Abstract
Shepherd's purse (Capsella bursa-pastoris (L.) Medik.), a wild herb as a traditional herbal medicine, has been proved with multiple healthy benefits. In this study, the chemical constituents of shepherd's purse were identified by UPLC-QTOF-MS/MS. The antioxidative and anti-inflammatory potential of shepherd's purse extract (SPE) were also investigated applying lipopolysaccharide- (LPS-) induced inflammation in RAW 264.7 macrophages and a carrageenan-induced mice paw edema model. Twenty-four chemical compounds were identified mainly including phenolic acids and flavonoids. The data also indicated SPE inhibited the productions of NO, PGE2, TNF-α, and IL-6 stimulated with LPS. In addition, SPE inhibited the increase of reactive oxygen species (ROS) and upregulated the expression of heme oxygenase-1 (HO-1). We further found that SPE inhibited the phosphorylation of P38 MAPK and activation of NF-κB. In vivo mice model also indicated that SPE showed strong antioxidative and anti-inflammatory activity.
1. Introduction
Inflammation which existed in obesity, elder bodies, is accompany with many diverse chronic diseases, such as insulin resistance, type 2 diabetes, vascular disease, chronic renal failure, several cancers, endocrine [1–3]. To counteract this chronic inflammatory status, nonsteroidal anti-inflammatory drugs (NSAIDs) were usually proposed as a treatment strategy [4]. However, the side effects associated with long-term use of NSAIDs and steroids stimulate the development of novel anti-inflammatory therapies [5, 6]. Thus, the functional foods, for instance, some edible wild herbs, which with special health benefits, unique flavor, and also with high nutritional values, may be a good choice for improvement of the chronic low-grade inflammation and its related diseases. More importantly, these functional foods also showed higher biosafety and also can be easily and well accepted.
Shepherd's purse (Capsella bursa-pastoris (L.) Medik.), a wild herb (Figure 1) with high nutritional value and has been eaten raw or cooked as a vegetable for thousands of years in many countries, is getting more and more attention. Shepherd's purse has been used as traditional herbal medicine for a long history which has been recorded in TCM ancient books “Ben Cao Gang Mu,” “Ming Yi Bie Lu,” and so on. Previous studies found that shepherd's purse contained a wide range of chemicals including flavonoids, alkaloids, polypeptides, choline, acetylcholine, histamine, tyramine, fatty acids, sterols, organic acids, amino acids, sulforaphane, many trace elements, vitamins, and many other compounds [7–11]. Furthermore, pharmacological studies also proved that shepherd's purse with various bioactivities, including anti-inflammatory, antioxidative, antiallergic, AChE inhibitory activity, and anticancer effects in previous studies [12–16]. Choi et al. prepared a sulforaphane-containing solution component from shepherd's purse and found it had significant anti-inflammatory activity [13]. Lan et al. found that the EtOAc extract of Capsella bursa-pastoris which with apigenin-7-O-β-D-glucopyranoside, luteolin-7-O-β-D glucopyranoside, α-adenosine, and uridine showed stronger anti-inflammatory activities in carrageenan-induced paw edema experiment and egg-albumin-induced inflammation experiment [17]. Even though little studies found shepherd's purse with anti-inflammatory activity, the chemical composition, antioxidative and anti-inflammatory activities of the extract of shepherd's purse, and its underlying mechanisms have not been systematically studied. Therefore, the aim of the present study was to systematically investigate the chemical composition, anti-oxidative and anti-inflammatory activities of shepherd's purse extract, and their underlying mechanisms using LPS-induced RAW 264.7 cells and an in vivo carrageenan-induced mouse paw edema model.
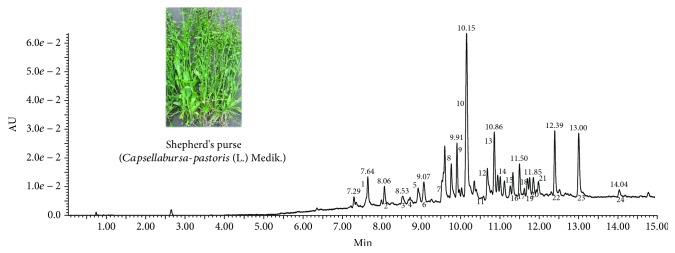
Figure 1.
The picture of shepherd's purse and DAD chromatogram at 320 nm of shepherd's purse extracts.
2. Material and Methods
2.1. Plant Materials and Preparation of Shepherd's Purse Extracts (SPE)
Fresh shepherd's purse was collected from Xiaogan, Hubei province of China, in March 2017. The specimen of the whole plant was deposited in College of Food Science and Technology in Huazhong Agricultural University (the voucher specimen number: 2016-02). The raw materials were dry in the shade and then were pulverized with a grinder. For extraction, 100 g raw materials were soaked with 2000 mL 95% ethanol at 100°C for 1 h for twice. The extract solution was combined and concentrated under reduced pressure and then freeze-dried using a vacuum freeze drying. The yield of extract was about 12.8% (w/w). The extracts were stored at −20°C for further use.
2.2. UPLC-QTOF-MS/MS System and Conditions
Chemical analysis of the SPE was performed by UPLC-QTOF-MS/MS analysis that was equipped with Waters Acquity UPLC system and MS system (Waters Corp., MA, USA). The UPLC analysis was performed with an Acquity UPLC BEH C18 column (2.1 × 100 mm, 1.7 μm). The mobile phases composed of water with 0.01% formic acid (A) and methanol (B); the elution was performed with a gradient procedure according to the following conditions: 0-0.5 min, 1% B; 0.5-30 min, 1% B -99% B, with a flow rate of 0.4 mL/min. 1 mg/mL of SPE in ethanol was prepared and filtered through 0.22 μm nylon micropore membranes prior to use. The injection volume was 1 μL. Parameters for ESI MS are as follows: negative mode; source temperature 120°C; desolvation gas flow 800 L/h; desolvation temperature 450°C; cone gas flow 50 L/h; sampling cone and capillary voltages were 30 and 2500 V, respectively. A scan ranges from m/z 100 to 1500 were applied.
2.3. Antioxidant Activity of SPE
The radical scavenging ability of SPE was evaluated using ABTS assay. The stock solution of ABTS+ was prepared by admixing ABTS (7 mM) with K2S2O8 solution. To obtain the ABTS+ working solution, the above stock solution was further diluted with water until the acceptable absorbance (0.7 ± 0.02) achieved at 734 nm. Ascorbic acid (Vc) was selected as a positive control and Vc equivalent antioxidant capacity was calculated. 10 μL sample with different concentration and 200 μL of the working solution were mixed thoroughly, incubated for about 10 min, and the absorbances were determined at 734 nm using a microplate reader.
2.4. Cell Culture
The mouse macrophage cell line RAW 264.7 (ATCC, USA) was grown in DMEM culture medium (ATCC, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco, USA) in a 5% CO2 humidified incubator at 37°C.
2.5. Cell Viability Assay
RAW 264.7 macrophages were seeded with a density of 4 × 103 cells/well into a 96-well plate. After incubation overnight, the cells were treated with SPE (0-320 μg/mL) and LPS for 20 h. Then, 20 μL of 5 mg/mL of methylthiazole tetrazolium (MTT) was added into each well and then incubated for another 4 h. After that, the supernatant was discarded and 100 μL DMSO was added. Plates were shaken for 1 min and the absorbance was measured at 570 nm using a microplate reader (Thermo Fisher, USA).
2.6. Determination of NO and Proinflammatory Cytokines
RAW 264.7 macrophages were treated as previously described [18]. Briefly, the cells were stimulated with 1 μg/mL of LPS with or without SPE for 16 h. The cell-free supernatant was collected with different treatment times (1, 2, 4, 8, and 16 h). NO concentration was measured using Griess reagent (Sigma, USA) and NaNO2 were applied as standard. The contents of PGE2, TNF-α, and IL-6 were measured using specific ELISA kit (Cayman Chemical, Ann Arbor, Michigan, USA; R&D, Minneapolis, MN, USA) according to the manufacturer's guidelines.
2.7. Measurements of ROS Production
The intracellular generation of ROS was determined using a 2′, 7′-dichlorofluorescein diacetate (DCFH-DA) as previously described [18]. RAW 264.7 macrophage cells were first incubated with LPS and SPE for 16 h; after that, cells were treated with 20 μM DCFH-DA at 37°C for 30 min in the dark. After that, DCF fluorescence intensity was measured by microplate fluorometer at wavelengths of 488 nm (excitation) and 535 nm (emission).
2.8. Western Blot Analysis
The treatment method was the same as described above. For iNOS, COX 2, and HO-1 determination, macrophages were stimulated with 1 μg/mL LPS and SPE for 16 h. For signaling molecule analysis (NF-κB and MAP kinase signaling), cells were treated with 1 μg/mL LPS and SPE for just one hour. Protein samples with or without MG132, a proteasome inhibitor, were also collected for 0.25, 0.5, and 1 h. After that, the cells were harvested and protein was collected. The detailed information of the western blot method was the same as previous reports [19]. The blots were detected using enhanced chemiluminescence assay kit (GE Healthcare, UK) and visualized by the chemiluminescent method (BioRad Laboratories, Hercules, CA, USA). β-Actin was used as a control.
2.9. Carrageenan-Induced Mouse Hind Paw Edema
C57BL/6 mice (20-22 g) were obtained from Laboratory Animal Center of Huazhong Agricultural University (Wuhan, China). All the procedures were approved by the Experimental Animal Review Committee of Huazhong Agricultural University of China. First, 100-400 mg/kg of SPE were administered orally; after 1 h, 30 μL of 1% carrageenan was injected into their right hind paw to induce edema. The thickness of the paw was evaluated at 1, 2, and 4 h. At last, mice were euthanized and the paw tissues were collected and kept at −80°C for the next study. The inflammatory cytokines including IL 6 and TNF-α were measured using specific ELISA kits (R&D, Minneapolis, MN, USA). SOD activity and MDA content were investigated using special test kits (Nanjing Jiancheng Bioengineering Institute, China).
2.10. Statistical Analysis
All data were presented as means±S.D. Statistical significance was analyzed using one-way ANOVA with Tukey multiple comparison test applied GraphPad Prism 5 Software (GraphPad Software, San Diego, CA). p < 0.05 was recognized as statistically significant.
3. Results
3.1. UPLC-QTOF-MS/MS Analysis of SPE
In the present study, a qualitative analysis of the composition of SPE was performed using UPLC–DAD-ESI-QTOF-MS. The DAD chromatogram at 320 nm of SPE was shown in Figure 1. As listed in Table 1, twenty-four chemical compounds were identified in the SPE. The compounds of SPE were tentatively identified with the MS data and by comparing with published literatures [20–31]. In brief, these compounds including 7 phenolic acids and their derivatives, 17 flavonoids belonged to the groups of flavones, flavonols, and flavanones.
Table 1.
Identification of compounds in shepherd's purse extract by UPLC-QTOF-MS/MS.
| No. | tR (min) | [M-H]− (m/z) | Major fragment ions (m/z) | Tentative identification | References |
|---|---|---|---|---|---|
| 1 | 7.64 | 353.0882 | 191.0553 | 5-O-Caffeolyquinic acid | [21] |
| 160.8414 | |||||
| 135.0285 | |||||
|
| |||||
| 2 | 8.06 | 367.1030 | 193.0493 | 1-O-Feruloylquinic acid | [22] |
| 134.0362 | |||||
|
| |||||
| 3 | 8.51 | 337.0929 | 191.0554 | 4-p-Coumaroylquinic acid | [23] |
| 173.0448 | |||||
|
| |||||
| 4 | 8.77 | 337.0924 | 191.0552 | 5-p-Coumaroylquinic acid | [24] |
|
| |||||
| 5 | 8.91 | 337.0924 | 173.0450 | 3-p-Coumaroylquinic acid | [23] |
|
| |||||
| 6 | 9.07 | 163.0392 | 163.0386 | p-Coumaric acid | [25] |
| 119.0492 | |||||
|
| |||||
| 7 | 9.56 | 367.1028 | 298.0486 | 5-O-Feruloylquinic acid | [24] |
| 191.0551 | |||||
| 173.0448 | |||||
|
| |||||
| 8 | 9.60 | 579.1343 | 459.0821 | Luteolin-6-C-pentoside-8-C-hexoside | [22] |
| 429.0771 | |||||
| 357.0613 | |||||
| 327.0504 | |||||
| 309.0403 | |||||
| 285.0396 | |||||
|
| |||||
| 9 | 9.91 | 447.0930 | 357.0610 | Luteolin-6-O-glucoside | [26] |
| 327.0508 | |||||
| 298.0470 | |||||
| 285.0399 | |||||
| 269.0452 | |||||
|
| |||||
| 10 | 10.15 | 563.1392 | 473.0954 | Apigenin-6-C-hexoside-8-C-pentoside | [27] |
| 443.1048 | |||||
| 383.0755 | |||||
| 353.0662 | |||||
|
| |||||
| 11 | 10.57 | 431.1925 | 293.0453 | Kaempferol-O-rhamnoside | [28] |
| 284.0307 | |||||
| 255.0252 | |||||
|
| |||||
| 12 | 10.68 | 609.1446 | 463.0859 | Quercetin-3-O-rutinoside | [29] |
| 301.0340 | |||||
| 300.0273 | |||||
| 271.0247 | |||||
| 255.0301 | |||||
|
| |||||
| 13 | 10.85 | 463.0882 | 300.0269 | Quercetin-3-O-glucoside | [28] |
| 301.0331 | |||||
| 271.0244 | |||||
|
| |||||
| 14 | 11.01 | 593.1501 | 443.0973 | Kaempferol-3-O-rutinoside | [30] |
| 323.0554 | |||||
| 285.0405 | |||||
|
| |||||
| 15 | 11.33 | 505.0986 | 300.0272 | Quercetin-3-(6-O-acetyl-beta-glucoside) | [28] |
| 271.0244 | |||||
| 255.0292 | |||||
|
| |||||
| 16 | 11.499 | 593.1499 | 285.0396 | Kaempferol-3-O-beta-D-rutinoside | [28] |
| 284.0320 | |||||
| 255.0298 | |||||
|
| |||||
| 17 | 11.62 | 725.1710 | 725.1678 | Kaempferol triglycoside | [26] |
| 605.1277 | |||||
| 429.0818 | |||||
| 327.0488 | |||||
| 309.0392 | |||||
| 285.0387 | |||||
|
| |||||
| 18 | 11.68 | 447.0938 | 447.0905 | Kaempferol-O-glucoside | [28] |
| 429.0782 | |||||
| 284.0313 | |||||
| 255.0290 | |||||
| 227.0341 | |||||
|
| |||||
| 19 | 11.75 | 623.1604 | 531.0240 | Isorhamnetin-3-rutinoside | [24] |
| 427.9770 | |||||
| 315.0495 | |||||
| 314.0418 | |||||
| 271.0243 | |||||
| 255.0283 | |||||
|
| |||||
| 20 | 11.85 | 755.1802 | 635.1353 | Diosmetin-7-O-triglycoside | [31] |
| 579.1293 | |||||
| 429.0812 | |||||
| 357.0258 | |||||
| 309.0400 | |||||
|
| |||||
| 21 | 11.93 | 623.1611 | 501.1388 | Quercetin rhamnoside glucuronate | [32] |
| 447.1135 | |||||
| 429.1028 | |||||
| 337.0923 | |||||
| 269.0453 | |||||
|
| |||||
| 22 | 12.39 | 607.1674 | 551.1418 | Chryseoriol- -rutinoside | [21] |
| 515.0217 | |||||
| 429.1035 | |||||
| 299.0561 | |||||
| 284.0328 | |||||
|
| |||||
| 23 | 13.00 | 461.1088 | 299.0558 | Chryseoriol-7-O-glucoside | [23] |
| 284.0325 | |||||
| 256.0376 | |||||
| 116.9280 | |||||
|
| |||||
| 24 | 14.04 | 299.1850 | 299.1852 | Chrysoeriol | [29] |
| 284.0325 | |||||
| 255.0302 | |||||
3.2. Effect of SPE on Viability of RAW 264.7 Macrophages
MTT assay was performed to calculate the cellular cytotoxicity of SPE with or without LPS. The results indicated SPE with no cytotoxicity even at a high concentration (160 μg/mL) on RAW 264.7 no matter the existence of LPS (Figure 2). Therefore, in this study 10, 20, 40, and 80 μg/mL of SPE were selected for next study.
Figure 2.
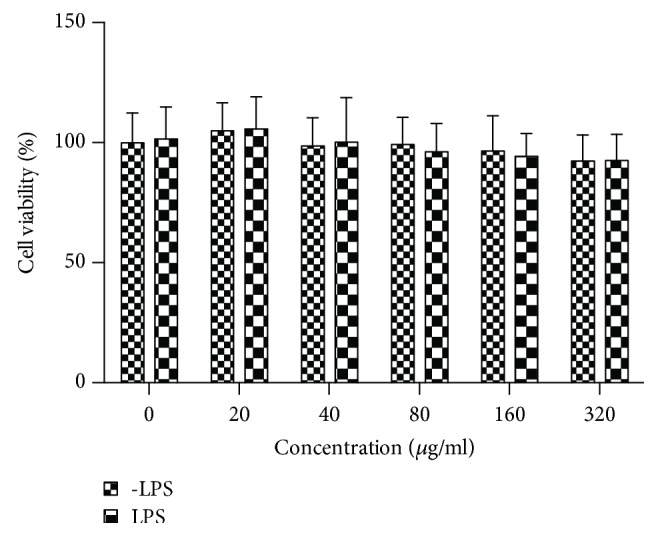
Effect of SPE on RAW 264.7 macrophage cell viability. Macrophages were cultured with SPE with or without LPS for 24 h, and cell viability was analyzed using the MTT assay.
3.3. Effect of SPE on the Inflammatory Cytokine Production and Their Related Gene Expression
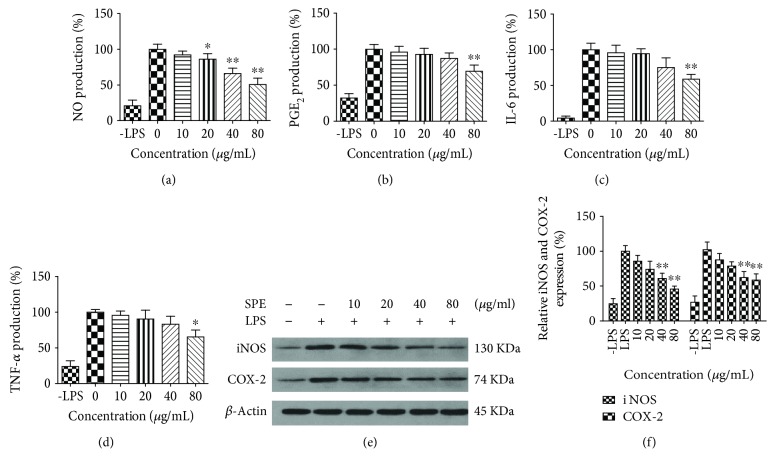
We first investigated whether SPE could inhibit the production of NO, which is the main proinflammatory mediator in LPS-induced inflammation in macrophages. As the results illustrated in Figure 3(a), LPS could induce large amount of NO; 40 μg/mL of SPE could significantly reduce the NO production (p < 0.05) with an IC50 of 91.09 μg/mL. For PGE2, similar to the NO production, with the increased concentration of SPE, the level of PGE2 was significantly decreased with an IC50 of 150.37 μg/mL (Figure 3(b)). Similarly, treatment with SPE resulted in a concentration-dependent reduction of IL 6 and TNF-α with IC50 of 129.4 and 136.2 μg/mL (Figures 3(c) and 3(d)). Further western blot assay (Figures 3(e) and 3(f)) also indicated that SPE showed significantly inhibitory effects on the expression of iNOS and COX-2.
Figure 3.
Influences of SPE on the production of proinflammatory cytokines and related protein expression. Mouse macrophages were induced with LPS in the presence of SPE for 16 h. Culture supernatant was removed and centrifuged to remove particulates and analyzed for production of these cytokines: (a) NO; (b) PGE2; (c) IL-6; and (d) TNF-α. (e) The expression levels of iNOS and COX2 were analyzed by western blot. (f) The band intensity of iNOS or COX2 was normalized with β-actin. ∗p < 0.05 and ∗∗p < 0.01 versus LPS alone group.
3.4. Effects of SPE on Proinflammatory Cytokine Secretion
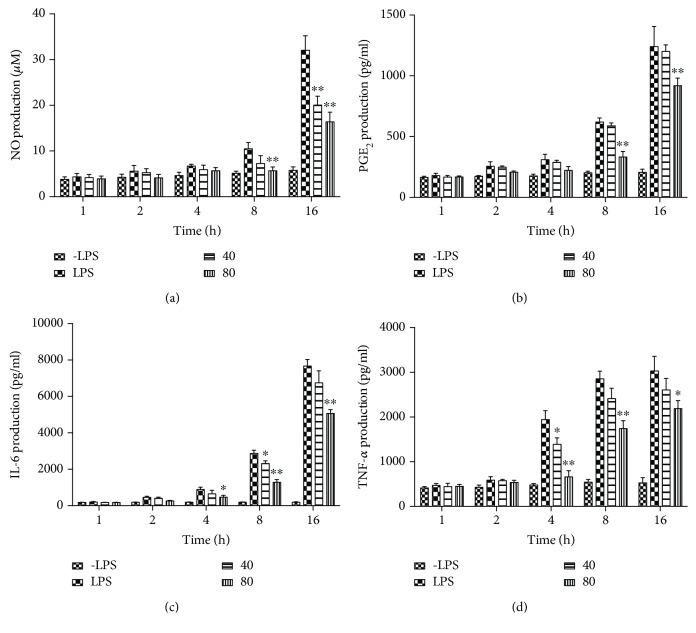
To further investigate the influences of SPE on the secretion of specific cytokines stimulated by LPS in different treatment times, the contents of NO, IL-6, PGE2, and TNF-α were measured at 1, 2, 4, 8, and 16 h. As the results illustrated in Figure 4, with the induction of LPS, the productions of all of these four main proinflammatory cytokines were increasing with the time increased of LPS stimulation. 80 μg/mL SPE could significantly inhibit the generation of these specific proinflammatory cytokines (p < 0.01) at 8 h and 16 h.
Figure 4.
Influences of SPE on the secretion of proinflammatory cytokines with different LPS stimulation time. (a) NO; (b) PGE2; (c) IL-6; and (d) TNF-α. Cells were incubated with 1 μg/ml LPS with the addition of 40 and 80 μg/ml of SPE at 1, 2, 4, 8, and 16 h timepoints; culture supernatant was removed and centrifuged to remove particulates and analyzed for production of these cytokines. ∗p < 0.05 and ∗∗p < 0.01 versus LPS alone group.
3.5. Effect of SPE on MAPK Phosphorylation and Activation of NF-κB
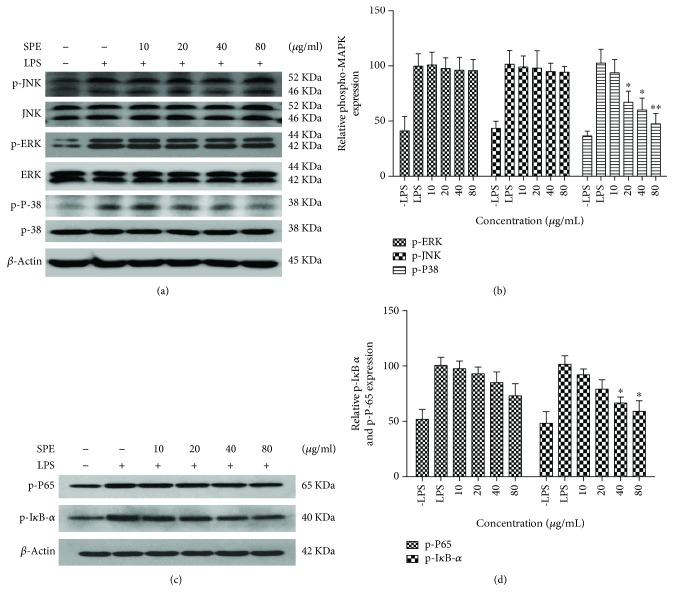
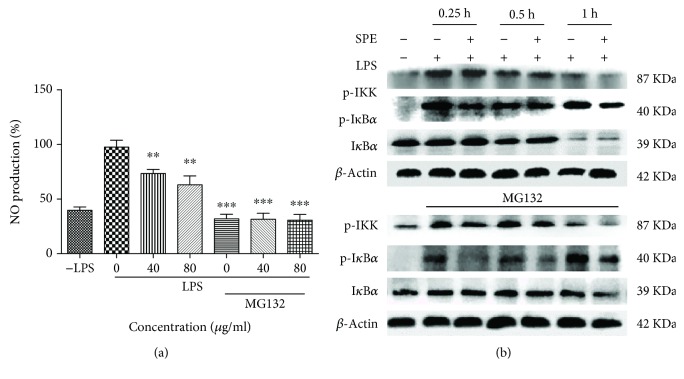
The NF-κB and MAP kinase signaling (p38, JNK, and ERK) pathways regulate the LPS-induced inflammatory response and also played key roles in the occurrence and development of inflammation [32]. To further clarify the underlying mechanism of the anti-inflammatory ability, influences of SPE on activation of NF-κB and phosphorylation of MAPKs were evaluated using western blot assays. As the data presented in Figures 5(a) and 5(b), SPE showed a significant reduction on the phosphorylation of p38. In this study, SB 203580, a p-38 MAPK inhibitor, also showed a significantly inhibitory activity on the NO production in LPS-induced RAW264.7 cells (32.06 ± 3.14 vs 18.51 ± 2.37 μM). Furthermore, the phosphorylation of IκB α and p65 appeared after LPS stimulation for 60 min (Figures 5(c) and 5(d)). The phosphorylation of p65 was significantly decreased with the treatment of SPE; the phosphorylation of IκB-α also decreased with the treatment of 80 μg/mL SPE even though there was no significant difference. These data indicated that the inhibitory effect of SPE on LPS-induced phosphorylation of p 38 MAPKs and activation of NF-κB was partly associated with its anti-inflammatory potential. In this study, we also used MG132, a proteasome inhibitor, to clarify the effect of SPE. As the results illustrated in Figure 6, MG132 showed significantly inhibitory effect on the LPS-induced inflammatory (Figure 6(a)). And as the results showed in Figure 6(b), LPS could significantly induce the phosphorylation of IKK and IκB and induce the degradation of IκB α. However, SPE could reverse this to reduce the development of inflammatory process.
Figure 5.
SPE inhibited the activation of LPS-induced MAPK (JNK, ERK, and p38) and NF-κB (IκB α and p65). Cells were incubated with 1 μg/ml LPS with the addition of different concentration of SPE for 1 h; the protein were extracted and the phosphorylation of JNK, ERK, p 38, p 65, and IκB α were analyzed with western blot assay. (a, c) Western blot assay of activation of MAPKs and NF-κB were analyzed by and (b, d) The band intensity of phosphorylation MAPKs and NF-κB were normalized with nonphosphorylation of MAPKs and β-actin. ∗p < 0.05 and ∗∗p < 0.01 versus LPS alone group.
Figure 6.
SPE reduced the activation of IKK and IκB α and reversed the degradation of IκB α during its inhibition of LPS-induced inflammation. (a) The NO production in the LPS induced RAW 264.7 cells with or without the treatment of SPE and MG 132. RAW 264.7 cells were first incubated with or without MG132 for 1 h and then treatment with LPS or LPS+SPE for 16 h. Culture supernatant was collected and NO production was measured using Griess reagent. (b) The phosphorylation of IKK and IκB α in macrophages. Apart treatment with LPS and SPE, Raw 264.7 cell were also incubated with or without MG132, at 0.25 h, 0.5 h, and 1 h; the proteins were extracted and were analyzed using western blot assay. ∗∗p < 0.01 and ∗∗∗p < 0.001 versus LPS alone.
3.6. Antioxidative Activities of SPE
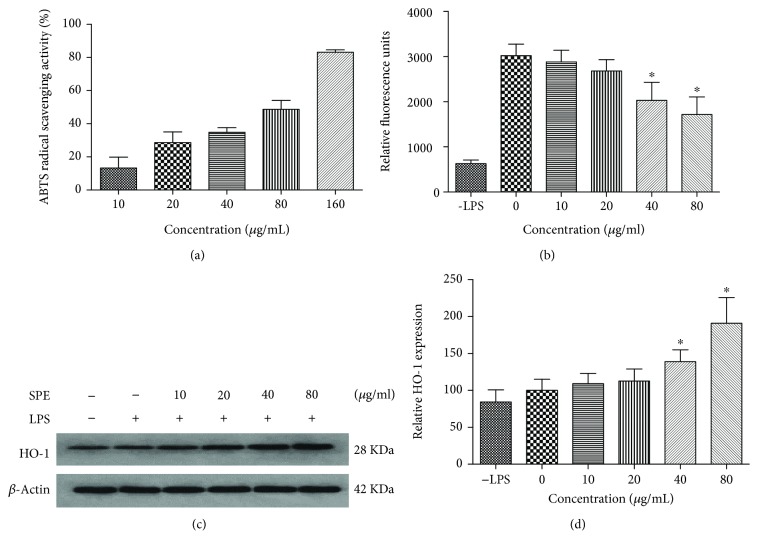
Previous studies have indicated that in vitro ABTS radical scavenging activity can potentially be used as marker for evaluating the anti-inflammatory activity of flavonoids [33]. Therefore, the antioxidant activity of SPE was firstly investigated using the ABTS assay. As the data presented in Figure 7(a), the ABTS radical scavenging activity of SPE increased with the increasing of SPE concentration with an EC50 value of 61.6 μg/mL. At the concentration of 160 μg/ml, about 80% of the ABTS free radical was scavenged. The Vc equivalent antioxidant capacity of SPE was calculated as 0.18 g per gram SPE. Figure 7(b) showed that with the stimulation of LPS, the intracellular ROS were accumulated in RAW 264.7 cells, whereas SPE showed a strong inhibitory effect on the ROS production.
Figure 7.
SPE showed strong antioxidative potential in vitro chemical and cell models. (a) The ABTS+ scavenging activity of SPE. (b) SPE inhibited ROS production in LPS-stimulated macrophages. (c, d) SPE enhanced the HO-1 expression. ∗p < 0.05 versus LPS alone group.
HO-1 has been reported as a stress-inducible protein induced by many stimuli such as oxidative stress and UV light [34, 35], and upregulation of the expression of HO-1 has been proved as a useful approach to improve oxidative injury and macrophage activation [35–38]. Therefore, the expression of HO-1 with SPE treatment was also evaluated in this study (Figures 7(c) and 7(d)). These results indicated that the expression of HO-1 was increased with the treatment of SPE.
3.7. In Vivo Anti-Inflammatory Activity of SPE
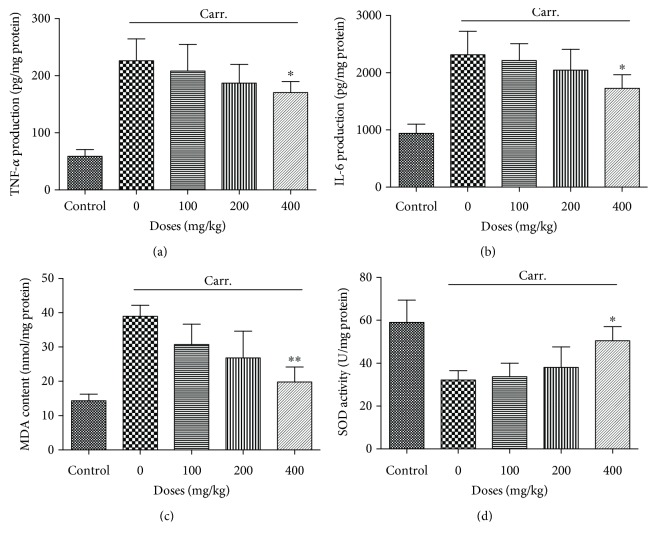
The anti-inflammation potential of SPE was further investigated using an in vivo mouse paw edema model. As the data illustrated in Figure 8, the paw thickness significantly increased after the carrageenan injection, and 400 mg/kg of SPE showed a significantly inhibitory activity of paw edema (Figure 8). With the oral administration of 400 mg/kg of SPE, the paw thickness significantly decreased, which was 0.30 ± 0.02 and 0.32 ± 0.02 cm compared with 0.37 ± 0.01 and 0.39 ± 0.03 cm at 2 h and 4 h, respectively. For TNF-α and IL-6, a large amount of TNF-α and IL-6 were induced with the injection of carrageenan (Figures 9(a) and 9(b)); 400 mg/kg of SPE could significantly decrease the generation of TNF-α and IL-6, which was 170.23 ± 19.58 and 1728.21 ± 237.69 pg/mg protein compared with 226.01 ± 38.70 and 2314.41 ± 409.04 pg/mg protein (p < 0.05), respectively. In addition, 400 mg/kg of SPE also could significantly decrease the MDA content (19.82 ± 4.36 vs 39.71 ± 5.30 nmol/mg protein, p < 0.01) (Figure 9(c)). These in vivo results were also in accordance with the result from cell culture model. Meanwhile, SOD activity assay also indicated that the decrease of SOD activity by the carrageenan injection was reversed with the treatment of SPE (50.53 ± 6.59 vs 32.19 ± 4.28 U/mg protein) (Figure 9(d)).
Figure 8.
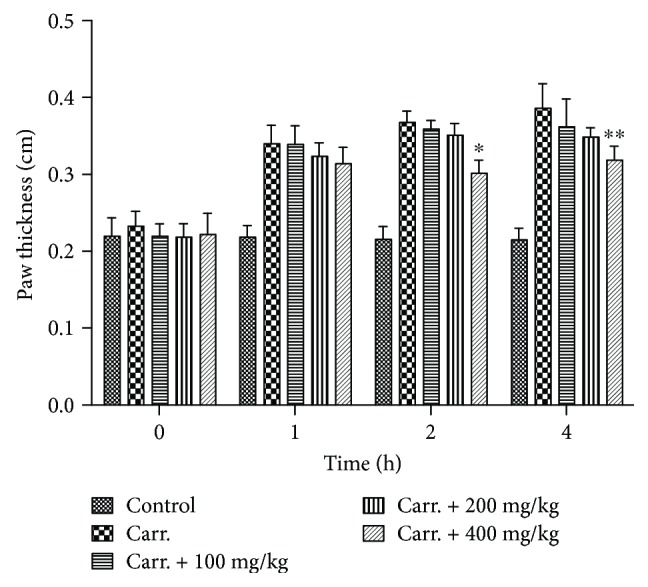
SPE inhibited the development of the paw edema induced by carrageenan injection. The edema model was induced with 30 μL of 1% carrageenan. Different doses of SPE were administered orally 1 h prior to carrageenan injection. ∗p < 0.05 and ∗∗p < 0.01 versus carrageenan group.
Figure 9.
Influence of SPE on the production of inflammatory biomarkers and oxidative stress parameters in paw tissue. The edema was induced with 30 μL of 1% carrageenan. Different doses of SPE were administered orally 1 h prior to carrageenan injection. (a) TNF-α expression levels. (b): IL-6 expression levels. (c) MDA level. (d) SOD activity in edema tissue. ∗p < 0.05 and ∗∗p < 0.01 versus model group.
4. Discussion
In recent years, the functional foods have received increasing attention worldwide. It can not only supply the nutrients but also provide many phytochemicals which play as a functional factor for human health, especially for these people with chronic disease or in the state of subhealth, such as chronic low-grade inflammation; these functional foods may be the best choice to improve their healthy rather than treatment with drugs [39, 40]. Previous studies have proved that shepherd's purse (Capsella bursa-pastoris) exerts multihealthy benefits, such as antimicrobial [41], anti-inflammatory [13], cardiovascular, reproductive, anticancer [16, 42], hepatoprotective, sedative, and other pharmacological effects [43]. Therefore, the chemical components of shepherd's purse ethanol extract were first characterized by UPLC-QTOF-MS/MS, and then the anti-inflammatory effects and its underlying mechanisms of SPE in LPS-induced RAW 264.7 inflammation model and in vivo mouse model were also systemically investigated in this study.
Macrophages play key roles in the immune system; the activation of macrophages induced the secretion of many inflammatory mediators, and also coupled with a high degree of oxidative stress [44]. SPE showed significantly inhibitory on the production of NO and PGE2. The overexpression of circulating inflammatory factors, including IL-6, IL-1β, TNF-α, and MCP-1, transforming growth factor (TGF)-a, TGF-b, and IFN-c also have been proved associated with low-grade, chronic inflammation [45, 46]. In this study, the results indicated that the production of TNF-α and IL-6 were significantly inhibited with the treatment of SPE from 4 h. These findings demonstrated that SPE could attenuate LPS-induced macrophage activation, which indicated that SPE possesses potential anti-inflammatory activity.
Inflammation is regulated by many proinflammatory mediators and cell signal pathways [47]. In this process, NF-κB, MAPKs, and Nrf 2/HO-1 pathway have been proved that played key roles in mediating the inflammatory responses [19, 48–52]. Therefore, these pathways are potential targets for pharmacological intervention in the treatment of inflammation [49, 50]. This study found that SPE could inhibit the phosphorylation of p38 MAPK and reduce the subsequent inflammatory response. Similarly, NF-κB is also an important signal pathway involved in immune and inflammatory responses [53, 54]. In addition, following treatment with SPE, LPS induced the phosphorylation of IκB and P-65 was also inhibited in RAW 264.7 macrophages with the treatment of SPE, which indicated that SPE could prevent the activation of NF-κB to exert its anti-inflammatory potential. Furthermore, there were very close connections between these two signal pathways. From the above results, we could find that both of the activation of p 38 MAPKs and NF-κB signal pathways were blocked by the treatment of SPE. Therefore, these data suggested SPE exerts its anti-inflammatory potential at least partly associated with its regulation on the activation of MAPKs and NF-κB pathways.
It has been proved that there was a connection between chronic inflammation and oxidative stress, and free radical-induced damage also could induce many chronic health problems [55–57]. In the inflammatory process, ROS have been proved to participate in the LPS-stimulated inflammation process by activating specific signaling pathways, resulting in the production of many specific cytokines [35, 57–60]. In addition, the upregulation of HO-1 was recognized as a pivotal response to different kinds of stress, it could exert its anti-inflammatory potential through inhibiting the excessive production of specific cytokines, and also through its regulation on macrophage switching to an M2-phenotype [61]. In this study, the data indicated SPE with strong antioxidative activity; meanwhile SPE could inhibit ROS production in macrophage cells whereas enhanced the expression of HO-1. These data proved the strong antioxidant activity also played an important role in the anti-inflammatory effect of SPE.
Carrageenan-induced paw edema animal model is usually applied to assess the different phases of inflammation reaction and evaluate the anti-inflammatory agents; it can induce acute inflammation, release of inflammatory mediators, and production of free radicals [62–64]. Therefore, this animal model was also used in this study. The results proved that SPE could inhibit the development of the carrageenan-induced edema, which was consisted with the in vitro findings. Meanwhile, the induction of inflammation by carrageenan was compared by generation of ROS and increased oxidative stress [64]. The animal study indicated that there was a significant increase of MDA content along with a distinct decrease of SOD activity with the injection of carrageenan in the model group. However, with the treatment of 400 mg/kg SPE, increase of SOD activity and the decrease of MDA in paw edema tissue were observed. These results indicated that SPE also showed strong anti-inflammatory and antioxidative potential in vivo.
In this study, about 24 chemical compounds were identified from the extracts of Capsella bursa-pastoris. As the HPLC-MS results, SPE contain a high amount of flavonoids, such as quercetin, kaempferol-7-O-rhamnopyranoside, quercetin-3-O-glucopyranoside, quercetin-6-C-glucopyranoside, and kaempferol-3-O-rutinoside; these results were also consistent with some previous findings [43, 65, 66]. Many studies had investigated the health benefits of these components, for example, antioxidative, ant-obesity, and anticancer activities [64, 67, 68]. Therefore, the flavonoid constituents presented in SPE could play key roles for its antioxidative and anti-inflammatory activity. However, since the extract contained many compounds, further works are still needed to be undertaken to investigate the anti-inflammatory properties of single compounds to further clear the anti-inflammatory potential of SPE.
5. Conclusion
In conclusion, in this study, the chemical composition, anti-inflammatory potential of shepherd's purse, and its underlying mechanisms were first systematically evaluated. Our findings indicated that Capsella bursa-pastoris (L.) Medik might reduce NO and PGE2 production and also inhibited the production of TNF-α and IL-6 in inflammatory development process. The underlying mechanism study proved that the anti-inflammatory potential of Capsella bursa-pastoris (L.) Medik was through the inhibition of the activation of p-38 MAPKs and NF-κB pathways. Taken together, besides the good nutritional value and delicious taste already described in the previous studies, Capsella bursa-pastoris (L.) Medik also showed special health benefits, suggesting that it may be interesting not only for human health but also as food additive.
Acknowledgments
This work was supported by the Natural Science Foundation of China (31701712), Hubei Provincial Natural Science Foundation of China (2017CFB197), Fundamental Research Funds for the Central Universities (2662016QD035), Longgang Science and Technology programs (no. 20160607142024828), and Shenzhen Innovation of Science and Technology Commission programs (nos. ZDSYS201506050935272).
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors have declared no conflict of interest.
Authors' Contributions
Jinming Peng and Tianyong Hu are co-first authors who contributed equally to this work.
References
- 1.Adler U. C. Low-grade inflammation in chronic diseases: an integrative pathophysiology anticipated by homeopathy? Medical Hypotheses. 2011;76(5):622–626. doi: 10.1016/j.mehy.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 2.Lee J., Ha S. J., Lee H. J., et al. Protective effect of Tremella fuciformis Berk extract on LPS-induced acute inflammation via inhibition of the NF-κB and MAPK pathways. Food & Function. 2016;7(7):3263–3272. doi: 10.1039/C6FO00540C. [DOI] [PubMed] [Google Scholar]
- 3.Castro A. M., Macedo-de la Concha L. E., Pantoja-Meléndez C. A. Low-grade inflammation and its relation to obesity and chronic degenerative diseases. Revista Médica del Hospital General de México. 2017;80(2):101–105. doi: 10.1016/j.hgmx.2016.06.011. [DOI] [Google Scholar]
- 4.Cevenini E., Caruso C., Candore G., et al. Age-related inflammation: the contribution of different organs, tissues and systems. How to face it for therapeutic approaches. Current Pharmaceutical Design. 2010;16(6):609–618. doi: 10.2174/138161210790883840. [DOI] [PubMed] [Google Scholar]
- 5.Süleyman H., Demircan B., Karagöz Y. Anti-inflammatory and side effects of cyclooxygenase inhibitors. Pharmacological Reports. 2007;59(3):247–258. [PubMed] [Google Scholar]
- 6.Manson S. C., Brown R. E., Cerulli A., Vidaurre C. F. The cumulative burden of oral corticosteroid side effects and the economic implications of steroid use. Respiratory Medicine. 2009;103(7):975–994. doi: 10.1016/j.rmed.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Guil-Guerrero J. L., Gimenez-Martinez J. J., Torija-Isasa M. E. Nutritional composition of wild edible crucifer species. Journal of Food Biochemistry. 1999;23(3):283–294. doi: 10.1111/j.1745-4514.1999.tb00020.x. [DOI] [Google Scholar]
- 8.Cha J. M., Suh W. S., Lee T. H., Subedi L., Kim S. Y., Lee K. R. Phenolic glycosides from Capsella bursa-pastoris (L.) Medik and their anti-inflammatory activity. Molecules. 2017;22(6, article 1023) doi: 10.3390/molecules22061023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pehlivan M., Akgulo H., yayla F. The some nutrient and trace elements content of wild plants using as ethno botanical and grown in the Gaziantep region. Journal of Applied Pharmaceutical Science. 2013;3(4):143–145. [Google Scholar]
- 10.Wang Q. H., Wu E. Q., Dai N. Y. T. Study on chemical constituents of Capsella bursa-pastoris. Chemical composition and anti-inflammatory effects of the EtOAc extract from Capsella bursa-pastoris (L.) Medic. Natural Product Research and Development. 2014;26:50–52. [Google Scholar]
- 11.Al-Snafi A. E. The chemical constituents and pharmacological effects of Capsella bursa-pastoris - a review. International Journal of Pharmacology & Toxicology. 2015;5(2):76–81. [Google Scholar]
- 12.Goun E. A., Petrichenko V. M., Solodnikov S. U., et al. Anticancer and antithrombin activity of Russian plants. Journal of Ethnopharmacology. 2002;81(3):337–342. doi: 10.1016/S0378-8741(02)00116-2. [DOI] [PubMed] [Google Scholar]
- 13.Choi W. J., Kim S. K., Park H. K., Sohn U. D., Kim W. Anti-inflammatory and anti-superbacterial properties of sulforaphane from shepherd's purse. Korean J Physiol Pharmacol. 2014;18(1):33–39. doi: 10.4196/kjpp.2014.18.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi H. K., Shin E. J., Park S. J., et al. Ethanol extract of Capsella bursa-pastoris improves hepatic steatosis through inhibition of histone acetyltransferase activity. Journal of Medicinal Food. 2017;20(3):251–257. doi: 10.1089/jmf.2016.3877. [DOI] [PubMed] [Google Scholar]
- 15.Kuroda K., Akao M., Kanisawa M., Miyaki K. Inhibitory effect of Capsella bursa-pastoris extract on growth of Ehrlich solid tumor in mice. Cancer Research. 1976;36(6):1900–1903. [PubMed] [Google Scholar]
- 16.Lee K. E., Shin J. A., Hong I. S., Cho N. P., Cho S. D. Effect of methanol extracts of Cnidium officinale Makino and Capsella bursa-pastoris on the apoptosis of HSC-2 human oral cancer cells. Experimental and Therapeutic Medicine. 2013;5(3):789–792. doi: 10.3892/etm.2012.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lan X., Qing-Hu W., Baiyinmuqier B., Agula B. Chemical composition and anti-inflammatory effects of the EtOAc extract from Capsella bursa-pastoris (L.) Medic. African Journal of Pharmacy and Pharmacology. 2017;11(15):186–190. doi: 10.5897/ajpp2017.4762. [DOI] [Google Scholar]
- 18.Li K. K., Shen S. S., Deng X., et al. Dihydrofisetin exerts its anti-inflammatory effects associated with suppressing ERK/p 38 MAPK and heme oxygenase-1 activation in lipopolysaccharide-stimulated RAW 264.7 macrophages and carrageenan-induced mice paw edema. International Immunopharmacology. 2018;54:366–374. doi: 10.1016/j.intimp.2017.11.034. [DOI] [PubMed] [Google Scholar]
- 19.Li K. K., Peng J. M., Zhu W., Cheng B. H., Li C. M. Gallocatechin gallate (GCG) inhibits 3T3-L1 differentiation and lipopolysaccharide induced inflammation through MAPK and NF-κB signaling. Journal of Functional Foods. 2017;30:159–167. doi: 10.1016/j.jff.2017.01.016. [DOI] [Google Scholar]
- 20.Martins N., Barros L., Santos-Buelga C., Silva S., Henriques M., Ferreira I. C. Decoction, infusion and hydroalcoholic extract of cultivated thyme: antioxidant and antibacterial activities, and phenolic characterisation. Food Chemistry. 2015;167:131–137. doi: 10.1016/j.foodchem.2014.06.094. [DOI] [PubMed] [Google Scholar]
- 21.Lv Z. L., Dong J., Zhang B. L. Rapid identification and detection of flavonoids compounds from bamboo leaves by LC-(ESI)-IT-TOF/MS. BioResources. 2012;7(2):1405–1418. doi: 10.15376/biores.7.2.1405-1418. [DOI] [Google Scholar]
- 22.Lin L. Z., Lu S., Harnly J. M. Detection and quantification of glycosylated flavonoid malonates in celery, Chinese celery, and celery seed by LC-DAD-ESI/MS. Journal of Agricultural and Food Chemistry. 2007;55(4):1321–1326. doi: 10.1021/jf0624796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bergantin C., Maietti A., Cavazzini A., et al. Bioaccessibility and HPLC-MS/MS chemical characterization of phenolic antioxidants in red chicory (Cichorium intybus) Journal of Functional Foods. 2017;33:94–102. doi: 10.1016/j.jff.2017.02.037. [DOI] [Google Scholar]
- 24.Zhao Y., Li X., Zeng X., Huang S., Hou S., Lai X. Characterization of phenolic constituents in Lithocarpus polystachyus. Analytical Methods. 2014;6(5):1359–1363. doi: 10.1039/c3ay41288a. [DOI] [Google Scholar]
- 25.Llorent-Martínez E. J., Ortega-Barrales P., Zengin G., et al. Evaluation of antioxidant potential, enzyme inhibition activity and phenolic profile of Lathyrus cicera and Lathyrus digitatus: potential sources of bioactive compounds for the food industry. Food and Chemical Toxicology. 2017;107, Part B:609–619. doi: 10.1016/j.fct.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Zhu M., Dong X., Guo M. Phenolic profiling of Duchesnea indica combining macroporous resin chromatography (MRC) with HPLC-ESI-MS/MS and ESI-IT-MS. Molecules. 2015;20(12):22463–22475. doi: 10.3390/molecules201219859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun Q., Zhu J., Cao F., Chen F. Anti-inflammatory properties of extracts from Chimonanthus nitens Oliv. leaf. PLoS One. 2017;12(7, article e0181094) doi: 10.1371/journal.pone.0181094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sánchez-Rabaneda F., Jáuregui O., Lamuela-Raventós R. M., Bastida J., Viladomat F., Codina C. Identification of phenolic compounds in artichoke waste by high-performance liquid chromatography-tandem mass spectrometry. Journal of Chromatography A. 2003;1008(1):57–72. doi: 10.1016/S0021-9673(03)00964-6. [DOI] [PubMed] [Google Scholar]
- 29.Ferreres F., Gil-Izquierdo A., Andrade P. B., Valentão P., Tomás-Barberán F. A. Characterization of C-glycosyl flavones O-glycosylated by liquid chromatography-tandem mass spectrometry. Journal of Chromatography A. 2007;1161(1-2):214–223. doi: 10.1016/j.chroma.2007.05.103. [DOI] [PubMed] [Google Scholar]
- 30.Karioti A., Giocaliere E., Guccione C., et al. Combined HPLC-DAD-MS, HPLC-MS (n) and NMR spectroscopy for quality control of plant extracts: the case of a commercial blend sold as dietary supplement. Journal of Pharmaceutical and Biomedical Analysis. 2014;88:7–15. doi: 10.1016/j.jpba.2013.07.040. [DOI] [PubMed] [Google Scholar]
- 31.Torres-Carro R., Isla M. I., Thomas-Valdes S., Jiménez-Aspee F., Schmeda-Hirschmann G., Alberto M. R. Inhibition of pro-inflammatory enzymes by medicinal plants from the Argentinean highlands (Puna) Journal of Ethnopharmacology. 2017;205:57–68. doi: 10.1016/j.jep.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 32.Gaestel M., Kotlyarov A., Kracht M. Targeting innate immunity protein kinase signalling in inflammation. Nature Reviews Drug Discovery. 2009;8(6):480–499. doi: 10.1038/nrd2829. [DOI] [PubMed] [Google Scholar]
- 33.Chanput W., Krueyos N., Ritthiruangdej P. Anti-oxidative assays as markers for anti-inflammatory activity of flavonoids. International Immunopharmacology. 2016;40:170–175. doi: 10.1016/j.intimp.2016.08.038. [DOI] [PubMed] [Google Scholar]
- 34.Lee C. J., Lee S. S., Chen S. C., Ho F. M., Lin W. W. Oregonin inhibits lipopolysaccharide-induced iNOS gene transcription and upregulates HO-1 expression in macrophages and microglia. British Journal of Pharmacology. 2005;146(3):378–388. doi: 10.1038/sj.bjp.0706336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asehnoune K., Strassheim D., Mitra S., Kim J. Y., Abraham E. B. Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-κB. The Journal of Immunology. 2004;172(4):2522–2529. doi: 10.4049/jimmunol.172.4.2522. [DOI] [PubMed] [Google Scholar]
- 36.Abraham N. G., Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacological Reviews. 2008;60(1):79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- 37.Lee J. W., Bae C. J., Choi Y. J., et al. 3, 4, 5-trihydroxycinnamic acid inhibits lipopolysaccharide (LPS) - induced inflammation by Nrf 2 activation in vitro and improves survival of mice in LPS-induced endotoxemia model in vivo. Molecular and Cellular Biochemistry. 2014;390(1-2):143–153. doi: 10.1007/s11010-014-1965-y. [DOI] [PubMed] [Google Scholar]
- 38.Yang H. L., Lin S. W., Lee C. C., et al. Induction of Nrf2-mediated genes by Antrodia salmonea inhibits ROS generation and inflammatory effects in lipopolysaccharide-stimulated RAW264.7 macrophages. Food & Function. 2015;6(1):229–240. doi: 10.1039/c4fo00869c. [DOI] [PubMed] [Google Scholar]
- 39.Lee M. Y., Lee J. A., Seo C. S., et al. Anti-inflammatory activity of Angelica dahurica ethanolic extract on RAW264.7 cells via upregulation of heme oxygenase-1. Food and Chemical Toxicology. 2011;49(5):1047–1055. doi: 10.1016/j.fct.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 40.Wang X., Zhang C., Peng Y., et al. Chemical constituents, antioxidant and gastrointestinal transit accelerating activities of dried fruit of Crataegus dahurica. Food Chemistry. 2018;246:41–47. doi: 10.1016/j.foodchem.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 41.Soleimanpour S., Sedighinia F. S., Afshar A. S., Zarif R., Asili J., Ghazvini K. Synergistic antibacterial activity of Capsella bursa-pastoris and Glycyrrhiza glabra against oral pathogens. Jundishapur Journal of Microbiology. 2013;6(8):p. 7262. doi: 10.5812/jjm.7262. [DOI] [Google Scholar]
- 42.Yildirim A. B., Karakas F. B., Turker A. U. In vitro antibacterial and antitumor activities of some medicinal plant extracts, growing in Turkey. Asian Pacific Journal of Tropical Medicine. 2012;6(8):616–624. doi: 10.1016/s1995-7645(13)60106-6. [DOI] [PubMed] [Google Scholar]
- 43.Grosso C., Vinholes J., Silva L. R., et al. Chemical composition and biological screening of Capsella bursa-pastoris. Revista Brasileira de Farmacognosia. 2011;21(4):635–643. doi: 10.1590/S0102-695X2011005000107. [DOI] [Google Scholar]
- 44.Khajuria V., Gupta S., Sharma N., et al. Anti-inflammatory potential of hentriacontane in LPS stimulated RAW 264.7 cells and mice model. Biomedicine & Pharmacotherapy. 2017;92:175–186. doi: 10.1016/j.biopha.2017.05.063. [DOI] [PubMed] [Google Scholar]
- 45.Youn G. S., Lee K. W., Choi S. Y., Park J. Overexpression of HDAC6 induces pro-inflammatory responses by regulating ROS-MAPK-NF-κB/AP-1 signaling pathways in macrophages. Free Radical Biology & Medicine. 2016;97:14–23. doi: 10.1016/j.freeradbiomed.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 46.Germolec D. R., Frawley R. P., Evans E. Markers of inflammation. Methods in Molecular Biology. 2010;598:53–73. doi: 10.1007/978-1-60761-401-2_5. [DOI] [PubMed] [Google Scholar]
- 47.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454(7203):428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 48.Choo Y. Y., Lee S., Nguyen P. H., et al. Caffeoylglycolic acid methyl ester, a major constituent of sorghum, exhibits anti-inflammatory activity via the Nrf 2/heme oxygenase-1 pathway. RSC Advances. 2015;5(23):17786–17796. doi: 10.1039/C4RA13847C. [DOI] [Google Scholar]
- 49.Gupta S. C., Sundaram C., Reuter S., Aggarwal B. B. Inhibiting NF-κB activation by small molecules as a therapeutic strategy. Biochimica et Biophysica Acta. 2010;1799(10–12):775–787. doi: 10.1016/j.bbagrm.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang F., Guan H., Liu D., Wu X., Fan M., Han J. Flavonoids from sea buckthorn inhibit the lipopolysaccharide-induced inflammatory response in RAW 264.7 macrophages through the MAPK and NF-κB pathways. Food & Function. 2017;8(3):1313–1322. doi: 10.1039/C6FO01873D. [DOI] [PubMed] [Google Scholar]
- 51.Bhat N. R., Zhang P., Lee J. C., Hogan E. L. Extracellular signal-regulated kinase and p 38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. The Journal of Neuroscience. 1998;18(5):1633–1641. doi: 10.1523/JNEUROSCI.18-05-01633.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Waetzig V., Czeloth K., Hidding U., et al. c-Jun N-terminal kinases (JNKs) mediate pro-inflammatory actions of microglia. Glia. 2005;50(3):235–246. doi: 10.1002/glia.20173. [DOI] [PubMed] [Google Scholar]
- 53.Li Q., Verma I. M. NF-κB regulation in the immune system. Nature Reviews Immunology. 2002;2(10):725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 54.Salminen A., Kauppinen A., Kaarniranta K. Phytochemicals suppress nuclear factor-κB signaling: impact on health span and the aging process. Current Opinion in Clinical Nutrition and Metabolic Care. 2012;15(1):23–28. doi: 10.1097/MCO.0b013e32834d3ae7. [DOI] [PubMed] [Google Scholar]
- 55.Persson T., Popescu B. O., Cedazo-Minguez A. Oxidative stress in Alzheimer’s disease: why did antioxidant therapy fail? Oxidative Medicine and Cellular Longevity. 2014;2014:11. doi: 10.1155/2014/427318.427318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li H., Ge Y., Luo Z., et al. Evaluation of the chemical composition, antioxidant and anti-inflammatory activities of distillate and residue fractions of sweet basil essential oil. Journal of Food Science and Technology. 2017;54(7):1882–1890. doi: 10.1007/s13197-017-2620-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qi S., Feng Z., Li Q., Qi Z., Zhang Y. Myricitrin modulates NADPH oxidase-dependent ROS production to inhibit endotoxin-mediated inflammation by blocking the JAK/STAT1 and NOX2/p47phox pathways. Oxidative Medicine and Cellular Longevity. 2017;2017:20. doi: 10.1155/2017/9738745.9738745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kacimi R., Giffard R. G., Yenari M. A. Endotoxin-activated microglia injure brain derived endothelial cells via NF-κB, JAK-STAT and JNK stress kinase pathways. Journal of Inflammation. 2011;8(1):p. 7. doi: 10.1186/1476-9255-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou H. Y., Shin E. M., Guo L. Y., et al. Anti-inflammatory activity of 4-methoxyhonokiol is a function of the inhibition of iNOS and COX-2 expression in RAW 264.7 macrophages via NF-κB, JNK and p38 MAPK inactivation. European Journal of Pharmacology. 2008;586(1–3):340–349. doi: 10.1016/j.ejphar.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 60.Sun G. Y., Chen Z., Jasmer K. J., et al. Quercetin attenuates inflammatory responses in BV-2 microglial cells: role of MAPKs on the Nrf 2 pathway and induction of heme oxygenase-1. PLoS One. 2015;10(10, article e0141509) doi: 10.1371/journal.pone.0141509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Naito Y., Takagi T. Heme oxygenase-1 and anti-inflammatory M2 macrophages. Archives of Biochemistry and Biophysics. 2014;564:83–88. doi: 10.1016/j.abb.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 62.Huang M. H., Wang B. S., Chiu C. S., et al. Antioxidant, antinociceptive, and anti-inflammatory activities of Xanthii Fructus extract. Journal of Ethnopharmacology. 2011;135(2):545–552. doi: 10.1016/j.jep.2011.03.057. [DOI] [PubMed] [Google Scholar]
- 63.Ben Salem M., Affes H., Athmouni K., et al. Chemicals compositions, antioxidant and anti-inflammatory activity of Cynara scolymus leaves extracts, and analysis of major bioactive polyphenols by HPLC. Evidence-based Complementary and Alternative Medicine. 2017;2017:14. doi: 10.1155/2017/4951937.4951937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chang T. N., Huang S. S., Chang Y. S., et al. Analgesic effects and mechanisms of anti-inflammation of taraxeren-3-one from Diospyros maritima in mice. Journal of Agricultural and Food Chemistry. 2011;59(17):9112–9119. doi: 10.1021/jf201375u. [DOI] [PubMed] [Google Scholar]
- 65.Song N., Xu W., Guan H., Liu X., Wang Y., Nie X. Several flavonoids from Capsella bursa-pastoris (L.) Medic. Asina Journal Of Traditional Medicines. 2007;2(5):218–222. [Google Scholar]
- 66.Kubínová R., Spačková V., Svajdlenka E., Lučivjanská K. Antioxidant activity of extracts and HPLC analysis of flavonoids from Capsella bursa-pastoris (L.) Medik. Ceska a Slovenska Farmacie. 2013;62(4):174–176. [PubMed] [Google Scholar]
- 67.Zhao L., Zhang Q., Ma W., Tian F., Shen H., Zhou M. A combination of quercetin and resveratrol reduces obesity in high-fat diet-fed rats by modulation of gut microbiota. Food & Function. 2017;8(12):4644–4656. doi: 10.1039/C7FO01383C. [DOI] [PubMed] [Google Scholar]
- 68.Cho Y. H., Kim N. H., Khan I., et al. Anti-inflammatory potential of quercetin-3-O-β-D-(“2”-galloyl)-glucopyranoside and quercetin isolated from Diospyros kaki calyx via suppression of MAP signaling molecules in LPS-induced RAW 264.7 macrophages. Journal of Food Science. 2016;81(10):C2447–C2456. doi: 10.1111/1750-3841.13497. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.