Abstract
The prevalence of excess gestational weight gain is increasing worldwide and is associated with pregnancy complications, including gestational diabetes mellitus, pre-eclampsia, preterm birth, macrosomia, and development of obesity in offspring. Whereas gestational weight gain positively correlates with the gain in fat mass (FM), fat-free mass (FFM) gain is relatively consistent across pregnancies. Commonly used methods to assess body composition include anthropometry, densitometry (air displacement plethysmography, underwater weighing), and hydrometry (isotope dilution, bioimpedance analysis). While these techniques can be applied to pregnancy, they require specific adjustments to assumptions inherent within each method, most importantly to accommodate for the hydration of FFM which is transient throughout gestation. Here we discuss the application of the abovementioned methods to pregnant women and the relevant adjustments needed to more accurately calculate FM based on body weight, body volume, or total body water. We also present a novel application of classical data to provide FFM density estimates for pregnant women at any stage of pregnancy. Use of these adjustments will help standardize assumptions on FFM hydration and minimize error in FM estimation. Techniques still fail, however, to fully distinguish tissue gains between mother and fetus. To fill this important gap, imaging techniques such as ultrasound and magnetic resonance imaging are being used more frequently and will provide more insight into fetal development, fetal adiposity, and depot specificity of maternal FM acquisition. Efforts to synchronize protocols are necessary to allow seamless comparison of data to advance the understanding of maternal body composition changes that contribute to pregnancy-related complications.
Introduction
Pregnancy is a period during which the female body undergoes significant changes in its composition to support fetal growth and development. These phenotypic and physiologic changes are recognized in the form of gestational weight gain (GWG), which includes gains in maternal and fetal mass, as well as growth of placental tissue and alterations in amniotic fluid. Epidemiological data shows a U-shaped association between GWG and the risk for adverse pregnancy outcomes including preterm birth and impaired fetal growth, infant death, and offspring weight status in adolescence [1–3]. Based on these data, the Institute of Medicine (IOM) developed universal guidelines for healthy GWG that are determined by a woman’s pre-gravid weight category (i.e., body mass index (BMI)) [4].
Despite having well-established weight gain guidance for pregnant women since 1990, only about one-third of pregnant women achieve a healthy weight gain [5, 6]. Excess GWG, or weight gain that exceeds the recommended guidelines, is becoming increasingly prevalent among pregnancies worldwide. The most recent US data revealed that excess GWG occurred in ~50% of all pregnancies in 2015 [5]. Similar epidemiological data has been reported for women in Europe [6], New Zealand, and Australia [7]. There is also an increased prevalence of excess GWG among women who are overweight or obese at conception (61% and 55%, respectively) [5]. Because GWG is positively associated with gains in fat mass (FM) but not fat-free mass (FFM) [8, 9], it is important to better understand how to foster healthy GWG in pregnant women so that pregnancy-related comorbidities for both mother and infant can be diminished [10, 11].
In research and clinical settings, the primary focus for evaluating maternal body composition is to assess changes in FM and FFM before, during, and after pregnancy. While there are a few non-invasive, low burdensome methods available, certain methods are clearly more superior to others. The benefits of easy-to-use and cost-effective techniques such as skinfold thickness and bioelectrical impedance are evident; however, to truly understand the etiology of adverse pregnancy outcomes, more advanced and validated methods are required. Methods based on densitometry and hydrometry provide valuable estimates for FM and FFM but are challenged by the need to adjust for FFM hydration, which is subject to change during pregnancy. The accuracy of FM and FFM measurements can be improved by combining methods, such as densitometry and hydrometry. The key shortcoming of these methods is that FM is estimated as a single whole-body component. There is an obvious need to also understand changes in adipose tissue distribution throughout pregnancy given its likely differential impact on metabolic health and thereby for adverse outcomes [12, 13]. Imaging technologies are beginning to shed light on important considerations and best practices for assessing body composition in pregnancy. Nonetheless, these approaches also have limited use due to cost, the need for specialized expertise and equipment, and potential safety concerns that prohibit assessments in early pregnancy.
The objective of this review is to provide an overview of different methodologies to assess body composition in pregnancy. The theory of each method is discussed alongside important adjustments required to apply the techniques to pregnant women.
Body composition changes in pregnancy
Maternal body composition changes throughout pregnancy in order to support healthy development of the fetus. In the first several months of gestation, the changes in maternal body composition reflect preparation of the female body for fetal development. Specifically, the uterus and breast tissue that comprises the maternal unit grows and blood volume expands. In late pregnancy, a more pronounced growth of the fetal unit (e.g., fetus, amniotic fluid, and placenta) occurs alongside continued growth of the maternal tissue and further blood volume expansion. At the time of delivery, the fetal unit will contribute up to approximately one-third of the total GWG [14]. Additionally, distinct changes in maternal body composition occur in both early and late pregnancy. A healthy weight gain (0.5–2 kg) reveals that only small changes in maternal FM and FFM occur during early gestation compared to the relatively large and variable changes in FM and FFM observed in late gestation [4].
Maternal unit
Changes in FFM
The maternal unit accumulates approximately 2 L of blood and 2 L of extracellular fluid and expands the uterus and breast tissue by ~2 kg [15]. Various studies have investigated protein accrual during pregnancy and found the amounts to be <1 kg [8, 16, 17]. Changes in bone mass are poorly investigated, but assessment of calcium balance indicates negligible gain or loss of bone mass for most women [18]. The large proportion of water accumulated relative to GWG leads to an increase in the hydration of FFM throughout pregnancy [11, 19, 20]. Indeed, water accumulation is highly variable ranging from 67% to 80% of FFM [21, 22], which can impact the density of FFM. Studies are needed to better understand how FFM hydration is impacted by non-modifiable factors, such as maternal age, race, and pre-existing comorbid conditions, as well as modifiable factors, such as BMI and adiposity [23, 24].
Changes in FM
Maternal FM is the most variable component of GWG. Changes in maternal FM range from −10 to +15 kg at term [25] and positively correlate with GWG [9]. Owing to the lack of frequent body composition assessments, there is limited data available on the timing of FM gain throughout pregnancy. Butte et al. showed a linear increase in FM throughout pregnancy at a rate of approximately 2 kg per trimester [8], while other groups have reported smaller gains in early pregnancy [26]. Of note, most of the women studied throughout pregnancy are of normal weight (18.5 ≤ BMI < 24.9 kg/m2) at conception, and thus longitudinal changes in FM in women with pre-gravid overweight (25.0 ≤ BMI < 30.0 kg/m2) or obesity (BMI ≥ 30.0 kg/m2) are lacking.
Fetal unit
The fetal unit comprises the placenta, amniotic fluid, and fetus. Placenta growth occurs in two phases; a linear increase up to week 24 (~150 g), followed by a steeper, linear increase during the third trimester, and reaching a plateau in growth at term (~600–700 g, range 500–1000 g) [4, 27–30]. The placenta is comprised of ~88% water, 11% protein, and only 1% fat [4]. Amniotic fluid also accumulates up to 3 L throughout a healthy pregnancy [31], and thus significantly contributes to the increased water accumulation during pregnancy. The fetus grows at a relatively consistent rate to ~1 kg by the 28th week of gestation. Over the next 12 weeks, the fetus gains more than two-thirds of its final weight (~2.5 kg) [4]. Therefore, it is later in pregnancy where deviations in fetal growth occur producing variability in birth weight [4]. The average FM of an infant at birth is ~350 g [32], most of which is accrued in late pregnancy [33]. Studies describe a linear relationship between GWG and infant adiposity [34, 35] and suggest that >14% body fat reflects overweight/obesity in the neonate [34]. Additionally, a large proportion of the weight accrued in the fetal unit is attributed to water [36]. The water content of FFM in infants (~80%) is significantly higher compared to adults (~73%) due to a smaller contribution of muscle mass to the overall FFM depot [33].
GWG, body composition and pre-gravid BMI
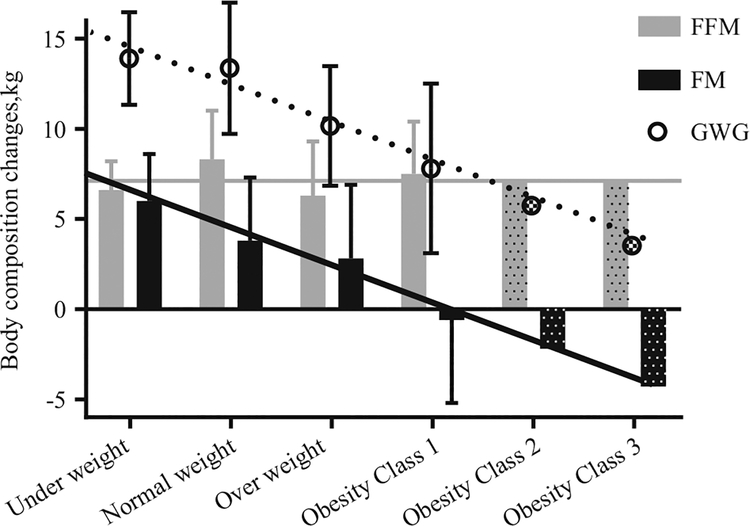
The amount and composition of healthy GWG significantly differs between women who are underweight, normal weight, overweight, and obese and is inversely related to the pre-gravid BMI class [4]. Specifically, the IOM guidelines recommend a total weight gain of 12.5–18 kg for women who are underweight, 11.5–16 kg for those who are normal weight, 7–11.5 kg for overweight women, and 5–9 kg for women who are obese at conception [4]. Current weight gain guidelines have not been expanded to include recommended gains in FM and FFM; however, according to rigorous body composition studies, among women who gain an appropriate amount of weight according to these recommendations, changes in FFM appear to be relatively constant and independent of BMI class (6–8 kg, Fig. 1). Thus smaller weight gains among heavier women are almost exclusively explained by smaller gains in FM (−0.6 kg FM in obese vs +6 kg FM in underweight) [9].
Fig. 1.
Whole-body composition changes in pregnancy by BMI class. Body weight and body composition changes for women with recommended total weight gain [4] are shown by each BMI class (underweight, normal weight, overweight, obese) and partitioned into fat-free mass (FFM; gray bars) and fat mass (FM; black bars) as reported by Lederman et al. [9]. For obesity class 2 and 3, data are estimated from linear regression of the change in composition components based on data across the other BMI groups (change in FM (kg) = 8.2 −2.1×[BMI category factor], where BMI category factors are: underweight = 1, normal weight = 2, overweight = 3, obesity class 1 = 4, class 2 = 5, class 3 = 6), R2 = 0.99, P < 0.001): the gray line shows the estimated change in FFM based on the 2009 IOM report [4] and the black line shows a linear trend line for FM change extrapolated from the other BMI classes. Theoretically, it may be likely that healthy gestational weight gain in women with obesity class 2 and 3 would be lower than for women with obesity class 1 and may include a loss of maternal FM
To date, there are no GWG recommendations for women within different degrees of obesity due to the lack of available data. Therefore, the present guidelines recommend similar weight gain and hence FM gain for all women with obesity. In an attempt to shed light on possible weight gain recommendations for women considered obesity class 2 and 3 prior to pregnancy, we constructed linear models for the changes in GWG, FFM, and FM based on published data for women underweight through to obesity class 1 [9] (Fig. 1). Using these linear models, we predicted changes in body weight and body composition for women with obesity class 2 and 3 and with an appropriate total GWG (7 kg, 5 kg, and 3 kg for obesity class 1, 2, and 3, respectively). Assuming the gain in FFM would be comparable to the other BMI classes, and the inverse association between pre-gravid BMI and FM gain continues (for every increase in pre-gravid BMI class, gain of FM is 2 kg less), the linear model of published data suggests that maternal FM could be lost during pregnancy in cases of morbid obesity (BMI > 40 kg/m2) to optimize health outcomes in obese pregnancies. Future advances in body composition research in pregnant women will hopefully provide more insight to the changes in FFM and FM in women with morbid obesity and those with optimal pregnancy outcomes.
Models of maternal body composition
The assessment of body composition in pregnancy generally partitions the body into two compartments, FM and FFM, where FFM includes the combined mass of total body water (TBW), bone, protein, and non-bone mineral mass. This is referred to as a 2-compartment model (2C) of body composition. More advanced models of body composition assessment such as 3-compartment models (3C) that partition the FFM component into TBW and lean body mass are also used in pregnant women. Unfortunately, neither the 2C or 3C models can further partition the total FM and FFM depots into the maternal and fetal units. Imaging techniques such as magnetic resonance imaging (MRI) can determine individual organ volumes and therefore may provide separate measures of maternal and fetal tissues in the future.
2C models for estimating body composition in pregnancy
A 2C model of body composition can be obtained in multiple ways. Anthropometric measures such as skinfold thickness can be used to estimate percent body fat by prediction equations and combined with weight to obtain FM and FFM. The most commonly used approach to determine body composition are densitometry-based methods that measure body volume and derive body density and FM, e.g., underwater weighing (UWW) and air displacement plethysmography (ADP). Alternatively, deuterium dilution is used to measure TBW (hydrometry) and estimate FM and FFM by using assumptions for the TBW:FFM ratio. Lastly, bioimpedance analysis (BIA) provides an estimate of FM and FFM based on their distinct electrical conductivities.
Skinfold thickness assessment
Anthropometric measurements of body composition via skinfold thickness assessment is non-invasive and suitable for field research given its reliance on mobile and non-specialized equipment. Skinfold thickness protocols typically include measurements at 4–7 sites (e.g., triceps, biceps, subscapular, supra-iliac/iliac crest, mid-thigh, mid-calf). The simple summation of skinfold thickness measurements at particular areas of the body are used to approximate total body subcutaneous fat [37]. Skinfolds together with maternal weight and circumferences (e.g., wrist) have been combined to develop prediction equations for estimating percent body fat in pregnant women [38, 39]. Prediction equations are specific to rigid time points in gestation and are also specific to maternal age and race. Most equations are old and their validation studies do not include many women with obesity and thereby the applicability of skinfold equations for percent fat in women with obesity is not known [39].
The accuracy of skinfold thickness measures to estimate percent body fat is debated. Some published equations using skinfold thicknesses claim to explain a high percentage of the variability in predicting FM or percent body fat in pregnancy [38, 39], while other reports show only mediocre correlations [40–42]. Estimating FM from a skinfold protocol has numerous limitations. First, pregnancy presents a unique anatomical challenge for obtaining accurate skinfold thickness measures at the same site across pregnancy due to expansion and stretch of the skin. Second, skinfold assessment is highly subjective, and thus extensive training is necessary to ensure a high level of reliability both within a pregnant woman over time and between pregnant women. Additionally, skinfold thickness during pregnancy is often greater in underweight compared to overweight women [43], greater in primiparous compared to multiparous women [44], and the increase in thickness differs by site throughout gestation [45]. These are critical factors that need to be considered when selecting a published equation to estimate FM or percent body fat. Withstanding these limitations, when precision is maintained, repeated evaluation of skinfold thickness throughout pregnancy can be useful in both research and clinical settings.
Recently, advancements in digital technology have contributed to the development of digital anthropometry, which is discussed by Heymsfield et al. in a companion review in this issue [46]. Briefly, three-dimensional optical imaging can measure all circumferences of the body, body surface area, and segmental body volume by non-invasive and, hopefully in the future, low-cost methods that can be used in clinical, research, and even home settings. While this technique is permittable in pregnancy, validation studies have not yet included pregnant women [46].
Underwater weighing
UWW is a non-invasive technique that uses Archimedes principle of buoyance to measure body volume [47]: the weight of a body that is under water equals the weight of the displaced water, and therefore:
where BV = body volume, BWair = body weight in air, BWwater = body weight under water, and dWater = density water at measured temperature.
For the measurement of body volume with UWW, subjects are submerged under water and weighed while they hold their breath following exhalation for ~10 s. The measurement is corrected for residual lung volume, which is ideally measured by the helium dilution technique [48]. Practical limitations to this method include discomfort during complete submersion with the nostrils occluded for 2–3 min and difficulty in executing the breathing protocol correctly. Nonetheless, UWW is non-invasive, precise, and can be performed longitudinally without undue risk for pregnant women.
Air displacement plethysmography
ADP is becoming a widely adopted body composition assessment method with increasing use in pregnancy. The ‘BOD POD’ technology (COSMED, Concord, CA, USA), utilizes a dual-chamber model that is based on Boyle’s law, where small contrasts in volume and pressure in each chamber are measured [49]. With correction for thoracic gas volume by either measurement or estimation, the proprietary software of the BOD POD estimates body volume [50]. An accurate measurement of body volume requires any residual air from the body surface to be minimized and therefore individuals need to wear tightly fitted clothing (e.g., lycra swimsuit). Advantages of ADP include high level of safety and participant tolerance thereby allowing for repeated measurements across pregnancy. Moreover, the protocol is easy for the participant to follow and provides an assessment of body volume in <5 min.
Nonetheless, the direct applicability of ADP to pregnant women is inhibited by various factors. First, the BOD POD is expensive to purchase although the ongoing costs to operate and maintain are fairly low. Similar to UWW, the BOD POD is not portable and thus is not suitable for field research. Second, we learned that obtaining an actual measurement of thoracic gas volume by the BOD POD software is difficult for pregnant women. Half of measurement attempts have been unsuccessful in our studies of overweight and obese women. When reported in other studies, a considerable number of subjects also failed to produce acceptable measurements (11/38 or 29% in ref. [51], 6/41 or 15% in ref. [52]). Therefore, for longitudinal studies of body composition, it is important that either the measured or estimated thoracic gas volume is consistently applied for each woman across assessments to limit this potential source of error in the FM and FFM estimations. Studies have also shown that thoracic gas volume declines throughout pregnancy by ~100 mL per trimester [50, 53, 54] and, if ignored, would lead to an overestimation of FM particularly in late pregnancy. Thus thoracic gas volume needs to be adjusted for the trimester-specific decline in lung volume in all women since this is not accounted for in the BOD POD software. Lastly, the current BOD POD software does not include any 2C models specific for use in pregnant women, that is, which contain the appropriate adjustment for density of FFM relative to gestation age.
Deuterium dilution
The principle of hydrometry is to use a stable isotope (e.g., deuterium) to estimate TBW and hence FFM. A known dose of deuterium oxide (D2O), an isotope of water, is ingested and equilibrates within the TBW pool of the body [55]. The size of the TBW pool determines the maximal concentration (=enrichment) of the isotope (D) and can thus be calculated:
where TBW = total body water.
Specifically, a baseline urine (or saliva) sample is collected to account for naturally present isotope in the TBW pool that is in equilibrium with urine. Subsequently, a known quantity of D2O is ingested (30 g for adults) and allowed to mix within TBW pool for 2–5 h. Urine samples are frequently collected during this period to determine when the enrichment reaches a plateau. This plateau represents the maximal enrichment of the isotope that is used to calculate TBW [55].
Using the measure of TBW, FFM can be calculated from a measured or estimated ratio of TBW and FFM. The ratio of TBW:FFM defines the hydration of FFM (hFFM) [56], which is used to calculate FM as follows:
where BW = body weight, TBW = total body water, and hFFM = hydration fat-free mass [56].
Advantages of hydrometry measurements to estimate FFM in pregnancy include the use of the stable isotope deuterium that is not harmful to pregnant women or the fetus. With newer, portable devices for measuring isotope enrichment (e.g., Fourier Transform Infrared Spectrometry), deuterium dilution is possible in field research or in low-resource settings [55]. The primary shortcoming of the technique is that the procedure takes approximately 4–5 h to complete. Disadvantages, like other 2C models in pregnancy, include the relative assumptions that need to be applied for pregnant women. Additionally, derived hydration values are likely population specific and cannot be easily applied beyond the estimated population.
Bioelectrical impedance analysis
BIA is a cheap and practical body composition methodology for measuring TBW and body composition. Specifically, BIA is a non-invasive method that relies on assumptions and relationships in the electrical properties of biological tissues. BIA is performed by placing electrodes on the ankle and wrist while the subject is supine in a horizontal position [57, 58] and passing a low amperage current throughout the body. The electrical conductivity of current is determined by the amount of water contained in different biological tissues. Tissues with high water content such as skeletal muscle are more conductive than adipose tissue or bone, which have less water content. The volume of a conductive tissue then can be calculated from the resistance of the electrical signal throughout body parts. In pregnant women, BIA has been shown to provide valid estimates of TBW as compared to deuterium dilution early in pregnancy (14 weeks); however, BIA may underestimate TBW in late pregnancy (32 weeks) [57]. An inherent problem with BIA are fluid shifts throughout the day that can affect BIA measurements more than isotope dilution [58]. Standardization of time of day is therefore important. Nonetheless, there are increasing numbers of body composition studies in pregnant women with estimates of FFM and FM from BIA [59–62], suggesting their ease of use and applicability to research studies. Additional validation studies are needed.
Pregnancy adjustments necessary for 2C models of body composition
To estimate body composition with the 2C model using densitometry, first, body weight is divided by measured body volume to derive total body density:
where dB = total body density, BW = body weight, and BV=body volume.
Body density can then be partitioned into FM and FFM using an equation such as Siri [22] with individual densities of fat and lean tissues to yield an estimate for FM:
where dB=total body density, dFM=density fat mass, and dFFM=density fat-free mass [22].
The density of FM in the pre-gravid state is 0.9 kg/L and remains fairly constant throughout pregnancy [22]. In contrast, the drastic increases in blood volume and extra-cellular fluid with advancing gestation cause a decline in the density of FFM as pregnancy progresses. A number of studies have published estimates for the hydration [19, 23, 24, 26, 39, 63–66] and/or density [19, 23, 39, 52, 63–65] of FFM at specific time points in pregnancy (Fig. 2a, b). Failing to account for increasing hydration and decreasing density of FFM throughout pregnancy (i.e., Siri) or adopting estimates from others results in different FM and FFM estimates (Table 1). Specifically, failure to account for the increase in FFM hydration in hydrometry models would overestimate the actual FFM in pregnant women, and subsequently, FM would be underestimated. On the contrary, overestimating FFM density in densitometry models underestimates FFM and overestimates FM. Indeed, Marshall et al. [52] showed recently that BOD POD software, which does not account for pregnancy-specific changes in FFM hydration, overestimated FM by 3.1 ± 4.8 kg as compared to a 3C model. In contrast, when the group included FFM density relative to gestation age, the FM estimate was considerably improved and only 1.1 ± 4.7 kg above the reference (35.5 ± 13.1 kg, n = 33) [52]. The importance of using accurate and ideally measured hydration constants (versus estimations) on the calculation of FM and FFM is evident (Table 1).
Fig. 2.
Published values for FFM hydration and density throughout pregnancy. Hydration (a) and density (b) of FFM are shown for specific time points in pregnancy as published by the presented studies. Individual values are calculated by using body weight, body volume, and total body water (3C models). Published values are used as estimates/reference when individually measured values are not available (2C models). The exponential regression lines are only based on the data by van Raaij et al. [19], which are most commonly used in 2C models, and allow for estimation of FFM hydration/density for any given time throughout gestation, , where GA = gestational age; R2 = 0.998, P < 0.00, and , where GA = gestational age; R2=0.999, P < 0.001)
Table 1.
Effect of adopting different hydration constants on FM and FFM calculation at 30 weeks gestation
| Normal weighta | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weight (kg) |
Hydrometry | Densitometry | ||||||||||
| TBW (L) | Hydration Constant |
FFM (kg) |
FM (kg) |
FM (%) | BV (L) | dBody (kg/L) |
Density FFM |
FFM (kg) |
FM (kg) |
FM (%) | ||
| Normal weighta | ||||||||||||
| Siri [22] | 76 | 45.0 | 0.724 | 62.2 | 13.8 | 18.2 | 71.9 | 1.057 | 1.100 | 62.1 | 13.9 | 18.3 |
| van Raaij et al. [19] | 76 | 45.0 | 0.740 | 60.8 | 15.2 | 20.0 | 71.9 | 1.057 | 1.093 | 64.1 | 11.9 | 15.7 |
| Fidanza [64] | 76 | 45.0 | 0.752 | 59.8 | 16.2 | 21.3 | 71.9 | 1.057 | 1.092 | 64.2 | 11.8 | 15.5 |
| Catalano et al. [63] | 76 | 45.0 | 0.762 | 59.1 | 16.9 | 22.3 | 71.9 | 1.057 | 1.089 | 65.1 | 10.9 | 14.4 |
| Obese weightb | ||||||||||||
| Siri[22] | 92 | 40.2 | 0.724 | 55.5 | 36.5 | 39.6 | 91.0 | 1.011 | 1.100 | 55.6 | 36.5 | 39.6 |
| van Raaij et al. [19] | 92 | 40.2 | 0.740 | 54.3 | 37.7 | 41.0 | 91.0 | 1.011 | 1.093 | 57.3 | 34.7 | 37.7 |
| Fidanza [64] | 92 | 40.2 | 0.752 | 53.5 | 38.5 | 41.9 | 91.0 | 1.011 | 1.092 | 57.4 | 34.6 | 37.6 |
| Catalano et al. [63] | 92 | 40.2 | 0.762 | 52.8 | 39.2 | 42.7 | 91.0 | 1.011 | 1.089 | 58.2 | 33.8 | 36.7 |
FFM fat-free mass, FM fat mass, TBW total body water, BV body volume, dBody density body
Normal weight data calculated for a 34-year-old female with pre-gravid normal BMI of 24.8 kg/m2 (e.g., weight 65 kg; height 162 cm), assuming a 11 kg weight gain and TBW of 45.0 L at 30 weeks gestation
Obese weight data calculated for a 34-year-old female with pre-gravid normal BMI of 32.4 kg/m2 (e.g., weight 85 kg; height 162 cm), assuming a 7 kg weight gain and TBW of 40.2 L at 30 weeks gestation
The most commonly used reference to estimate dFFM for women throughout pregnancy is published by van Raaij et al. [19]. However, FFM density values are provided only in 10-week intervals throughout gestation, and thus subsequent studies relying on this data have been limited in the period of gestation where body composition assessments are being estimated (Fig. 2b). To expand the utility of the data published by van Raaij et al. [19], we developed a regression equation that estimates FFM density as a function of gestational age (in weeks):
where GA = gestational age; R2=0.999, P < 0.001, and
where GA = gestational age; R2 = 0.998, P < 0.001.
3C models for estimating body composition in pregnancy
The 3C model eliminates the need to assume the individual hydration of FFM and thereby 3C provide more accurate measurements of FM than 2C models [52, 65]. Indeed, when using a 4C model to estimate body adiposity that combines measurements of weight, body volume, TBW, and bone mineral density as reference, measurements of FM in late pregnancy show significantly higher precision from 3C models as compared to dFFM-adjusted 2C models from BOD POD and deuterium dilution (SD of the difference to the 4C reference: 3C: ±0.89 kg, 2C (BOD POD): ±3.0 kg, and 2C (deuterium dilution): ±2.3 kg), whereas the average agreements with 4C were comparable [52]. Thus the accuracy of maternal body composition assessments can be clearly improved by the use of 3C models when FFM hydration is measured for each woman. Using the equations developed by Siri and Catalano for 3C models [22, 63], FM is calculated using body volume, body weight, and TBW:
where FM = fat mass, TBW = total body water, BW=body weight, and BV = body volume.
The hydration and density of FFM can be calculated individually:
where FFM = fat-free mass, TBW = total body water, BW = body weight, and TBV = total body volume.
An alternative approach to measure body composition in pregnancy is to measure FFM by its total potassium content. While 2C models for non-pregnant women assume the potassium content as 2.3 g/kg of FFM [45, 67], due to the increased hydration of FFM potassium content is lower in pregnancy (2.1 g/kg FFM [65]). Therefore, pregnancy-specific 3C models with a measure of total body potassium and TBW propose FM can be calculated as [45, 65]:
where BW=body weight, TBW=total body water, and TBK=total body potassium [45, 65].
Similar to the densitometry/hydrometry 3C model above, estimates obtained by hydrometry/potassium do not differ significantly from a 4C model of maternal body composition although a larger variation was observed for the latter [65].
Multiple compartment models for determination of AT depots and the fetal unit
The chief limitation of using body composition methodology that relies on 2C and 3C models to estimate body composition in pregnancy is the lack of information that can be obtained for the individual contributions of the maternal and fetal units and, possibly more important, the distribution of FM. Imaging technology can potentially overcome this shortcoming. Sonography or ultrasound is routinely used to obtain images of the fetus throughout gestation to assess growth and normal development and has been extended to derive measures of fetal skinfold thickness in utero [68–73]. Numerous studies are now also using ultrasound to derive measures of maternal skinfold thickness suggesting that it overcomes many of the technical issues inherent with skinfold calipers but validation studies are lacking [74, 75]. While designed to measure bone mineral density, dual-energy X-ray absorptiometry (DXA) can also provide measurements of total and regional-specific FM and FFM, and DXA is now the most commonly used method to measure total body composition in non-pregnant individuals [76]. However, since DXA scanners emit radiation the procedure is considered harmful to a developing fetus and is prohibited during pregnancy [77]. Measurement of maternal body composition with DXA immediately before and/or after gestation, however, can still be highly valuable for understanding body composition changes across pregnancy [8, 9, 65]. During pregnancy, whole-body imaging can be accomplished with MRI; however, partitioning of the maternal and fetal units is still complex.
Ultrasound
Ultrasound is a simple technique that involves the production of sound waves at varying frequencies to measure adipose tissue depot (expansion) during pregnancy. While the estimates for skinfold thickness obtained by ultrasound correlate highly with estimates by a caliper [75], ultrasound estimates may be superior in the increased accuracy and precision. Specifically, ultrasound avoids the need for skin extension and compression when using a skinfold caliper in pregnant women [74, 75]. In addition, ultrasound may enable the measurement of visceral adipose tissue thickness, whereas skinfold thickness only provides an estimate for subcutaneous fat [78–82]. Importantly, while it is used to measure visceral adipose tissue, we are not aware of any study that has validated the ultrasound against other objective methods such as MRI, which would be considered the gold standard. Ultrasound is also limited in its ability to derive site-specific measurements of adipose tissue thickness only rather than measurements of whole-body composition. Research is needed to assess the validity of FM measured by ultrasound throughout the course of pregnancy before this growing body of literature can be considered alongside data derived from validated techniques used in 2C and 3C models.
Beyond maternal adipose tissue, fetal ultrasonography is currently the most widespread method used to assess fetal size [83] and adiposity using single-site measurements of fetal subcutaneous fat in the extremities and abdomen [68–73]. Two independent studies show that predictions of newborn adiposity assessed by fetal ultrasound in late gestation (~30 weeks) were accurate when compared to fetal adiposity measured by DXA or ADP at birth [72, 73]. Measurement of fetal fat deposition by ultrasound, however, is limited to mid/late pregnancy due to insufficient fetal FM earlier in gestation.
Magnetic resonance imaging
MRI is an in vivo imaging technique that uses a powerful magnetic field to measure tissues and organs. For body composition, MRI is useful because with whole-body or regional scanning protocols, adipose tissue and skeletal muscle mass can be acquired, as can individual organ sizes. MRI is currently used as a diagnostic tool in pregnancy to evaluate and diagnose medical conditions, such as appendicitis [84], intestinal obstruction [85], cardiac displacement [86], and placental and fetal brain abnormalities [87]. Indeed, the utility of MRI in clinical research offers advances in the assessment of maternal body composition in pregnancy. Sohlström and Forsum were one of the first groups to evaluate whole-body composition by MRI in pregnant women [88, 89]. The group found body fat assessed by MRI was 1.4 ± 2.9% less compared to assessment by UWW. To date, there are no publications reporting the use of MRI to assess changes in body composition throughout pregnancy; however, two studies are currently underway (LIFT: NCT#01616147; MomEE: NCT#01954342).
In addition to the likely increased precision of FM estimates with MRI, the great advantage of MRI over other methods is the ability to localize adipose tissue distribution [88], as well as ectopic fat accumulation in the liver [90] and the fetus [91, 92]. To improve data quality and facilitate synergy of findings, attempts are being made to develop MRI scanning protocols for abdominal fat distribution [93]. Anatomical landmarks are used to standardize the location of MRI slice(s) in the abdominal cavity. In pregnancy, changes in maternal posture and location of abdominal organs pose a potential source of variance for obtaining measures of abdominal fat. A recent study in obese women compared different approaches for calculating abdominal subcutaneous and visceral fat area ratios in the second trimester [94]. Comparing measurements by varying slice thickness and slice distance from anatomical landmarks, the group concluded that abdominal slices should be centered above the uterine fundus to produce reliable MRI measurements of subcutaneous and visceral fat between women. This approach, however, would only be applicable when scanning women at the same time point in gestation. Given that the height of the uterine fundus increases throughout pregnancy, for longitudinal studies this approach would induce large variance in FM estimates within and between women.
MRI is deemed safe in pregnancy; however, non-diagnostic scanning protocols are recommended only from the second trimester and onwards. The obvious limitation of MRI protocols is cost. Many clinical centers charge approximately $600 USD per 30 min of scanning time (sufficient for an abdominal scan or lower-quality whole-body scan) with the cost for image analysis added as an extra expense. Manufacturer differences between scanners can introduce problems in standardizing hardware and software among clinical centers as well. In addition, limitations to the field of view will limit the accuracy of abdominal scanning in some women, particularly later in gestation.
Summary
Advancing assessment of body composition in pregnant women is important to divulge any interaction between FM and adverse outcomes for the mother and her fetus. Methods such as BIA, ADP, and isotope dilution (D2O) offer evaluation of body composition in two compartments—FM and FFM—but without consideration of changes in FFM hydration are prone to errors. Using published estimates for FFM hydration is common, but these estimates are specific to set time periods and have never been published as continuum. Our novel application of the most widely reported FFM density estimates for pregnant women allow for body composition assessment at any stage of pregnancy. A limitation of this data is the homogeneity of exclusively healthy Dutch women, and the effect of age, race, BMI, and adiposity requires more research. Indeed, measuring the hydration of FFM is superior to an estimate and can be obtained by combining measures of weight, body volume (ADP), and TBW (BIA, isotope dilution). The increased attention to MRI in pregnancy in the coming years will advance our understanding of changes in fat distribution and in conjunction with ultrasound may, for the first time, disentangle the body composition of the mother from that of the fetus. More efforts are necessary to establish common protocols that account for changes of anatomical landmarks that are used to define body compartments and adipose tissue depots. Lastly, expanding the available body composition data in pregnant women obtained from use of validated techniques would allow researchers to better compare body composition changes to objective measures of energy intake and energy expenditure [95] and to provide improved estimates of energy requirements for healthy fetal growth. The marriage of maternal body composition to energy balance will enable the development energy intake recommendations for pregnant women, with the ultimate objective to help facilitate an appropriate amount of weight gain throughout pregnancy.
Acknowledgments
Funding This work is supported by National Institute of Diabetes and Digestive and Kidney Diseases (U01DK094418, R01DK099175).
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Bodnar LM, Siminerio LL, Himes KP, Hutcheon JA, Lash TL, Parisi SM, et al. Maternal obesity and gestational weight gain are risk factors for infant death. Obesity (Silver Spring). 2016;24:490–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oken E, Rifas-Shiman SL, Field AE, Frazier AL, Gillman MW. Maternal gestational weight gain and offspring weight in adolescence. Obstet Gynecol. 2008;112:999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viswanathan M, Siega-Riz AM, Moos MK, Deierlein A, Mumford S, Knaack J, et al. Outcomes of maternal weight gain. Evid Rep Technol Assess (Full Rep). 2008:1–223. [PMC free article] [PubMed] [Google Scholar]
- 4.IOM (Institute of Medicine) and NRC (National Research Council). Weight gain during pregnancy: reexamining the guidelines. Washington, DC, USA: The National Academies Press; 2009. [PubMed] [Google Scholar]
- 5.Deputy NP, Sharma AJ, Kim SY. Gestational weight gain - United States, 2012 and 2013. MMWR Morb Mortal Wkly Rep. 2015;64:1215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devlieger R, Benhalima K, Damm P, Van Assche A, Mathieu C, Mahmood T, et al. Maternal obesity in Europe: where do we stand and how to move forward?: a scientific paper commissioned by the European Board and College of Obstetrics and Gynaecology (EBCOG). Eur J Obstet Gynecol Reprod Biol. 2016;201:203–8. [DOI] [PubMed] [Google Scholar]
- 7.Chung JG, Taylor RS, Thompson JM, Anderson NH, Dekker GA, Kenny LC, et al. Gestational weight gain and adverse pregnancy outcomes in a nulliparous cohort. Eur J Obstet Gynecol Reprod Biol. 2013;167:149–53. [DOI] [PubMed] [Google Scholar]
- 8.Butte NF, Ellis KJ, Wong WW, Hopkinson JM, Smith EO. Composition of gestational weight gain impacts maternal fat retention and infant birth weight. Am J Obstet Gynecol. 2003;189:1423–32. [DOI] [PubMed] [Google Scholar]
- 9.Lederman SA, Paxton A, Heymsfield SB, Wang J, Thornton J, Pierson RN Jr. Body fat and water changes during pregnancy in women with different body weight and weight gain. Obstet Gynecol. 1997;90(4 Pt 1):483–8. [DOI] [PubMed] [Google Scholar]
- 10.Adamo KB, Ferraro ZM, Brett KE. Can we modify the intrauterine environment to halt the intergenerational cycle of obesity? Int J Environ Res Public Health. 2012;9:1263–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hytten FE, Thomson AM, Taggart N. Total body water in normal pregnancy. J Obstet Gynaecol Br Commonw. 1966;73:553–61. [DOI] [PubMed] [Google Scholar]
- 12.Balani J, Hyer S, Johnson A, Shehata H. The importance of visceral fat mass in obese pregnant women and relation with pregnancy outcomes. Obstet Med. 2014;7:22–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartha JL, Marin-Segura P, Gonzalez-Gonzalez NL, Wagner F, Aguilar-Diosdado M, Hervias-Vivancos B. Ultrasound evaluation of visceral fat and metabolic risk factors during early pregnancy. Obesity (Silver Spring). 2007;15:2233–9. [DOI] [PubMed] [Google Scholar]
- 14.Pitkin RM. Nutritional support in obstetrics and gynecology. Clin Obstet Gynecol. 1976;19:489–513. [DOI] [PubMed] [Google Scholar]
- 15.de Haas S, Ghossein-Doha C, van Kuijk SM, van Drongelen J, Spaanderman ME. Physiological adaptation of maternal plasma volume during pregnancy: a systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2017;49:177–87. [DOI] [PubMed] [Google Scholar]
- 16.Forsum E, Sadurskis A, Wager J. Resting metabolic rate and body composition of healthy Swedish women during pregnancy. Am J Clin Nutr. 1988;47:942–7. [DOI] [PubMed] [Google Scholar]
- 17.Stevens-Simon C, McAnarney ER, Roghmann KJ, Forbes GB. Composition of gestational weight gain in adolescent pregnancy. J Matern Fetal Med. 1997;6:79–86. [DOI] [PubMed] [Google Scholar]
- 18.Purdie DW, Aaron JE, Selby PL. Bone histology and mineral homeostasis in human pregnancy. Br J Obstet Gynaecol. 1988;95:849–54. [DOI] [PubMed] [Google Scholar]
- 19.van Raaij JM, Peek ME, Vermaat-Miedema SH, Schonk CM, Hautvast JG. New equations for estimating body fat mass in pregnancy from body density or total body water. Am J Clin Nutr. 1988;48:24–9. [DOI] [PubMed] [Google Scholar]
- 20.Hytten FE, Robertson EG. Maternal water metabolism in pregnancy. Proc R Soc Med. 1971;64:1072. [PMC free article] [PubMed] [Google Scholar]
- 21.Chumlea WC, Guo SS, Kuczmarski RJ, Flegal KM, Johnson CL, Heymsfield SB, et al. Body composition estimates from NHANES III bioelectrical impedance data. Int J Obes Relat Metab Disord. 2002;26:1596–609. [DOI] [PubMed] [Google Scholar]
- 22.Siri WE. Body composition from fluid spaces and density: analysis of methods. 1961. Nutrition. 1993;9:480–91. [PubMed] [Google Scholar]
- 23.Lof M, Forsum E. Hydration of fat-free mass in healthy women with special reference to the effect of pregnancy. Am J Clin Nutr. 2004;80:960–5. [DOI] [PubMed] [Google Scholar]
- 24.Prentice AM, Goldberg GR, Davies HL, Murgatroyd PR, Scott W. Energy-sparing adaptations in human pregnancy assessed by whole-body calorimetry. Br J Nutr. 1989;62:5–22. [DOI] [PubMed] [Google Scholar]
- 25.Lederman SA, Paxton A, Heymsfield SB, Wang J, Thornton J, Pierson RN Jr.. Maternal body fat and water during pregnancy: do they raise infant birth weight? Am J Obstet Gynecol. 1999;180(Pt 1):235–40. [DOI] [PubMed] [Google Scholar]
- 26.Kopp-Hoolihan LE, van Loan MD, Wong WW, King JC. Fat mass deposition during pregnancy using a four-component model. J Appl Physiol (1985). 1999;87:196–202. [DOI] [PubMed] [Google Scholar]
- 27.Bleker OP, Hoogland HJ. Short review: ultrasound in the estimation of human intrauterine placental growth. Placenta. 1981;2:275–8. [DOI] [PubMed] [Google Scholar]
- 28.Almog B, Shehata F, Aljabri S, Levin I, Shalom-Paz E, Shrim A. Placenta weight percentile curves for singleton and twins deliveries. Placenta. 2011;32:58–62. [DOI] [PubMed] [Google Scholar]
- 29.Thompson JM, Irgens LM, Skjaerven R, Rasmussen S. Placenta weight percentile curves for singleton deliveries. BJOG. 2007;114:715–20. [DOI] [PubMed] [Google Scholar]
- 30.Wallace JM, Bhattacharya S, Horgan GW. Gestational age, gender and parity specific centile charts for placental weight for singleton deliveries in Aberdeen, UK. Placenta. 2013;34:269–74. [DOI] [PubMed] [Google Scholar]
- 31.Ounpraseuth ST, Magann EF, Spencer HJ, Rabie NZ, Sandlin AT. Normal amniotic fluid volume across gestation: comparison of statistical approaches in 1190 normal amniotic fluid volumes. J Obstet Gynaecol Res. 2017;43:1122–31. [DOI] [PubMed] [Google Scholar]
- 32.Catalano PM, Thomas A, Huston-Presley L, Amini SB. Increased fetal adiposity: a very sensitive marker of abnormal in utero development. Am J Obstet Gynecol. 2003;189:1698–704. [DOI] [PubMed] [Google Scholar]
- 33.Eriksson B, Lof M, Eriksson O, Hannestad U, Forsum E. Fat-free mass hydration in newborns: assessment and implications for body composition studies. Acta Paediatr. 2011;100:680–6. [DOI] [PubMed] [Google Scholar]
- 34.Hull HR, Thornton JC, Ji Y, Paley C, Rosenn B, Mathews P, et al. Higher infant body fat with excessive gestational weight gain in overweight women. Am J Obstet Gynecol. 2011;205:211 e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koo B, Walters J, Hockman E, Koo W. Body composition of newborn twins: intrapair differences. J Am Coll Nutr. 2002;21:245–9. [DOI] [PubMed] [Google Scholar]
- 36.Lederman SA. Pregnancy In: Heymsfield SB, Lohman TG, Wang Z, Going SB, editor. Human body composition. 2nd ed Champaign, IL, USA: Human Kinetics; 2005. [Google Scholar]
- 37.Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32:77–97. [DOI] [PubMed] [Google Scholar]
- 38.Huston Presley L, Wong WW, Roman NM, Amini SB, Catalano PM. Anthropometric estimation of maternal body composition in late gestation. Obstet Gynecol. 2000;96:33–7. [DOI] [PubMed] [Google Scholar]
- 39.Paxton A, Lederman SA, Heymsfield SB, Wang J, Thornton JC, Pierson RN Jr. Anthropometric equations for studying body fat in pregnant women. Am J Clin Nutr. 1998;67:104–10. [DOI] [PubMed] [Google Scholar]
- 40.Forsum E, Sadurskis A, Wager J. Estimation of body fat in healthy Swedish women during pregnancy and lactation. Am J Clin Nutr. 1989;50:465–73. [DOI] [PubMed] [Google Scholar]
- 41.Butte NF, Wills C, Smith EO, Garza C. Prediction of body density from skinfold measurements in lactating women. Br J Nutr. 1985;53:485–9. [DOI] [PubMed] [Google Scholar]
- 42.Durnin JV, McKillop FM, Grant S, Fitzgerald G. Energy requirements of pregnancy in Scotland. Lancet. 1987;2:897–900. [DOI] [PubMed] [Google Scholar]
- 43.Ehrenberg HM, Huston-Presley L, Catalano PM. The influence of obesity and gestational diabetes mellitus on accretion and the distribution of adipose tissue in pregnancy. Am J Obstet Gynecol. 2003;189:944–8. [DOI] [PubMed] [Google Scholar]
- 44.Taggart NR, Holliday RM, Billewicz WZ, Hytten FE, Thomson AM. Changes in skinfolds during pregnancy. Br J Nutr. 1967;21:439–51. [DOI] [PubMed] [Google Scholar]
- 45.Pipe NG, Smith T, Halliday D, Edmonds CJ, Williams C, Coltart TM. Changes in fat, fat-free mass and body water in human normal pregnancy. Br J Obstet Gynaecol. 1979;86:929–40. [DOI] [PubMed] [Google Scholar]
- 46.Heymsfield SB, Bourgeois B, Ng BK, Sommer MJ, Li X, Shepherd JA. Digital anthopometry: a critical review. Eur J Clin Nutr. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Francis KT. Body-composition assessment using underwater weighing techniques. Phys Ther. 1990;70:657–62. [DOI] [PubMed] [Google Scholar]
- 48.Tantucci C, Bottone D, Borghesi A, Guerini M, Quadri F, Pini L. Methods for measuring lung volumes: is there a better one? Respiration. 2016;91:273–80. [DOI] [PubMed] [Google Scholar]
- 49.Dempster P, Aitkens S. A new air displacement method for the determination of human body composition. Med Sci Sports Exerc. 1995;27:1692–7. [PubMed] [Google Scholar]
- 50.Crapo RO, Morris AH, Clayton PD, Nixon CR. Lung volumes in healthy nonsmoking adults. Bull Eur Physiopathol Respir. 1982;18:419–25. [PubMed] [Google Scholar]
- 51.Henriksson P, Lof M, Forsum E. Assessment and prediction of thoracic gas volume in pregnant women: an evaluation in relation to body composition assessment using air displacement plethysmography. Br J Nutr. 2013;109:111–7. [DOI] [PubMed] [Google Scholar]
- 52.Marshall NE, Murphy EJ, King JC, Haas EK, Lim JY, Wiedrick J, et al. Comparison of multiple methods to measure maternal fat mass in late gestation. Am J Clin Nutr. 2016;103:1055–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jensen D, Webb KA, Davies GA, O’Donnell DE. Mechanical ventilatory constraints during incremental cycle exercise in human pregnancy: implications for respiratory sensation. J Physiol. 2008;586:4735–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Knuttgen HG, Emerson K Jr. Physiological response to pregnancy at rest and during exercise. J Appl Physiol (1985). 1974;36:549–53. [DOI] [PubMed] [Google Scholar]
- 55.International Atomic Energy Agency. Introduction to body composition assessment using the deuterium dilution technique with analysis of saliva samples by Fourier transform infrared spectrometry. Vienna: IAEA; 2010. [Google Scholar]
- 56.Pace N, Rathbun EN. Studies on body composition .3. the body water and chemically combined nitrogen content in relation to fat content. J Biol Chem. 1945;158:685–91. [Google Scholar]
- 57.Lof M, Forsum E. Evaluation of bioimpedance spectroscopy for measurements of body water distribution in healthy women before, during, and after pregnancy. J Appl Physiol (1985). 2004;96:967–73. [DOI] [PubMed] [Google Scholar]
- 58.Lukaski HC, Bolonchuk WW, Hall CB, Siders WA. Validation of tetrapolar bioelectrical impedance method to assess human body composition. J Appl Physiol (1985). 1986;60:1327–32. [DOI] [PubMed] [Google Scholar]
- 59.Boyle VT, Thorstensen EB, Thompson JMD, McCowan LME, Mitchell EA, Godfrey KM, et al. The relationship between maternal 25-hydroxyvitamin D status in pregnancy and childhood adiposity and allergy: an observational study. Int J Obes (Lond). 2017;41:1755–60. [DOI] [PubMed] [Google Scholar]
- 60.Facca TA, Mastroianni-Kirsztajn G, Sabino ARP, Passos MT, Dos Santos LF, Fama EAB, et al. Pregnancy as an early stress test for cardiovascular and kidney disease diagnosis. Pregnancy Hypertens. 2017, 10.1016/j.preghy.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 61.Piuri G, Ferrazzi E, Bulfoni C, Mastricci L, Di Martino D, Speciani AF. Longitudinal changes and correlations of bioimpedance and anthropometric measurements in pregnancy: Simple possible bed-side tools to assess pregnancy evolution. J Matern Fetal Neonatal Med. 2017;30:2824–30. [DOI] [PubMed] [Google Scholar]
- 62.Kugananthan S, Gridneva Z, Lai CT, Hepworth AR, Mark PJ, Kakulas F. et al. Associations between maternal body composition and appetite hormones and macronutrients in human milk. Nutrients. 2017;9:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Catalano PM, Wong WW, Drago NM, Amini SB. Estimating body composition in late gestation: a new hydration constant for body density and total body water. Am J Physiol. 1995;268(Pt 1): E153–8. [DOI] [PubMed] [Google Scholar]
- 64.Fidanza F The density of fat-free body mass during pregnancy. Int J Vitam Nutr Res. 1987;57:104. [PubMed] [Google Scholar]
- 65.Hopkinson JM, Butte NF, Ellis KJ, Wong WW, Puyau MR, Smith EO. Body fat estimation in late pregnancy and early postpartum: comparison of two-, three-, and four-component models. Am J Clin Nutr. 1997;65:432–8. [DOI] [PubMed] [Google Scholar]
- 66.Seitchik J Total body water and total body density of pregnant women. Obstet Gynecol. 1967;29:155–66. [PubMed] [Google Scholar]
- 67.Lukaski HC. Methods for the assessment of human body composition: traditional and new. Am J Clin Nutr. 1987;46:537–56. [DOI] [PubMed] [Google Scholar]
- 68.Bernstein IM, Goran MI, Amini SB, Catalano PM. Differential growth of fetal tissues during the second half of pregnancy. Am J Obstet Gynecol. 1997;176(1 Pt 1):28–32. [DOI] [PubMed] [Google Scholar]
- 69.Larciprete G, Valensise H, Vasapollo B, Novelli GP, Parretti E, Altomare F, et al. Fetal subcutaneous tissue thickness (SCTT) in healthy and gestational diabetic pregnancies. Ultrasound Obstet Gynecol. 2003;22:591–7. [DOI] [PubMed] [Google Scholar]
- 70.Moyer-Mileur LJ, Slater H, Thomson JA, Mihalopoulos N, Byrne J, Varner MW. Newborn adiposity measured by plethysmography is not predicted by late gestation two-dimensional ultrasound measures of fetal growth. J Nutr. 2009;139:1772–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schwartz J, Galan H. Ultrasound in assessment of fetal growth disorders: is there a role for subcutaneous measurements? Ultrasound Obstet Gynecol. 2003;22:329–35. [DOI] [PubMed] [Google Scholar]
- 72.Ikenoue S, Waffarn F, Sumiyoshi K, Ohashi M, Ikenoue C, Buss C, et al. Association of ultrasound-based measures of fetal body composition with newborn adiposity. Pediatr Obes. 2017;12(Suppl 1):86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moore GS, Allshouse AA, Fisher BM, Kahn BF, Hernandez TL, Reece MS, et al. Can fetal limb soft tissue measurements in the third trimester predict neonatal adiposity? J Ultrasound Med. 2016;35:1915–24. [DOI] [PubMed] [Google Scholar]
- 74.Kuczmarski RJ, Fanelli MT, Koch GG. Ultrasonic assessment of body composition in obese adults: overcoming the limitations of the skinfold caliper. Am J Clin Nutr. 1987;45:717–24. [DOI] [PubMed] [Google Scholar]
- 75.Stevens-Simon C, Thureen P, Barrett J, Stamm E. Skinfold caliper and ultrasound assessments of change in the distribution of subcutaneous fat during adolescent pregnancy. Int J Obes Relat Metab Disord. 2001;25:1340–5. [DOI] [PubMed] [Google Scholar]
- 76.Seabolt LA, Welch EB, Silver HJ. Imaging methods for analyzing body composition in human obesity and cardiometabolic disease. Ann NY Acad Sci. 2015;1353:41–59. [DOI] [PubMed] [Google Scholar]
- 77.Pregnancy and medical radiation [press release], 2000.
- 78.Kinoshita T, Itoh M. Longitudinal variance of fat mass deposition during pregnancy evaluated by ultrasonography: the ratio of visceral fat to subcutaneous fat in the abdomen. Gynecol Obstet Invest. 2006;61:115–8. [DOI] [PubMed] [Google Scholar]
- 79.Straughen JK, Trudeau S, Misra VK. Changes in adipose tissue distribution during pregnancy in overweight and obese compared with normal weight women. Nutr Diabetes. 2013;3:e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.De Souza LR, Berger H, Retnakaran R, Vlachou PA, Maguire JL, Nathens AB, et al. Hepatic fat and abdominal adiposity in early pregnancy together predict impaired glucose homeostasis in mid-pregnancy. Nutr Diabetes. 2016;6:e229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.De Souza LR, Berger H, Retnakaran R, Maguire JL, Nathens AB, Connelly PW, et al. First-trimester maternal abdominal adiposity predicts dysglycemia and gestational diabetes mellitus in mid-pregnancy. Diabetes Care. 2016;39:61–4. [DOI] [PubMed] [Google Scholar]
- 82.Pontual AC, Figueiroa JN, De Souza LR, Ray JG, Alves JG. Visceral adiposity in the first half of pregnancy in association with glucose, lipid and insulin profiles in later pregnancy: a cohort study. Matern Child Health J. 2016;20:1720–5. [DOI] [PubMed] [Google Scholar]
- 83.Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements--a prospective study. Am J Obstet Gynecol. 1985;151:333–7. [DOI] [PubMed] [Google Scholar]
- 84.Tsai R, Raptis C, Fowler KJ, Owen JW, Mellnick VM. MRI of suspected appendicitis during pregnancy: interradiologist agreement, indeterminate interpretation and the meaning of nonvisualization of the appendix. Br J Radiol. 2017;90:20170383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Daimon A, Terai Y, Nagayasu Y, Okamoto A, Sano T, Suzuki Y, et al. A case of intestinal obstruction in pregnancy diagnosed by MRI and treated by intravenous hyperalimentation. Case Rep Obstet Gynecol. 2016;2016:8704035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Holmes S, Kirkpatrick ID, Zelop CM, Jassal DS. MRI evaluation of maternal cardiac displacement in pregnancy: implications for cardiopulmonary resuscitation. Am J Obstet Gynecol. 2015;213:401 e1–5. [DOI] [PubMed] [Google Scholar]
- 87.Girardi G MRI-based methods to detect placental and fetal brain abnormalities in utero. J Reprod Immunol. 2016;114:86–91. [DOI] [PubMed] [Google Scholar]
- 88.Sohlstrom A, Forsum E. Changes in adipose tissue volume and distribution during reproduction in Swedish women as assessed by magnetic resonance imaging. Am J Clin Nutr. 1995;61:287–95. [DOI] [PubMed] [Google Scholar]
- 89.Sohlstrom A, Wahlund LO, Forsum E. Adipose tissue distribution as assessed by magnetic resonance imaging and total body fat by magnetic resonance imaging, underwater weighing, and body-water dilution in healthy women. Am J Clin Nutr. 1993;58:830–8. [DOI] [PubMed] [Google Scholar]
- 90.Forbes S, Barr SM, Reynolds RM, Semple S, Gray C, Andrew R, et al. Convergence in insulin resistance between very severely obese and lean women at the end of pregnancy. Diabetologia. 2015;58:2615–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Anblagan D, Deshpande R, Jones NW, Costigan C, Bugg G, Raine-Fenning N, et al. Measurement of fetal fat in utero in normal and diabetic pregnancies using magnetic resonance imaging. Ultrasound Obstet Gynecol. 2013;42:335–40. [DOI] [PubMed] [Google Scholar]
- 92.Berger-Kulemann V, Brugger PC, Reisegger M, Klein K, Hachemian N, Koelblinger C, et al. Quantification of the subcutaneous fat layer with MRI in fetuses of healthy mothers with no underlying metabolic disease vs. fetuses of diabetic and obese mothers. J Perinat Med. 2011;40:179–84. [DOI] [PubMed] [Google Scholar]
- 93.Ward LC, Poston L, Godfrey KM, Koletzko B. Assessing early growth and adiposity: report from an EarlyNutrition Academy workshop. Ann Nutr Metab. 2013;63:120–30. [DOI] [PubMed] [Google Scholar]
- 94.Takahashi K, Ohkuchi A, Furukawa R, Matsubara S, Suzuki M. Establishing measurements of subcutaneous and visceral fat area ratio in the early second trimester by magnetic resonance imaging in obese pregnant women. J Obstet Gynaecol Res. 2014;40:1304–7. [DOI] [PubMed] [Google Scholar]
- 95.Poppitt SD, Prentice AM, Goldberg GR, Whitehead RG. Energy-sparing strategies to protect human fetal growth. Am J Obstet Gynecol. 1994;171:118–25. [DOI] [PubMed] [Google Scholar]