Abstract
Hormone replacement therapy (HRT) can alleviate estrogen deficiency symptoms especially during menopause. The present study aimed at investigating the effect of soy isoflavones as HRT on immunological and bone health-related parameters with a special focus on the interactions between immunological status and metabolism. Thirty healthy cyclic female Wistar rats were used in this experiment. Ten females were sham-operated, and 20 females were subjected to ovariectomy. Overiectomized (OVX) female rats were randomly divided into 2 groups: the control group (G1, OVX/casein) was fed a casein-based diet, and the second group (G2, OVX/soy) was fed a high soy isoflavone diet. Both groups were compared to a sham-operated group (G3, sham/casein). Treatments continued for 7 weeks. Feed intake, weight gain, and lymphoid organ relative weights were recorded. Some metabolic, immunological, and bone health-related parameters were measured. Moreover, nitric oxide (NO), malondialdehyde (MDA), and total antioxidant capacity (TAC) were determined. Bone histopathology and immunohistochemistry to estrogen receptor alpha (ERα) were done. Feeding soy to OVX females reduced feed intake, weight gain, relative lymphoid organ weight, and T-lymphocytes transformation. Soy isoflavone administration normalized nearly all metabolic and immunological parameters to a level comparable to the sham group via oxidative stress amelioration and bone ERα promotion. Soy isoflavones seemed to be good HRT in estrogen deprivation which modulated the appetite, weight gain, lipid profile, proinflammation, and bone turnover.
1. Introduction
The estrogen hormone contributes a substantial role in different aspects of body homeostasis and anabolism [1]. It exerts these effects via unexpected regulatory roles on oxidative stress [2], immune function [3], and several metabolic aspects including bone cells as well as adipose tissue [4]. The estrogen hormone gives signals through two main distinguished receptors: estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ) [5]. These receptors are widely spread all over the different body tissues such as reproductive [6], nervous [7], fat [8], liver [9], immune [10], cardiovascular [11], and bone tissues [12].
Estrogen hormone deficiency or depletion has been associated with several metabolic [13] and immunological alterations [14]. These alterations include dyslipidemia, increased appetite, and bone loss [13] that predispose metabolic syndrome along with predisposing autoimmunity and proinflammation [14]. The latter two are characterized by generalized defects in lymphocyte selection and homeostasis along with upregulation in cytokine production [15]. Homeostatic alterations due to estrogen depletion and menopause led researchers to suggest hormone replacement therapy (HRT) to combat their adverse effects.
Several endocrinological, metabolic, and immunological factors as well as oxidative stress were implied in the pathogenesis of these abnormalities along with the interactions with estrogen receptors [16]. Ghrelin is a stomach hormone acting centrally to promote appetite and body weight gain [17]. Moreover, resistin, tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6) are adipokine peptides, produced by adipocytes and cytokines that function in metabolic and immunological crosstalk [18]. Both ghrelin and adipokine transcription seemed to be influenced by the estrogen hormone [19]. The calcitonin hormone is beheld as a mediator for estrogen hormone action in bone tissue [20].
Soy isoflavones, a subclass of phytoestrogens, are compounds found in several legumes including soybeans and their products. They include several isoforms divided into four chemical forms: glucoside (genistin, daidzin, and glycitin), aglycone (genistein, daidzein, and glycitein), acetylglucoside (acetylgenistin, acetyldaidzin, and acetylglycitin), and malonylglucoside (malonylgenistin, malonyldaidzin, and malonylglycitin). Isoflavones are considered selective estrogen receptor modulators (SERMs) as they can interact with two estrogen receptor subtypes: ERα and ERβ [21]. They are widely used as dietary supplement in both animals and human diets [9, 22]. Several studies investigated their usage as HRT in case of estrogen depletion [22–26]. Epidemiological data demonstrated the protective effect of isoflavones against age-related chronic diseases [27] and cardiovascular diseases [28] as well as anti-breast cancer effects which were demonstrated by lower incidence in an eastern Asian population where soy predominates diet [29]. Isoflavones also have different biological influences in both animals and humans. These effects include antitumor [30], antimenopausal osteoporosis [31] and antidiabetic, antidyslipidemic [32], and anti-inflammatory effects [33] as well as protective effects against coronary heart diseases [34]. In addition, they exert a myriad of immunological [35, 36], metabolic [37], antioxidant power, and hepatoprotective effects in laboratory animal models [9]. Therefore, the current study aimed at investigating the effects of soy isoflavones as HRT on estrogen deprivation-associated metabolic, immunological, and bone health disturbances in OVX female Wistar rats. The study focused on the possible implication of isoflavones on ghrelin, adipokines, calcitonin, and some immunological parameters in estrogen deprivation conditions.
2. Material and Methods
2.1. Chemicals
The following chemicals were purchased from Sigma-Aldrich Co., Egypt: Roswell Park Memorial Institute- (RPMI-) 1640 media, trypan blue, fetal calf serum (FCS), phytohaemagglutinin (PHA), tetrazolium dye, and trichloroacetic acid. Ficoll was obtained from Biowest Co., France. Hydrochloric acid, methanol, and acetic acid used in the current study were of HPLC grade and purchased from Fisher Scientific Co., USA. Both genistein and daidzein were obtained from Fujicco Co., Japan, and used as HPLC standards.
2.2. Animals, Ovariectomy, and Experimental Procedure
Thirty healthy cyclic female Wistar rats were purchased from a lab animal house at the Faculty of Veterinary Medicine, Suez Canal University. They were 2 months old with a body weight ranging 95-105 g. Rats were kept for 2 weeks for acclimation at natural daylight rhythm and allowed free access to a casein-based diet and water ad libitum. All the experimental animals received humane care that accorded with the approved guidelines of the research ethical committee at the Faculty of Veterinary Medicine, Suez Canal University (protocol no. 2018058).
Ten females were sham-operated under 1% thiopental sodium 30 mg/kg intravenous anesthesia. The remaining 20 females were subjected to ovariectomy via midline incision according to the method of Lasota and Danowska-Klonowska [38]. All rats were gavaged with amoxicillin (Amoun Pharmaceutical Co., Egypt) of 10 mg/kg body weight as Ibiamox® in the form of oral suspension for 3 days after surgical intervention [39].
Three weeks after ovariectomy, OVX female rats were randomly divided into 2 groups, 10 rats each. The control group (G1, OVX/casein) was fed a casein-based diet (0% soy that contained 0 μg/g genistein and daidzein as determined by HPLC). The second group (G2, OVX/soy) was fed a high soy isoflavone diet (26.41% soy that contained 1500 μg/g genistein and 800 μg/g daidzein). The sham-operated group was fed a casein-based diet (G3, sham/casein).
All diets were formulated according to NRC [40] to fulfill all the nutritional requirements of adult rats (Table 1) and were given for 7 weeks.
Table 1.
Diet composition.
| Ingredients | Control (%) | High isoflavones (%) |
|---|---|---|
| Yellow corn | 40.59 | 35.04 |
| Corn gluten | 15.00 | — |
| Soybean∗ | — | 26.41 |
| Casein | 5.00 | 5. 00 |
| Sucrose | 22.43 | 22.32 |
| Starch | 7.63 | 4.16 |
| Cellulose | 1.30 | — |
| Corn oil | 5.00 | — |
| Soybean oil | — | 5.00 |
| Methionine | 0.30 | 0.43 |
| Lysine | 0.26 | — |
| Tryptophan | 0.39 | — |
| Ground limestone | 0.58 | 0.70 |
| Dicalcium phosphate | 1.09 | 0.57 |
| Common salt | 0.13 | 0.13 |
| Premix∗∗ | 0.30 | 0.30 |
| Total | 100.00 | 100.00 |
| Calculated values | ||
| CP %∗∗∗ | 17.11 | 17.11 |
| ME (kcal/kg) | 3708.26 | 3703.05 |
| C/P ratio | 216.70 | 216.50 |
| Ca | 0.50 | 0.50 |
| P | 0.30 | 0.30 |
| Dietary HPLC analysis data | ||
| Genistein (μg/g) | 0 | 1500 |
| Daidzein | 0 | 800 |
∗Soybean was autoclaved at 110°C for 30 minutes according to Westfall and Hauge [121] to inactivate the trypsin inhibitor, tannins, saponins, phytate, protease inhibitors, lectins, and goitrogens. ∗∗Each 3 kg contains the following vitamins and minerals: vit. A 12 mIU, vit. D3 2 mIU, vit. E 1000 mg, vit. k3 1000 mg, vit. B1 1000 mg, vit. B2 5000 mg, vit. B6 1500 mg, vit. B12 10 mg, biotin 50 mg, pantothenic acid 10000 mg, nicotinic acid 30000 mg, folic acid 1000 mg, manganese 60000 mg, zinc 50000 mg, iron 30000 mg, copper 4000 mg, iodine 300 mg, selenium 100 mg, cobalt 100 mg, and carrier (CaCO3) to 3 kg (Golden premix- Selim Pharm Elasher, Egypt.). ∗∗∗Analyzed according to Helrick [122].
2.3. High-Performance Liquid Chromatography (HPLC) Analysis
Dietary isoflavones were subjected to extraction from experimental diets through mixing 1 g of each diet with 20 mL of HCL solution 0.1 mol/L and 80 mL methanol. These ingredients were subjected to sonication for 20 minutes then left for 2 hours at room temperature. The later ingredients were filtered with a filter paper (Clifton, USA). The obtained filtrate was subjected to centrifugation at 10000 rpm for 5 minutes. The supernatant genistein and daidzein were separated and quantified by high-performance liquid chromatography (HPLC) using a reversed-phase column (#50164-U, Sigma-Aldrich Co., Egypt) by using a gradient mobile phase. Solvent A was 0.1% acetic acid, 10% methanol, and 89.9% water; solvent B was 0.1% acetic acid and 99.9% methanol. The solvent B amount was linearly increased from 20% at 0 min to 30% at 2 min to 70% at 30 min. Genistein and daidzein were detected at 260 nm, then they were quantified by comparison with external standards [41].
2.4. Feed Intake, Weight Gain, and Lymphoid Organ Weight
Feed intake and body weight gain were recorded/week according to Helmy et al. [39]. Cumulative feed intake and cumulative body weight gain were also calculated. Thymus and spleen were excised from each experimental rat and weighed. The relative thymus and spleen weights were calculated by dividing spleen or thymus weight (g) over body weight (g), then the obtained value was multiplied by 100.
2.5. Sampling
At the end of the experimental period, three retro-orbital blood samples were drawn under effect of diethyl ether anesthesia from overnight-fasted rats. The first sample was collected in ethylenediaminetetraacetic acid (EDTA), the second sample in lithium heparin, and the third sample in plain tubes. These samples were used for leukocyte count (total and differential), lymphocytes transformation test (LTT), and serum separation, respectively. Sera were separated from plain tubes, collected, and stored at −80°C. The tibia of each experimental animal was dissected and directly immersed in 10% neutral buffered formalin.
2.6. Lipid Profile, Bone Biomarkers, and Ghrelin Level
Serum levels of high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), and total cholesterol (TC) were estimated by the use of enzymatic calorimetric kits (ELITech Diagnostic, France) according to Tietz [42]. Low-density lipoprotein cholesterol (LDL-C) was estimated using an enzymatic calorimetric kit purchased from QAC Co., Spain, according to the manufacturer's protocol. Ionized calcium levels were calculated according to the equation described by Căpriță et al. [43]. X = [0.9 + (0.55 × Y–0.3 × Z)], where X is ionized calcium (mg/dL), Y is total Ca (mg/dL), and Z is albumin (mg/dL).
Both total calcium and total albumin were estimated according to Tietz [42] using enzymatic calorimetric kits (BIOLABO Reagents Co., Maizy, France, and Biodiagnostic, Egypt, respectively). Inorganic phosphorous levels (mg/dL) and alkaline phosphatase activity (ALP) (IU/L) were determined via commercial kits (Biodiagnostic, Egypt, and BIOLABO Reagents Co., Maizy, France, respectively) [42]. Serum ghrelin concentrations were analyzed by the radioimmunoassay method using a standardized rat RIA ghrelin kit (Phoenix Pharmaceuticals Inc., USA). The analytical procedures were done according to the manufacturer's enclosed protocol.
2.7. Enzyme-Linked Immunoassay (ELISA), Lipid Peroxidation, and Total Antioxidant Capacity (TAC)
Serum calcitonin and resistin concentrations were determined using commercial rat ELISA kits (Phoenix Pharmaceuticals Inc., USA, and BioVendor Co., Czech, respectively). Serum TNF-α (IBL Co., Japan), IL-2, C-reactive protein (CRP) (IBL Co., USA), cyclooxygenase-2 (COX-2) (IBL Co. Japan), and nitric oxide (NO) (Antibodies Online, Germany) levels were assayed using rat ELISA kits. All procedures were done according to the manufacturer's instructions. Malondialdehyde (MDA), a lipid peroxidation biomarker, was calorimetrically assayed using a commercial kit (BioVision, USA) according to Ohkawa et al. [44]. Serum total antioxidant capacity (TAC) was determined via a calorimetric kit (LDN, Germany). All steps were carried out according to the manufacturers' protocol.
2.8. Leukocyte Counts and Lymphocytes Transformation Test (LTT)
Blood samples collected in EDTA tubes were subjected to total (TLC) and differential leukocyte (DLC) counts according to Feldman et al. [45]. Freshly obtained lithium heparinized blood samples were immediately transferred to the laboratory in ice bags. Lymphocytes were separated using Ficoll at 2400 rpm for 40 minutes in a cooling centrifuge. Separated lymphocytes were washed and suspended in RPMI-1640 medium. The viable lymphocyte cell count was adjusted 2 × 106/mL using trypan blue and a hemocytometer slide [46]. The viable lymphocytes were suspended in RPMI-1640 medium supplemented with 10% FCS. Lymphocytes were assayed for their transformation ability against PHA mitogen (15 μg/mL) using methyl thiazolyl tetrazolium (MTT) staining procedures [47].
2.9. Histopathology and Immunohistochemistry (IHC)
Formalin-fixed tibia were subjected to decalcification in 5% trichloroacetic acid for 20 days then dehydrated and stained with H&E. All histopathological procedures were performed according to Bancroft and Gamble [48]. Paraffin-embedded tibia were subjected to immunohistochemistry (IHC) using a primary antibody for ERα (Thermo Scientific Co., UK) according to the methodology of Helmy et al. [39]. The percentages of the IHC-stained area (IHC area %) were obtained using ImageJ software according to Elgawish et al. [49].
2.10. Statistical Analysis
The obtained data were expressed as means ± SEM and subjected to analysis by one-way ANOVA using SPSS (IBM SPSS Statistics, version 22, USA). Differences among means were tested at the 5% probability level using Duncan's multiple range test.
3. Results
3.1. Feed Intake, Weight Gain, and Lymphoid Organ Weight
Cumulative feed intake significantly (p < 0.05) declined in G2 (OVX/soy) compared to G1 (OVX/casein). The sham-operated group (G3) showed a significant reduction in cumulative feed intake than did both ovarictomized groups (G1 and G2). Cumulative weight gains significantly (p < 0.05) reduced in G2 (OVX/soy) compared with those in G1 (OVX/casein). However, there was no difference (p > 0.05) observed between the OVX/soy group and the sham-operated one (Table 2). The relative weights of thymus reduced (p < 0.05) in soy-fed OVX females than in casein-fed OVX females and sham ones. Spleen weights showed nonsignificant alterations among the tested groups (Table 2).
Table 2.
Effect of soy isoflavones on feed intake (g/day/female), cumulative feed intake (g/female), weight gain (g/week), cumulative weight gain (g), and relative thymus and spleen weight (g%) among experimental groups.
| Parameters | G1 (OVX/casein) | G2 (OVX/soy) | G3 (sham/casein) | |
|---|---|---|---|---|
| Feed intake (g/day/female) | 1st week | 19.34 ± 1.11a | 17.20 ± 1.17a | 12.57 ± 0.68b |
| 2nd week | 18.75 ± 1.20a | 12.92 ± 0.86b | 11.65 ± 1.15b | |
| 3rd week | 17.40 ± 0.67a | 14.63 ± 0.56ab | 12.87 ± 1.5 1b | |
| 4th week | 16.42 ± 0.84a | 16.22 ± 1.03a | 11.77 ± 1.33b | |
| 5th week | 21.93 ± 2.77a | 18.51 ± 0.80ab | 13.21 ± 1.39b | |
| 6th week | 21.97 ± 3.44a | 18.37 ± 0.95ab | 13.80 ± 1.42b | |
| 7th week | 21.33 ± 1.36a | 16.68 ± 3.14a | 9.75 ± 0.57b | |
| Cumulative feed intake (g/female) | 956.22 ± 6.15a | 801.47 ± 5.41b | 599.48 ± 11.55c | |
|
| ||||
| Weight gain (g/week) | 2nd week | 19.70 ± 1.08a | 11.73 ± 1.91b | 9.47 ± 1.28b |
| 3rd week | 10.45 ± 1.58a | 10.65 ± 0.88a | 8.90 ± 1.10a | |
| 4th week | 12.19 ± 1.11a | 8.02 ± 0.80b | 5.37 ± 0.98b | |
| 5th week | 7.08 ± 1.12a | 6.73 ± 0.63a | 5.73 ± 0.37a | |
| 6th week | 6.32 ± 0.81a | 3.62 ± 0.40b | 2.97 ± 0.49b | |
| 7th week | 5.33 ± 0.96a | 1.05 ± 0.52b | 1.07 ± 0.47b | |
| Cumulative weight gain (g) | 44.12 ± 5.13a | 31.60 ± 5.01b | 23.45 ± 2.39b | |
|
| ||||
| Relative weight (g%) | Thymus | 0.35 ± 0.04a | 0.16 ± 0.02b | 0.32 ± 0.05a |
| Spleen | 0.58 ± 0.04a | 0.50 ± 0.09a | 0.45 ± 0.06a | |
a-cMeans in the same row with different superscripts are significantly different (p < 0.05); values are presented as means ± SEM.
3.2. Lipid Profile, Bone Biomarkers, and Ghrelin Level
Table 3 shows improvement in lipid profile in OVX females fed a soy diet. HDL-C significantly (p < 0.05) improved in the soy OVX group than in the casein OVX group. There was no significant difference in HDL-C between G2 and the sham-operated group. Serum levels of LDL-C, TG, and TC declined significantly (p < 0.05) in G2 than in G1. However, nonsignificant changes were observed between G2 and G3. Serum-ionized Ca and phosphorus levels were reduced (p < 0.05), while ALP activity was elevated (p < 0.05) in the soy OVX group than in the casein OVX group (Table 3). Ghrelin hormone level significantly (p < 0.05) reduced in G2 when compared to G1. No significant difference was observed between G2 and G3 (Table 3).
Table 3.
Effect of soy isoflavones on serum lipid profile, ghrelin, calcitonin, ionized calcium, inorganic phosphorus, and alkaline phosphatase (ALP) among experimental groups.
| Parameters | G1 (OVX/casein) | G2 (OVX/soy) | G3 (sham/soy) |
|---|---|---|---|
| HDL-C (mg/dL) | 10.19 ± 0.41a | 12.58 ± 0.44b | 14.01 ± 0.20b |
| LDL-C (mg/dL) | 65.72 ± 1.96a | 55.08 ± 0.94b | 51.31 ± 4.72b |
| TG (mg/dL) | 109.80 ± 4.85a | 83.65 ± 8.49b | 83.15 ± 2.21b |
| TC (mg/dL) | 66.85 ± 2.41a | 58.32 ± 1.27b | 55.89 ± 2.87b |
| Ghrelin (pg/mL) | 545.80 ± 3.49a | 370.80 ± 6.55b | 369.50 ± 13.87b |
| Calcitonin (pg/mL) | 172.46 ± 5.98b | 249.90 ± 4.19a | 252.83 ± 2.50a |
| Resistin (ng/mL) | 0.87 ± 0.05a | 0.59 ± 0.04b | 0.61 ± 0.04b |
| Ionized Ca+2 (mg/dL) | 6.77 ± 0.35a | 5.77 ± 0.19b | 5.19 ± 0.30b |
| Phosphorus (mg/dL) | 6.04 ± 0.12a | 5.10 ± 0.33b | 4.98 ± 0.37b |
| ALP (IU/L) | 126.00 ± 5.12b | 190.80 ± 12.88a | 179.3 ± 3.57a |
a-bMeans in the same row with different superscripts are significantly different (p < 0.05); values are presented as means ± SEM.
3.3. Enzyme-Linked Immunoassay (ELISA), Lipid Peroxidation, and Total Antioxidant Capacity (TAC)
Soy feeding to OVX female rats exhibited an elevated (p < 0.05) calcitonin level compared to casein-fed ones nearly equal to the sham group level. Resistin level revealed a significant (p < 0.05) reduction in G2 than in G1; however, there was no significant difference observed between G2 and G3 (Table 3). Serum TNF-α, IL-2, CRP, COX-2, and NO significantly (p < 0.05) reduced in G2 than in G1 while their values nonsignificantly differed with G3 (Table 4). The level of MDA revealed a highly significant (p < 0.01) elevation in G1 compared to G2 and G3. The level of TAC revealed a significant (p < 0.01) promotion in G2 than in G1. Both MDA and TAC showed a nonsignificant variation between G2 and G3 (Table 4).
Table 4.
Effect of soy isoflavones on lymphocyte transformation test (LTT), nitric oxide (NO), total antioxidant capacity (TAC), malondialdehyde (MDA), and inflammatory mediators among experimental groups.
| Parameters | G1 (OVX/casein) | G2 (OVX/soy) | G3 (sham/soy) |
|---|---|---|---|
| LTT | 0.65 ± 0.06a | 0.27 ± 0.04b | 0.51 ± 0.06a |
| NO (μM/L) | 29.41 ± 0.85a | 21.29 ± 0.91b | 21.84 ± 0.66b |
| TAC (mM/L) | 0.34 ± 0.12a | 0.82 ± 0.08b | 0.72 ± 0.04b |
| MDA (nM/L) | 1.97 ± 0.13a | 1.61 ± 0.07b | 1.53 ± 0.09b |
| TNF-alpha (pg/mL) | 8.99 ± 0.30a | 5.73 ± 0.56b | 5.25 ± 0.46b |
| IL-2 (pg/mL) | 5.74 ± 0.54a | 3.54 ± 0.27b | 3.39 ± 0.39b |
| COX-2 (ng/L) | 9.75 ± 0.42a | 6.70 ± 0.59b | 7.50 ± 0.44b |
| CRP (mg/L) | 1.56 ± 0.03a | 1.09 ± 0.07b | 1.04 ± 0.08b |
a-bMeans in the same row with different superscripts are significantly different (p ≤ 0.05); values are presented as means ± SEM.
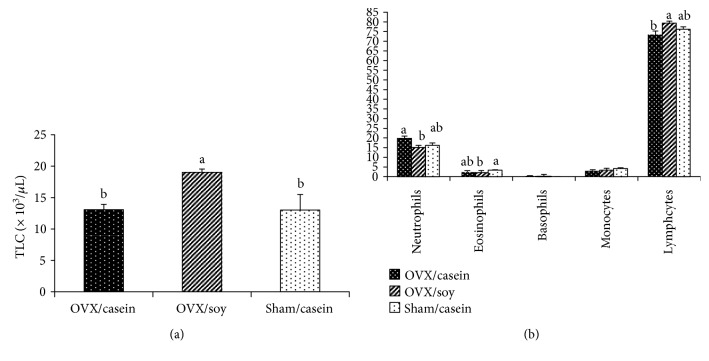
3.4. Leukocyte Counts and Lymphocytes Transformation Test (LTT)
Blood TLC was significantly (p < 0.05) elevated in the soy OVX group than in the casein OVX group and sham-operated ones (Figure 1(a)). Neutrophils % showed a significant (p < 0.05) reduction in G2 than in G1; however, sham-operated rats in G3 did not show any significant variation when compared with those of G1 and G2. Eosinophils % demonstrated a significant (p < 0.05) decline in G2 than in G3. Casein-fed OVX rats showed nonsignificant changes when compared with soy OVX and sham-operated ones. Basophils % and monocytes % revealed nonsignificant changes among tested groups. Lymphocytes % showed a significant (p < 0.05) increment in G2 than in G1 rats. Sham-operated rats in G3 did not reveal any significant change in lymphocytes % as compared with those of G1 and G2 (Figure 1(b)). Lymphocytes transformation exhibited significant (p < 0.05) suppression in G2 than in both G1 and G3. A nonsignificant difference was observed between G1 and G3 (Table 4).
Figure 1.
Effect of soy isoflavones on total (a) and differential leukocyte count (b) among experimental groups. Bars with different superscripts are significantly different at p < 0.05; values are presented as means ± SEM.
3.5. Histopathology and Immunohistochemistry
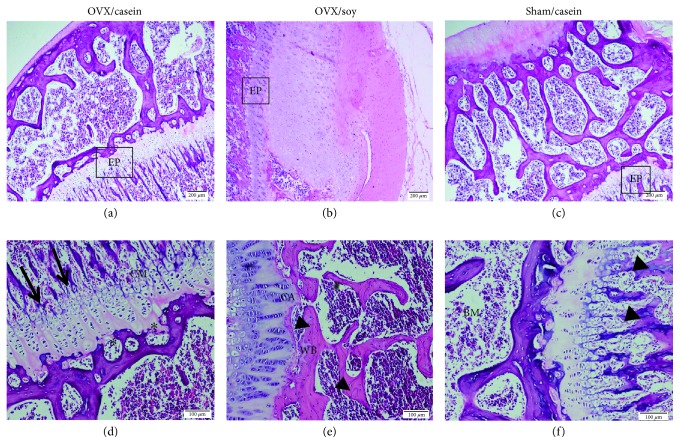
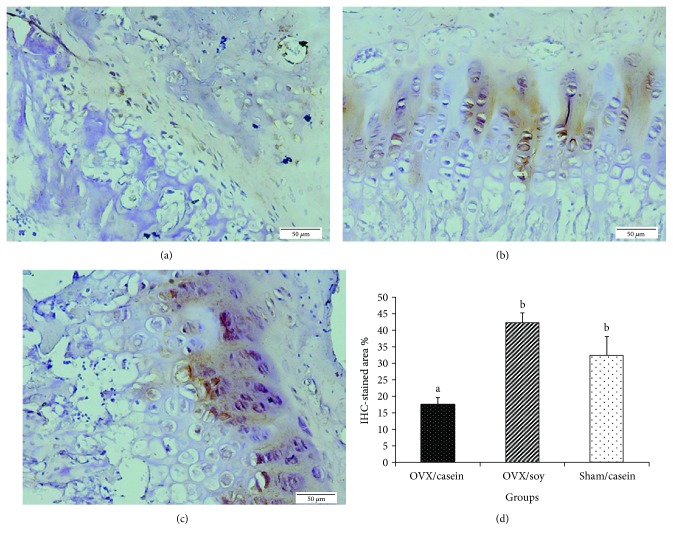
A histological examination of tibia from the treated and control groups are shown in Figure 2. After 7 weeks of ovariectomy, females fed a casein-based diet revealed changes in the growth of epiphyseal plate structure. The architecture of the growth plate showed fewer proliferative chondroblastic cells with few and thinner trabeculae as compared with sham and ovarictomized females fed a high soy isoflavone diet (Figures 2(a) and 2(d)). Also, the zone of cartilage ossification appeared to be resorbed. After 49 days of treatment with soy (Figures 2(b) and 3(e)), the tibia morphology was almost identical to that of the intact sham-operated group (Figures 2(c) and 2(f)). The epiphyseal plate was well developed and contained a typical arrangement of a proliferative, chondroblastic pattern. Besides, newly formed woven bones were observed which are a microscopic evidence of new bone formation, as well as well-developed bone trabeculae and ossification between cartilage and the zone of bone deposition. Soy isoflavones significantly (p < 0.001) increased the IHC-stained area % than the casein-fed OVX group, while there was no significant difference observed between G2 and G3 (Figure 3).
Figure 2.
Photomicrographs of epiphyseal bone and epiphyseal plate of rat tibia stained with H&E. (a, b, c) Scale bar: 200 μm. (d, e, f) Scale bar: 100 μm. (a, d) OVX/casein group, (b, e) OVX/soy group, and (c, f) sham/casein group. BM: bone marrow; WB: woven bone; CM: zone of cartilage maturation and hypertrophy; CA: zone of cartilage ossification between cartilage and the zone of bone deposition. Arrows represent resorbed bone trabeculae. Head arrows represent well-developed bone trabeculae. Asterisks represent resorbed bone trabeculae.
Figure 3.
A photomicrograph of a section from tibia bone showing estrogen receptor–α expression. (a) OVX/casein group, (b) OVX/soy group, (c) sham/casein group, and (d) a bar chart showing a comparison of the three groups in the estrogen receptor-α IHC-stained area % (IHC stain, scale bar: 50 μm).
4. Discussion
Metabolic and immunological disorders are common fate to estrogen deficiency or deprivation. In this case, the research for hormone replacement supplement is mandatory [25]. The usage of soy isoflavones as HRT to alleviate adverse effects of estrogen deficiency was tested in the present study. The level of soy isoflavones that was ingested by OVX females at the present study falls within the same range of soy ingested by the Asian population. Those people consumed 20 to 50 g of soy daily. Dietary isoflavone analysis by HPLC in the current study revealed 800 μg/g daidzein and 1500 μg/g genistein that were together equal to the Asian people intake which was estimated to be about 20 to 80 mg of phytoestrogens/day [50]. Moreover, dietary murine genistein equivalent to 1000 or 1500 μg/g was reported to produce serum genistein concentrations that matched the physiological range of humans under dietary phytoestrogens regimes [51]. Dietary genistein at 1500 μg/g was also reported by some publications to have immunological [52] and antilipogenic effects [53].
Cumulative feed intake and weight gains significantly declined in OVX females fed soy than casein-fed OVX ones to a level comparable to the sham-operated group. On the parallel side, ghrelin hormone followed the same trend of decrement. The possible explanation is that the absence of ovarian estrogen in OVX females led to a downregulation of hypothalamic estrogen receptors (ERs) restricting feed intake and modulating energy expenditure [54]. Hence, feed intake and body weight gain increased. Feeding soy isoflavones, as SERM, could result in overregulation of hypothalamic ERs that restricted feed intake and subsequent body weight gains to a level comparable to sham-operated females with intact ovaries. Moreover, soy isoflavones dramatically reduced the orexigenic ghrelin hormone level; therefore, it controlled the appetite-inducing action of ovariectomy [16] in this group. Our results were in partial agreement with Cederroth et al. [37] who demonstrated reduced feed intake and body weight gain without change in ghrelin hormone level in soy-fed mice.
Dietary soy had direct influences on lipid metabolism as it diminished TG, TC, and LDL-C and promoted HDL-C. These results were consistent with previous records of Tolba [55] and Yousefinejad et al. [56]. The observed hypolipidemic effect in G2 may be ascribed to the reduction in ghrelin hormone level which is considered a potent growth hormone secretagogue [57]. Ghrelin can promote white adipose tissue lipogenesis through a hypothalamic-mediated mechanism [58]. Thus, its reduction led to the observed hypolipidemia. Furthermore, soy isoflavones have the ability to decrease intestinal cholesterol absorption via increase in bile acid excretion [59]. Also, the capacity of soy isoflavones to decrease the lipid profile is related to AMPK activation which enhances fatty acid oxidation in liver and adipocytes [37].
Thymus relative weights significantly reduced in the soy OVX group than in OVX/casein and sham ones. However, splenic relative weights showed nonsignificant alterations. Our results coincided with those of Kakehashi et al. [60], Nishide et al. [61], and Ebaid et al. [36]. It is not surprising to find a similarity between dietary isoflavone exposure and estrogen's hormone action in mediating thymic atrophy [62] as well as suppression of LTT [63]. Isoflavones can overregulate and bind ERs especially ERα that exerts a potential restricting role to T-lymphocytes proliferation [64]. Soy genistein can also inhibit protein tyrosine kinases that subsequently suppress several white blood cells signaling cascades especially IL-2. These signaling cascades are involved in thymocytes and T-lymphocytes differentiation as well as their proliferation [65].
The dietary soy isoflavones significantly increased TLC with lymphocytosis at the expense of neutrophils than casein-fed rats. Current results were parallel to records of Jenkins et al. [66] and Cheng et al. [67]. Soy isoflavones mimicked estrogen in this group, therefore causing downregulation of adhesion molecules and chemokines that altered leukocytes recruitment and chemotaxis and thus exerting their anti-inflammatory action [68]. The elevation of lymphocytes percent on expense of neutrophils augmented the compensative effect of soy isoflavones to oxidative stress induced by gonadal removal. The relation between neutrophils and lymphocytes was used as indicator for inflammation [69], oxidative stress [70], and cortisol production [71]. The eosinopenia that happened in the soy-treated group was suggestive for the antiallergic effect of isoflavones. Administration of dietary soy isoflavones seemed to resemble estrogen action that could regulate eosinophils recruitment and cause their degranulation [72].
Ovariectomy accelerated oxidative stress that is demonstrated by increased NO, as an oxidative stress biomarker, and lipid peroxidation (MDA) with reduction in TAC that was normalized to the sham group level in the soy group. Our results were similar to those of Wang and Wu [73], Tang et al. [74], and Onuegbu et al. [75]. Oxidative stress is a casual factor for several metabolic and immunological disorders [76]. Lipid profile abnormalities observed in G1 could be the principle cause for lipid peroxidation and generation of excess reactive oxygen species (ROS) [9]. Soy isoflavone phenolic rings can act directly via free radical scavenging or indirectly via modulation of the pro- and antioxidant intracellular enzyme expression [77]. Furthermore, soy isoflavones can reduce inducible NO synthase enzyme and hence affect all physiological pathways that NO is involved in [78]. One of these pathways is leukocyte chemotactic response [79] which is manifested here by increased TLC in soy-treated rats.
Moreover, the function of NO as an intracellular messenger in chemokine signaling pathways [80] decreased and was manifested by decreasing levels of TNF-α and IL-2 in the soy OVX group to a level comparable to sham ones. These decrements were in accordance with results of Shalaby and Elgawish [81], Azadbakht et al. [26], and Gaffer et al. [35]. The scavenging effect of soy isoflavones to ROS which was demonstrated by restoration of TAC as well as reduction in MDA could entangle ROS-mediated NF-κB/TNF-α signaling activation [82]. In addition, soy isoflavones could promote ER expression that has a reciprocal antagonism NF-κB activity [83]. The latter promotes TNF-α production [82]. Moreover, ER promotion by soy isoflavones [65] could inhibit protein tyrosine kinase and topoisomerase II [63, 84]. These two enzymes are essential for IL-2 production. These results could briefly explain the decrease in LTT values where IL-2 plays a substantial role in T-cell proliferation in an autocrine manner [65, 85].
Our study demonstrated a significant reduction in serum resistin level in the soy-fed OVX group to a level around to that of sham-operated rats. Current results were harmonized with those of Chen et al. [86] and Zhang et al. [87]. Resistin is produced from adipose tissue and induces an inflammatory activation, with the production of TNF-α by macrophages through the NF-κB pathway [88]. These findings augmented the idea that resistin level is regulated and correlated to the level of TNF-α [89] as well as its pro-inflammatory potential [90]. This decrease in resistin levels could be implied for the effect of soy isoflavones on peroxisome proliferator-activated receptors (PPARs) in endothelial and mononuclear cells [91]. PPAR is a major factor involved in de novo fatty acid synthesis, adipocyte differentiation, and lipid accumulation [92]. Furthermore, PPARs cause resistin repression through direct binding to the resistin promoter [93]. The normalization of ovariectomy-induced resistin elevation by soy feeding was augmented with the reduction of body weight gain in such group. This was suggestive for the restoration of low abdominal fat mass, normal glucose metabolism, and insulin sensitivity that were impaired after ovariectomy (data not shown). The resistin hormone had a positive correlation with CRP level that is considered an inflammatory biomarker [94]; thus, CRP was significantly reduced in the soy OVX group than in the casein-fed OVX one. Decreased body weight gain was also attributed to CRP reduction due to a strong correlation between these two parameters [95]. The antioxidant potential of soy isoflavone polyphenolic ring had a negative influence on serum CRP as antioxidants negatively influence CRP production [96]. Furthermore, it is logic to find the downregulation of CRP level together with reduced TNF-α and IL-2 where CRP is produced by hepatic cells in response to such cytokines [97]. The decrement in CRP after soy isoflavone treatment was in harmony with results of Fanti et al. [98] and Jin et al. [99].
COX-2 is a critical proinflammatory enzyme that converts arachidonic acid to prostaglandins that have been implicated in pain and inflammation [100]. The reduction in COX-2 level in G2 could explain the anti-inflammatory effect of soy isoflavones in OVX rats. These results were generally consistent with those reported by Hooshmand et al. [101], Valles et al. [102], and Khan et al. [103]. Isoflavone modulation to estrogen receptors, as SERM, is involved in regulation of COX-2 production and its bioactivity [104]. Isoflavones especially genistein had a repressing action on NF-κB that in turn represses COX-2 genesis [101, 105]. Moreover, the antioxidant power of soy isoflavones has an inhibitory effect on the activation of protein kinase C [106] and activator protein-1 [107] that play a role in COX-2 promoter activity [100].
Ovariectomy hastens bone turnover, manifested by increased levels of ionized Ca2+ and phosphorous while decreased ALP activity denoted osteoblast activity. It also reduced chondroblastic cell proliferation with fewer and thinner trabeculae as well as resorption in the zone of cartilage ossification. These changes were also accompanied with reduced calcitonin hormone level that is known to be decreased in gonadal hormone deficiency [108]. The reduction in bone mass was attributed to depletion of ovarian estrogen and its bone receptors as observed in IHC. Soy feeding to OVX females promoted an IHC-stained area % of ERα, which decreased osteoclast activity while promoting osteoblast activity [109] through upregulation of calcitonin hormone level [20]. Therefore, they decreased serum-ionized Ca2+ and phosphorous levels favoring their deposition in the bone matrix as well as elevated ALP activity [110] to a level near the sham group. Elevated ALP activity may indicate active bone formation, as it is a byproduct of osteoblast activity [111]. In addition, the reduction in ghrelin level in G2 could be related to the decreased bone density as it directly influenced osteoblast function or indirectly via the growth hormone insulin-like growth factor axis [112]. Our results concerning bone restoration, calcitonin, ionized Ca2+, phosphorous, and ALP activity were in agreement with Zhong and Yamaguchi [113], Lee et al. [114], Wafay et al. [115], Nurrochmad et al. [116], and Hassan et al. [117]. Both TNF-α and IL-2 are present in the bone microenvironment, and their levels are indicative for bone health. Elevation of such cytokines led to progression of bone turnover and resorption [118]. The secretion of such cytokines was inhibited through the estrogenic influence of soy isoflavones. Their estrogenic action is attributed to reduction in bone turnover and increased osteoblastic activity [119] of OVX females in order to restore estrogen depletion. The estrogenic effect of soy isoflavones seemed to be overlapped with its ROS scavenging effect at the bone level. The antioxidant power of isoflavone polyphenols was able to scavenge excessive nitric oxide and MDA as well as promotion of TAC. These effects neutralized the ovariectomy of ROS that incriminated in the pathogenesis of bone loss excessive activity of osteoclasts and bone mineralization [120]. Therefore, a histopathological picture of the Soy/OVX group showed ossification improvements by presenting a well-developed epiphyseal plate that contained proliferative chondroblasts, besides the presence of newly formed woven bone and well-developed bone trabeculae.
5. Conclusion
Ovariectomy as a model for estrogen depletion resulted in a myriad of metabolic alterations and bone turnover that were promoted by excessive ROS production. Feeding soy isoflavones improved the lipid profile and subsequently the antioxidant reserve that exerted an anti-inflammatory effect and improved bone mineralization via the calcitonin hormone. Moreover, soy feeding restored ER deficiency that is implicated in appetite promotion, proinflammation, and bone loss, thus overcoming these deleterious effects.
Data Availability
No data were used to support this study.
Conflicts of Interest
The authors of this manuscript declare that there are no financial or scientific conflicts of interest to disclose.
Supplementary Materials
Supplementary material contains a graphical abstract that describes the experimental design and results with implications of these results.
References
- 1.Weitzmann M. N., Pacifici R. Estrogen deficiency and bone loss: an inflammatory tale. The Journal of Clinical Investigation. 2006;116(5):1186–1194. doi: 10.1172/JCI28550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White R. E., Gerrity R., Barman S. A., Han G. Estrogen and oxidative stress: a novel mechanism that may increase the risk for cardiovascular disease in women. Steroids. 2010;75(11):788–793. doi: 10.1016/j.steroids.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao L., Huang S., Mei S., et al. Pharmacological activation of estrogen receptor beta augments innate immunity to suppress cancer metastasis. Proceedings of National Academy of Sciences of the United States of America. 2018;115(16):E3673–E3681. doi: 10.1073/pnas.1803291115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trenti A., Tedesco S., Boscaro C., Trevisi L., Bolego C., Cignarella A. Estrogen, angiogenesis, immunity and cell metabolism: solving the puzzle. International Journal of Molecular Sciences. 2018;19(3) doi: 10.3390/ijms19030859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuiper G. G., Enmark E., Pelto-Huikko M., Nilsson S., Gustafsson J. A. Cloning of a novel receptor expressed in rat prostate and ovary. Proceedings of National Academy of Sciences of the United States of America. 1996;93(12):5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang H., Eriksson H., Sahlin L. Estrogen receptors α and β in the female reproductive tract of the rat during the estrous cycle. Biology of Reproduction. 2000;63(5):1331–1340. doi: 10.1095/biolreprod63.5.1331. [DOI] [PubMed] [Google Scholar]
- 7.Almey A., Milner T. A., Brake W. G. Estrogen receptors in the central nervous system and their implication for dopamine-dependent cognition in females. Hormones and Behavior. 2015;74:125–138. doi: 10.1016/j.yhbeh.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blüher M. Importance of estrogen receptors in adipose tissue function. Molecular Metabolism. 2013;2(3):130–132. doi: 10.1016/j.molmet.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelany O. E., Khaled H. E., el-Nahla A. M., Abdelrazek H. M. A., Abdel-Daim M. M. Hepatoprotective and metabolic effects of dietary soy phytoestrogens against hyper caloric diet in cyclic female albino rats is mediated through estradiol receptors beta. Biomedical and Pharmacology Journal. 2017;10(3):1061–1069. doi: 10.13005/bpj/1203. [DOI] [Google Scholar]
- 10.Yakimchuk K., Jondal M., Okret S. Estrogen receptor α and β in the normal immune system and in lymphoid malignancies. Molecular and Cellular Endocrinology. 2013;375(1-2):121–129. doi: 10.1016/j.mce.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 11.Pugach E. K., Blenck C. L., Dragavon J. M., Langer S. J., Leinwand L. A. Estrogen receptor profiling and activity in cardiac myocytes. Molecular and Cellular Endocrinology. 2016;431:62–70. doi: 10.1016/j.mce.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Windahl S. H., Andersson G., Gustafsson J. A. Elucidation of estrogen receptor function in bone with the use of mouse models. Trends in Endocrinology & Metabolism. 2002;13(5):195–200. doi: 10.1016/S1043-2760(02)00594-5. [DOI] [PubMed] [Google Scholar]
- 13.Ezzat-Zadeh Z., Kim J.-S., Chase P. B., Arjmandi B. H. The cooccurrence of obesity, osteoporosis, and sarcopenia in the ovariectomized rat: a study for modeling osteosarcopenic obesity in rodents. Journal of Aging Research. 2017;2017:11. doi: 10.1155/2017/1454103.1454103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cutolo M., Capellino S., Sulli A., et al. Estrogens and autoimmune diseases. Annals of the New York Academy of Sciences. 2006;1089(1):538–547. doi: 10.1196/annals.1386.043. [DOI] [PubMed] [Google Scholar]
- 15.Davidson A., Diamond B. Autoimmune diseases. The New England Journal of Medicine. 2001;345(5):340–350. doi: 10.1056/NEJM200108023450506. [DOI] [PubMed] [Google Scholar]
- 16.Lizcano F., Guzmán G. Estrogen deficiency and the origin of obesity during menopause. BioMed Research International. 2014;2014:11. doi: 10.1155/2014/757461.757461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakazato M., Murakami N., Date Y., et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409(6817):194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 18.Kang Y. E., Kim J. M., Joung K. H., et al. The roles of adipokines, proinflammatory cytokines, and adipose tissue macrophages in obesity-associated insulin resistance in modest obesity and early metabolic dysfunction. PLoS One. 2016;11(4, article e0154003) doi: 10.1371/journal.pone.0154003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stefańska A., Odrowąż-Sypniewska G. Metabolic syndrome, adipokines and sex hormone concentrations in middle-aged women. Medical Research Journal. 2014;2(3):77–83. [Google Scholar]
- 20.Agnusdei D., Civitelli R., Camporeale A., Gennari C. Calcitonin and estrogens. Journal of Endocrinological Investigation. 1990;13(8):625–630. doi: 10.1007/BF03349583. [DOI] [PubMed] [Google Scholar]
- 21.Brzezinski A., Debi A. Phytoestrogens: the “natural” selective estrogen receptor modulators? European Journal of Obstetrics & Gynecology and Reproductive Biology. 1999;85(1):47–51. doi: 10.1016/S0301-2115(98)00281-4. [DOI] [PubMed] [Google Scholar]
- 22.Ahsan M., Mallick A. K. The effect of soy isoflavones on the menopause rating scale scoring in perimenopausal and postmenopausal women: a pilot study. Journal of Clinical and Diagnostic Research. 2017;11(9):FC13–FC16. doi: 10.7860/JCDR/2017/26034.10654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ţiţ D. M., Pallag A., Iovan C., Furău G., Furău C., Bungău S. Somatic-vegetative symptoms evolution in postmenopausal women treated with phytoestrogens and hormone replacement therapy. Iranian Journal of Public Health. 2017;46(11):1528–1534. [PMC free article] [PubMed] [Google Scholar]
- 24.Bumbu A., Paşca B., Ţiţ D., Bungău S., Bumbu G. The effects of soy isoflavones and hormonal replacing therapy on the incidence and evolution of postmenopausal female urinary incontinence. Farmácia. 2016;64(3):419–422. [Google Scholar]
- 25.Aguiar P. M., de Paula Barbosa A. Use of soy isoflavones on hormone replacement therapy during climacteric. African Journal of Pharmacy and Pharmacology. 2014;8(42):1071–1078. [Google Scholar]
- 26.Azadbakht L., Kimiagar M., Mehrabi Y., Esmaillzadeh A., Hu F. B., Willett W. C. Soy consumption, markers of inflammation, and endothelial function: a cross-over study in postmenopausal women with the metabolic syndrome. Diabetes Care. 2007;30(4):967–973. doi: 10.2337/dc06-2126. [DOI] [PubMed] [Google Scholar]
- 27.Xiao C. W. Health effects of soy protein and isoflavones in humans. The Journal of Nutrition. 2008;138(6):1244S–1249S. doi: 10.1093/jn/138.6.1244S. [DOI] [PubMed] [Google Scholar]
- 28.Gil-Izquierdo A., Penalvo J. L., Gil J. I., et al. Soy isoflavones and cardiovascular disease epidemiological, clinical and -omics perspectives. Current Pharmaceutical Biotechnology. 2012;13(5):624–631. doi: 10.2174/138920112799857585. [DOI] [PubMed] [Google Scholar]
- 29.Chen M., Rao Y., Zheng Y., et al. Association between soy isoflavone intake and breast cancer risk for pre- and post-menopausal women: a meta-analysis of epidemiological studies. PLoS One. 2014;9(2, article e89288) doi: 10.1371/journal.pone.0089288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stubert J., Gerber B. Isoflavones – mechanism of action and impact on breast cancer risk. Breast Care. 2009;4(1):22–29. doi: 10.1159/000200980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taku K., Melby M. K., Nishi N., Omori T., Kurzer M. S. Soy isoflavones for osteoporosis: an evidence-based approach. Maturitas. 2011;70(4):333–338. doi: 10.1016/j.maturitas.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Mezei O., Banz W. J., Steger R. W., Peluso M. R., Winters T. A., Shay N. Soy isoflavones exert antidiabetic and hypolipidemic effects through the PPAR pathways in obese Zucker rats and murine RAW 264.7 cells. The Journal of Nutrition. 2003;133(5):1238–1243. doi: 10.1093/jn/133.5.1238. [DOI] [PubMed] [Google Scholar]
- 33.Yu J., Bi X., Yu B., Chen D. Isoflavones: anti-inflammatory benefit and possible caveats. Nutrients. 2016;8(6):p. 361. doi: 10.3390/nu8060361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wenzel U., Fuchs D., Daniel H. Protective effects of soy-isoflavones in cardiovascular disease. Identification of molecular targets. Hämostaseologie. 2008;28(1-2):85–88. doi: 10.1055/s-0037-1616927. [DOI] [PubMed] [Google Scholar]
- 35.Gaffer G. G., Elgawish R. A., Abdelrazek H. M. A., Ebaid H. M., Tag H. M. Dietary soy isoflavones during pregnancy suppressed the immune function in male offspring albino rats. Toxicology Reports. 2018;5:296–301. doi: 10.1016/j.toxrep.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ebaid H. M., Elgawish R. A. R., Abdelrazek H. M. A., Gaffer G., Tag H. M. Prenatal exposure to soy isoflavones altered the immunological parameters in female rats. International Journal of Toxicology. 2016;35(3):274–283. doi: 10.1177/1091581815625595. [DOI] [PubMed] [Google Scholar]
- 37.Cederroth C. R., Vinciguerra M., Gjinovci A., et al. Dietary phytoestrogens activate AMP-activated protein kinase with improvement in lipid and glucose metabolism. Diabetes. 2008;57(5):1176–1185. doi: 10.2337/db07-0630. [DOI] [PubMed] [Google Scholar]
- 38.Lasota A., Danowska-Klonowska D. Experimental osteoporosis-different methods of ovariectomy in female white rats. Roczniki Akademii Medycznej w Białymstoku. 2004;49(Supplement 1):129–131. [PubMed] [Google Scholar]
- 39.Helmy S., Emarah H., Abdelrazek H. Estrogenic effect of soy phytoestrogens on the uterus of ovariectomized female rats. Clinical Pharmacology & Biopharmaceutics. 2014;S2(e001) doi: 10.4172/2167-065X.S2-001. [DOI] [Google Scholar]
- 40.NRC. Nutrient Requirements of Laboratory Animals. 4th. Washington, DC, USA: National Academic Press; 1995. [Google Scholar]
- 41.Thiagarajan D. G., Bennink M. R., Bourquin L. D., Kavas F. A. Prevention of precancerous colonic lesions in rats by soy flakes, soy flour, genistein, and calcium. The American Journal of Clinical Nutrition. 1998;68(6):1394S–1399S. doi: 10.1093/ajcn/68.6.1394S. [DOI] [PubMed] [Google Scholar]
- 42.Tietz N. W. Tietz Clinical Guide to Laboratory Tests. WB Saunders Company; 1990. [Google Scholar]
- 43.Căpriță R., Caprita A., Cretescu I. Estimation of ionized calcium and corrected total calcium concentration based on serum albumin level. Scientific Papers Animal Science and Biotechnologies. 2013;46(1):180–184. [Google Scholar]
- 44.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 45.Feldman B., Zinki J., Jain V. Schalm’s Veterinary Hematology. 5th. Lippincot Wilkins; 2000. [Google Scholar]
- 46.Hudson L., Hay F. Practical Immunology. Blackwell Scientific Publication; 1980. [Google Scholar]
- 47.Abdelrazek H., Yusuf M., Ismail S., Elgawish R. Effect of probiotic strains mixture administration on serum interleukins concentration, lymphocyte proliferation and DNA damage in rams. Journal of Animal and Feed Sciences. 2015;24(4):302–307. doi: 10.22358/jafs/65612/2015. [DOI] [Google Scholar]
- 48.Bancroft J. D., Gamble M. Theory and Practice of Histological Techniques: Elsevier Health Sciences. Philadelphia, PA, USA: Churchill Livingstone/Elsevier; 2008. [Google Scholar]
- 49.Elgawish R. A. R., Rahman H. G. A., Abdelrazek H. M. A. Green tea extract attenuates CCl4-induced hepatic injury in male hamsters via inhibition of lipid peroxidation and p53-mediated apoptosis. Toxicology Reports. 2015;2:1149–1156. doi: 10.1016/j.toxrep.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adlercreutz H., Honjo H., Higashi A., et al. Urinary excretion of lignans and isoflavonoid phytoestrogens in Japanese men and women consuming a traditional Japanese diet. The American Journal of Clinical Nutrition. 1991;54(6):1093–1100. doi: 10.1093/ajcn/54.6.1093. [DOI] [PubMed] [Google Scholar]
- 51.Yellayi S., Zakroczymski M. A., Selvaraj V., et al. The phytoestrogen genistein suppresses cell-mediated immunity in mice. Journal of Endocrinology. 2003;176(2):267–274. doi: 10.1677/joe.0.1760267. [DOI] [PubMed] [Google Scholar]
- 52.Wei J., Bhatt S., Chang L. M., Sampson H. A., Masilamani M. Isoflavones, genistein and daidzein, regulate mucosal immune response by suppressing dendritic cell function. PLoS One. 2012;7(10, article e47979) doi: 10.1371/journal.pone.0047979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Naaz A., Yellayi S., Zakroczymski M. A., et al. The soy isoflavone genistein decreases adipose deposition in mice. Endocrinology. 2003;144(8):3315–3320. doi: 10.1210/en.2003-0076. [DOI] [PubMed] [Google Scholar]
- 54.Xu Y., Nedungadi T. P., Zhu L., et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metabolism. 2011;14(4):453–465. doi: 10.1016/j.cmet.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tolba E. A.-E. H. T. Dietary phytoestrogens reduce the leptin level in ovariectomized female rats. International Journal of Chemical, Environmental & Biological Sciences. 2013;1(3) [Google Scholar]
- 56.Yousefinejad A., Siassi F., Javanbakht M. H., et al. Effect of genistein and L-carnitine and their combination on lipid profile and inflammatory cytokines in experimental nephrotic syndrome. Reports of Biochemistry and Molecular Biology. 2018;7(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- 57.Khatib N., Gaidhane S., Gaidhane A. M., et al. Ghrelin: ghrelin as a regulatory peptide in growth hormone secretion. Journal of Clinical and Diagnostic Research. 2014;8(8):MC13–MC17. doi: 10.7860/JCDR/2014/9863.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sangiao-Alvarellos S., Vázquez M. J., Varela L., et al. Central ghrelin regulates peripheral lipid metabolism in a growth hormone-independent fashion. Endocrinology. 2009;150(10):4562–4574. doi: 10.1210/en.2009-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Greaves K. A., Wilson M. D., Rudel L. L., Williams J. K., Wagner J. D. Consumption of soy protein reduces cholesterol absorption compared to casein protein alone or supplemented with an isoflavone extract or conjugated equine estrogen in ovariectomized cynomolgus monkeys. The Journal of Nutrition. 2000;130(4):820–826. doi: 10.1093/jn/130.4.820. [DOI] [PubMed] [Google Scholar]
- 60.Kakehashi A., Tago Y., Yoshida M., et al. Hormonally active doses of isoflavone aglycones promote mammary and endometrial carcinogenesis and alter the molecular tumor environment in Donryu rats. Toxicological Sciences. 2012;126(1):39–51. doi: 10.1093/toxsci/kfs016. [DOI] [PubMed] [Google Scholar]
- 61.Nishide Y., Tadaishi M., Kobori M., et al. Possible role of S-equol on bone loss via amelioration of inflammatory indices in ovariectomized mice. Journal of Clinical Biochemistry and Nutrition. 2013;53(1):41–48. doi: 10.3164/jcbn.12-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kohen F., Abel L., Sharp A., et al. Estrogen-receptor expression and function in thymocytes in relation to gender and age. Developmental Immunology. 1998;5(4):277–285. doi: 10.1155/1998/62380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Atluru S., Atluru D. Evidence that genistein, a protein-tyrosine kinase inhibitor, inhibits CD28 monoclonal-antibody-stimulated human T cell proliferation. Transplantation. 1991;51(2):448–450. doi: 10.1097/00007890-199102000-00035. [DOI] [PubMed] [Google Scholar]
- 64.Pierdominici M., Maselli A., Colasanti T., et al. Estrogen receptor profiles in human peripheral blood lymphocytes. Immunology Letters. 2010;132(1-2):79–85. doi: 10.1016/j.imlet.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 65.Atluru D., Jackson T. M., Atluru S. Genistein, a selective protein tyrosine kinase inhibitor, inhibits interleukin-2 and leukotriene B4 production from human mononuclear cells. Clinical Immunology and Immunopathology. 1991;59(3):379–387. doi: 10.1016/0090-1229(91)90033-7. [DOI] [PubMed] [Google Scholar]
- 66.Jenkins D. J. A., Kendall C. W. C., Connelly P. W., et al. Effects of high- and low-isoflavone (phytoestrogen) soy foods on inflammatory biomarkers and proinflammatory cytokines in middle-aged men and women. Metabolism. 2002;51(7):919–924. doi: 10.1053/meta.2002.33352. [DOI] [PubMed] [Google Scholar]
- 67.Cheng W.-C., Lo S.-C., Tsai K.-S., et al. Effects of high-dose phytoestrogens on circulating cellular microparticles and coagulation function in postmenopausal women. Journal of the Formosan Medical Association. 2015;114(8):710–716. doi: 10.1016/j.jfma.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 68.Ugochukwu C. N. C., Ebong P. E., Eyong E. U. Biochemical implication of long term administration of Halofantrine hydrochloride (Halfan) on estradiol levels of female Wistar rats. Pakistan Journal of Nutrition. 2008;7(2):227–230. doi: 10.3923/pjn.2008.227.230. [DOI] [Google Scholar]
- 69.Zahorec R. Ratio of neutrophil to lymphocyte counts-rapid and simple parameter of systemic inflammation and stress in critically ill. Bratislavske lekarske listy. 2001;102(1):5–14. [PubMed] [Google Scholar]
- 70.Kulaksizoglu B., Kulaksizoglu S. Relationship between neutrophil/lymphocyte ratio with oxidative stress and psychopathology in patients with schizophrenia. Neuropsychiatric Disease and Treatment. 2016;12:1999–2005. doi: 10.2147/NDT.S110484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dionigi R., Dominioni L., Benevento A., et al. Effects of surgical trauma of laparoscopic vs. open cholecystectomy. Hepato-Gastroenterology. 1994;41(5):471–476. [PubMed] [Google Scholar]
- 72.Keselman A., Heller N. Estrogen signaling modulates allergic inflammation and contributes to sex differences in asthma. Frontiers in Immunology. 2015;6:p. 568. doi: 10.3389/fimmu.2015.00568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang B., Wu C. Dietary soy isoflavones alleviate dextran sulfate sodium‑induced inflammation and oxidative stress in mice. Experimental and Therapeutic Medicine. 2017;14(1):276–282. doi: 10.3892/etm.2017.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tang X.-L., Liu X.-J., Tian Q., Zhang W. Dynamic oxidative stress and DNA damage induced by oestrogen deficiency and protective effects of puerarin and 17β-oestradiol in ovariectomized rats. Basic & Clinical Pharmacology & Toxicology. 2012;111(2):87–91. doi: 10.1111/j.1742-7843.2012.00864.x. [DOI] [PubMed] [Google Scholar]
- 75.Onuegbu A. J., Olisekodiaka J. M., Irogue S. E., et al. Consumption of soymilk reduces lipid peroxidation but may lower micronutrient status in apparently healthy individuals. Journal of Medicinal Food. 2018;21(5):506–510. doi: 10.1089/jmf.2017.0094. [DOI] [PubMed] [Google Scholar]
- 76.Seifried H. E., Anderson D. E., Fisher E. I., Milner J. A. A review of the interaction among dietary antioxidants and reactive oxygen species. The Journal of Nutritional Biochemistry. 2007;18(9):567–579. doi: 10.1016/j.jnutbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 77.Valsecchi A. E., Franchi S., Panerai A. E., Rossi A., Sacerdote P., Colleoni M. The soy isoflavone genistein reverses oxidative and inflammatory state, neuropathic pain, neurotrophic and vasculature deficits in diabetes mouse model. European Journal of Pharmacology. 2011;650(2-3):694–702. doi: 10.1016/j.ejphar.2010.10.060. [DOI] [PubMed] [Google Scholar]
- 78.Jantaratnotai N., Utaisincharoen P., Sanvarinda P., Thampithak A., Sanvarinda Y. Phytoestrogens mediated anti-inflammatory effect through suppression of IRF-1 and pSTAT1 expressions in lipopolysaccharide-activated microglia. International Immunopharmacology. 2013;17(2):483–488. doi: 10.1016/j.intimp.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 79.Trifilieff A., Fujitani Y., Mentz F., Dugas B., Fuentes M., Bertrand C. Inducible nitric oxide synthase inhibitors suppress airway inflammation in mice through down-regulation of chemokine expression. The Journal of Immunology. 2000;165(3):1526–1533. doi: 10.4049/jimmunol.165.3.1526. [DOI] [PubMed] [Google Scholar]
- 80.Cherla R. P., Ganju R. K. Stromal cell-derived factor 1α-induced chemotaxis in T cells is mediated by nitric oxide signaling pathways. The Journal of Immunology. 2001;166(5):3067–3074. doi: 10.4049/jimmunol.166.5.3067. [DOI] [PubMed] [Google Scholar]
- 81.Shalaby A., Elgawish R. A. R. Influence of dietary soy phytoestrogens on cytokine production and immunoglobulin in ovariectomized rats. International Journal of Advances in Chemical Engineering and Biological Sciences. 2015;2(2) doi: 10.15242/ijacebs.er1215037. [DOI] [Google Scholar]
- 82.Karin M., Delhase M. The IκB kinase (IKK) and NF-κB: key elements of proinflammatory signalling. Seminars in Immunology. 2000;12(1):85–98. doi: 10.1006/smim.2000.0210. [DOI] [PubMed] [Google Scholar]
- 83.Evans M. J., Eckert A., Lai K., Adelman S. J., Harnish D. C. Reciprocal antagonism between estrogen receptor and NF-κB activity in vivo. Circulation Research. 2001;89(9):823–830. doi: 10.1161/hh2101.098543. [DOI] [PubMed] [Google Scholar]
- 84.Chiaffarino F., Biffi M., Luciano A., Gromo G., Leoni F. Involvement of multiple protein kinases in CD3-mediated activation of human T lymphocytes. Cellular Immunology. 1994;153(1):39–51. doi: 10.1006/cimm.1994.1004. [DOI] [PubMed] [Google Scholar]
- 85.Smith K. A. Interleukin-2: inception, impact, and implications. Science. 1988;240(4856):1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- 86.Chen S. W., Zhang H. M., Zhang L. S., Feng X. F. Effects of soy isoflavone on gene expression of resistin in insulin-resistance rats. Sichuan da xue xue bao Yi xue ban. 2006;37(5):717–720. [PubMed] [Google Scholar]
- 87.Zhang H.-M., Chen S.-W., Zhang L.-S., Feng X.-F. The effects of soy isoflavone on insulin sensitivity and adipocytokines in insulin resistant rats administered with high-fat diet. Natural Product Research. 2008;22(18):1637–1649. doi: 10.1080/14786410701869598. [DOI] [PubMed] [Google Scholar]
- 88.Silswal N., Singh A. K., Aruna B., Mukhopadhyay S., Ghosh S., Ehtesham N. Z. Human resistin stimulates the pro-inflammatory cytokines TNF-α and IL-12 in macrophages by NF-κB-dependent pathway. Biochemical and Biophysical Research Communications. 2005;334(4):1092–1101. doi: 10.1016/j.bbrc.2005.06.202. [DOI] [PubMed] [Google Scholar]
- 89.Lehrke M., Reilly M. P., Millington S. C., Iqbal N., Rader D. J., Lazar M. A. An inflammatory cascade leading to hyperresistinemia in humans. PLoS Medicine. 2004;1(2, article e45) doi: 10.1371/journal.pmed.0010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reilly M. P., Lehrke M., Wolfe M. L., Rohatgi A., Lazar M. A., Rader D. J. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation. 2005;111(7):932–939. doi: 10.1161/01.CIR.0000155620.10387.43. [DOI] [PubMed] [Google Scholar]
- 91.Rius C., Abu-Taha M., Hermenegildo C., et al. Trans- but not Cis-resveratrol impairs angiotensin-II–mediated vascular inflammation through inhibition of NF-κB activation and peroxisome proliferator-activated receptor-γ upregulation. The Journal of Immunology. 2010;185(6):3718–3727. doi: 10.4049/jimmunol.1001043. [DOI] [PubMed] [Google Scholar]
- 92.Jump D. B., Botolin D., Wang Y., Xu J., Christian B., Demeure O. Fatty acid regulation of hepatic gene transcription. The Journal of Nutrition. 2005;135(11):2503–2506. doi: 10.1093/jn/135.11.2503. [DOI] [PubMed] [Google Scholar]
- 93.Patel L., Buckels A. C., Kinghorn I. J., et al. Resistin is expressed in human macrophages and directly regulated by PPARγ activators. Biochemical and Biophysical Research Communications. 2003;300(2):472–476. doi: 10.1016/S0006-291X(02)02841-3. [DOI] [PubMed] [Google Scholar]
- 94.Shetty G. K., Economides P. A., Horton E. S., Mantzoros C. S., Veves A. Circulating adiponectin and resistin levels in relation to metabolic factors, inflammatory markers, and vascular reactivity in diabetic patients and subjects at risk for diabetes. Diabetes Care. 2004;27(10):2450–2457. doi: 10.2337/diacare.27.10.2450. [DOI] [PubMed] [Google Scholar]
- 95.Festa A., D’Agostino R., Jr., Howard G., Mykkänen L., Tracy R. P., Haffner S. M. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2000;102(1):42–47. doi: 10.1161/01.CIR.102.1.42. [DOI] [PubMed] [Google Scholar]
- 96.Kooshki A., Samadipour E., Akbarzadeh R. The association between serum C-reactive protein and macronutrients and antioxidants intake in hemodialysis patients. Journal of Medicine and Life. 2015;8:43–46. [PMC free article] [PubMed] [Google Scholar]
- 97.Du Clos T. W. Function of C-reactive protein. Annals of Medicine. 2000;32(4):274–278. doi: 10.3109/07853890009011772. [DOI] [PubMed] [Google Scholar]
- 98.Fanti P., Asmis R., Stephenson T. J., Sawaya B. P., Franke A. A. Positive effect of dietary soy in ESRD patients with systemic inflammation—correlation between blood levels of the soy isoflavones and the acute-phase reactants. Nephrology Dialysis Transplantation. 2006;21(8):2239–2246. doi: 10.1093/ndt/gfl169. [DOI] [PubMed] [Google Scholar]
- 99.Jin M., Shen M. H., Jin M. H., Jin A. H., Yin X. Z., Quan J. S. Hypoglycemic property of soy isoflavones from hypocotyl in Goto-Kakizaki diabetic rats. Journal of Clinical Biochemistry and Nutrition. 2018;62(2):148–154. doi: 10.3164/jcbn.17-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Smith W. L., DeWitt D. L., Garavito R. M. Cyclooxygenases: structural, cellular, and molecular biology. Annual Review of Biochemistry. 2000;69(1):145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 101.Hooshmand S., Soung D. Y., Lucas E. A., Madihally S. V., Levenson C. W., Arjmandi B. H. Genistein reduces the production of proinflammatory molecules in human chondrocytes. The Journal of Nutritional Biochemistry. 2007;18(9):609–614. doi: 10.1016/j.jnutbio.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 102.Valles S. L., Dolz-Gaiton P., Gambini J., et al. Estradiol or genistein prevent Alzheimer’s disease-associated inflammation correlating with an increase PPARγ expression in cultured astrocytes. Brain Research. 2010;1312:138–144. doi: 10.1016/j.brainres.2009.11.044. [DOI] [PubMed] [Google Scholar]
- 103.Khan A. Q., Khan R., Rehman M. U., et al. Soy isoflavones (daidzein & genistein) inhibit 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced cutaneous inflammation via modulation of COX-2 and NF-κB in Swiss albino mice. Toxicology. 2012;302(2-3):266–274. doi: 10.1016/j.tox.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 104.Hermenegildo C., Oviedo P. J., García-Pérez M. A., Tarín J. J., Cano A. Effects of phytoestrogens genistein and daidzein on prostacyclin production by human endothelial cells. The Journal of Pharmacology and Experimental Therapeutics. 2005;315(2):722–728. doi: 10.1124/jpet.105.090456. [DOI] [PubMed] [Google Scholar]
- 105.Laughton M. J., Evans P. J., Moroney M. A., Hoult J. R. S., Halliwell B. Inhibition of mammalian 5-lipoxygenase and cyclo-oxygenase by flavonoids and phenolic dietary additives: relationship to antioxidant activity and to iron ion-reducing ability. Biochemical Pharmacology. 1991;42(9):1673–1681. doi: 10.1016/0006-2952(91)90501-U. [DOI] [PubMed] [Google Scholar]
- 106.Lee S. F., Lin J. K. Inhibitory effects of phytopolyphenols on TPA‐induced transformation, PKC activation, and c‐jun expression in mouse fibroblast cells. Nutrition and Cancer. 1997;28(2):177–183. doi: 10.1080/01635589709514572. [DOI] [PubMed] [Google Scholar]
- 107.Huang T.-S., Lee S.-C., Lin J.-K. Suppression of c-Jun/AP-1 activation by an inhibitor of tumor promotion in mouse fibroblast cells. Proceedings of National Academy of Sciences of the United States of America. 1991;88(12):5292–5296. doi: 10.1073/pnas.88.12.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Filipović B., Šošić-Jurjević B., Nestorović N., et al. The thyroid C cells of ovariectomized rats treated with estradiol. Histochemistry and Cell Biology. 2003;120(5):409–414. doi: 10.1007/s00418-003-0578-4. [DOI] [PubMed] [Google Scholar]
- 109.Cheng M. Z., Rawlinson S. C. F., Pitsillides A. A., et al. Human osteoblasts’ proliferative responses to strain and 17β-estradiol are mediated by the estrogen receptor and the receptor for insulin-like growth factor I. Journal of Bone and Mineral Research. 2002;17(4):593–602. doi: 10.1359/jbmr.2002.17.4.593. [DOI] [PubMed] [Google Scholar]
- 110.Garcí-Pérez M. A., del Val R., Noguera I., et al. Estrogen receptor agonists and immune system in ovariectomized mice. International Journal of Immunopathology and Pharmacology. 2006;19(4):807–819. doi: 10.1177/039463200601900410. [DOI] [PubMed] [Google Scholar]
- 111.Rodan G., Rodan S. Advances in Bone and Mineral Research. Amsterdam, Netherlands: WA Peck, Excerpts, Media; 1984. [Google Scholar]
- 112.Delhanty P. J. D., van der Eerden B. C. J., van Leeuwen J. P. T. M. Ghrelin and bone. BioFactors. 2014;40(1):41–48. doi: 10.1002/biof.1120. [DOI] [PubMed] [Google Scholar]
- 113.Zhong J., Yamaguchi M. Synergistic effect of genistein and casein phosphopeptides on bone components in young and elderly female rats. Journal of Health Science. 2000;46(6):474–479. doi: 10.1248/jhs.46.474. [DOI] [Google Scholar]
- 114.Lee Y.-B., Lee H. J., Kim K. S., et al. Evaluation of the preventive effect of isoflavone extract on bone loss in ovariectomized rats. Bioscience, Biotechnology, and Biochemistry. 2004;68(5):1040–1045. doi: 10.1271/bbb.68.1040. [DOI] [PubMed] [Google Scholar]
- 115.Wafay H. A., Abdel-Moniem M., Megahed H. A., Elmalt H. The effect of soy isoflavones and nondigestive oligosccharides on bone turnover markers. International Journal of Pharmacy and Pharmaceutical Sciences. 2013;5:152–156. [Google Scholar]
- 116.Nurrochmad A., Leviana F., Wulancarsari C. G., Lukitaningsih E. Phytoestrogens of Pachyrhizus erosus prevent bone loss in an ovariectomized rat model of osteoporosis. International Journal of Phytomedicine. 2010;2(4) [Google Scholar]
- 117.Hassan N. M., Hassan R., Setta L. A., El-moniem M. A., Ahmed H., Hammouda F. Potent role of dietary phytoestrogen plants cultivated in Egypt against osteoporosis in ovariectomized rats. Australian Journal of Basic and Applied Sciences. 2010;4(2):359–369. [Google Scholar]
- 118.Gür A., Denli A., Nas K., et al. Possible pathogenetic role of new cytokines in postmenopausal osteoporosis and changes during calcitonin plus calcium therapy. Rheumatology International. 2002;22(5):194–198. doi: 10.1007/s00296-002-0223-x. [DOI] [PubMed] [Google Scholar]
- 119.Pacifici R. Estrogen, cytokines, and pathogenesis of postmenopausal osteoporosis. Journal of Bone and Mineral Research. 1996;11(8):1043–1051. doi: 10.1002/jbmr.5650110802. [DOI] [PubMed] [Google Scholar]
- 120.Domazetovic V., Marcucci G., Iantomasi T., Brandi M. L., Vincenzini M. T. Oxidative stress in bone remodeling: role of antioxidants. Clinical Cases in Mineral and Bone Metabolism. 2017;14(2):209–216. doi: 10.11138/ccmbm/2017.14.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Westfall R. J., Hauge S. M. The nutritive quality and the trypsin inhibitor content of soybean flour heated at various temperatures. The Journal of Nutrition. 1948;35(3):379–389. doi: 10.1093/jn/35.3.379. [DOI] [PubMed] [Google Scholar]
- 122.Helrick K. Official Methods of Analysis: AOAC. Arilington, VA, USA: Association of official analytical chemists, Inc; 1990. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material contains a graphical abstract that describes the experimental design and results with implications of these results.
Data Availability Statement
No data were used to support this study.