Abstract
The mechanisms by which the sensitivity of naive CD4+ T cells to stimulation by the cognate antigen via the T cell antigen receptor (TCR) determines their differentiation into distinct helper T cell subsets remain elusive. Here we demonstrate functional collaboration of the ubiquitin E3 ligases Itch and WWP2 in regulating the strength of the TCR signal. Mice lacking both Itch and WWP2 in T cells showed spontaneous autoimmunity and lung inflammation. CD4+ T cells deficient in Itch and WWP2 exhibited hypo-responsiveness to TCR stimulation and a bias toward differentiation into the TH2 subset of helper T cells. Itch and WWP2 formed a complex and cooperated to enhance TCR-proximal signaling by catalyzing the conjugation of atypical ubiquitin chains to the phosphatase SHP-1 and reducing the association of SHP-1 with the tyrosine kinase Lck. These findings indicate that targeted ubiquitination regulates the strength of the TCR signal and differentiation toward the TH2 lineage.
Helper T cells have a central role in adaptive immunity and are involved not only in host defense against different types of infectious agents but also in the pathogenesis of autoimmunity or inflammatory diseases. When naive CD4+ T cells encounter antigen presented in the context of major histocompatibility complex on antigen-presenting cells, they differentiate into various subsets of helper T cells, such as TH1 cells, TH2 cells, TH9 cells, TH17 cells, regulatory T cells and follicular helper T cells1. The differentiation of naive CD4+ T cells into each helper T cell subset is tightly controlled by a specific set of cytokines and transcription factors. Th2 cells are crucial for host immunity to extracellular parasites and also induce allergic inflammatory responses by producing type 2 cytokines, such as interleukin 4 (IL-4), IL-5 and IL-13. Because IL-4 drives expression of the transcription factor GATA-3 via activation of the transcription factor STAT6, and GATA-3 further amplifies IL-4 production, this regulatory loop is thought to be a key element in determining the TH2 lineage2. In addition to the cytokine milieu, the early ‘decision’ between TH1 differentiation and TH2 differentiation is also affected by the strength of the signal mediated by the T cell antigen receptor (TCR)3,4. Stimulation of naive CD4+ T cells with a low dose of antigen drives TH2 differentiation by inducing GATA-3 expression in a manner independent of IL-4 and STAT6, whereas stronger TCR signals generated by stimulation with a high dose of peptide direct naive CD4+ T cells toward development into Th1 cells5. However, the mechanisms by which the strength of TCR signaling regulates helper T cell ‘decisions’ is poorly understood.
The Nedd4 family of E3 ubiquitin ligases modulates CD4+ T cell function6–8. In humans, the Nedd4 family comprises nine E3 ligases that contain a conserved amino-terminal C2 domain, a variable number of WW domains9 and a carboxy-terminal HECT domain. Among these, Itch (AIP4) and WWP2 (AIP2) contain four WW domains and are nearly identical to each other. Despite the structural homology, the substrates and interacting proteins identified so far for WWP2 and Itch do not overlap10,11. In genetic studies, mice deficient in Itch (the itchy strain) have a skin-scratching phenotype, multiple-organ inflammation12 and deficient TH2 differentiation6. Single-nucleotide polymorphism autozygosity mapping has linked Itch deficiency to multisystem autoimmune diseases and asthma in humans13. WWP2 is dispensable for thymic or peripheral CD4+ T cell development at steady state14. In the present study, we investigated whether Itch and WWP2 cooperated during the activation and differentiation of CD4+ T cells. Using a combination of biochemical, genetic and proteomic approaches, we found that Itch and WWP2 cooperated to regulate TH2 differentiation by enhancing the strength of the TCR signal via inducing atypical ubiquitination of the phosphatase SHP-1.
Results
Itch interacts with WWP2.
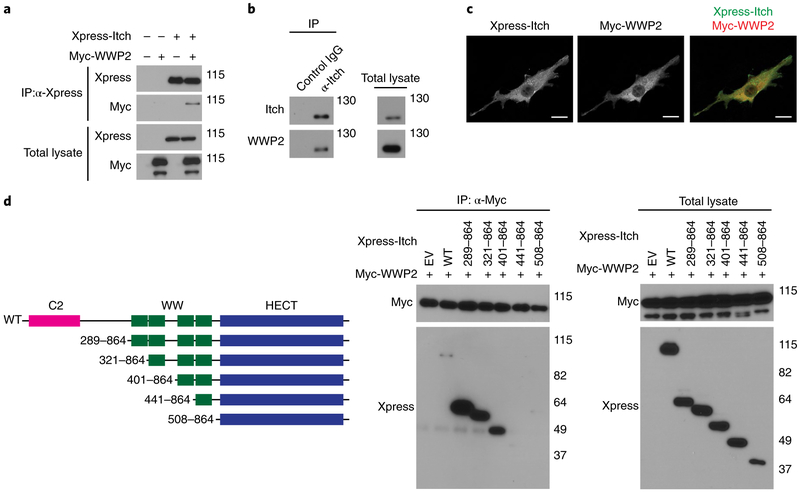
Itch and WWP2 share a high level of structural homology. Amino-acid sequence alignment indicated that the WW and HECT domains of Itch were approximately 59–86% identical to those of WWP2 (Supplementary Fig. 1a), and preliminary proteomics analysis indicated that Itch and WWP2 formed a protein complex (data not shown). To investigate whether Itch and WWP2 form a complex in vivo and collaborate to regulate CD4+ T cell homeostasis, we assessed whether Itch associated with WWP2. Xpress-tagged Itch and Myc-tagged WWP2 co-immunoprecipitated when ectopically expressed in HEK293T human embryonic kidney T cells (Fig. 1a and Supplementary Fig. 1b). Further proteomics analysis demonstrated that FLAG-tagged Itch bound to endogenous WWP2 in HEK293T cells (Supplementary Table 1). In Jurkat human T cells, Itch immunoprecipitated with WWP2 (Fig. 1b), suggestive of an endogenous physiological interaction between Itch and WWP2 in T cells. This interaction was not further enhanced in response to TCR stimulation (Supplementary Fig. 1c). By confocal microscopy, Itch and WWP2 ectopically expressed in NIH3T3 mouse fibroblasts exhibited a punctate distribution (Fig. 1c), as previously reported15,16, with the perinuclear region showing enrichment for both Itch and WWP2 (Fig. 1c). Through the use of Xpress-tagged full-length Itch and several Itch mutant proteins lacking C2 and various WW domains (Fig. 1d), we observed that Itch mutants consisting of amino acids 441–864 or 508–864, which lacked the third WW domain of Itch, did not associate with WWP2, whereas Itch lacking the C2 domain or the first or second WW domain associated with WWP2, similar to full-length Itch (Fig. 1d). Thus, Itch and WWP2 interacted with each other in HEK293T cells in a manner dependent on the third WW domain of Itch.
Fig. 1 ∣. Itch interacts with WWP2 through its third WW domain.
a, Immunoblot analysis of HEK293T cells transfected with expression vectors for Xpress-tagged Itch and/or Myc-tagged WWP2 or not (above lanes), assessed after immunoprecipitation (IP) with anti-Xpress (α-Xpress) or no immunoprecipitation (Total lysate) (left margin); right margin, molecular size in kilodaltons (throughout). b, Immunoblot analysis of the interaction of endogenous Itch with WWP2 in Jurkat T cells, assessed after immunoprecipitation with anti-Itch or the control antibody IgG (above lanes; left) or no immunoprecipitation (right). c, Confocal microscopy of the intracellular localization of Xpress-tagged Itch and Myc-tagged WWP2 in NIH3T3 cells transfected to express those molecules. Scale bars, 20 μm. d, Full-length (WT) Itch and its deletion mutants lacking functional domains (left; ranges (left margin) indicate amino acids present in construct), and immunoblot analysis of the interaction of Itch and WWP2 in HEK293T cells co-transfected (above lanes) to express Myc-tagged WWP2 plus empty vector (EV) or Xpress-tagged full-length Itch or various deletion mutants (middle and right), assessed after immunoprecipitation with anti-Myc (middle) or no immunoprecipitation (right). Data are representative of two to four independent experiments.
Loss of Itch and WWP2 leads to auto-inflammation.
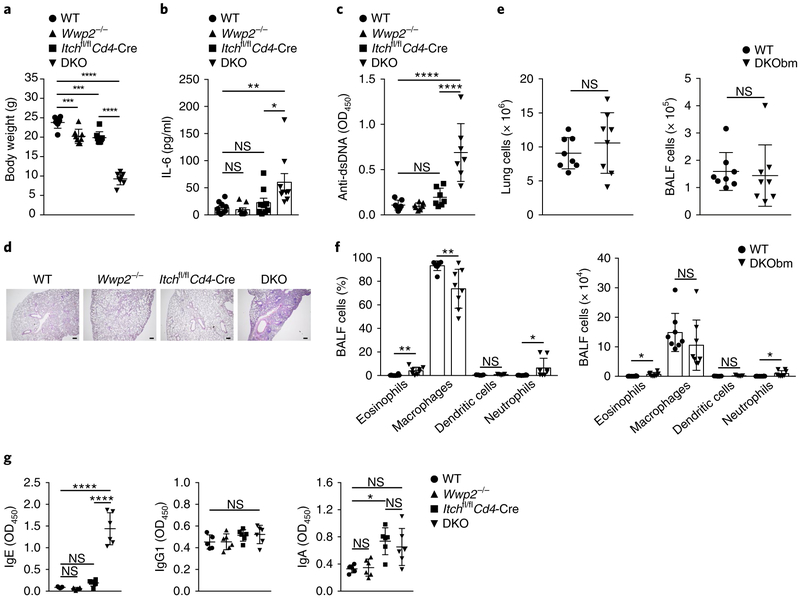
To determine if the E3 ligases Itch and WWP2 cooperated in vivo, we generated Wwp2−/−Itchf/fCd4-Cre mice (called ‘DKO mice’ here), which lack Itch and WWP2 in T cells and lack WWP2 expression systemically. Because the number of DKO pups born per breeding pair was significantly lower than expected, we also generated DKO bone-marrow chimeras by transferring cells from whole (unfractionated) DKO bone marrow into lethally irradiated B6.SJL congenic wild-type mice (to generate ‘DKObm’ mice). At 6 weeks of age, the DKO mice showed significantly lower body weight than that of wild-type, Itchf/fCd4-Cre or Wwp2−/− mice (Fig. 2a). DKO mice showed profound anemia and elevated amounts of IL-6 in the serum (Fig. 2b and Supplementary Fig. 2a), indicative of an inflammatory state, and augmented titers of autoantibodies to nuclear antigens such as double-stranded DNA and histone (Fig. 2c and Supplementary Fig. 2b). Histological investigation revealed that DKO mice developed spontaneous lung inflammation manifested by myeloid-cell infiltration and tissue damage (Fig. 2d and Supplementary Fig. 2c). Although the absolute number of cells in the lungs and bronchoalveolar lavage fluid of DKObm mice was similar to that of control chimeras generated with wild-type bone marrow, the number of eosinophils and neutrophils was greater in the bronchoalveolar lavage fluid of DKObm mice (Fig. 2e,f and Supplementary Fig. 2d). DKO mice exhibited higher titers of immunoglobulin E (IgE) in the serum than that of their wild-type, Itchf/fCd4-Cre or Wwp2−/− counterparts (Fig. 2g), while the abundance of other immunoglobulin subclasses was similar among these four groups (Fig. 2g and Supplementary Fig. 2e). Thus, loss of Itch and WWP2 in T cells led to autoimmune and inflammatory manifestations that were more severe than those of mice with single deficiency in Itch or WWP2.
Fig. 2 ∣. Accelerated autoimmunity and inflammation in DKO mice.
a, Body weight of 6-week-old male wild-type (WT) mice, Wwp2−/−, Itchf/fCd4-Cre and Itchf/fCd4-Cre Wwp2−/− (DKO) mice (key; n = 8 per group). b, ELISA of IL-6 in the serum of 6- to 8-week-old mice as in a (key; n = 9 per group). c, ELISA of antibodies to double-stranded DNA (Anti-dsDNA) in the serum of 6-week-old mice as in a (key; n = 7 per group), presented as optical density at 450 nm (OD450). d, Lung sections from 6-week-old mice as in a (above images), stained with hematoxylin and eosin. Scale bars, 100 μm.
e, Absolute number of cells in the lungs (left) or bronchoalveolar lavage fluid (BALF) (right) of wild-type or DKObm mice (key; n = 8 per group) at 6-8 weeks after cell transplantation. f, Frequency (left) and number (right) of eosinophils, macrophages, dendritic cells and neutrophils (horizontal axis) in the bronchoalveolar lavage fluid of mice as in e (key; n = 8 per group (eosinophils, macrophages and neutrophils) or n = 5 per group (dendritic cells)).
g, ELISA of the immunoglobulin subclasses IgE, IgG1 and IgA in serum from 6-week-old wild-type mice (n = 5), Wwp2−/− mice (n = 6), Itchf/fCd4-Cre mice (n = 6) or DKO mice (n = 6) as in a (key). Each symbol (a-c,e-g) represents an individual mouse; small horizontal lines (c,e,g) indicate the mean (±s.d.). NS, not significant (P > 0.05); *P < 0.05, **P < 0.01, *** P < 0.001 and ****p < 0.0001 (one-way analysis of variance (ANOVA) with Bonferroni’s multiple-comparisons test (a-c,g) or unpaired two-tailed Student’s t-test (e,f)). Data are pooled from or representative of two to four independent experiments (mean±s.d. in a,f; mean±s.e.m. in b).
Itch and WWP2 cooperate in TH2 differentiation.
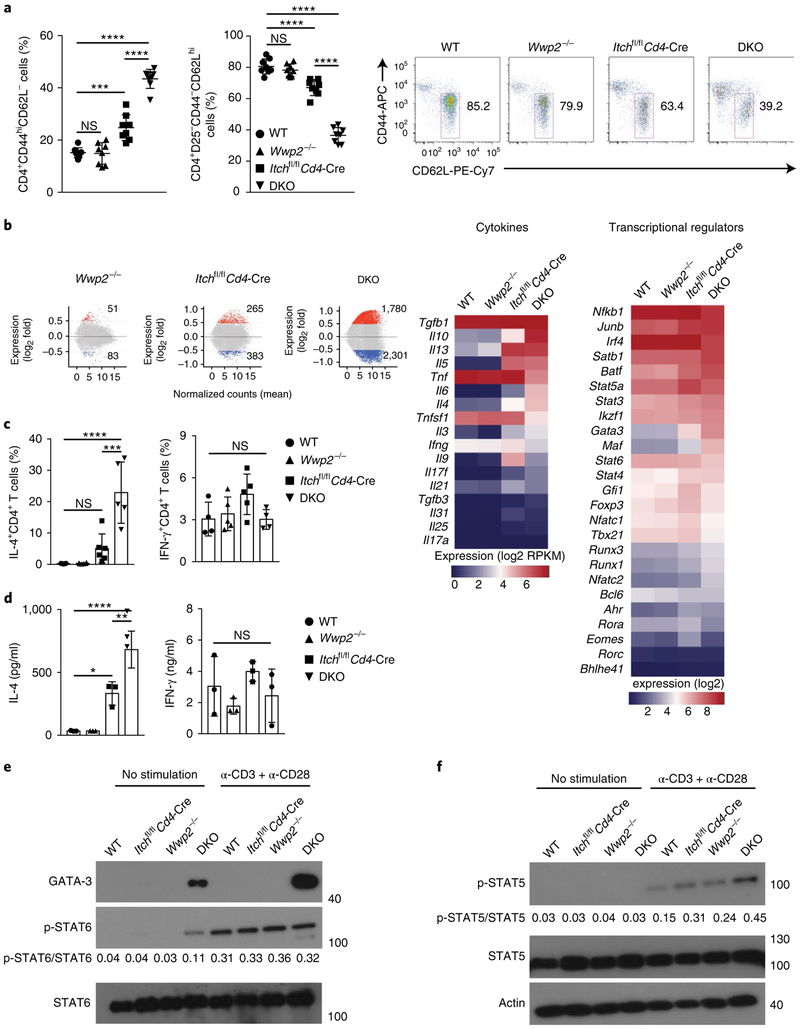
Next we investigated whether Itch and WWP2 collaboratively regulated TH2 differentiation. We first analyzed memory-like and naive CD4+ T cell populations in the spleen of 6- to 7-week-old mice. Although the spleen of Itchf/fCd4-Cre mice contained more memory-like CD4+ T cells than did that of wild-type mice, there was a significantly more conversion of naive CD4+ T cells into memory-like CD4+ T cells in the spleen of DKO mice than in that of wild-type mice (Fig. 3a and Supplementary Fig. 3a). We did not observe a significant difference among wild-type, Itchf/fCd4-Cre and Wwp2−/− and DKO mice in the frequency of CD4+Foxp3+ T cells in the spleen (Supplementary Fig. 3b).
Fig. 3 ∣. Itch and WWP2 collaboratively regulate TH2 differentiation.
a, Frequency of splenic memory-like (CD44hiCD62L−) CD4+ T cells (left) and naive (CD25−CD44−CD62Lhi) CD4+ T cells (middle) from 6- to 7-week-old wild-type, Wwp2−/−, Itchf/fCd4-Cre and DKO mice (key; n = 8 per group), and flow cytometry of splenic naive CD4+ T cells from such mice, showing gating used (right). Numbers adjacent to outlined areas (right) indicate percent CD4+CD25− CD44−CD62Lhi cells. b, Gene-expression profiles (left) of splenic CD4+ T cells stimulated for 4h with PMA and ionomycin, showing genes expressed differentially (mean of normalized counts) in Wwp2−/−, Itchf/fCd4-Cre or DKO cells (above plots) relative to their expression in wild-type cells, and expression of genes encoding selected cytokines (middle) or transcriptional regulators (right) in cells as at left (above plots), presented as log2 reads per kilobase of exon per million mapped reads (RPKM) values. c, Frequency of CD4+ T cells with intracellular staining of IL-4 (left) or IFN-γ (right) among splenocytes obtained from mice as in a (key; n = 5–6 per group (IL-4) or n = 4–5 per group (IFN-γ)) and stimulated for 5h in vitro with PMA and ionomycin in the presence of the protein-transport inhibitor GolgiStop. d, ELISA of IL-4 (left) or IFN-γ (right) in supernatants of CD4+ T cells sorted from mice as in a (key; n = 3 per group) and stimulated for 48h in vitro with anti-CD3 plus anti-CD28. e,f, Immunoblot analysis of GATA-3 (e) and phosphorylated (p-) and total STAT6 (e) or STAT5 (f), as well as actin (loading control) (left margin), in lysates of CD4+ T cells sorted from mice as in a (above lanes) and left unstimulated (left) or stimulated for 16h with anti-CD3 plus anti-CD28 (right; α-CD3 + α-CD28); numbers below lanes indicate the ratio of phosphorylated protein to total protein. Each symbol (a,c,d) represents an individual mouse; small horizontal lines (a) indicate the mean (±s.d.). *P < 0.05, **P < 0.01, *** P < 0.001 and ****P < 0.0001 (one-way ANOVA with Bonferroni’s multiple-comparisons test). Data are pooled from or representative two to six independent experiments (mean±s.d. in c,d).
RNA-based next-generation sequencing analysis of CD4+ T cells stimulated with the phorbol ester PMA and ionomycin indicated that 1,780 genes were upregulated and 2,301 genes were downregulated in DKO CD4+ T cells relative to their expression in wild-type CD4+ T cells (Fig. 3b). Genes encoding proteins involved in TH2 differentiation, such as Il4 and Gata3, were upregulated in Itch−/− and DKO CD4+ T cells relative to their expression in wild-type CD4+ T cells (Fig. 3b), with a much more prominent effect in DKO CD4+ T cells. On the other hand, the expression of TH1 cell–specific genes, such as Ifng and Tbx21, in DKO T cells was not different from that in wild-type T cells (Fig. 3b). The upregulation of TH2 cell–specific genes in DKO CD4+ T cells was confirmed by qRT-PCR (Supplementary Fig. 3c,d). We further analyzed the expression of a set of TH2 cell–specific genes that have been identified by genome-wide gene-expression analysis17. Among these, the expression of GATA-3-regulated genes was particularly upregulated in DKO CD4+ T cells relative to their expression in wild-type CD4+ T cells (Supplementary Fig. 3e). When cytokine production in CD4+ T cells was assessed by flow cytometry and ELISA, DKO CD4+ T cells secreted significantly more IL-4 than did Itchf/fCd4-Cre CD4+ T cells (Fig. 3c,d and Supplementary Fig. 3f). Of note, DKO naive CD4+ T cells showed substantial production of IL-4 (Supplementary Fig. 3g), which suggested that Itch and WWP2 collaboratively regulated TH2 differentiation, particularly during its early phase.
Expression of GATA-3 and phosphorylation of STAT6 are indicators of the TH2 differentiation of CD4+ T cells. We detected expression of GATA-3 protein in unstimulated DKO CD4+ T cells or those stimulated with antibody to the TCR invariant chain CD3 (anti-CD3) plus antibody to the co-receptor CD28 (anti-CD28), but not in wild-type, Itchf/fCd4-Cre or Wwp2−/− CD4+ T cells (Fig. 3e). We detected phosphorylation of STAT6 in unstimulated DKO CD4+ T cells, while after stimulation, the phosphorylation of STAT6 in wild-type CD4+ T cells was similar to that in DKO CD4+ T cells (Fig. 3e). In addition to GATA-3, STAT5 has a key role during TH2 cell differentiation18. DKO CD4+ T cells showed higher expression of STAT5’s target genes than that of wild-type CD4+ T cells (Supplementary Fig. 3h). We also found greater phosphorylation of STAT5 in DKO CD4+ T cells than in wild-type, Itchf/fCd4-Cre or Wwp2−/− CD4+ T cells, after stimulation with anti-CD3 plus anti-CD28 (Fig. 3f). Overall, these data suggested that Itch and WWP2 cooperated to negatively regulate the differentiation of TH2 cells.
Itch and WWP2 control proximal TCR signaling.
We next investigated whether Itch and WWP2 modulated the TCR-mediated activation of T cells. We observed much less production of IL-2 by DKO CD4+ T cells after stimulation with anti-CD3 plus anti-CD28 than by their wild-type counterparts (Fig. 4a and Supplementary Fig. 4a). We also observed that the tyrosine-phosphorylation of TCR-proximal signaling components such as ZAP70, LAT and PLCγ 1 induced by anti-CD3 plus anti-CD28 was markedly compromised in DKO CD4+ T cells compared with that in their wild-type counterparts (Fig. 4b). Similar results were obtained with Jurkat T cells in which Itch and Wwp2 were deleted by CRISPR-Cas9 gene editing (Fig. 4c and Supplementary Fig. 4b,c). Notably, the association of Lck with CD3ζ induced by anti-CD3 was attenuated in DKO Jurkat T cells (Fig. 4d). In addition, overexpression of Itch and WWP2 (as protein) in Jurkat T cells resulted in enhanced phosphorylation of TCR-proximal signaling molecules (Fig. 4e). Because ubiquitination is required for TCR turnover through the regulation of membrane trafficking19, we next investigated whether deletion of Itch and WWP2 affected intracellular trafficking of the TCR after stimulation. The kinetics of the downregulation, internalization and recycling of the TCR complexes in wild-type CD4+ T cells were similar to those in DKO CD4+ T cells (Fig. 4f and Supplementary Fig. 4d,e). These data indicated that Itch and WWP2 might act synergistically in regulating TCR signaling via modulating the formation of signaling complexes at the TCR-proximal level.
Fig. 4 ∣. Itch and WWP2 cooperate to promote proximal TCR signaling.
a, ELISA of IL-2 in supernatants of naive CD4+ T cells sorted from wild-type, Wwp2−/−, Itchf/fCd4-Cre or DKO mice (key; n = 3 per group) and stimulated for 48h in vitro with anti-CD3 plus anti-CD28. b, Immunoblot analysis of phosphorylated and total ZAP70, LAT and PLCγ1, and total Itch and WWP2 (left margin), in lysates of CD4+ T cells sorted from mice as in a (above blots) and stimulated for 0, 5 or 15 min (above lanes) with anti-CD3 plus anti-CD28; numbers below lanes indicate the ratio of phosphorylated protein to total protein (left margin). c, Immunoblot analysis of phosphorylated and total CD3ζ, LAT and PLCγ1 (left margin) in lysates of wild-type and DKO Jurkat T cells (above blots) stimulated for 0, 5 or 15 min (above lanes) with anti-CD3; numbers below lanes, as in b. d, Immunoblot analysis of lysates of wild-type and DKO Jurkat T cells (above lanes) transfected to express FLAG-tagged CD3ζ and left unstimulated or stimulated for 5 min with anti-CD3 (above blots), assessed after immunoprecipitation with anti-FLAG or no immunoprecipitation (far left margin). e, Immunoblot analysis of phosphorylated and total proteins (left margin) in lysates of Jurkat T cells transfected with various combinations (above lanes) of expression vectors for FLAG-tagged WWP2 and Xpress-tagged Itch and stimulated for 0, 2 or 15 min (above lanes) with anti-CD3; numbers below lanes, as in b. f, Frequency of CD4+CD45.2+ T cells with downmodulation of TCRβ among such cells sorted from wild-type or DKObm mice (key; n = 3 per group) and stimulated for 0-8h (horizontal axis) in vitro with anti-CD3 plus anti-CD28, followed by analysis of cell-surface TCRβ expression. Each symbol (a) represents an individual mouse. **P < 0.01 and ***P < 0.001 (one-way ANOVA with Bonferroni’s multiple-comparisons test (a) or unpaired two-tailed Student’s t-test (f)). Data are pooled from or representative of two to three independent experiments (mean ± s.d. in a,f).
Itch and WWP2 promote the polyubiquitination of SHP-1.
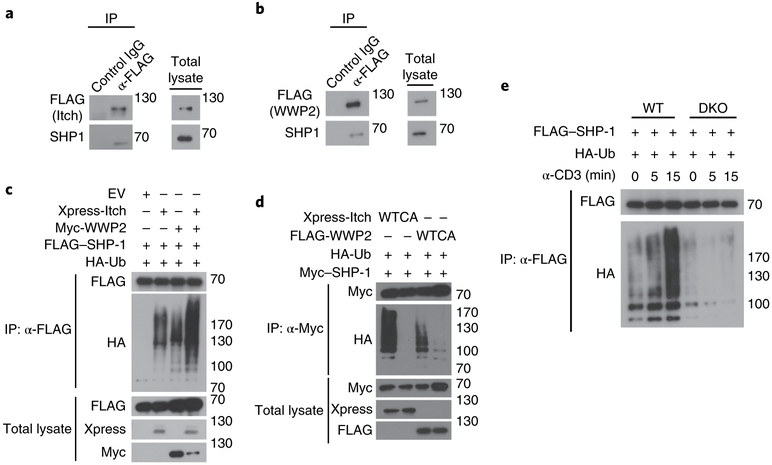
To delineate the regulatory mechanisms by which Itch and WWP2 regulate proximal signaling through the TCR, we used a proteomics approach to identify the proteins that interact with these two E3 ligases in T cells. Mass spectrometry of immunocomplexes purified by affinity chromatography of FLAG-tagged Itch or FLAG-tagged WWP2 transiently expressed in Jurkat T cells indicated an interaction between Itch and WWP2 (Supplementary Tables 2 and 3). In addition, the Itch- and WWP2-precipitated immunocomplexes contained TCR signaling–related components such as tyrosine kinases, phosphatases and adaptors (Supplementary Tables 2 and 3), indicative of the involvement of Itch and WWP2 in the regulation of TCR-proximal signaling. Among these, SHP-1, a non-transmembrane tyrosine phosphatase that modulates TCR- and/or TH2 cell–related signaling pathways20–22, showed a higher score (for peptide matching) and greater sequence coverage for both E3 ligases than that of any other identified TCR-signaling-related molecule (Supplementary Tables 2 and 3). In co-immunoprecipitation assays, Itch and WWP2 interacted with SHP-1 in Jurkat T cells and HEK293T cells (Fig. 5a,b and Supplementary Fig. 5a,b).
Fig. 5 ∣. Itch and WWP2 promote the polyubiquitination of SHP-1.
a,b, Immunoblot analysis of lysates of Jurkat T cells transfected to express FLAG-tagged Itch (a) or WWP2 (b), assessed after immunoprecipitation with anti-FLAG or the control antibody IgG (above lanes, left) or without immunoprecipitation (right). c,d, Immunoblot analysis of an in vivo ubiquitination assay of SHP-1 in HEK293T cells transfected with various combinations (above lanes) of empty vector and expression vectors for Xpress-tagged Itch, Myc-tagged WWP2, FLAG-tagged SHP-1 and hemagglutinin-tagged ubiquitin (HA-Ub) (c) or with expression vectors for Xpress-tagged Itch or FLAG-tagged WWP2 (each wild-type (WT) or catalytically inactive (with the active-site cysteine replaced with alanine (CA)), and hemagglutinin-tagged ubiquitin and Myc-tagged SHP-1 (d), then lysed under denaturing conditions and immunoprecipitated with anti-FLAG (c) or anti-Myc (d) or assessed without immunoprecipitation (left margin). e, Immunoblot analysis of the ubiquitination of SHP-1 in lysates of wild-type and DKO Jurkat T cells (above blots) transfected to express FLAG-tagged SHP-1 together with HA-tagged ubiquitin, stimulated for 0, 5 or 15 min (above lanes) with anti-CD3, assessed after immunoprecipitation with anti-FLAG (left margin). Data are representative of three to four independent experiments.
We then investigated whether Itch and WWP2 ubiquitinated SHP-1. The conjugation of ubiquitin chains to SHP-1 was markedly increased in the presence of Itch and WWP2 (Fig. 5c) and was reduced by the co-expression of a catalytically inactive mutant of Itch or of WWP2 in which an active-site cysteine in the HECT domain was replaced with alanine (Fig. 5d). To exclude the possibility of non-specific effects on the ubiquitination of SHP-1 due to overexpression of Itch or WWP2, we assessed whether Cbl-b, a member of the RING family of E3 ubiquitin ligases, ubiquitinated SHP-1. Unlike the overexpression of Itch and WWP2, overexpression of Cbl-b did not promote the ubiquitination of SHP-1 in HEK293T cells (Supplementary Fig. 5c). In addition, simultaneous depletion of endogenous Itch and WWP2 by small interfering RNA–mediated knockdown substantially abolished the ubiquitination of SHP-1 in HEK293T cells relative to that in HEK293T cells treated with control (non-targeting) small interfering RNA (Supplementary Fig. 5d). Stimulation of the TCR with anti-CD3 induced the polyubiquitination of SHP-1 in wild-type Jurkat T cells; however, this was markedly impaired in DKO Jurkat T cells at 5–15 min of stimulation (Fig. 5e). Thus, SHP-1 was a substrate for Itch- and WWP2-mediated ubiquitination in T cells.
Itch and WWP2 mediate atypical ubiquitin conjugation of SHP-1.
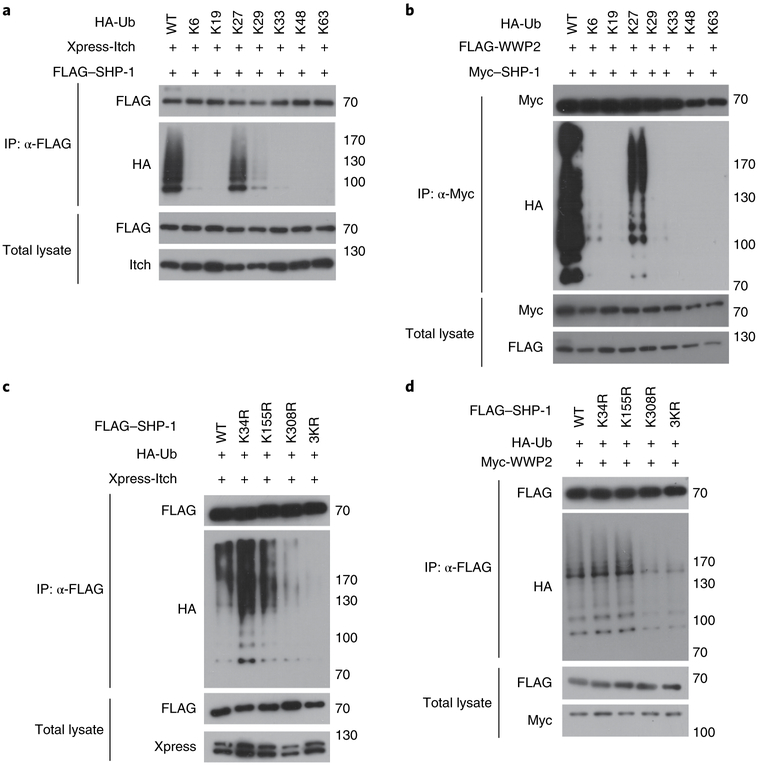
We next investigated which type of ubiquitin-chain linkage was catalyzed by Itch or WWP2 on SHP-1. We transfected HEK293 T cells to express wild-type ubiquitin or various ubiquitin mutants (in which only one of each of the seven lysine residues remained, with the other lysine residues being replaced with arginine), together with SHP-1 and either Itch or WWP2. Among all the ubiquitin mutants tested, we observed that the mutant Ub-K27 (with lysine only at position 27) was sufficient for Itch- or WWP2-mediated conjugation of polyubiquitin to SHP-1, although Itch also promoted conjugation of the Ub-K29 mutant (with lysine only at position 29) at much lower extent than its conjugation of the Ub-K27 mutant (Fig. 6a,b). We next mapped the sites on SHP-1 for Itch- and WWP2-mediated ubiquitination. Mass spectrometry of affinity-purified FLAG-tagged SHP-1 in HEK293T cells overexpressing Itch and WWP2 identified three different lysine residues, at positions 34, 155 and 308, as the ubiquitination sites of mouse SHP-1 (Supplementary Table 4), all of which were highly conserved among mammalian species (Supplementary Fig. 6). The Itch- or WWP2-mediated polyubiquitination of the mutant 3KR–SHP-1 (with replacement of all three lysine residues with arginine) was much lower than that of wild-type SHP-1 (Fig. 6c,d). We also saw less ubiquitination of the mutant K308R-SHP-1 (with replacement of the lysine at position 308 with arginine) than that of wild-type SHP-1, which suggested that the lysine at position 308 might be a major site for the ubiquitination of SHP-1 by Itch and WWP2. Together these data indicated that Itch and WWP2 catalyzed the conjugation of atypical ubiquitin chains to SHP-1 at multiple lysine residues.
Fig. 6 ∣. Characterization of the ubiquitination of SHP-1 by Itch and WWP2.
a,b, Immunoblot analysis of ubiquitin-chain formation on SHP-1 in lysates of HEK293T cells transfected with expression vectors for HA-tagged wild-type ubiquitin (WT) or mutant ubiquitin (with retention of one lysine residue in each; above lanes), plus either Xpress-tagged Itch and FLAG-tagged SHP-1 (a) or FLAG-tagged WWP2 and Myc-tagged SHP-1 (b), assessed after immunoprecipitation with anti-FLAG (a) or anti-Myc (b) or without immunoprecipitation (left margin). c,d, Immunoblot analysis of an in vivo assay of the ubiquitination of SHP-1 in HEK293T cells transfected with expression vectors for FLAG-tagged wild-type SHP-1 (WT) or mutant SHP-1 with replacement of various lysine residues with arginine (above lanes), plus HA-tagged ubiquitin and either Xpress-tagged Itch (c) or Myc-tagged WWP2 (d), assessed after immunoprecipitation with anti-FLAG or without immunoprecipitation (left margin). Data are representative of three independent experiments.
Ubiquitination of SHP-1 reduces its association with Lck.
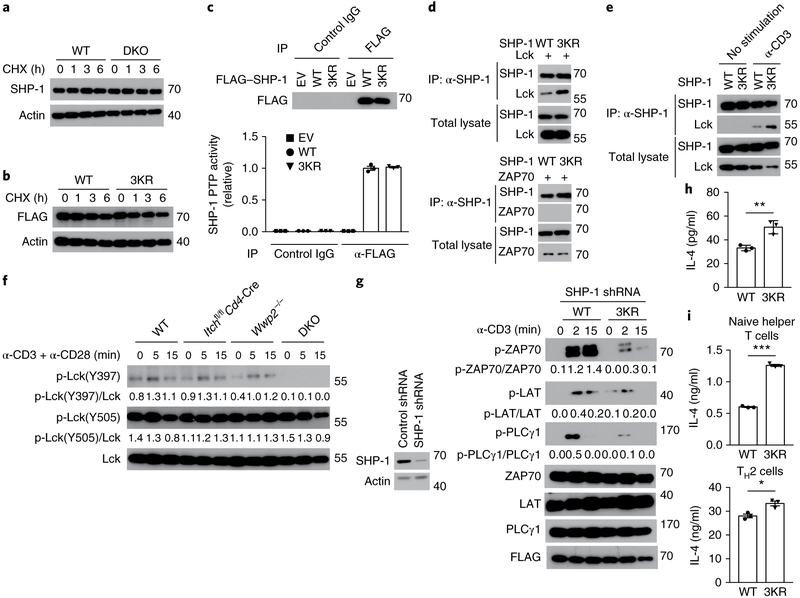
To elucidate the molecular mechanism underlying the Itch- and WWP2-mediated ubiquitination of SHP-1 in the regulation of TCR signaling, we investigated whether the ubiquitination of SHP-1 regulated its stability as a protein. We found no difference between cyclohexamide-treated wild-type Jurkat T cells and cyclohexamide-treated DKO Jurkat T cells in their rate of SHP-1 turnover (Fig. 7a), which indicated that endogenous SHP-1 seemed to be relatively stable. Consistent with that, the stability of 3KR–SHP-1 protein overexpressed in Jurkat T cells was unaffected compared with that of wild-type SHP-1 (Fig. 7b). There was no difference between Jurkat T cells treated with the proteasomal inhibitor MG132 and their untreated counterparts in their expression of endogenous SHP-1 protein (Supplementary Fig. 7a,b). To investigate whether ubiquitination of SHP-1 was involved in the regulation of its phosphatase activity, we assessed the tyrosine-phosphatase activity of wild-type SHP-1 or 3KR–SHP-1 complexes immunoprecipitated from cell lysates. The activity of 3KR–SHP-1 was similar to that of wild-type SHP-1 (Fig. 7c), which indicated that the ubiquitination of SHP-1 did not affect its phosphatase activity. Using purified fusion proteins of glutathione S-transferase and SHP-1 in vitro, we did not observe a difference between the tyrosine-phosphatase activity of wild-type SHP-1 and that of 3KR–SHP-1 (Supplementary Fig. 7c). Phosphorylation of the carboxy-terminal tyrosine residues at positions 536 (Tyr536) and 564 (Tyr564) is reported to increase the phosphatase activity of SHP-121. The total tyrosine-phosphorylation of 3KR–SHP-1 was similar to that of wild-type SHP-1 (Supplementary Fig. 7d,e), while the phosphorylation of SHP-1 at position Tyr564 in wild-type Jurkat T cells was similar to that in DKO Jurkat T cells (Supplementary Fig. 7f), which suggested that the Itch- and WWP2-mediated ubiquitination of SHP-1 did not affect its stability as a protein or its phosphatase activity.
Fig. 7 ∣. SHP-1 ubiquitination regulates the function of Lck.
a, Immunoblot analysis of SHP-1 and actin (loading control) in lysates of wild-type and DKO Jurkat T cells (above blots) treated for 0, 1, 3 or 6 h (above lanes) with 50 μg/ml of cycloheximide (CHX). b, Immunoblot analysis of lysates of Jurkat T cells expressing wild-type SHP-1 or 3KR–SHP-1 (above blots) and treated with cycloheximide as in a. c, Protein tyrosine-phosphatase (PTP) activity of 3KR–SHP-1 among proteins immunoprecipitated, with the control antibody IgG or anti-Flag (below plot), from lysates of Jurkat T cells transfected with empty vector or expression vector for FLAG-tagged wild-type SHP-1 or 3KR–SHP-1 (key), presented relative to the activity of wild-type-SHP-1 (bottom), and immunoblot analysis of such immunoprecipitates (above lanes) (top). d, Immunoblot analysis of the interaction of SHP-1 and Lck in lysates of HEK293T cells transfected with expression vectors for wild-type SHP-1 or 3KR–SHP-1 plus either Lck (top group) or ZAP70 (bottom group) (above lanes), assessed after immunoprecipitation with anti-SHP-1 or without immunoprecipitation (left margin). e, Immunoblot analysis of the interaction of SHP-1 and Lck in Jurkat T cells transfected with expression vectors for wild-type SHP-1 or 3KR–SHP-1 (above lanes) and left unstimulated or stimulated for 5 min with anti-CD3 (above blots), assessed after immunoprecipitation with anti-SHP-1 or without immunoprecipitation (left margin). f, Immunoblot analysis of Lck phosphorylated at Tyr397 (p-Lck(Y397)) or Tyr505 (p-Lck(Y505)) and total Lck in CD4+ T cells sorted from wild-type, Wwp2−/−, Itchf/fCd4-Cre or DKO mice (above blots) and stimulated for 0, 5 or 15 min (above lanes) with anti-CD3 plus anti-CD28; numbers below lanes indicate the ratio of phosphorylated protein to total protein. g, Immunoblot analysis of phosphorylated and total signaling molecules (left margin), and FLAG (loading control), in lysates of Jurkat T cells expressing short hairpin RNA targeting SHP-1 (SHP-1 shRNA) together with FLAG-tagged wild-type SHP-1 or 3KR–SHP-1 (above blots) and stimulated for 0, 2 or 15 min (above lanes) with anti-CD3 (numbers below lanes, as in f) (right blots), and immunoblot analysis of SHP-1 in Jurkat T cells expressing control (non-targeting) or SHP-1-targeting short hairpin RNA (above lanes) (left). h, ELISA of IL-4 in supernatants of GFP+ (transduced) CD4+ T cells sorted from bone marrow chimeras reconstituted with bone marrow cells transduced with retroviral vector encoding green fluorescent protein (GFP) and either wild-type SHP-1 or 3KR–SHP-1 (horizontal access), then stimulated for 48 h with anti-CD3 plus anti-CD28. i, ELISA of IL-4 in supernatants of naive CD4+ T cells transduced to express wild-type SHP-1 or 3KR–SHP-1 (horizontal axis) under neutral conditions (top) or TH2 conditions (bottom) and then allowed to ‘rest’ for 3 d, followed by sorting of GFP+ (transduced) cells and re-stimulation for 24 h with anti-CD3 plus anti-CD28. Each symbol (c,h,i) represents an individual technical replicate (c,i) or mouse (h). *P < 0.05, **P < 0.01 and *** P < 0.001 (unpaired two-tailed Student’s t-test). Data are pooled from or representative of two to three independent experiments (mean ± s.e.m. (c,i) or mean ± s.d. (h) of n = 3 technical replicates).
SHP-1 has been shown to function in the dephosphorylation and inactivation of Lck in T cells stimulated with weak or antagonistic ligands22. Because DKO T cells were hypo-responsive to TCR stimulation, we investigated whether ubiquitination of SHP-1 regulated TCR signaling through modulation of Lck activity. When overexpressed in HEK293T cells, 3KR–SHP-1 showed enhanced binding to Lck relative to that of wild-type SHP-1 (Fig. 7d), while neither 3KR–SHP-1 nor wild-type SHP-1 interacted with ZAP70 (Fig. 7d). More association between 3KR–SHP-1 and endogenous Lck than between wild-type SHP-1 and Lck was also observed in Jurkat T cells (Fig. 7e), which indicated that ubiquitination of SHP-1 might regulate its binding to Lck. To determine whether the enhanced interaction between SHP-1 and Lck affected the activity of Lck, we compared the phosphorylation of Lck at Tyr394 with its phosphorylation at Tyr505, which either increases (Tyr394) or decreases (Tyr505) the activity of Lck, in CD4+ T cells from wild-type, Itchf/fCd4-Cre, Wwp2−/− or DKO mice. The phosphorylation of Lck at Tyr394 was much lower in DKO CD4+ T cells than in any other CD4+ T cell tested, while there was no difference among all the groups of cells in its phosphorylation at Tyr505 (Fig. 7f). To further assess the effect of the ubiquitination of SHP-1 in TCR signaling, we assessed the phosphorylation of TCR-proximal components in Jurkat T cells in which endogenous SHP-1 was stably knocked down by short hairpin RNA and wild-type SHP-1 or 3KR–SHP-1 was transiently overexpressed. Jurkat T cells expressing 3KR–SHP-1 showed less phosphorylation of ZAP70, LAT and PLCγ1 than that of Jurkat T cells expressing wild-type SHP-1 (Fig. 7g). These data indicated that Itch- and WWP2-mediated ubiquitination of SHP-1 was required for proper TCR-proximal signaling through the modulation of Lck’s activity.
To investigate the effect of the polyubiquitination of SHP-1 on the activation CD4+ T cells, we reconstituted lethally irradiated wild-type mice with whole bone marrow cells infected with a bicistronic retrovirus expressing wild-type SHP-1 or 3KR–SHP-1 and green fluorescent protein (to generate ‘wild-type SHP-1 chimeras’ or ‘3KR–SHP-1 chimeras’, respectively). Thymocyte development in wild-type SHP-1 chimeras was similar to that in 3KR-SHP1 chimeras at 6–8 weeks after reconstitution (Supplementary Fig. 7g,h). In addition, thymocyte development was normal in mixed–bone marrow chimeras reconstituted with bone marrow cells from wild-type mice and DKO mice (Supplementary Fig. 7i–k). Moreover, the frequency of memory-like and naive CD4+ T cells in 3KR–SHP-1 chimeras was similar to that in wild-type SHP-1 chimeras at 6–8 weeks after reconstitution (Supplementary Fig. 7l), although CD4+ T cells sorted from the spleen of 3KR–SHP-1 chimeras produced more IL-4 than did those sorted from the spleen of wild-type SHP-1 chimeras (Fig. 7h). To further confirm that finding, we transduced naive CD4+ T cells with retrovirus encoding wild-type SHP-1 or 3KR–SHP-1 in either ‘neutral’ conditions (anti-CD3 plus anti-CD28) or TH2 conditions and assessed IL-4 production by the cells after stimulation with anti-CD3 plus anti-CD28. CD4+ T cells transduced with 3KR–SHP-1 produced more IL-4 than did those transduced with wild-type SHP-1, under both culture conditions (Fig. 7i). Together these findings indicated that Itch- and WWP2-mediated ubiquitination of SHP-1 negatively controlled the differentiation of naive CD4+ T cells into IL-4-producing cells through modulation of proximal TCR signaling.
Discussion
Here we found that mice lacking Itch and WWP2 showed signs of autoimmunity and lung inflammation due to biased differentiation toward the TH2 lineage and hypo-responsiveness after TCR stimulation. Itch and WWP2 interacted with each other, and SHP-1 acted as a substrate for Itch- and WWP2-mediated atypical polyubiquitination involved in promoting TCR signaling and inhibiting TH2 differentiation. Thus, our findings have identified a previously unrecognized function for molecular interplay between E3 ubiquitin ligases in TCR-mediated TH2 differentiation.
Although IL-4 is a critical factor for TH2 differentiation during the induction and polarization phase, and several types of cells have been proposed as potential IL-4-producing cells, the exact initial source of IL-4 is still not clear23. Here we found that Itch- and WWP2-deficient CD4+ T cells produced more IL-4, which led to severe lung inflammation and augmented IgE production, even in young mice. These data emphasize a CD4+ T cell-intrinsic contribution to TH2 differentiation. In addition to the IL-4 milieu, the strength of the TCR signal is a crucial factor for TH1-TH2 cell-fate ‘decisions’4,24–26. TCR-mediated activation of the kinase ERK seems to have a role in this process5,27, but how TCR-proximal signaling is regulated has remained largely unknown. DKO CD4+ T cells showed attenuated TCR-proximal signaling, enhanced induction of GATA-3 and activation of STAT5, while loss of either Itch or WWP2 alone did not have that effect. This suggests that Itch and WWP2 cooperatively function in regulating TCR signal strength at a TCR-proximal level to limit TH2 differentiation.
Proteomics approaches and biochemical analysis identified SHP-1 as a common interacting protein for Itch and WWP2. Mice of the motheaten (me/me) strain carrying a mutation of the locus encoding SHP-1 that ablates the expression of SHP-1 exhibit severe autoimmunity and systemic inflammation28,29, similar to the phenotype of the itchy strain of mice12. In T cells, SHP-1 interacts with several proteins and functions as a negative regulator of TCR signaling21. T cell–specific deletion of SHP-1 augments IL-4-mediated TH2 differentiation20. However, the precise mechanism by which SHP-1 regulates TH2 differentiation has remained obscure.
The tyrosine kinase Lck, which phosphorylates CD3ζ and ZAP70, is a key element in the initiation of TCR signaling, and dephosphorylation of Lck by SHP-1 might be part of a negative regulatory circuit that regulates TCR signaling21,30. However, whether SHP-1 directly targets Lck has remained controversial31–33. We found that the Itch- and WWP2-mediated ubiquitination of SHP-1 did not affect its stability as a protein, while such ubiquitination was required for the regulation of Lck through binding to Lck. In addition, Itch and WWP2 promoted mainly K27-mediated ubiquitination of SHP-1 but not K48- or K63-mediated ubiquitination of SHP-1. Thus, defective ubiquitination of SHP-1 in DKO CD4+ T cells enhanced the interaction between SHP-1 and Lck and attenuated TCR-proximal signaling, with weaker TCR signaling eventually directing TH2 differentiation.
Lck is constitutively active in resting cells, and TCR activation does not augment the proportion of active Lck34, which suggests that both the conformational state of active Lck and its spatial distribution at the plasma membrane are critical for the propagation of TCR signaling. The physiological role of K27-linked ubiquitin chains, although not fully understood, seems to be in signaling-complex assembly35. Although we observed that loss of Itch and WWP2 led to reduced activity of Lck in stimulated CD4+ T cells, this defect might have been independent of the phosphatase activity of SHP-1. On the basis of our data, we speculate that Itch- and WWP2-mediated ubiquitination of SHP-1 is involved in regulating the access of active Lck to CD3ζ at the plasma membrane of resting CD4+ T cells.
Combined loss of Itch and WWP2 predisposed mice to autoimmunity and more-prominent TH2 differentiation, indicative of a synergistic effect of these two E3 ubiquitin ligases on the activation of CD4+ T cells. Co-expression of Itch and WWP2 resulted in more conjugation of polyubiquitin to SHP-1 than did expression of either E3 ligase alone, and loss of Itch or WWP2 alone in CD4+ T cells had minimal to no effect on the development of spontaneous autoimmunity and inflammation. Published studies have shown that distinct E3 ligases act sequentially on common substrates by ‘tag-teaming’ with different ubiquitin chains36–38. It is also possible that Itch and WWP2 might mutually modulate their E3 ligase activity for the ubiquitination of SHP-1, serving either as an E3 ligase for the other or as a scaffolding protein that coordinates the assembly of regulatory components for their E3 ligase activity. It remains to be investigated how Itch and WWP2 ubiquitinate SHP-1 in a concerted manner.
Methods
Methods, including statements of data availability and any associated accession codes and references, are available at https://doi.org/10.1038/s41590-018-0137-8.
Mice.
Itchf/f Cd4-Cre and Wwp2−/− mice have been described previously39,40. To generate Wwp2−/− Itchf/f Cd4-Cre mice (DKO mice), Wwp2−/− Itchf/f mice were crossed with Wwp2−/− Itchf/+ Cd4-Cre mice. For the generation of bone marrow chimeras, 2 × 106 unfractionated bone marrow cells freshly harvested from wild-type or DKO mice were transplanted by retro-orbital injection into lethally irradiated B6.SJL congenic mice (given a single dose of 1,100 rads). For the generation of mixed–bone marrow chimeras, 4 × 106 bone marrow cells, mixed at a ratio of 3:1 (CD45.1+ wild-type/CD45.2+ DKO) were transplanted into lethally irradiated B6.SJL congenic mice. For the generation of bone marrow chimeras expressing SHP-1, bone marrow cells were infected with retrovirus expressing wild-type SHP-1 or 3KR–SHP-1 and then were injected into lethally irradiated B6 mice. Transplanted mice were kept on antibiotics until they were analyzed at 6–8 weeks after transplantation. All mice were maintained under specific pathogen-free conditions. All the animal procedures were conducted in accordance with approved protocols by the Animal Care and Use committee at La Jolla Institute for Allergy and Immunology, and Tsinghua University.
Reagents and antibodies.
For flow cytometry, cell stimulation and ELISA, antibodies to mouse CD3ε (145-2C11), human CD3 (OKT3),CD8 (53-6.7), CD11b (M1/70), CD11c (N418), CD28 (37.51), CD45.1 (A20), CD45.2 (104), Ly6G (1A8), I-A/I-E (M5/114.15.2),IL-2 (JES6-1A12), IL-4 (11B11), IFN-γ (XMG1.2) IgA (RMA-1), IgE (RME-1), IgG1 (RMG1-1) and IgG2a (RMG2a-62) were from BioLegend; antibodies to mouse CD4 (RM4-5) CD25 (PC61.5), CD44 (IM7), CD62L (MEL-14), CD69 (H1.2F3), TCRβ (H57-597) and Foxp3 (FJK-16S) were from eBioscience; and antibody to Siglec-F (E50-2440) was obtained from BD Biosciences. For immunoblot analysis, antibodies to Myc (sc-789 and sc-40 (9E10)), HA (sc-805), SHP-1 (sc-287), GATA-3 (sc-268), WWP2 (sc-11896), CD3ζ (sc-1239 (6B10.2)) and Lck (sc-433 (3A5)) were from Santa Cruz Biotechnology; antibodies to STAT5 phosphorylated at Tyr694 (9359), STAT5 (9363), STAT6 phosphorylated at Tyr641 (9361), STAT6 (9362), ZAP70/Syk phosphorylated at Tyr319 and Tyr352 (2701), ZAP70 (2705), PLCγ1 phosphorylated at Tyr783 (2821), PLCγ1 (5690), Lck phosphorylated at Tyr505 (2751) and SHP-1 phosphorylated at Tyr564 (8849) were from Cell Signaling; antibodies to LAT phosphorylated at Tyr191 (07-278) and LAT (06-807) were from Millipore; antibodies to Itch (611199 (32/Itch)) and CD247 phosphorylated at Tyr142 (558402 (K25-407.69)) were from BD Biosciences; antibodies to actin (A2228 (AC-74)) and FLAG (F1804 (M2), F7425) were from Sigma-Aldrich; antibody to WWP2 (A302-936) was obtained from Bethyl Laboratories; anti-Xpress (R910-25) was from Invitrogen; and antibody to Lck phosphorylated at Tyr394 (number) was from ThermoFisher Scientific. For immunofluorescent staining, Alexa Fluor 488–conjugated goat antimouse IgG (H + L) (A11001) and Alexa Fluor 555-conjugated goat anti-rabbit IgG (H + L) (A21428) were obtained from Invitrogen. Cycloheximide and MG132 were purchased from Sigma-Aldrich. Human WWP2-targeting small interfering RNA (siRNA) (SI00093744) and negative control siRNA (1027310) were from QIAGEN. Human Itch-targeting siRNA was described elsewhere41.
Generation of Itch and WWP2 double-knockout Jurkat T cells.
Jurkat T cells lacking endogenous Itch and WWP2 were generated by CRISPR-Cas9–based genome editing, as previously described42. Jurkat T cells were infected with lentivirus carrying hWWP2 single-guide RNA (sgRNA). After 48 h, the cells were selected with 2 μg puromycin for 7 d. Then, selected cells were sequentially infected with lentivirus carrying hItch sgRNA and mAmetrin cassette. The DKO Jurkat T cell line was established by sorting of single mAmetrin-expressing cell into 96-well plate and was validated by genomic sequencing and immunoblot analysis. The sites targeted by the sgRNAs are shown in Supplementary Fig. 4b.
Real-time quantitative PCR and RNA-based next-generation sequencing (RNA-seq).
For real-time quantitative PCR analysis, total RNA was isolated from sorted cells using the RNeasy Mini Kit (QIAGEN) and cDNA was synthesized with a Superscript III first strand synthesis kit (Invitrogen) according to the manufacturer’s protocol. Real-time PCR reactions were performed with technical replicates using iTaqTM SYBR Green Supermix with ROX (Bio-Rad) and run with a Lightcycler 2.0 (Roche). Relative gene expression was normalized to the expression of the gene encoding β-actin. The gene-specific primers for Il13 (QT00099554) were obtained from QIAGEN. The other specific primers used to amplify the target genes are listed as follows: Il4, forward (5′-AGATCATCGGCATTTTGAACG-3′) and reverse (5′-TTTGGCACATCCATCTCCG-3′); Il-5, forward (5′-CGCTCACCGAGCTCTGTTG-3′) and reverse (5′-CCAATGCATAGCTGGTGATTTTT-3′); Ifng, forward (5′-GATGCATTCATGAGTATTGCCAAGT-3′) and reverse (5′-GTGGACCACTCGGATGAGCTC-3′); Tbx21, forward (5′-CAACAACCCCTTTGCCAAAG) and reverse (5′-TCCCCCAAGCAGTTGACAGT-3′); Gata3, forward (5′-AGGGACATCCTGCGCGAACTGT-3′) and reverse (5′-CATCTTCCGGTTTCGGGTCTGG-3′); and Actb (encoding β-actin), forward (5′-ATCCTGGCCTCGCTGTCCAC-3′) and reverse (5′-GGGCCGGACTCGTCATAC-3′). For RNA-seq, total RNA was isolated from CD4+ T cells stimulated with PMA (phorbol 12-myristate 13-acetate) and ionomycin. The quality and concentration of the cDNA libraries generated were determined by Agilent 2100 Bio analyzer (Agilent Technology). The libraries were sequenced on BGISEQ-500 platform (BGI) using 50-bp pair-end reads. Genes expressed differentially in single-knockout or DKO cells relative to their expression in wild-type cells were defined as a change in expression of 1.41-fold (upregulated) or 0.7-fold (downregulated). For heat-map generation, expression values of transcripts were calculated as log2-transformed reads per kilobase of exon per million mapped reads (RPKM).
ELISA.
For detection of IL-2, IFN-γ and IL-4, cells were stimulated with anti-CD3 (100313; BioLegend) plus anti-CD28 (102111; BioLegend) in 96-well plates. Then, the culture supernatants were harvested at the appropriate time points. For the detection of autoantibodies, immunoglobulins and IL-6, serum was collected from mice 6–8 weeks of age by retro-orbital ‘bleed’. Antibodies to double-stranded DNA or histone were measured as previously described43,44. IL-6 levels were determined by an eBioscience Mouse IL-6 ELISA Ready-SET-GO! Kit according to the manufacturer’s instructions (eBioscience).
Immunoprecipitation, immunoblot analysis and in vivo ubiquitination assay.
For immunoprecipitation (IP), cells were lysed for 30 min in IP buffer containing 0.5% Triton X-100, 20 mM Tris H-Cl (pH 7.4), 150 mM NaCl, 10 mM NaF, 2 mM Na3VO4 and protein inhibitor cocktail (Roche). Equal amounts of proteins were cleared by centrifugation and immunoprecipitated with the appropriate antibodies and Protein A/G PLUS-agarose (Santa Cruz Biotechnology) for 2 h to overnight at 4 °C. Immunoprecipitates were washed with IP buffer, boiled in SDS sample buffer and subjected to immunoblot analysis as previously described45. For in vivo ubiquitination assays, cells were transfected with the appropriate expression vectors and were lysed in lysis buffer containing 1% TritonX-100, 20 mM Tris H-Cl (pH7.4), 150 mM NaCl, 10 mM NaF, 1% SDS, 5 mM N-ethylmaleimide and protease inhibitor cocktail by boiling for 5 min. Lysates were diluted for ten times with SDS-free lysis buffer, centrifuged and immunoprecipitated with the appropriate antibodies.
PTP activity assay.
For assay of immunocomplex protein tyrosine-phosphatase (PTP) activity, Jurkat T cells transfected to express FLAG-tagged wild-type SHP-1 or FLAG-3KR–SHP-1 were lysed and immunoprecipitated with anti-FLAG (F1804 and F7425; Sigma-Aldrich). Immunoprecipitates were washed with PTP reaction buffer containing 25 mM HEPES (pH 7.4), 0.1 mM EDTA, 5 mM DTT and 100 μg/ml BSA. The immunocomplex was resupended in PTP reaction buffer containing 50 μM DifMUP (Thermo SCIENTIFIC) and was incubated for 30 min at 25 °C. The DifMUP fluorescence signal was measured at an excitation wavelength of 358 nm and an emission wavelength of 455 nm with a plate reader. For the in vitro PTP activity assay, glutathione S-transferase–fused wild-type-SHP-1 or 3KR–SHP-1 was purified from transformed BL21DE3 cells (a chemically competent Escherichia coli cell line for protein expression) with glutathione-Sepharose columns. Purified proteins were used at the appropriate concentration in the assay.
Cell culture, expression constructs, transfection and retroviral transduction.
HEK293T and NIH3T3 cells were cultured in DMEM supplemented with 10% FCS and 1% penicillin-streptomycin. Jurkat T cells were cultured in RPMI 1640 medium with 10% FCS. Plasmids encoding Itch, inactive Itch ligase (CA), CD3ζ, Lck and ubiquitin were previously described45,46. The cDNA encoding WWP2 (NM_025830.3) or SHP-1 (NM_013545.3) was isolated from mouse spleen. The pLKO.1 lentiviral vector encoding human SHP-1–targeting or negative control short hairpin RNA were obtained from RNAi libraries at Tsinghua University. For the generation of SHP-1 KR mutants, point mutations were generated by site-directed mutagenesis. Retroviral plasmid encoding wild-type SHP-1 or 3KR–SHP-1 was cloned into retroviral vector pMSCV-IRES-GFP. For transfection, Neofect DNA transfection reagents (Neofect Biotech) or TransIT-LT1 transfection reagent (Mirus Bio) were (was) used for HEK293T cells or NIH3T3 cells, respectively. For siRNA-mediated gene silencing, transfection was sequentially carried out twice with 200 nM siRNA using Lipofectamine 2000 (Life Technologies), followed by the appropriate plasmids. Jurkat T cells were transfected by electroporation (Gene-Pulser Xcell; BioRad) as previously described39. For retrovirus-mediated gene transfer to naive CD4+ T cells, PLAT-E cells were transfected with retroviral plasmid. After overnight incubation, medium was replaced with RPMI 1640 medium and culture supernatant containing retroviral particles was collected 48 and 72 h after transfection. At each time point, retroviral supernatants were collected, filtrated and supplemented with 100 U/ml IL-2 and 5 μg/ml polybrene and then were used to spin-infect naive CD4+ T cells previously activated with anti-CD3 and anti-CD28 (identified above). Spin infection was performed at 2000 rpm for 90 min at 24 °C. After a second round of infection, cells were washed and cultured in the presence of 100 U/ml IL-2 for another 3 d before re-stimulation.
Analysis of cell populations in the bronchoalveolar lavage fluid (BALF) and lungs.
The bone marrow chimeras were euthanized and the tracheas were cannulated. The lungs were flushed twice with 1-ml aliquots of PBS. The aliquots were combined and centrifuged at 300 g for 5 min to isolate BAL cells. For isolation of lung cells, the lungs were minced and digested with 2 mg/ml type IV collagenase (Roche) and 0.02 mg/ml DNase I (Roche) for 60 min at 37 °C. Then digested lungs were filtrated through a 70-μm cell strainer to obtain single-cell suspensions. Red blood cells were lysed with ACK buffer. BAL and lung cell populations were defined as follows: eosinophils (CD45.2+Siglec-F+CD11b+), macrophages (CD45.2+Siglec-F+CD11c+), dendritic cells (CD45.2+CD11c+I-AhiI-Ehi) and neutrophils (CD45.2+CD11b+Ly6G+).
Mass-spectrometry proteomics.
Jurkat T cells were transiently transfected with empty expression vector (FLAG only) or expression vector for FLAG-tagged Itch or WWP2. After 48 h, cells were lysed with IP buffer as described above. Cell lysates were cleared by centrifugation and incubated with anti-FLAG M2 Affinity Gel (Sigma-Aldrich) for 2 h at 4 °C with gentle rotation. Then, agarose beads were washed three times with IP buffer and immunocomplexes were eluted with 150 μg/ml FLAG peptide (Sigma-Aldrich), followed by precipitation with chloroform and ice-cold methanol. The concentrated proteins were solubilized in SDS sample buffer, separated by SDS-PAGE and stained with silver using a ProteoSilver Silver Stain Kit (Sigma-Aldrich). Each lane of the stained gel was cut into eight to ten slices, and individual gel slices were subjected to in-gel digestion with trypsin, followed by analysis by nanoscale liquid chromatography coupled-to tandem mass spectrometry (LC-MS/MS). The generated tandem mass spectrometry spectra were searched against the Uniprot database using Proteome Discoverer software 1.4 (Thermo Scientific). For identification of sites for the ubiquitination of SHP-1 by Itch and WWP2, HEK293T cells were co-transfected with expression plasmids for Itch, WWP2 and FLAG-tagged SHP-1. After 48 h, FLAG-tagged SHP-1 was affinity-purified as described above, and eluted proteins were separated by SDS-PAGE followed by Coomassie Brilliant Blue staining. The SHP-1 band was excised and subjected to LC-MS/MS analysis.
Immunofluorescent staining.
NIH3T3 cells were seeded onto poly-l-lysine–coated glass coverslips overnight. Then, the cells were co-transfected with cDNA encoding Itch or WWP2. After 48 h, the cells were fixed with 4% formaldehyde for 15 min and were permeabilized with 0.1% Tritonx-100 for 5 min and blocked with 3% BSA for 30 min, followed by antibody incubation. Coverslips were mounted using Prolong Gold Antifade Reagent (Invitrogen). The images were captured by a Zeiss LSM 780 confocal microscope with an × 63 oil objective (Carl Zeiss) and were processed using ZEN 2012 SP1 software (Carl Zeiss).
Flow cytometry.
For the detection of cell-surface molecules, single-cell suspensions from the spleens and lungs were stained in FACS buffer containing 0.5% BSA with the appropriate single-color fluorochrome-conjugated antibodies. Foxp3 staining was performed using a Foxp3 staining kit (eBioscience). For intracellular cytokine staining, cells were stimulated with PMA and ionomycin in the presence of BD GoligiStop (BD Bioscience). After cell-surface staining, cells were fixed, permeabilized and stained using a BD Cytofix/Cytoperm Kit according to the manufacturer’s instructions (BD Bioscience). Cells were acquired on BD LSR II, LSRFortessa or FACSAria II (BD Biosciences).
Receptor-turnover analysis.
For assessment of TCRβ down-modulation, isolated CD4+ T cells were stimulated for the appropriate times with anti-CD3 plus anti-CD28 (identified above). Then, cells were stained with APC-conjugated anti-TCRβ (H57-597, eBioscience) and analyzed by flow cytometry. For assessment of TCRβ internalization, isolated CD4+ T cells were incubated with APC-conjugated anti-TCRβ (identified above) and stimulated cross-linked with goat antibody to Armenian hamster IgG (127-005-160, Jackson ImmunoResearch Laboratories). Then cells were stimulated at 37 °C for the indicated times. At each time point, half of cells were treated twice with ice-cold acidic buffer (1% BSA in PBS at pH 3.0), and wash with neutralization buffer (1% BSA, 0.5% NaN3 in PBS at pH 7.4). Both treated and untreated cells were analyzed for APC fluorescence. For assessment of TCRβ recycling, isolated CD4+ T cells were incubated with APC-conjugated anti-TCRβ (identified above) and stimulated cross-linked with goat anti-hamster IgG (identified above) for 30 min. Then cells were treated with ice-cold acidic buffer, washed with neutralization buffer to remove uninternalized antibodies and incubated to allow for the recycling of internalized receptor at 37 °C for the indicated time. At each time point, half of the cells were treated with ice-cold acid buffer and subsequently wash with neutralization buffer. Both treated and untreated cells were analyzed for APC fluorescence. Percentages of TCRβ turnover were based on MFI of TCRβ expression and calculated as previously described19,47.
Statistical analysis.
The data are presented as mean ± s.d. except when indicated otherwise. n represents the number of animals per experiment. To assess the statistical significance, the P values were calculated using unpaired two-tailed Student’s t-test. For comparisons of multiple groups, one-way ANOVA with Bonferroni’s test was used. Statistical analysis was performed using GraphPad Prism 7.0. We considered P values of < 0.05 to be significant.
Reporting Summary.
Further information on experimental design is available in the Nature Research Reporting Summary linked to this article.
Data availability.
The data that support the findings of this study are available from the corresponding author upon request. Additional supplementary information for this paper is available in Supplementary Dataset 1.
Supplementary Material
Acknowledgements
We thank L.G. Glimcher (Dana-Farber Cancer Institute) for the Wwp2−/− mouse strain; H. Deng for mass spectrometry; Z. Yang for purification of glutathione S-transferase fusion proteins; H. Li for imaging analysis, J. Greenbaum, A. Sethi and G. Tian for RNA-seq analysis; and members of the Liu laboratory for discussions. Supported by the Mochida Memorial Foundation for Medical and Pharmaceutical Research (fellowship to D.A.), the National Natural Science Foundation of China (81725010 and XBD19000000 to W. Zou), the Ministry of Science and Technology of China (YFA0505802, YFC0903900, NSFC81630041), the US National Institutes of Health (RO1AI123398 and R21AI122258) and the Tsinghua-Peking Center for Life Sciences (Y.-C.L.).
Footnotes
Competing interests
The authors declare no competing interests.
Additional information
Supplementary information is available for this paper at https://doi.org/10.1038/s41590-018-0137-8.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhu J & Paul WE Peripheral CD4+ T-cell differentiation regulated by networks of cytokines and transcription factors. Immunol. Rev. 238, 247–262 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ho IC, Tai TS & Pai SY GATA3 and the T-cell lineage: essential functions before and after T-helper-2-cell differentiation. Nat. Rev. Immunol. 9, 125–135 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Constant S, Pfeiffer C, Woodard A, Pasqualini T & Bottomly K Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells. J. Exp. Med. 182, 1591–1596 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamane H & Paul WE Early signaling events that underlie fate decisions of naive CD4+ T cells toward distinct T-helper cell subsets. Immunol. Rev. 252, 12–23 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamane H, Zhu J & Paul WE Independent roles for IL-2 and GATA-3 in stimulating naive CD4+ T cells to generate a Th2-inducing cytokine environment. J. Exp. Med. 202, 793–804 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang D et al. Dysregulation of T lymphocyte function in itchy mice: a role for Itch in TH2 differentiation. Nat. Immunol. 3, 281–287 (2002). [DOI] [PubMed] [Google Scholar]
- 7.Heissmeyer V et al. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat. Immunol. 5, 255–265 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Yang B et al. Nedd4 augments the adaptive immune response by promoting ubiquitin-mediated degradation of Cbl-b in activated T cells. Nat. Immunol. 9, 1356–1363 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rotin D & Kumar S Physiological functions of the HECT family of ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 10, 398–409 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Aki D, Zhang W & Liu YC The E3 ligase Itch in immune regulation and beyond. Immunol. Rev. 266, 6–26 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Chen A et al. The HECT-type E3 ubiquitin ligase AIP2 inhibits activation-induced T-cell death by catalyzing EGR2 ubiquitination. Mol. Cell Biol. 29, 5348–5356 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hustad CM et al. Molecular genetic characterization of six recessive viable alleles of the mouse agouti locus. Genetics 140, 255–265 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lohr NJ et al. Human ITCH E3 ubiquitin ligase deficiency causes syndromic multisystem autoimmune disease. Am. J. Hum. Genet. 86, 447–453 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Y et al. E3 ligase WWP2 negatively regulates TLR3-mediated innate immune response by targeting TRIF for ubiquitination and degradation. Proc. Natl. Acad. Sci. USA 110, 5115–5120 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angers A, Ramjaun AR & McPherson PS The HECT domain ligase itch ubiquitinates endophilin and localizes to the trans-Golgi network and endosomal system. J. Biol. Chem. 279, 11471–11479 (2004). [DOI] [PubMed] [Google Scholar]
- 16.Martin-Serrano J, Eastman SW, Chung W & Bieniasz PD HECT ubiquitin ligases link viral and cellular PPXY motifs to the vacuolar protein-sorting pathway. J. Cell Biol. 168, 89–101 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sasaki T et al. Genome-wide gene expression profiling revealed a critical role for GATA3 in the maintenance of the Th2 cell identity. PLoS One 8, e66468 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu J, Cote-Sierra J, Guo L & Paul WE Stat5 activation plays a critical role in Th2 differentiation. Immunity 19, 739–748 (2003). [DOI] [PubMed] [Google Scholar]
- 19.Naramura M et al. c-Cbl and Cbl-b regulate T cell responsiveness by promoting ligand-induced TCR down-modulation. Nat. Immunol. 3, 1192–1199 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Johnson DJ et al. Shp1 regulates T cell homeostasis by limiting IL-4 signals. J. Exp. Med. 210, 1419–1431 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lorenz U SHP-1 and SHP-2 in T cells: two phosphatases functioning at many levels. Immunol. Rev. 228, 342–359 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stefanová I et al. TCR ligand discrimination is enforced by competing ERK positive and SHP-1 negative feedback pathways. Nat. Immunol. 4, 248–254 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Paul WE & Zhu J How are TH2-type immune responses initiated and amplified? Nat. Rev. Immunol. 10, 225–235 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Constant SL & Bottomly K Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu. Rev. Immunol. 15, 297–322 (1997). [DOI] [PubMed] [Google Scholar]
- 25.Tubo NJ & Jenkins MK TCR signal quantity and quality in CD4+ T cell differentiation. Trends Immunol. 35, 591–596 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Panhuys N, Klauschen F & Germain RN T-cell-receptor-dependent signal intensity dominantly controls CD4+ T cell polarization in vivo. Immunity 41, 63–74 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jorritsma PJ, Brogdon JL & Bottomly K Role of TCR-induced extracellular signal-regulated kinase activation in the regulation of early IL-4 expression in naive CD4+ T cells. J. Immunol. 170, 2427–2434 (2003). [DOI] [PubMed] [Google Scholar]
- 28.Shultz LD et al. Mutations at the murine motheaten locus are within the hematopoietic cell protein-tyrosine phosphatase (Hcph) gene. Cell 73, 1445–1454 (1993). [DOI] [PubMed] [Google Scholar]
- 29.Tsui HW, Siminovitch KA, de Souza L & Tsui FW Motheaten and viable motheaten mice have mutations in the haematopoietic cell phosphatase gene. Nat. Genet. 4, 124–129 (1993). [DOI] [PubMed] [Google Scholar]
- 30.Salmond RJ, Filby A, Qureshi I, Caserta S & Zamoyska R T-cell receptor proximal signaling via the Src-family kinases, Lck and Fyn, influences T-cell activation, differentiation, and tolerance. Immunol. Rev. 228, 9–22 (2009). [DOI] [PubMed] [Google Scholar]
- 31.Choi S et al. THEMIS enhances TCR signaling and enables positive selection by selective inhibition of the phosphatase SHP-1. Nat. Immunol. 18, 433–441 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu G et al. Themis sets the signal threshold for positive and negative selection in T-cell development. Nature 504, 441–445 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paster W et al. A THEMIS:SHP1 complex promotes T-cell survival. EMBO J. 34, 393–409 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nika K et al. Constitutively active Lck kinase in T cells drives antigen receptor signal transduction. Immunity 32, 766–777 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Q et al. The E3 ubiquitin ligase AMFR and INSIG1 bridge the activation of TBK1 kinase by modifying the adaptor STING. Immunity 41, 919–933 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Harreman M et al. Distinct ubiquitin ligases act sequentially for RNA polymerase II polyubiquitylation. Proc. Natl. Acad. Sci. USA 106, 20705–20710 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hwang CS, Shemorry A, Auerbach D & Varshavsky A The N-end rule pathway is mediated by a complex of the RING-type Ubr1 and HECT-type Ufd4 ubiquitin ligases. Nat. Cell Biol. 12, 1177–1185 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scott DC et al. Two distinct types of E3 ligases work in unison to regulate substrate ubiquitylation. Cell 166, 1198–1214 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao N et al. The E3 ubiquitin ligase Itch is required for the differentiation of follicular helper T cells. Nat. Immunol. 15, 657–666 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zou W et al. The E3 ubiquitin ligase Wwp2 regulates craniofacial development through mono-ubiquitylation of Goosecoid. Nat. Cell Biol. 13, 59–65 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei W, Li M, Wang J, Nie F & Li L The E3 ubiquitin ligase ITCH negatively regulates canonical Wnt signaling by targeting dishevelled protein. Mol. Cell Biol. 32, 3903–3912 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shalem O et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 343, 84–87 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin DA et al. Autoimmunity stimulated by adoptively transferred dendritic cells is initiated by both αβ and γδ T cells but does not require MyD88 signaling. J. Immunol. 179, 5819–5828 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mihara M et al. CTLA4Ig inhibits T cell-dependent B-cell maturation in murine systemic lupus erythematosus. J. Clin. Invest. 106, 91–101 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang H et al. K33-linked polyubiquitination of T cell receptor-ζ regulates proteolysis-independent T cell signaling. Immunity 33, 60–70 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Couture C et al. Activation of p56lck by p72syk through physical association and N-terminal tyrosine phosphorylation. Mol. Cell Biol. 14, 5249–5258 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Myers MD, Dragone LL & Weiss A Src-like adaptor protein down-regulates T cell receptor (TCR)-CD3 expression by targeting TCRζ for degradation. J. Cell Biol. 170, 285–294 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request. Additional supplementary information for this paper is available in Supplementary Dataset 1.