelial cells augmented the proliferation rate, the accumulation of macrophages, which rendered the animals susceptible to injury-induced neointimal formation [40]. In addition, loss of endothelial KLF4 exacerbated mouse PAH induced by chronic hypoxia [8]. In this study, S-nitrosation of KLF4 leads to decreased expression of its target genes eNOS, ASS1 and thrombomodulin (Fig. 2), which might impair the protective effects of KLF4. By using wire myograph, we observed that overexpression of KLF4 improved pulmonary artery vasorelaxation, which was impaired after GSNO treatment (Fig. 4). However, GSNO treatment still impaired the vasorelaxation in response to higher concentrations of ACh. Such a result may indicate broader effects of GSNO. Previous studies showed that GSNO caused S-nitrosation and ensuing inhibition of eNOS itself [41] and soluble guanylyl cyclase, the NO receptor [42]. These results may help interpreting the additional action of GSNO. We also examined the effect of GSNO on the vasodilation of pulmonary arteries from wild-type mice. As shown in Fig. S5, GSNO also impaired arterial relaxation in WT-Control mice. This observation is probably due to S-nitrosation of endogenous KLF4 and other vasodilatory molecules such as eNOS and sGC [41], [42].
Multiple post-translational modifications of KLF4 expanded its functional diversity required for essential roles of KLF4 in physiology and diseases. Ubiquitination induced destabilization of KLF4 through proteasome degradation pathway [17]. Acetylation enhanced KLF4 transcriptional activity [18]. Phosphorylation of KLF4 by ERK1/2 inhibited the capacity of KLF4 to regulate transcription [15], and initiate the interaction of KLF4 with nuclear export factor XPO1, leading to nuclear export of KLF4 [43]. Here, we found that S-nitrosation inhibited KLF4 transactivity and diminished its nuclear localization (Fig. 2). In mouse KLF4, Cys437 is contained within the second nuclear localization sequence (NLS), a region that sufficiently facilitates the transport of KLF4 into the nucleus [44]. It is thus conceivable that SNO-modification of this residue might have prevented KLF4 from entering the nucleus. Besides, it is also possible that S-nitrosation increased the nuclear export of KLF4 as well. Because Cys437 residue is also located in the zinc finger DNA binding domains [45], it has been proposed that S-nitrosation at this site may result in zinc release, leading to conformational change and disruption of the interaction with DNA [46]. We compared the expression levels of the KLF4 target genes between the ECs transfected with wildtype or mutated (C437A) KLF4. The results showed that, unlike the suppressive effects of GSNO on the target genes induction by wildtype KLF4, GSNO failed to suppress the target genes induction by mutant KLF4 in which the cysteine at 437 was replaced with alanine (Fig. S3A-F). This data did corroborate the notion that S-nitrosation at C437 may contribute to the decreased transcriptional capacity of KLF4. We also tried to examine whether the mutant block KLF4 nuclear translocation. However, the mutant KLF4 protein under basal condition appeared to have more cytosolic retention. This result was beyond our anticipation. We suspect that disrupting C437, a residue within the zinc finger domain and the nuclear localizing signal, may also affect the regular trafficking of this protein. Nevertheless, GSNO exposure reduced protein levels of wildtype but not the mutant KLF4 in nuclear fraction, it providing a fundamental support to the role of S-nitrosation at C437 in KLF4 function (Fig. S3G, H). Analysis of the protein sequence of KLF4 revealed conserved cysteine residues within zinc finger motif among human, rat, and mouse [13]. Thus, it is indicated that S-nitrosation is an evolutionarily conserved mechanism by which activity of KLF4 is suppressed.
The S-nitrosation-based modification of KLF4 is potentially important as a physiological mechanism. Firstly, such a SNO-based regulation put KLF4 under a tight check to accommodate the redox state in micro-environment. Particularly, this modification may orchestrate the activity of endothelial KLF4 with the bioavailability of NO at the interface between the circulation and vessel wall. KLF4 transcriptionally induce the expression of eNOS and increases NO release. Reciprocally, accumulated NO can S-nitrosate KLF4 and decrease its DNA binding capacity and the nuclear localization. Given that S-nitrosation is a reversible process, the SNO-signaling may represent a negative feedback loop to dynamically regulate KLF4 function. However, nitrosative stress resulting from dysregulated production of reactive nitrogen and oxygen species may trigger excessive S-nitrosation and render it into an irreversible modification [22].
Oxidative and nitrosative stress is implicated in endothelial dysfunction during the development of PAH [36] and may explain our findings that KLF4 is over-nitrosated in the lung tissues of chronic hypoxia-induced rat PAH (Fig. 6). This is consistent with the notion that SNO modification is hypoxia-dependent as with ryanodine receptor in skeletal muscle and hemoglobin in pulmonary circulation [23], [47]. In ECs, SNO-KLF4 was significantly increased by ET-1, a potent vasoconstrictor elevated in PAH [48]. In fact, ET-1 could trigger nitrosative stress in ECs (Fig. 5). As we showed that KLF4 improved the endothelium-dependent relaxation in mouse pulmonary arteries and this effect was compromised after GSNO exposure (Fig. 4), it is likely that S-nitrosation of KLF4 may play a role in the detrimental vascular effects of ET-1. Conversely, the clinically used ET-1 receptor antagonist Bosentan effectively prevented KLF4 from the ET-1 induced S-nitrosation (Fig. 5), corroborating a link between the SNO-KLF4 and endothelial dysfunction in PAH. Nevertheless, functional roles of this new PTM mode of KLF4 in physiological processes and diseases warrant further investigation.
Sources of funding
This work was supported by grants from the National Science Foundation of China (81830015, 31430045, 81470373, 81670408, 31570857 and 91849203), the National Key Research and Development Program of China (2017YFA0504000).
Declarations of interest
None.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2019.101099.
Contributor Information
Chang Chen, Email: changchen@moon.ibp.ac.cn.
Nanping Wang, Email: npwang@dmu.edu.cn.
Appendix A. Supplementary material
Supplementary material
References
- 1.Chen X., Whitney E.M., Gao S.Y., Yang V.W. Transcriptional profiling of Krüppel-like factor 4 reveals a function in cell cycle regulation and epithelial differentiation. J. Mol. Biol. 2003;326(3):665–677. doi: 10.1016/S0022-2836(02)01449-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fan Y., Lu H., Liang W., Hu W., Zhang J., Chen Y.E. Overexpression of Krüppel-like factor 4 in the human colon cancer cell line RKO leads to reduced tumorigenecity. J. Mol. Cell Biol. 2017;9(5):352–363. [Google Scholar]
- 3.Shields J.M., Christy R.J., Yang V.W. Identification and characterization of a gene encoding a gut-enriched Krüppel-like factor expressed during growth arrest. J. Biol. Chem. 1996;271(33):20009–20017. doi: 10.1074/jbc.271.33.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamik A., Lin Z., Kumar A., Balcells M., Sinha S., Katz J., Feinberg M.W., Gerzsten R.E., Edelman E.R., Jain M.K. Kruppel-like factor 4 regulates endothelial inflammation. J. Biol. Chem. 2007;282(18):13769–13779. doi: 10.1074/jbc.M700078200. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida T., Kaestner K.H., Owens G.K. Conditional deletion of Kruppel-like factor 4 delays downregulation of smooth muscle cell differentiation markers but accelerates neointimal formation following vascular injury. Circ. Res. 2008;102(12):1548–1557. doi: 10.1161/CIRCRESAHA.108.176974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou G., Hamik A., Nayak L., Tian H., Shi H., Lu Y., Sharma N., Liao X., Hale A., Boerboom L., Feaver R.E., Gao H., Desai A., Schmaier A., Gerson S.L., Wang Y., Atkins G.B., Blackman B.R., Simon D.I., Jain M.K. Endothelial Kruppel-like factor 4 protects against atherothrombosis in mice. J. Clin. Invest. 2012;122(12):4727–4731. doi: 10.1172/JCI66056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salmon M., Johnston W.F., Woo A., Pope N.H., Su G., Upchurch G.R.J., Owens G.K., Ailawadi G. KLF4 regulates abdominal aortic aneurysm morphology and deletion attenuates aneurysm formation. Circulation. 2013;128(11 Suppl. 1):S163–S174. doi: 10.1161/CIRCULATIONAHA.112.000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shatat M.A., Tian H., Zhang R., Tandon G., Hale A., Fritz J.S., Zhou G., Martínez-González J., Rodríguez C., Champion H.C., Jain M.K., Hamik A. Endothelial Kruppel-like factor 4 modulates pulmonary arterial hypertension. Am. J. Respir. Cell Mol. Biol. 2014;50(3):647–653. doi: 10.1165/rcmb.2013-0135OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCormick S.M., Eskin S.G., McIntire L.V., Teng C.L., Lu C.M., Russell C.G., Chittur K.K. DNA microarray reveals changes in gene expression of shear stressed human umbilical vein endothelial cells. Proc. Natl. Acad. Sci. USA. 2001;98(16):8955–8960. doi: 10.1073/pnas.171259298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y., Zhao B., Zhang Y., Tang Z., Shen Q., Zhang Y., Zhang W., Du J., Chien S., Wang N. Kruppel-like factor 4 is induced by rapamycin and mediates the anti-proliferative effect of rapamycin in rat carotid arteries after balloon injury. Br. J. Pharmacol. 2012;165(7):2378–2388. doi: 10.1111/j.1476-5381.2011.01734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park C.S., Shen Y., Lewis A., Lacorazza H.D. Role of the reprogramming factor KLF4 in blood formation. J. Leukoc. Biol. 2016;99(5):673–685. doi: 10.1189/jlb.1RU1215-539R. [DOI] [PubMed] [Google Scholar]
- 12.Villarreal G.J., Zhang Y., Larman H.B., Gracia-Sancho J., Koo A., García-Cardeña G. Defining the regulation of KLF4 expression and its downstream transcriptional targets in vascular endothelial cells. Biochem Biophys. Res. Commun. 2010;391(1):984–989. doi: 10.1016/j.bbrc.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghaleb A.M., Yang V.W. Kruppel-like factor 4 (KLF4): what we currently know. Gene. 2017;611:27–37. doi: 10.1016/j.gene.2017.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H.X., Han M., Bernier M., Zheng B., Sun S.G., Su M., Zhang R., Fu J.R., Wen J.K. Kruppel-like factor 4 promotes differentiation by transforming growth factor-beta receptor-mediated Smad and p38 MAPK signaling in vascular smooth muscle cells. J. Biol. Chem. 2010;285(23):17846–17856. doi: 10.1074/jbc.M109.076992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim M.O., Kim S.H., Cho Y.Y., Nadas J., Jeong C.H., Yao K., Kim D.J., Yu D.H., Keum Y.S., Lee K.Y., Huang Z., Bode A.M., Dong Z. ERK1 and ERK2 regulate embryonic stem cell self-renewal through phosphorylation of Klf4. Nat. Struct. Mol. Biol. 2012;19(3):283–290. doi: 10.1038/nsmb.2217. [DOI] [PubMed] [Google Scholar]
- 16.Lim K.H., Kim S.R., Ramakrishna S., Baek K.H. Critical lysine residues of Klf4 required for protein stabilization and degradation. Biochem. Biophys. Res. Commun. 2014;443(4):1206–1210. doi: 10.1016/j.bbrc.2013.12.121. [DOI] [PubMed] [Google Scholar]
- 17.Chen Z.Y., Wang X., Zhou Y., Offner G., Tseng C.C. Destabilization of Kruppel-like factor 4 protein in response to serum stimulation involves the ubiquitin-proteasome pathway. Cancer Res. 2005;65(22):10394–10400. doi: 10.1158/0008-5472.CAN-05-2059. [DOI] [PubMed] [Google Scholar]
- 18.Evans P.M., Zhang W., Chen X., Yang J., Bhakat K.K., Liu C. Kruppel-like factor 4 is acetylated by p300 and regulates gene transcription via modulation of histone acetylation. J. Biol. Chem. 2007;282(47):33994–34002. doi: 10.1074/jbc.M701847200. [DOI] [PubMed] [Google Scholar]
- 19.Tahmasebi S., Ghorbani M., Savage P., Yan K., Gocevski G., Xiao L., You L., Yang X.J. Sumoylation of Kruppel-like factor 4 inhibits pluripotency induction but promotes adipocyte differentiation. J. Biol. Chem. 2013;288(18):12791–12804. doi: 10.1074/jbc.M113.465443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu D., Gur M., Zhou Z., Gamper A., Hung M.C., Fujita N., Lan L., Bahar I., Wan Y. Interplay between arginine methylation and ubiquitylation regulates KLF4-mediated genome stability and carcinogenesis. Nat. Commun. 2015;6:8419. doi: 10.1038/ncomms9419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.JS S. Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell. 1994;78(6):931–936. doi: 10.1016/0092-8674(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 22.Hess D.T., Matsumoto A., Kim S.O., Marshall H.E., Stamler J.S. Protein S-nitrosylation: purview and parameters. Nat. Rev. Mol. Cell Biol. 2005;6(2):150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez D.R., Beigi F., Treuer A.V., Hare J.M. Deficient ryanodine receptor S-nitrosylation increases sarcoplasmic reticulum calcium leak and arrhythmogenesis in cardiomyocytes. Proc. Natl. Acad. Sci. USA. 2007;104(51):20612–20617. doi: 10.1073/pnas.0706796104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foster M.W., McMahon T.J., Stamler J.S. S-nitrosylation in health and disease. Trends Mol. Med. 2003;9(4):160–168. doi: 10.1016/s1471-4914(03)00028-5. [DOI] [PubMed] [Google Scholar]
- 25.Gaston B.M., Carver J., Doctor A., Palmer L.A. S-nitrosylation signaling in cell biology. Mol. Interv. 2003;3(5):253–263. doi: 10.1124/mi.3.5.253. [DOI] [PubMed] [Google Scholar]
- 26.Wang X., Fang X., Zhou J., Chen Z., Zhao B., Xiao L., Liu A., Li Y.S., Shyy J.Y., Guan Y., Chien S., Wang N. Shear stress activation of nuclear receptor PXR in endothelial detoxification. Proc. Natl. Acad. Sci. USA. 2013;110(32):13174–13179. doi: 10.1073/pnas.1312065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei H.L., Zhang C.Y., Jin H.F., Tang C.S., Du J.B. Hydrogen sulfide regulates lung tissue-oxidized glutathione and total antioxidant capacity in hypoxic pulmonary hypertensive rats. Acta Pharmacol. Sin. 2008;29(6):670–679. doi: 10.1111/j.1745-7254.2008.00796.x. [DOI] [PubMed] [Google Scholar]
- 28.Lin L., Han Q., Xiong Y., Li T., Liu Z., Xu H., Wu Y., Wang N., Liu X. Krupple-like-factor 4 attenuates lung fibrosis via inhibiting epithelial-mesenchymal transition. Sci. Rep. 2017;7(1):15847. doi: 10.1038/s41598-017-14602-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y., Tian X.Y., Mao G., Fang X., Fung M.L., Shyy J.Y., Huang Y., Wang N. Peroxisome proliferator-activated receptor-gamma ameliorates pulmonary arterial hypertension by inhibiting 5-hydroxytryptamine 2B receptor. Hypertension. 2012;60(6):1471–1478. doi: 10.1161/HYPERTENSIONAHA.112.198887. [DOI] [PubMed] [Google Scholar]
- 30.Zhang F., Cheng J., Hackett N.R., Lam G., Shido K., Pergolizzi R., Jin D.K., Crystal R.G., Rafii S. Adenovirus E4 gene promotes selective endothelial cell survival and angiogenesis via activation of the vascular endothelial-cadherin/Akt signaling pathway. J. Biol. Chem. 2004;279(12):11760–11766. doi: 10.1074/jbc.M312221200. [DOI] [PubMed] [Google Scholar]
- 31.Huang B., Chen C. Detection of protein S-nitrosation using irreversible biotinylation procedures (IBP) Free Radic. Biol. Med. 2010;49(3):447–456. doi: 10.1016/j.freeradbiomed.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Yin R., Fang L., Li Y., Xue P., Li Y., Guan Y., Chang Y., Chen C., Wang N. Pro-inflammatory Macrophages suppress PPARgamma activity in Adipocytes via S-nitrosylation. Free Radic. Biol. Med. 2015;89:895–905. doi: 10.1016/j.freeradbiomed.2015.10.406. [DOI] [PubMed] [Google Scholar]
- 33.Kelleher Z.T., Matsumoto A., Stamler J.S., Marshall H.E. NOS2 regulation of NF-kappaB by S-nitrosylation of p65. J. Biol. Chem. 2007;282(42):30667–30672. doi: 10.1074/jbc.M705929200. [DOI] [PubMed] [Google Scholar]
- 34.Garbán H.J., Márquez-Garbán D.C., Pietras R.J., Ignarro L.J. Rapid nitric oxide-mediated S-nitrosylation of estrogen receptor: regulation of estrogen-dependent gene transcription. Proc. Natl. Acad. Sci. USA. 2005;102(7):2632–2636. doi: 10.1073/pnas.0409854102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chester A.H., Yacoub M.H. The role of endothelin-1 in pulmonary arterial hypertension. Glob. Cardiol. Sci. Pract. 2014;2014(2):62–78. doi: 10.5339/gcsp.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tabima D.M., Frizzell S., Gladwin M.T. Reactive oxygen and nitrogen species in pulmonary hypertension. Free Radic. Biol. Med. 2012;52(9):1970–1986. doi: 10.1016/j.freeradbiomed.2012.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brendeford E.M., Andersson K.B., Gabrielsen O.S. Nitric oxide (NO) disrupts specific DNA binding of the transcription factor c-Myb in vitro. FEBS Lett. 1998;425(1):52–56. doi: 10.1016/s0014-5793(98)00196-3. [DOI] [PubMed] [Google Scholar]
- 38.Li F., Sonveaux P., Rabbani Z.N., Liu S., Yan B., Huang Q., Vujaskovic Z., Dewhirst M.W., Li C.Y. Regulation of HIF-1alpha stability through S-nitrosylation. Mol. Cell. 2007;26(1):63–74. doi: 10.1016/j.molcel.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou G., Hamik A., Nayak L., Tian H., Shi H., Lu Y., Sharma N., Liao X., Hale A., Boerboom L., Feaver R.E., Gao H., Desai A., Schmaier A., Gerson S.L., Wang Y., Atkins G.B., Blackman B.R., Simon D.I., Jain M.K. Endothelial Kruppel-like factor 4 protects against atherothrombosis in mice. J. Clin. Invest. 2012;122(12):4727–4731. doi: 10.1172/JCI66056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshida T., Yamashita M., Horimai C., Hayashi M. Deletion of Kruppel-like factor 4 in endothelial and hematopoietic cells enhances neointimal formation following vascular injury. J. Am. Heart Assoc. 2014;3(1):e000622. doi: 10.1161/JAHA.113.000622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ravi K., Brennan L.A., Levic S., Ross P.A., Black S.M. S-nitrosylation of endothelial nitric oxide synthase is associated with monomerization and decreased enzyme activity. Proc. Natl. Acad. Sci. USA. 2004;101(8):2619–2624. doi: 10.1073/pnas.0300464101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sayed N., Baskaran P., Ma X., van den Akker F., Beuve A. Desensitization of soluble guanylyl cyclase, the NO receptor, by S-nitrosylation. Proc. Natl. Acad. Sci. USA. 2007;104(30):12312–12317. doi: 10.1073/pnas.0703944104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dhaliwal N.K., Miri K., Davidson S., Tamim El Jarkass H., Mitchell J.A. KLF4 nuclear export requires ERK activation and initiates exit from naive pluripotency. Stem Cell Rep. 2018;10(4):1308–1323. doi: 10.1016/j.stemcr.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shields J.M., Yang V.W. Two potent nuclear localization signals in the gut-enriched Krüppel-like factor define a subfamily of closely related Krüppel proteins. J. Biol. Chem. 1997;272(29):18504–18507. doi: 10.1074/jbc.272.29.18504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schuetz A., Nana D., Rose C., Zocher G., Milanovic M., Koenigsmann J., Blasig R., Heinemann U., Carstanjen D. The structure of the Klf4 DNA-binding domain links to self-renewal and macrophage differentiation. Cell Mol. Life Sci. 2011;68(18):3121–3131. doi: 10.1007/s00018-010-0618-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kröncke K.D. Zinc finger proteins as molecular targets for nitric oxide-mediated gene regulation. Antioxid. Redox Signal. 2001;3(4):565–575. doi: 10.1089/15230860152542934. [DOI] [PubMed] [Google Scholar]
- 47.McMahon T.J., Ahearn G.S., Moya M.P., Gow A.J., Huang Y.C., Luchsinger B.P., Nudelman R., Yan Y., Krichman A.D., Bashore T.M., Califf R.M., Singel D.J., Piantadosi C.A., Tapson V.F., Stamler J.S. A nitric oxide processing defect of red blood cells created by hypoxia: deficiency of S-nitrosohemoglobin in pulmonary hypertension. Proc. Natl. Acad. Sci. USA. 2005;102(41):14801–14806. doi: 10.1073/pnas.0506957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giaid A., Yanagisawa M., Langleben D., Michel R.P., Levy R., Shennib H., Kimura S., Masaki T., Duguid W.P., Stewart D.J. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N. Engl. J. Med. 1993;328(24):1732–1739. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material