Abstract
Obstructive sleep apnea (OSA) is the most common sleep-disordered breathing condition. Patients with OSA symptoms are often not diagnosed clinically, which is a concern, given the health and safety risks associated with unmanaged OSA. The availability of fewer practicing medical specialists combined with longer travel distances to access health care services results in barriers to diagnosis and treatment in rural communities. This study aimed to (1) determine whether the proportion of adults reporting OSA symptoms in the absence of a sleep apnea diagnosis in rural populations varied by travel distance to specialist medical care and (2) assess whether any distance-related patterns were attributable to differences in the frequency of diagnosis among adults who likely required this specialist medical care. We used a cross-sectional epidemiologic study design, augmented by analysis of follow-up survey data. Our study base included adults who completed a 2010 baseline questionnaire for the Saskatchewan Rural Health Study. Follow-up occurred until 2015. 6525 adults from 3731 households constituted our sample. Statistical models used log-binomial regression. Rural adults who reported the largest travel distances (≥250 km) to specialist medical care were 1.17 (95% CI: 1.07, 1.29) times more likely to report OSA symptoms in the absence of a sleep apnea diagnosis than those who reported the smallest (<100 km; referent) distances. However, the proportion of sleep apnea diagnoses was low and unaffected by reported travel distance among adults who likely required this specialist medical care. Our findings suggest factors other than travel distance may be contributing to the low sleep apnea diagnostic rate. This remains important as undiagnosed and untreated OSA has serious implications on the health of people and populations, but effective treatments are available. Health care access barriers to the diagnosis and treatment of OSA require evaluation to inform health care planning and delivery.
1. Introduction
Obstructive sleep apnea (OSA) is the most common sleep-disordered breathing condition [1, 2]. It is estimated that 5.4 million Canadian adults have symptoms of OSA, yet most symptomatic adults are not clinically diagnosed [1–4]. OSA is characterized by periodic obstructions of the upper airway during sleep, leading to complete cessation (apnea) or reduction (hypopnea) in airflow [5–7]. Sleep-disturbed breathing may result in reduction of hemoglobin oxygen concentration (hypoxemia) and fragmented sleep [5, 8]. Patient reports of excessive daytime sleepiness and loud snoring are common diagnostic symptoms [7, 9]. If untreated, symptoms may interfere with activities of daily living and increase risks for occupational injury [1, 10–12], motor vehicle crashes [13, 14], and cardiovascular diseases [15–24]. Effective treatments for OSA exist [25–37] and can substantially improve health outcomes and overall quality of life. The most common treatment, continuous positive airway pressure (CPAP), assists in mitigating health and safety risks [14, 23, 38–44].
Rural populations may be particularly at risk for OSA due to high levels of obesity, physical inactivity, and other related risk factors [45–50]. Rural patients with OSA symptoms are often not diagnosed clinically [1], which is a concern, as the increased general health risks experienced by rural vs. urban residents [46, 51–57] may be exacerbated specifically by the presence of undiagnosed OSA. Explanations for such clinical patterns warrant focused study.
Access to health care services is an important determinant of health [58, 59], and health care use is known to be lower in rural than in urban communities [60–63]. The availability of fewer practicing medical specialists combined with longer travel distances to access health care services results in barriers to diagnosis and treatment [47, 55, 56, 64–67]. Care for patients with clinical suspicion of OSA begins with the family physician and, if necessary, the family doctor then refers the patient for diagnostic testing at a sleep clinic [68]. At the specialist level, long travel distance may be an important barrier to the diagnosis and treatment of sleep apnea in rural populations, given modest numbers of sleep laboratories [3, 69]. Travel distance is an example of the availability and accommodation dimension of health care access (as per Levesque's model [70]). Even with access to a family doctor, travel distance to specialist care is still a relevant concern for sleep apnea because referral to a sleep medicine specialist for overnight, in-laboratory diagnostic testing is the gold standard for diagnosis [71, 72].
Lack of diagnosis and treatment for OSA is concerning, and evidence-informed changes to health service planning in rural communities surrounding the diagnosis and treatment of OSA may be particularly impactful. While a number of studies have evaluated the impact of rural residence and/or travel burden on the use of specialized health care services (e.g., mental health services, screening for colorectal, and breast and cervical cancer) and clinical diagnoses such as cancer [60–63, 66, 67, 73, 74], to our knowledge, no Canadian studies have specifically examined the impacts of travel distance to specialist medical services on sleep apnea diagnosis. We had the opportunity to address this gap in knowledge using baseline and follow-up data from a large population-based rural health study conducted in Saskatchewan from 2010 to 2015 [75]. Our hope was to contribute to policy decisions whereby the diagnosis and effective treatment of rural people with sleep apnea would be improved.
2. Materials and Methods
2.1. Data Source: The Saskatchewan Rural Health Study
The Saskatchewan Rural Health Study is a population-based cohort study. The baseline phase occurred in 2010 and the follow-up phase began in 2014 and ended in 2015. Both phases involved administration of a survey developed based on the population health framework [75, 76] that documented individual and contextual factors that characterized and influenced respiratory health, including sleep-disordered breathing [75].
2.1.1. Baseline Sample
Rural municipalities and towns (population ranging from 500 to 5000 [75]) were selected from four quadrants of the province (northwest, northeast, southwest, and southeast). A random sample of 36 of the 297 rural municipalities in Saskatchewan (nine per quadrant) and 16 of Saskatchewan's 145 towns were selected to participate. Local councils for 32 of the rural municipalities and 15 of the towns agreed to participate on behalf of their residents and provided mailing addresses. Eligible towns and municipalities were those that (1) were located at least 60 km from an urban center, meeting the Statistics Canada definition of “rural” [75, 77]; (2) were located outside the commuting zone of larger urban centers (areas with a population of 10,000 or more [75, 77]); (3) did not recently participate in the Saskatchewan Farm Injury Cohort Study, another large cohort study [75, 78]; and (4) were not included in the pilot study conducted to refine the baseline questionnaire and inform methods to optimize response rates [75, 79]. A registry of mailing addresses compiled from taxation lists was used to determine household eligibility. Households with unknown or duplicate addresses were excluded, as were homeowners with a mailing address outside the study area and deceased homeowners. Eligible adults were all those aged 18 years and older living in included households.
2.1.2. Baseline Data Collection
Data were collected by self-administered questionnaire using Dillman's total design method [80, 81] to optimize response rates. The household response rate was 42% [75]. A key informant was asked to provide household-level information and then to complete a section for each adult (aged 18 years and older) living in the household. Data were collected for 8261 adults from 4624 households in farm and nonfarm rural communities in Saskatchewan. Our study base included adult participants who completed the baseline questionnaire.
2.1.3. Follow-Up Phase: Sample and Data Collection
The baseline sample was subsequently contacted for participation in the follow-up phase of the study [82]. Consenting adults comprised this sample. Follow-up data were collected for 4867 adults from 2797 households by a similar survey (household response rate = 60%; individual response rate = 59%) [82].
2.2. Study Design
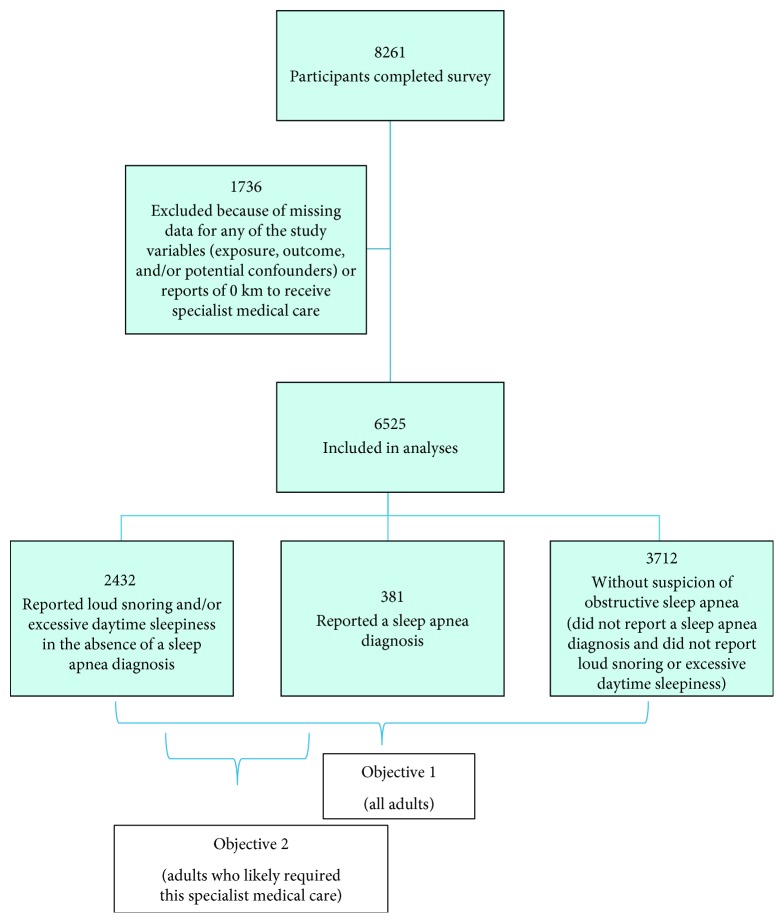
Cross-sectional analyses of baseline data were performed. Adults were classified according to reports of a sleep apnea diagnosis and the common OSA symptoms of loud snoring and excessive daytime sleepiness as assessed by the Epworth Sleepiness Scale (ESS) score [7, 9]. Out of the 8261 participants in the baseline sample, 6525 adults from 3731 households provided complete information for all variables of interest in our study (exposure, outcome, and/or potential confounders) and were included in analyses (Figure 1).
Figure 1.
Sample according to self-reports of a sleep apnea diagnosis and obstructive sleep apnea symptoms (loud snoring and excessive daytime sleepiness measured by an Epworth Sleepiness Scale score of greater than 10 out of 24).
Analysis of follow-up survey data aimed to assess whether, after five years, the frequency of an incident sleep apnea diagnosis among adults reporting OSA symptoms in the absence of a sleep apnea diagnosis at baseline was attributable to travel distance to specialist medical care. Out of the 4867 participants in the follow-up phase sample, 1459 adults from 1180 households met the inclusion criteria for this analysis and provided complete information for all variables of interest in our study.
3. Measurement of Key Variables
3.1. Objective 1
3.1.1. Exposure: Travel Distance to Specialist Medical Care
Travel distance to specialist medical care was assessed using an original survey item developed to evaluate access to health care services that asked: “How far do you travel to receive medical or surgical specialist services (in km)?” [75]. Distance quartiles were established: (1) <100 km, (2) 100–189 km, (3) 190–249 km, and (4) ≥250 km.
3.1.2. Outcome
The outcome was self-reports of OSA symptoms (loud snoring and/or excessive daytime sleepiness) in the absence of a sleep apnea diagnosis and was assessed using the survey items for sleep apnea diagnosis and OSA symptoms.
3.1.3. Sleep Apnea Diagnosis
This diagnosis was determined using the survey item adapted from the Canadian Community Health Survey that asked, “Has a doctor ever said you (yes or no) had any of the following chest illnesses (ever in your life and during the past 12 months): h. Sleep Apnea” [83].
3.1.4. OSA Symptoms
A dichotomous “loud snoring” variable (yes or no) was created based on the following 2 original survey items: (1) “Do you snore?” and (2) “If you snore, is your snoring: Slightly louder than breathing? As loud as talking? Louder than talking? Very loud—can be heard in adjacent rooms?” [75]. Loud snoring was identified in adults who reported snoring that was “as loud as talking,” “louder than talking,” or “very loud” [84].
The ESS score was used to assess excessive daytime sleepiness [75]. The scale described eight situations and asked, “How likely are you to doze off or fall asleep in the situations described below, in contrast to just feeling tired? This refers to your usual way of life in recent times. Even if you have not done some of these things recently, try to work out how they would have affected you. Please check one response choice for each situation.” Likelihood of dozing off or falling asleep was scored on a 4-point (0–3) Likert scale. The eight responses were summed, and a score greater than 10 out of 24 was considered abnormal and indicative of excessive daytime sleepiness [7, 85, 86].
3.2. Objective 2
3.2.1. Exposure: Travel Distance to Specialist Medical Care
Travel distance to specialist medical care was assessed in the same way as described for objective 1.
3.2.2. Outcome
The outcome was reports of a sleep apnea diagnosis among adults who likely required this specialist medical care (e.g., adults reporting either OSA symptoms or a sleep apnea diagnosis), which was assessed using the survey item for sleep apnea diagnosis used for objective 1.
4. Minimum Detectable Relative Risks and Attributable Risk
The minimum detectable relative risk represents the smallest increase in risk for reporting the outcome between the farthest and closest distance quartiles that our study can detect with 80% power and an alpha level (two-sided) of 5%. We could detect a relative risk of 1.32 or greater for the first objective and 1.55 or greater for the second objective.
We calculated an attributable risk percent of 24%. This means that 24% of adults reporting the largest travel distances to specialist medical care (≥250 km) who also report OSA symptoms in the absence of a sleep apnea diagnosis could have potentially been diagnosed had their travel distances been shorter (e.g., improved access to specialist care). A priori, we considered that because of the health and safety risks associated with unmanaged OSA [1, 10–24, 38–44], combined with the availability of effective treatments [25–37], this makes this 24% increase in reports of OSA symptoms in the absence of a sleep apnea diagnosis clinically important. Providing these individuals with appropriate care may help manage symptoms and reduce health and safety risks at the individual and population levels [14, 23, 38–44].
4.1. Statistical Analyses
All analyses were conducted using SAS software version 9.4 (SAS Institute Inc., Cary, NC).
4.1.1. Sample Description
Characteristics of the study sample, as well as the subset of adults who likely required this specialist medical care, were described by each study variable according to (1) the primary outcome, reports of OSA symptoms in the absence of a sleep apnea diagnosis, and (2) travel distance to specialist medical care. Next, proportions of individuals reporting OSA symptoms in the absence of a sleep apnea diagnosis, both overall and within strata of each study variable, were examined. Rao-Scott chi-square tests [87], which adjust for the clustered nature of the data (individuals nested within households), were performed to test for statistical significance of differences in proportions. The same was done to compare proportions of individuals in the farthest distance quartile, both overall and within strata of each study variable.
4.1.2. Regression Analysis
Log-binomial regression models were created to estimate the strength of associations between (1) travel distance to specialist medical care and the proportion of adults reporting OSA symptoms in the absence of a sleep apnea diagnosis and (2) travel distance to specialist medical care and frequency of sleep apnea diagnosis among adults who likely required this specialist medical care. Sex was first explored as an effect modifier. Models then accounted for potential confounders (age, sex, “money left over at the end of the month,” and education level) as well as body mass index (BMI), heavy alcohol consumption, and smoking status, which were treated as proxies for baseline health behaviors (unmeasured potential confounders) to reduce any differences that might arise as reasons other than travel distance to specialist medical care. To account for clustering of adults within households, generalized estimating equations (exchangeable working correlation structure) were used to obtain robust variance estimates [88]. Relative risks and their 95% confidence intervals were estimated.
We also evaluated comparability of reported travel distance to specialist medical care and driving distance to the closest of Saskatchewan's sleep centers for all households using postal codes.
4.1.3. Sensitivity Analysis
A sensitivity analysis using multiple imputation [89] was performed to assess the potential impact of excluding participants with missing data.
5. Results
5.1. Objective 1
Six percent (381/6525) of the sample reported a sleep apnea diagnosis and an additional 37% (2432/6525) reported OSA symptoms in the absence of a sleep apnea diagnosis (Figure 1). Participants ranged in age from 18 to 101 years (mean 55.0 [±15.6] years), with a mean reported travel distance to specialist medical care of 183 [±113] km (Table 1). The largest reported travel distances (≥250 km) were significantly and positively associated with lower education levels and “not enough” money left over at the end of the month (Table 1). There was an increased proportion of adults reporting OSA symptoms in the absence of a sleep apnea diagnosis in association with increasing travel distance to specialist medical care (Table 1). Middle to older age, male sex, overweight and obese BMIs, secondary education or less, higher frequency of heavy alcohol consumption, and a history of smoking were also associated with higher proportions of adults reporting OSA symptoms in the absence of a sleep apnea diagnosis (Table 1).
Table 1.
Characteristics of the study sample (n = 6525; 3731 households).
| Characteristics | Proportion (%) | Obstructive sleep apnea symptoms in the absence of a sleep apnea diagnosis (n = 2432; 37%) | P valuea | Travel distance to specialist medical care ≥ 250 km (n=1651; 25%) | P valuea | ||
|---|---|---|---|---|---|---|---|
| n | Row % | n | Row % | ||||
| Exposure | |||||||
| Travel distance to specialist medical care (km) | |||||||
| <100 | 22 | 480 | 33.8 | 0.006 | — | — | — |
| 100–189 | 28 | 669 | 36.7 | — | — | ||
| 190–249 | 25 | 622 | 38.1 | — | — | ||
| ≥250 | 25 | 661 | 40.0 | — | — | ||
| Potential confounders | |||||||
| Age (years) b | |||||||
| 18–45 | 25 | 533 | 32.5 | <0.001 | 393 | 23.9 | 0.08 |
| 46–55 | 26 | 695 | 41.6 | 439 | 26.3 | ||
| 56–65 | 24 | 676 | 43.9 | 381 | 24.8 | ||
| >65 | 26 | 528 | 31.6 | 438 | 26.2 | ||
| Sex | |||||||
| Female | 50 | 922 | 28.5 | <0.001 | 830 | 25.6 | 0.05 |
| Male | 50 | 1510 | 45.9 | 821 | 25.0 | ||
| Body mass index (kg/m 2) | |||||||
| Normal (<25) | 29 | 479 | 25.3 | <0.001 | 463 | 24.5 | 0.38 |
| Overweight (25–29.9) | 41 | 1041 | 38.6 | 658 | 24.4 | ||
| Obese (≥30) | 30 | 912 | 47.1 | 530 | 27.4 | ||
| Education level | |||||||
| Postsecondary | 41 | 947 | 35.2 | 0.005 | 668 | 24.9 | 0.02 |
| Secondary or less | 59 | 1485 | 38.7 | 983 | 25.6 | ||
| Money left over at the end of the month | |||||||
| Some | 60 | 1452 | 37.3 | 0.14 | 937 | 24.1 | 0.008 |
| Just enough | 21 | 489 | 35.4 | 328 | 23.7 | ||
| Not enough | 19 | 491 | 39.3 | 386 | 30.9 | ||
| Heavy alcohol consumption, more than 5 drinks on one occasion | |||||||
| Never | 55 | 1217 | 34.0 | <0.001 | 934 | 26.1 | 0.21 |
| 1/month or less | 33 | 837 | 38.8 | 529 | 24.5 | ||
| 1/week or less | 10 | 320 | 48.1 | 161 | 24.2 | ||
| More than 1/week | 2 | 58 | 47.2 | 27 | 22.0 | ||
| Smoking status | |||||||
| Never | 52 | 1118 | 32.7 | <0.001 | 839 | 24.6 | 0.76 |
| Past | 36 | 981 | 42.3 | 596 | 25.7 | ||
| Current | 12 | 333 | 42.2 | 216 | 27.4 | ||
a P value from Rao-Scott chi-square tests for significant difference in proportions with obstructive sleep apnea symptoms in the absence of a sleep apnea diagnosis/travel distance to specialist medical care ≥250 km between levels of each variable. bProportions add to 101% due to rounding.
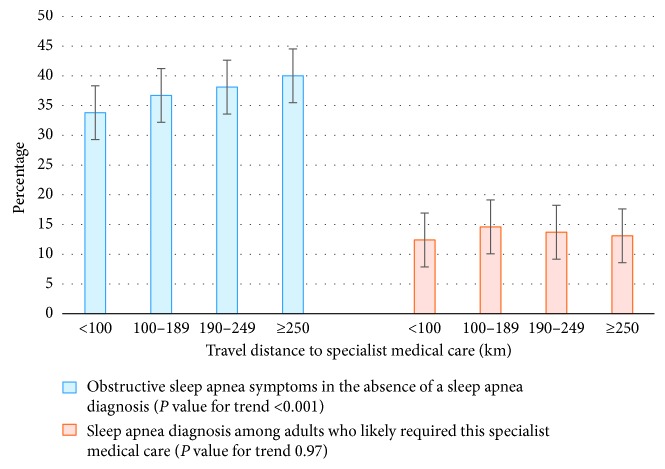
Adults who reported the largest travel distances (≥250 km) to access specialist medical care were 19% more likely to report OSA symptoms in the absence of a sleep apnea diagnosis than those who reported the smallest travel distances (<100 km) (RR = 1.19; 95% CI: 1.08, 1.30; Table 2). The relative risks and 95% CIs were not statistically different between males and females at the 5% level of significance, after adjustment for confounding variables (P=0.06). Sex was not found to be a meaningful effect modifier and was controlled for as a potential confounder. After adjustment for confounding variables, the effect of travel distance to specialist medical care was similar to the unadjusted effect (RR = 1.17; 95% CI: 1.07, 1.29; Table 2). There was a significant increasing trend (P < 0.001) between the proportion of adults reporting OSA symptoms in the absence of a sleep apnea diagnosis and travel distance to specialist medical care (Figure 2).
Table 2.
Results of multivariable log-binomial regression modeling likelihood of obstructive sleep apnea symptoms in the absence of a sleep apnea diagnosis in the study sample.
| Variable | Unadjusted model RR (95% CI) | Adjusted modela RR (95% CI) | P value |
|---|---|---|---|
| Exposure | |||
| Travel distance to specialist medical care (km) | |||
| <100 | 1.00 (referent) | 1.00 (referent) | 0.008 |
| 100–189 | 1.09 (0.99, 1.20) | 1.08 (0.99, 1.19) | |
| 190–249 | 1.13 (1.02, 1.25) | 1.10 (1.00, 1.21) | |
| ≥250 | 1.19 (1.08, 1.30) | 1.17 (1.07, 1.29) | |
| Potential confounders b | |||
| Age (years) | |||
| 18–45 | 1.00 (referent) | 1.00 (referent) | <0.001 |
| 46–55 | 1.28 (1.17, 1.40) | 1.18 (1.08, 1.28) | |
| 56–65 | 1.35 (1.23, 1.48) | 1.20 (1.10, 1.32) | |
| >65 | 0.98 (0.88, 1.08) | 0.91 (0.81, 1.01) | |
| Sex | |||
| Females | 1.00 (referent) | 1.00 (referent) | <0.001 |
| Males | 1.63 (1.53, 1.73) | 1.47 (1.37, 1.57) | |
| Body mass index (kg/m 2) | |||
| Normal (<25) | 1.00 (referent) | 1.00 (referent) | <0.001 |
| Overweight (25–29.9) | 1.53 (1.39, 1.67) | 1.35 (1.23, 1.48) | |
| Obese (≥30) | 1.87 (1.71, 2.04) | 1.59 (1.45, 1.74) | |
| Money left over at the end of the month | |||
| Some | 1.00 (referent) | 1.00 (referent) | 0.32 |
| Just enough | 0.95 (0.87, 1.03) | 0.94 (0.87, 1.02) | |
| Not enough | 1.05 (0.97, 1.15) | 0.99 (0.91, 1.08) | |
| Education level | |||
| Postsecondary | 1.00 (referent) | 1.00 (referent) | 0.73 |
| Secondary or less | 1.11 (1.04, 1.18) | 1.01 (0.95, 1.08) | |
| Heavy alcohol consumption, more than 5 drinks on one occasion | |||
| Never | 1.00 (referent) | 1.00 (referent) | 0.03 |
| 1/month or less | 1.15 (1.07, 1.23) | 1.00 (0.93, 1.07) | |
| 1/week or less | 1.44 (1.31, 1.58) | 1.14 (1.04, 1.25) | |
| More than 1/week | 1.40 (1.16, 1.68) | 1.03 (0.86, 1.24) | |
| Smoking status | |||
| Never | 1.00 (referent) | 1.00 (referent) | <0.001 |
| Past | 1.29 (1.20, 1.38) | 1.16 (1.09, 1.25) | |
| Current | 1.29 (1.17, 1.42) | 1.20 (1.09, 1.32) | |
aModel adjusted for age, sex, body mass index, money left over at the end of the month, education level, heavy alcohol consumption, and smoking status. Standard errors corresponding to confidence intervals were inflated to account for clustering of adults within households. bModels adjusted for the other 6 potential confounders and travel distance to specialist medical care. Standard errors corresponding to confidence intervals were inflated to account for clustering of adults within households.
Figure 2.
Proportion of adults reporting (1) obstructive sleep apnea symptoms in the absence of a sleep apnea diagnosis and (2) a sleep apnea diagnosis by travel distance to specialist medical care. The error bars represent the standard error of the mean.
Our comparison of reported travel distance to specialist medical care and driving distance to the closest of Saskatchewan's sleep centers for all households using postal codes found inconsistencies between reported travel distances and driving distance. For example, among the adults who reported distances between 100 km and 189 km to access specialist medical care, driving distance to the closest sleep center in the province was consistent with reported data only 36% of the time; driving distance was larger than reported distance 60% of the time. Among the adults who reported distances of 250 km or greater to access specialist medical care, driving distance to the closest sleep center in the province was smaller than reported distance 38% of the time (Table 3). Distance as determined by postal code may be more of an approximation than distances reported by rural dwellers whose way of life depends on extensive travel.
Table 3.
Agreement between reported travel distance to specialist medical care and driving distance as determined by postal codes, to the closest sleep center in Saskatchewan.
| Reported travel distance to specialist medical care (km) | Driving distance to the closest sleep center in Saskatchewan (km) | |||
|---|---|---|---|---|
| <100 | 100–189 | 190–249 | ≥250 | |
| <100 | 11% | 16% | 53% | 20% |
| 100–189 | 4% | 36% | 26% | 34% |
| 190–249 | 0% | 11% | 74% | 15% |
| ≥250 | 0% | 3% | 35% | 62% |
5.2. Objective 2
Forty-three percent (2813/6525) of the sample was identified as possibly requiring sleep specialist care (either reported OSA symptoms or a sleep apnea diagnosis) (Figure 1). This subset of the sample is described in Table 4. Age ranged from 18 to 101 (mean 55.5 [±13.6]) years. Mean travel distance to specialist medical care was 189 [±114] km; mean distance was similar among adults reporting a sleep apnea diagnosis and those reporting OSA symptoms in the absence of a sleep apnea diagnosis (188 [±113] km vs. 189 [±114] km, respectively). Eighty-one percent reported travel distances ≥100 km and 27% reported travel distances ≥250 km. Among adults who likely required this specialist medical care, travel distance to specialist medical care was not associated with the proportion of adults reporting OSA symptoms in the absence of a sleep apnea diagnosis. Younger age, female sex, normal BMI, “just enough” or “some” money left over at the end of the month, and lower to moderate frequency of heavy alcohol consumption were also associated with higher proportions of adults reporting OSA symptoms in the absence of a sleep apnea diagnosis.
Table 4.
Characteristics of adults who likely required this specialist medical care (n = 2813; 2230 households).
| Characteristics | Proportion (%) | Obstructive sleep apnea symptoms in the absence of a sleep apnea diagnosis (n = 2432; 86%) | P valuea | Travel distance to specialist medical care ≥ 250 km (n = 761; 27%) | P valuea | ||
|---|---|---|---|---|---|---|---|
| n | Row % | n | Row % | ||||
| Exposure | |||||||
| Travel distance to specialist medical care (km) | |||||||
| <100 | 19 | 480 | 87.6 | 0.71 | — | — | — |
| 100–189 | 28 | 669 | 85.4 | — | — | ||
| 190–249 | 26 | 622 | 86.3 | — | — | ||
| ≥250 | 27 | 661 | 86.9 | — | — | ||
| Potential confounders | |||||||
| Age (years) | |||||||
| 18–45 | 21 | 533 | 91.1 | 0.003 | 140 | 23.9 | 0.12 |
| 46–55 | 29 | 695 | 85.3 | 224 | 27.5 | ||
| 56–65 | 28 | 676 | 85.6 | 215 | 27.2 | ||
| >65 | 22 | 528 | 84.8 | 182 | 29.2 | ||
| Sex | |||||||
| Females | 36 | 922 | 89.9 | <0.001 | 269 | 26.2 | 0.24 |
| Males | 64 | 1510 | 84.5 | 492 | 27.5 | ||
| Body mass index (kg/m 2) | |||||||
| Normal (<25) | 19 | 479 | 91.4 | <0.001 | 128 | 24.4 | 0.52 |
| Overweight (25–29.9) | 41 | 1041 | 90.1 | 312 | 27.0 | ||
| Obese (≥30) | 40 | 912 | 80.5 | 321 | 28.3 | ||
| Education level | |||||||
| Postsecondary | 39 | 947 | 86.3 | 0.80 | 287 | 26.1 | 0.40 |
| Secondary or less | 61 | 1485 | 86.6 | 474 | 27.6 | ||
| Money left over at the end of the month | |||||||
| Some | 59 | 1452 | 87.5 | 0.005 | 423 | 25.5 | 0.19 |
| Just enough | 20 | 489 | 87.8 | 153 | 27.5 | ||
| Not enough | 21 | 491 | 82.4 | 185 | 31.0 | ||
| Heavy alcohol consumption, more than 5 drinks on one occasion | |||||||
| Never | 50 | 1217 | 86.2 | 0.03 | 402 | 28.5 | 0.26 |
| 1/month or less | 34 | 837 | 87.6 | 248 | 25.9 | ||
| 1/week or less | 13 | 320 | 87.0 | 97 | 26.4 | ||
| More than 1/week | 3 | 58 | 75.3 | 14 | 18.2 | ||
| Smoking status | |||||||
| Never | 46 | 1118 | 86.5 | 0.47 | 349 | 27.0 | 0.98 |
| Past | 41 | 981 | 85.8 | 314 | 27.5 | ||
| Current | 13 | 333 | 88.3 | 98 | 26.0 | ||
a P value from Rao-Scott chi-square tests for significant difference in proportions with obstructive sleep apnea symptoms in the absence of a sleep apnea diagnosis/travel distance to specialist medical care ≥250 km between levels of each variable.
Sample characteristics by each distance quartile are shown in Supplemental Table 1 (Appendix A). No significant trends were observed.
Among adults who likely required this specialist medical care, the proportion reporting a sleep apnea diagnosis was low and unaffected by reported travel distance to specialist medical care (RR = 1.06; 95% CI: 0.79, 1.42; Table 5). We found marginal evidence of effect modification by sex at the 5% level of significance (P=0.05). In the cross-sectional data, there was some evidence of a significant relationship between travel distance to specialist medical care and reports of a sleep apnea diagnosis among men who likely required this specialist medical care (P=0.04); however, this relationship was only borderline significant at the 5% level of significance and followed no clear or interpretable trend, and the analysis of follow-up survey data showed no evidence of such a relationship (P=0.37). Sex was not found to be a meaningful effect modifier and was controlled for as a potential confounder. After adjustment for confounding variables, the association between travel distance to specialist medical care and sleep apnea diagnosis remained statistically nonsignificant (RR = 1.09; 95% CI: 0.82, 1.46; Table 5). There was no trend between the proportion of adults reporting a sleep apnea diagnosis and travel distance to specialist medical care (Figure 2).
Table 5.
Results of multivariable log-binomial regression modeling probability of a sleep apnea diagnosis among adults who likely required this specialist medical care.
| Variable | Unadjusted model RR (95% CI) | Adjusted modela RR (95% CI) | P value |
|---|---|---|---|
| Exposure | |||
| Travel distance to specialist medical care (km) | |||
| <100 | 1.00 (referent) | 1.00 (referent) | 0.32 |
| 100–189 | 1.17 (0.88, 1.57) | 1.27 (0.95, 1.70) | |
| 190–249 | 1.11 (0.83, 1.48) | 1.21 (0.90, 1.62) | |
| ≥250 | 1.06 (0.79, 1.42) | 1.09 (0.82, 1.46) | |
| Potential confounders b | |||
| Age (years) | |||
| 18–45 | 1.00 (referent) | 1.00 (referent) | <0.001 |
| 46–55 | 1.66 (1.22, 2.25) | 1.71 (1.27, 2.32) | |
| 56–65 | 1.63 (1.19, 2.22) | 1.68 (1.23, 2.30) | |
| >65 | 1.71 (1.24, 2.36) | 1.99 (1.41, 2.81) | |
| Sex | |||
| Females | 1.00 (referent) | 1.00 (referent) | <0.001 |
| Males | 1.53 (1.24, 1.88) | 1.58 (1.27, 1.96) | |
| Body mass index (kg/m 2) | |||
| Normal (<25) | 1.00 (referent) | 1.00 (referent) | <0.001 |
| Overweight (25–29.9) | 1.16 (0.84, 1.60) | 1.09 (0.79, 1.50) | |
| Obese (≥30) | 2.28 (1.68, 3.08) | 2.24 (1.65, 3.05) | |
| Money left over at the end of the month | |||
| Some | 1.00 (referent) | 1.00 (referent) | 0.02 |
| Just enough | 0.97 (0.75, 1.26) | 1.01 (0.78, 1.30) | |
| Not enough | 1.41 (1.13, 1.75) | 1.39 (1.12, 1.73) | |
| Education level | |||
| Postsecondary | 1.00 (referent) | 1.00 (referent) | 0.05 |
| Secondary or less | 0.97 (0.80, 1.18) | 0.82 (0.68, 1.00) | |
| Heavy alcohol consumption, more than 5 drinks on one occasion | |||
| Never | 1.00 (referent) | 1.00 (referent) | 0.12 |
| 1/month or less | 0.90 (0.73, 1.012) | 0.89 (0.72, 1.11) | |
| 1/week or less | 0.94 (0.70, 1.28) | 0.93 (0.67, 1.28) | |
| More than 1/week | 1.79 (1.19, 2.67) | 1.65 (1.05, 2.58) | |
| Smoking status | |||
| Never | 1.00 (referent) | 1.00 (referent) | 0.60 |
| Past | 1.05 (0.86, 1.28) | 0.91 (0.74, 1.11) | |
| Current | 0.86 (0.63, 1.17) | 0.89 (0.65, 1.22) | |
aModel adjusted for age, sex, body mass index, money left over at the end of the month, education level, heavy alcohol consumption, and smoking status. Standard errors corresponding to confidence intervals were inflated to account for clustering of adults within households. bModels adjusted for the other 6 potential confounders and travel distance to specialist medical care. Standard errors corresponding to confidence intervals were inflated to account for clustering of adults within households.
5.2.1. Analysis of Follow-Up Survey Data
Findings were consistent with our cross-sectional analysis. For adults who reported OSA symptoms in the absence of a sleep apnea diagnosis at baseline, travel distance to specialist medical care was not associated with reports of an incident sleep apnea diagnosis after five years (RR = 1.01; 95% CI: 0.98, 1.05; Table 6).
Table 6.
Results of multivariable log-binomial regression modeling probability of an incident sleep apnea diagnosis after 5 years among adults with obstructive sleep apnea symptoms in the absence of a sleep apnea diagnosis (n = 1459; 1180 households).
| Exposure | Proportion (%) | Obstructive sleep apnea symptoms in the absence of a sleep apnea diagnosis (row %) | Unadjusted model RR (95% CI) | Adjusted modela RR (95% CI) | P value |
|---|---|---|---|---|---|
| Travel distance to specialist medical care, km | |||||
| <100 | 20 | 94.8 | 1.00 (referent) | 1.00 (referent) | 0.80 |
| 100–189 | 26 | 95.4 | 0.87 (0.44, 1.71) | 1.00 (0.97, 1.04) | 0.84b |
| 190–249 | 26 | 96.4 | 0.69 (0.34, 1.41) | 1.01 (0.98, 1.05) | |
| ≥250 | 28 | 96.1 | 0.74 (0.37, 1.47) | 1.01 (0.98, 1.05) | |
aModel adjusted for age, sex, body mass index, money left over at the end of the month, education level, heavy alcohol consumption, and smoking status. Standard errors corresponding to confidence intervals were inflated to account for clustering of adults within households. b P value for trend test.
5.3. Sensitivity Analysis
A sensitivity analysis using multiple imputation revealed minimal impact of excluding participants with missing data for any of the study variables (data not shown).
6. Discussion
Using data from a large population-based rural health study conducted in Saskatchewan, we found that as travel distance to specialist medical care increased, so did the proportion of rural adult residents that reported OSA symptoms in the absence of a sleep apnea diagnosis. Among adults who likely required this specialist medical care and who would optimally be screened, diagnosed, and/or clinically managed appropriately, the proportion of sleep apnea diagnoses was low and unaffected by reported travel distance.
This study adds to a body of evidence that focuses on the population health impacts of poor access to specialist medical care in rural populations [47, 55, 56, 60–67], and specifically presents a novel analysis of the impact of travel distance to specialist medical care on sleep apnea diagnosis. Health care use is known to be lower in rural than in urban communities [60–63], and this has been attributed to increased travel distances and limited access to primary care and medical specialists [47, 55, 56, 64–67]. Health care access barriers are one possible risk factor for inadequate diagnosis and treatment of medical conditions [58, 70]. Even in the presence of accessible primary care, travel distance to specialist medical care is still a relevant concern for cases of sleep apnea because referral to a sleep medicine specialist for overnight, in-laboratory diagnostic testing, or at-home testing is required for diagnosis [71, 72]. Our finding of an increased proportion of rural adults reporting OSA symptoms in the absence of a sleep apnea diagnosis in association with increased travel distance to specialist medical care suggests decreased use of health care services by adults in remote rural communities.
Strengths and limitations of our analysis warrant comment. Our modeling strategy assessed for effect modification by biological sex, and then controlled for key confounding variables to reduce any differences that might arise for reasons other than travel distance to specialist medical care. Our analysis of follow-up survey data lent temporal evidence to our finding that the trend of an increasing proportion of adults reporting OSA symptoms in the absence of a sleep apnea diagnosis in association with increasing travel distance to specialist medical care was not attributable to differences in the frequency of diagnosis among adults who likely required this specialist medical care. Our findings suggest that once referred to a specialist, travel distance to specialist care is not associated with the sleep apnea diagnostic rate in rural populations. The low sleep apnea diagnostic rate may reflect other health care access barriers.
Self-reported travel distance may have resulted in exposure misclassification. Reporting a travel distance in kilometers may be difficult, especially if unfamiliar with where to get specialist care in Saskatchewan and if not having gone to see a specialist recently or ever. Participants may have over or underestimated travel distance as a result, which is supported by our comparison of reported travel distance and driving distance as determined by postal code, to the closest sleep center in the province. Although our exposure measure referred to all medical or surgical specialist services and was not specific to sleep specialist care, Saskatchewan's only two publicly funded sleep centers are located within the cities of Saskatoon and Regina [90, 91]. It is likely that most specialist medical and surgical services are provided out of these centers, especially given the general lack of medical specialists practicing in rural communities [47, 55]. Reliance on self-report data through a mailed questionnaire may have also led to underreporting the presence and/or degree of OSA symptoms due to social desirability bias and/or a lack of awareness that symptoms may be consistent with OSA. This potential outcome misclassification would have biased effect estimates for the first objective towards the null, assuming symptom underreporting was nondifferential across distance quartiles, and reduced the sample size and, therefore, power of the second objective. Though our analyses controlled for most known potential confounders, some degree of residual confounding is possible from measurement imprecision with self-report data.
Our sample was limited to adults living in rural Saskatchewan. Inclusion of adults from urban areas would have provided a useful basis of comparison. Our study could only evaluate the impact of travel distance to specialist medical care on OSA diagnosis between varying degrees of remoteness from such care. Our use of a sample that was exclusively rural in nature may have biased study findings in that effects that may be evident when you compare rural to urban populations would have been missed. Furthermore, in terms of external validity, the sample may not be representative of all rural populations, and the observed trend, rather than the magnitude of risk estimates, may only be generalized to rural communities without a strong metropolitan influence zone that have similar environmental and occupational exposures as Saskatchewan.
Our findings may have implications for health policy surrounding the diagnosis and treatment of OSA. In rural Saskatchewan, the largest reported travel distances to access specialist medical care (≥250 km) were associated with a greater proportion of adults reporting OSA symptoms in the absence of a sleep apnea diagnosis and suggest decreased use of health care services in remote populations. However, among adults who likely required this specialist medical care, the proportion of sleep apnea diagnoses was low and unaffected by reported travel distance. Factors other than travel distance may therefore be contributing to the low sleep apnea diagnostic rate. This remains important as undiagnosed and untreated OSA has serious implications on the health of people and populations [1, 10–24, 38–44], but effective treatments are available [25–37]. Health care access is an important determinant of health [58, 59] and barriers to the multiple dimensions of health care access (as per Levesque's model [70]) that have been established for other conditions (e.g., access to primary care, transportation barriers, and/or financial barriers) should be evaluated in future research that focuses on OSA. Interventional work on potential strategies to address and ameliorate any identified barriers is also warranted and results may provide important evidence to inform health care planning and delivery, which may be particularly impactful in rural and remote communities. Studies of interventional strategies, be their policies that foster better health care access through telemedicine [92–94] and portable diagnostic devices for in-home testing [4, 95–103], or innovative community outreach strategies that potentially increase access, especially among vulnerable and remote populations [104–106], are especially warranted.
7. Conclusions
Long travel distances may pose barriers to seeking health care in rural communities. In rural Saskatchewan, there was an increasing proportion of adults reporting OSA symptoms in the absence of a sleep apnea diagnosis in association with increasing travel distance to specialist medical care, which suggests decreased use of health care services in remote populations. However, among adults who likely required this specialist medical care, the proportion of sleep apnea diagnoses was low and unaffected by reported travel distance. Other health care access barriers may therefore be contributing to the low sleep apnea diagnostic rate. This remains important as undiagnosed and untreated OSA has serious implications on the health of people and populations, but effective treatments are available. Health care access barriers to the diagnosis and treatment of OSA require evaluation. Interventional work on potential strategies to address and ameliorate any identified barriers is warranted and results may provide important evidence to inform health care planning and delivery, which may be particularly impactful in rural and remote communities. Studies of interventional strategies, be their policies that foster better access through telemedicine and portable diagnostic devices for in-home testing, or innovative community outreach strategies that potentially increase access, especially among vulnerable and remote populations, are especially warranted.
Acknowledgments
The principal investigator (CS) holds a Frederick Banting and Charles Best Canada Graduate Scholarship from the Canadian Institutes of Health Research (CIHR) Master's (CGS-M) program. The Saskatchewan Rural Health Study was funded by a grant from CIHR (MOP-187209-POP-CCAA-11829). We thank the Saskatchewan Rural Health Study Research Team for permission to use data from this rural health study for the present secondary analysis and the participants in that cohort for their ongoing contributions to scientific knowledge in their province.
Data Availability
Original microdata files for the Saskatchewan Rural Health Study used to support the findings of this study are available from Drs. Pahwa and Dosman at the Canadian Centre for Health and Safety in Agriculture, University of Saskatchewan, upon request.
Disclosure
This manuscript is based on the master's thesis of Catherine M. Spagnuolo.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Supplementary Materials
Supplemental Table 1. Characteristics of adults who likely required this specialist medical care (n = 2813; 2230 households) by distance quartile.
References
- 1.King N., Pickett W., Hagel L., Lawson J., Trask C., Dosman J. A. Impact of excessive daytime sleepiness on the safety and health of farmers in Saskatchewan. Canadian Respiratory Journal. 2014;21(6):363–369. doi: 10.1155/2014/609217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aurora R. N., Collop N. A., Jacobowitz O., Thomas S. M., Quan S. F., Aronsky A. J. Quality measures for the care of adult patients with obstructive sleep apnea. Journal of Clinical Sleep Medicine. 2015;11(3):357–83. doi: 10.5664/jcsm.4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Public Health Agency of Canada. What is the impact of sleep apnea on Canadians? http://www.phac-aspc.gc.ca/cd-mc/sleepapnea-apneesommeil/ff-rr-2009-eng.php, 2010.
- 4.Pang K. P., Terris D. J. Screening for obstructive sleep apnea: an evidence-based analysis. American Journal of Otolaryngology. 2006;27(2):112–118. doi: 10.1016/j.amjoto.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 5.American Academy of Sleep Medicine Task Force. Sleep–related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22(5):667–689. [PubMed] [Google Scholar]
- 6.Flemons W. W., Littner M. R., Rowley J. A., et al. Home diagnosis of sleep apnea: a systematic review of the literature. Chest. 2003;124(4):1543–1579. doi: 10.1378/chest.124.4.1543. [DOI] [PubMed] [Google Scholar]
- 7.Riha R. L. Diagnostic approaches to respiratory sleep disorders. Journal of Thoracic Disease. 2015;7(8):1373–1384. doi: 10.3978/j.issn.2072-1439.2015.08.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson M. L., Howard M. E., Barnes M. Cognition and daytime functioning in sleep-related breathing disorders. Progress in Brain Research. 2011;190:53–68. doi: 10.1016/b978-0-444-53817-8.00003-7. [DOI] [PubMed] [Google Scholar]
- 9.Miller J. N., Berger A. M. Screening and assessment for obstructive sleep apnea in primary care. Sleep Medicine Reviews. 2016;29:41–51. doi: 10.1016/j.smrv.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Dosman J. A., Hagel L., Skomro R., et al. Loud snoring is a risk factor for occupational injury in farmers. Canadian Respiratory Journal. 2013;20(1):42–46. doi: 10.1155/2013/469391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spengler S. E., Browning S. R., Reed D. B. Sleep deprivation and injuries in part-time Kentucky farmers: impact of self reported sleep habits and sleep problems on injury risk. Aaohn Journal. 2004;52(9):373–382. [PubMed] [Google Scholar]
- 12.Lindberg E., Carter N., Gislason T., Janson C. Role of snoring and daytime sleepiness in occupational accidents. American Journal of Respiratory and Critical Care Medicine. 2001;164(11):2031–2035. doi: 10.1164/ajrccm.164.11.2102028. [DOI] [PubMed] [Google Scholar]
- 13.Ellen R. L. B., Marshall S. C., Palayew M., Molnar F. J., Wilson K. G., Man-Son-Hing M. Systematic review of motor vehicle crash risk in persons with sleep apnea. Journal of Clinical Sleep Medicine. 2006;2(2):193–200. [PubMed] [Google Scholar]
- 14.Jennum P., Riha R. L. Epidemiology of sleep apnoea/hypopnoea syndrome and sleep-disordered breathing. European Respiratory Journal. 2009;33(4):907–914. doi: 10.1183/09031936.00180108. [DOI] [PubMed] [Google Scholar]
- 15.Knauert M., Naik S., Gillespie M. B., Kryger M. Clinical consequences and economic costs of untreated obstructive sleep apnea syndrome. World Journal of Otorhinolaryngology-Head and Neck Surgery. 2015;1(1):17–27. doi: 10.1016/j.wjorl.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gunnarsson S. I., Peppard P. E., Korcarz C. E., et al. Obstructive sleep apnea is associated with future subclinical carotid artery disease thirteen-year follow-up from the Wisconsin sleep cohort. Arteriosclerosis, Thrombosis, and Vascular Biology. 2014;34(10) doi: 10.1161/atvbaha.114.303965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maeder M. T., Schoch O. D., Rickli H. A clinical approach to obstructive sleep apnea as a risk factor for cardiovascular disease. Vascular Health and Risk Management. 2016;12:85–103. doi: 10.2147/vhrm.s74703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X., Ouyang Y., Wang Z., Zhao G., Liu L., Bi Y. Obstructive sleep apnea and risk of cardiovascular disease and all-cause mortality: a meta-analysis of prospective cohort studies. International Journal of Cardiology. 2013;169(3):207–214. doi: 10.1016/j.ijcard.2013.08.088. [DOI] [PubMed] [Google Scholar]
- 19.Redline S., Yenokyan G., Gottlieb D. J., et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. American Journal of Respiratory and Critical Care Medicine. 2010;182(2):269–277. doi: 10.1164/rccm.200911-1746oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young T., Peppard P., Palta M., et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Archives of Internal Medicine. 2014;157(15):1746. doi: 10.1001/archinte.1997.00440360178019. [DOI] [PubMed] [Google Scholar]
- 21.Peppard P. E., Young T., Palta M., Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. New England Journal of Medicine. 2000;342(19):1378–1384. doi: 10.1056/nejm200005113421901. [DOI] [PubMed] [Google Scholar]
- 22.Nieto F. J., Young T. B., Lind B. K., et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. JAMA. 2000;283(14):1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 23.Bauters F., Rietzschel E. R., Hertegonne K. B. C., Chirinos J. A. The link between obstructive sleep apnea and cardiovascular disease. Current Atherosclerosis Reports. 2016;18(1) doi: 10.1007/s11883-015-0556-z. [DOI] [PubMed] [Google Scholar]
- 24.Konecny T., Kara T., Somers V. K. Obstructive sleep apnea and hypertension: an update. Hypertension. 2014;63(2) doi: 10.1161/hypertensionaha.113.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jordan A. S., McSharry D. G., Malhotra A. Adult obstructive sleep apnoea. The Lancet. 2014;383(9918):736–747. doi: 10.1016/s0140-6736(13)60734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedman M., Schalch P., Lin H.-C., Kakodkar K. A., Joseph N. J., Mazloom N. Palatal implants for the treatment of snoring and obstructive sleep apnea/hypopnea syndrome. Otolaryngology-Head and Neck Surgery. 2008;138(2):209–216. doi: 10.1016/j.otohns.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 27.Huang T.-W., Cheng P.-W., Fang K.-M. Concurrent palatal implants and uvulopalatal flap: safe and effective office-based procedure for selected patients with snoring and obstructive sleep apnea syndrome. Laryngoscope. 2011;121(9):2038–2042. doi: 10.1002/lary.22129. [DOI] [PubMed] [Google Scholar]
- 28.Nordgård S., Stene B. K., Skjøstad K. W. Soft palate implants for the treatment of mild to moderate obstructive sleep apnea. Otolaryngology-Head and Neck Surgery. 2006;134(4):565–570. doi: 10.1016/j.otohns.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 29.Walker R. P., Levine H. L., Hopp M. L., Greene D., Pang K. Palatal implants: a new approach for the treatment of obstructive sleep apnea. Otolaryngology-Head and Neck Surgery. 2006;135(4):549–554. doi: 10.1016/j.otohns.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 30.Weaver T. E., Mancini C., Maislin G., et al. Continuous positive airway pressure treatment of sleepy patients with milder obstructive sleep apnea: results of the CPAP Apnea Trial North American Program (CATNAP) randomized clinical trial. American Journal of Respiratory and Critical Care Medicine. 2012;186(7):677–683. doi: 10.1164/rccm.201202-0200oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giles T. L., Lasserson T. J., Smith B., White J., Wright J. J., Cates C. J. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database of Systematic Reviews. 2006;3 doi: 10.1002/14651858.CD001106.pub2.CD001106 [DOI] [PubMed] [Google Scholar]
- 32.Kushida C. A., Berry R. B., Blau A., et al. Positive airway pressure initiation: a randomized controlled trial to assess the impact of therapy mode and titration process on efficacy, adherence, and outcomes. Sleep. 2011;34(8):1083–1092. doi: 10.5665/SLEEP.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bratton D. J., Gaisl T., Schlatzer C., Kohler M. Comparison of the effects of continuous positive airway pressure and mandibular advancement devices on sleepiness in patients with obstructive sleep apnoea: a network meta-analysis. Lancet Respiratory Medicine. 2015;3(11):869–878. doi: 10.1016/s2213-2600(15)00416-6. [DOI] [PubMed] [Google Scholar]
- 34.Ramar K., Dort L. C., Katz S. G., et al. Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015. Journal of Clinical Sleep Medicine. 2015;11(7):773–827. doi: 10.5664/jcsm.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phillips C. L., Grunstein R. R., Darendeliler M. A., et al. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. American Journal of Respiratory and Critical Care Medicine. 2013;187(8):879–887. doi: 10.1164/rccm.201212-2223oc. [DOI] [PubMed] [Google Scholar]
- 36.Fleetham J., Ayas N., Bradley D., et al. Canadian Thoracic Society guidelines: diagnosis and treatment of sleep disordered breathing in adults. Canadian Respiratory Journal. 2006;13(7):387–392. doi: 10.1155/2006/627096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Camacho M., Certal V., Abdullatif J., et al. Myofunctional therapy to treat obstructive sleep apnea: a systematic review and meta-analysis. Sleep. 2015;38(5):669–675. doi: 10.5665/sleep.4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marin J. M., Agusti A., Villar I., et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA. 2012;307(20):2169–2176. doi: 10.1001/jama.2012.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fava C., Dorigoni S., Dalle Vedove F., et al. Effect of CPAP on blood pressure in patients with OSA/hypopnea: a systematic review and meta-analysis. Chest. 2014;145(4):762–771. doi: 10.1378/chest.13-1115. [DOI] [PubMed] [Google Scholar]
- 40.Javaheri S., Barbe F., Campos-Rodriguez F., et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. Journal of the American College of Cardiology. 2017;69(7) doi: 10.1016/j.jacc.2016.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lewington S., Clarke R., Qizilbash N., Peto R., Collins R., Prospective Studies Collaboration Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. The Lancet. 2002;360(9349):1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 42.Campos-Rodriguez F., Martinez-Garcia M. A., Reyes-Nuñez N., Caballero-Martinez I., Catalan-Serra P., Almeida-Gonzalez C. V. Role of sleep apnea and continuous positive airway pressure therapy in the incidence of stroke or coronary heart disease in women. American Journal of Respiratory and Critical Care Medicine. 2014;189(12):1544–1550. doi: 10.1164/rccm.201311-2012oc. [DOI] [PubMed] [Google Scholar]
- 43.Leger D., Bayon V., Laaban J. P., Philip P. Impact of sleep apnea on economics. Sleep Medicine Reviews. 2012;16(5):455–462. doi: 10.1016/j.smrv.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Antonopoulos C. N., Sergentanis T. N., Daskalopoulou S. S., Petridou E. T. Nasal continuous positive airway pressure (nCPAP) treatment for obstructive sleep apnea, road traffic accidents and driving simulator performance: a meta-analysis. Sleep Medicine Reviews. 2011;15(5):301–310. doi: 10.1016/j.smrv.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 45.Beaulieu M. S. Demographic Changes in Canadian Agriculture. Ottawa, ON, Canada: Statistics Canada; 2015. [Google Scholar]
- 46.Brumby S., Chandrasekara A., McCoombe S., Kremer P., Lewandowski P. Cardiovascular risk factors and psychological distress in Australian farming communities. Australian Journal of Rural Health. 2012;20(3):131–137. doi: 10.1111/j.1440-1584.2012.01273.x. [DOI] [PubMed] [Google Scholar]
- 47.Hay D., Varga-Toth J., Hines E. Frontline Health Care in Canada: Innovations in Delivering Services to Vulnerable Populations. Ottawa, ON, Canada: Canadian Policy Research Networks Inc.; 2006. [Google Scholar]
- 48.Befort C. A., Nazir N., Perri M. G. Prevalence of obesity among adults from rural and urban areas of the United States: findings from NHANES (2005-2008) Journal of Rural Health. 2012;28(4):392–397. doi: 10.1111/j.1748-0361.2012.00411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wen M., Fan J. X., Kowaleski-Jones L., Wan N. Rural–urban disparities in obesity prevalence among working age adults in the United States: exploring the mechanisms. American Journal of Health Promotion. 2018;32(2):400–408. doi: 10.1177/0890117116689488. [DOI] [PubMed] [Google Scholar]
- 50.Trivedi T., Liu J., Probst J., Merchant A., Jhones S., Martin A. B. Obesity and obesity-related behaviors among rural and urban adults in the USA. Rural and Remote Health. 2015;15(4):p. 3267. [PubMed] [Google Scholar]
- 51.Statistics Canada. Saskatchewan provincial trends. Canola area surpassed spring wheat area in Saskatchewan. http://www.statcan.gc.ca/pub/95-640-x/2011001/p1/prov/prov-47-eng.htm, 2016.
- 52.Pickett W., Hartling L., Dimich-Ward H., et al. Surveillance of hospitalized farm injuries in Canada. Injury Prevention. 2001;7(2):123–128. doi: 10.1136/ip.7.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hagel L. M., Pickett W., Pahwa P., et al. Prevention of agricultural injuries: an evaluation of an education-based intervention. Injury Prevention. 2008;14(5):290–295. doi: 10.1136/ip.2008.018515. [DOI] [PubMed] [Google Scholar]
- 54.Pickett W., Hartling L., Brison R. J., Guernsey J. R. The Canadian agricultural injury surveillance program. Fatal work-related farm injuries in Canada, 1991-1995. Canadian Medical Association Journal. 1999;160(13):1843–1848. [PMC free article] [PubMed] [Google Scholar]
- 55.Laurent S. Rural Canada: Access to Health Care (PRB 02-45E) Ottawa, ON, Canada: Parliamentary Research Branch; 2002. [Google Scholar]
- 56.Jones C. A., Parker T. S., Ahearn M., Mishra A. K., Variyam J. N. Health Status and Health Care Access of Farm and Rural Populations. Washington, DC, USA: 2009. [Google Scholar]
- 57.Trivedi T., Liu J., Probst J. C., Martin A. B. The metabolic syndrome: are rural residents at increased risk? Journal of Rural Health. 2013;29(2):188–197. doi: 10.1111/j.1748-0361.2012.00422.x. [DOI] [PubMed] [Google Scholar]
- 58.Clarke J. Difficulty accessing health care services in Canada. 2016. Health at a Glance Catalogue no.82-624-X.
- 59.Public Health Agency of Canada. What makes Canadians healthy or unhealthy? https://www.canada.ca/en/public-health/services/health-promotion/population-health/what-determines-health/what-makes-canadians-healthy-unhealthy.html#healthservices, 2013.
- 60.Casey M. M., Call K. T., Klingner J. M. Are rural residents less likely to obtain recommended preventive healthcare services? American Journal of Preventive Medicine. 2001;21(3):182–188. doi: 10.1016/s0749-3797(01)00349-x. [DOI] [PubMed] [Google Scholar]
- 61.Chan S., Hixon B., Adkins M., Shinn J. B., Bush M. L. Rurality and determinants of hearing healthcare in adult hearing aid recipients. Laryngoscope. 2017;127(10):2362–2367. doi: 10.1002/lary.26490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cole A. M., Jackson J. E., Doescher M. Urban-rural disparities in colorectal cancer screening: cross-sectional analysis of 1998-2005 data from the centers for disease control’s behavioral risk factor surveillance study. Cancer Medicie. 2012;1(3):350–356. doi: 10.1002/cam4.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Teich J., Ali M. M., Lynch S., Mutter R. Utilization of mental health services by veterans living in rural areas. Journal of Rural Health. 2017;33(3):297–304. doi: 10.1111/jrh.12221. [DOI] [PubMed] [Google Scholar]
- 64.Chan L., Hart L. G., Goodman D. C. Geographic access to health care for rural medicare beneficiaries. Journal of Rural Health. 2006;22(2):140–146. doi: 10.1111/j.1748-0361.2006.00022.x. [DOI] [PubMed] [Google Scholar]
- 65.Fordyce M. A., Chen F. M., Doescher M. P., Hart L. G. Physician Supply and Distribution in Rural Areas of the United States. Seattle, WC, USA: The University of Washington Rural Health Research Center; 2007. [Google Scholar]
- 66.Mattson J. Transportation, Distance, and Health Care Utilization for Older Adults in Rural and Small Urban Areas. Bismarck, ND, USA: Transportation Research Record; 2010. [Google Scholar]
- 67.Karunanayake C. P., Rennie D. C., Hagel L., et al. Access to specialist care in rural saskatchewan: the saskatchewan rural health study. Healthcare. 2015;3(1):84–99. doi: 10.3390/healthcare3010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rotenberg B. W., George C. F., Sullivan K. M., Wong E. Wait times for sleep apnea care in Ontario: a multidisciplinary assessment. Canadian Respiratory Journal. 2010;17(4):170–174. doi: 10.1155/2010/420275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Evans J., Skomro R., Driver H., et al. Sleep laboratory test referrals in Canada: sleep apnea rapid response survey. Canadian Respiratory Journal. 2014;21(1):e4–e10. doi: 10.1155/2014/592947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Levesque J.-F., Harris M. F., Russell G. Patient-centred access to health care: conceptualising access at the interface of health systems and populations. International Journal for Equity in Health. 2013;12(1):p. 18. doi: 10.1186/1475-9276-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fleetham J., Ayas N., Bradley D., et al. Canadian Thoracic Society 2011 guideline update: diagnosis and treatment of sleep disordered breathing. Canadian Respiratory Journal. 2011;18(1):25–47. doi: 10.1155/2011/506189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kapur V. K., Auckley D. H., Chowdhuri S., et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American academy of sleep medicine clinical practice guideline. Journal of Clinical Sleep Medicine. 2017;13(3):479–504. doi: 10.5664/jcsm.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ambroggi M., Biasini C., Del Giovane C., Fornari F., Cavanna L. Distance as a barrier to cancer diagnosis and treatment: review of the literature. Oncologist. 2015;20(12):1378–1385. doi: 10.1634/theoncologist.2015-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Klitzman P., Armstrong B., Janicke D. M. Distance as a predictor of treatment attendance in a family based pediatric weight management program in rural areas. Journal of Rural Health. 2015;31(1):19–26. doi: 10.1111/jrh.12078. [DOI] [PubMed] [Google Scholar]
- 75.Pahwa P., Karunanayake C. P., Hagel L., et al. The Saskatchewan rural health study: an application of a population health framework to understand respiratory health outcomes. BMC Research Notes. 2012;5(1):p. 400. doi: 10.1186/1756-0500-5-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.The Federal, Provincial and Territorial Advisory Committee on Population Health. Strategies for Population Health: Investing in the Health of Canadians. Ottawa, ON, Canada: Health Canada; 1994. [Google Scholar]
- 77.Du Plessis V., Beshiri R., Bollman R. D., Clemenson H. Definitions of “Rural”. Vol. 1. Ottawa, Canada: Statistics Canada; 2002. [Google Scholar]
- 78.Pickett W., Day L., Hagel L., et al. The saskatchewan farm injury cohort: rationale and methodology. Public Health Reports. 2008;123(5):567–575. doi: 10.1177/003335490812300506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pahwa P., Karunanayake C., Hagel L., et al. Self-selection bias in an epidemiological study of respiratory health of a rural population. Journal of Agromedicine. 2012;17(3):316–325. doi: 10.1080/1059924x.2012.686381. [DOI] [PubMed] [Google Scholar]
- 80.Dillman D. A., Smyth J. D., Christian L. M. Internet, Phone, Mail, and Mixed-Mode Surveys : The Tailored Design Method. 4th. Hoboken, NJ, USA: John Wiley & Sons; 2014. [Google Scholar]
- 81.Hoddinott S. N., Bass M. J. The Dillman total design survey method: a sure-fire way to get high survey return rates. Canadian Family Physician. 1986;32:2366–2368. [PMC free article] [PubMed] [Google Scholar]
- 82.Pahwa P., Rana M., Pickett W., et al. Cohort profile: the saskatchewan rural health study—adult component. BMC Research Notes. 2017;10(1):p. 732. doi: 10.1186/s13104-017-3047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Statistics Canada. Canadian community health survey-annual component (CCHS) http://www23.statcan.gc.ca/imdb/p2SV.pl?Function=getSurvey&SDDS=3226#a2, 2016.
- 84.Gjevre J. A., Pahwa P., Karunanayake C., et al. Excessive daytime sleepiness among rural residents in Saskatchewan. Canadian Respiratory Journal. 2014;21(4):227–233. doi: 10.1155/2014/921541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Johns M., Hocking B. Daytime sleepiness and sleep habits of Australian workers. Sleep. 1997;20(10):844–849. doi: 10.1093/sleep/20.10.844. [DOI] [PubMed] [Google Scholar]
- 86.Lee S. J., Kang H. W., Lee L. H. The relationship between the Epworth Sleepiness Scale and polysomnographic parameters in obstructive sleep apnea patients. European Archives of Oto-Rhino-Laryngology. 2012;269(4):1143–1147. doi: 10.1007/s00405-011-1808-3. [DOI] [PubMed] [Google Scholar]
- 87.Rao J. N. K., Scott A. J. On simple adjustments to chi-square tests with sample survey data. Annals of Statistics. 1987;15(1):385–397. doi: 10.1214/aos/1176350273. [DOI] [Google Scholar]
- 88.Lipsitz S. R., Kim K., Zhao L. Analysis of repeated categorial data using generalized estimating equations. Statistics in Medicine. 1994;13(11):1149–1163. doi: 10.1002/sim.4780131106. [DOI] [PubMed] [Google Scholar]
- 89.Sterne J. A. C., White I. R., Carlin J. B., et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:p. b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saskatchewan Health Authority. Sleep Disorders Centre. https://www.saskatoonhealthregion.ca/locations_services/Services/cdm/Pages/Programs/Sleep-Disorders-Main.aspx, 2017.
- 91.Saskatchewan Health Authority. Sleep Disorder Centre. http://www.rqhealth.ca/department/respiratory/sleep-disorder-centre, 2018.
- 92.Banner D., Lear S., Kandola D., et al. The experiences of patients undertaking a “virtual” cardiac rehabilitation program. Studies in Health Technology and Informatics. 2015;209:9–14. [PubMed] [Google Scholar]
- 93.Raugi G. J., Nelson W., Miethke M., et al. Teledermatology implementation in a VHA secondary treatment facility improves access to face-to-face care. Telemedicine and e-Health. 2016;22(1):12–17. doi: 10.1089/tmj.2015.0036. [DOI] [PubMed] [Google Scholar]
- 94.Coma-Del-Corral M. J., Alonso-Álvarez M. L., Allende M., et al. Reliability of telemedicine in the diagnosis and treatment of sleep apnea syndrome. Telemedicine and e-Health. 2013;19(1):7–12. doi: 10.1089/tmj.2012.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bar A., Pillar G., Dvir I., Sheffy J., Schnall R. P., Lavie P. Evaluation of a portable device based on peripheral arterial tone for unattended home sleep studiesa. Chest. 2003;123(3):695–703. doi: 10.1378/chest.123.3.695. [DOI] [PubMed] [Google Scholar]
- 96.Pang K. P., Gourin C. G., Terris D. J. A comparison of polysomnography and the WatchPAT in the diagnosis of obstructive sleep apnea. Otolaryngology-Head and Neck Surgery. 2007;137(4):665–668. doi: 10.1016/j.otohns.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 97.Masa J. F., Duran-Cantolla J., Capote F., et al. Effectiveness of home single-channel nasal pressure for sleep apnea diagnosis. Sleep. 2014;37(12):1953–1961. doi: 10.5665/sleep.4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Reichert J. A., Bloch D. A., Cundiff E., Votteri B. A. Comparison of the NovaSom QSGe, a new sleep apnea home-diagnostic system, and polysomnography. Sleep Medicine. 2003;4:213–218. doi: 10.1016/s1389-9457(02)00234-4. [DOI] [PubMed] [Google Scholar]
- 99.Ficker J. H., Wiest G. H., Wilpert J., Fuchs F. S., Hahn E. G. Evaluation of a portable recording device (Somnocheck®) for use in patients with suspected obstructive sleep apnoea. Respiration. 2001;68(3):307–312. doi: 10.1159/000050515. [DOI] [PubMed] [Google Scholar]
- 100.Gjevre J. A., Taylor-Gjevre R. M., Skomro R., Reid J., Fenton M., Cotton D. Comparison of polysomnographic and portable home monitoring assessments of obstructive sleep apnea in Saskatchewan women. Canadian Respiratory Journal. 2011;18(5):271–274. doi: 10.1155/2011/408091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ng S. S. S., Chan T.-O., To K.-W., et al. Validation of Embletta portable diagnostic system for identifying patients with suspected obstructive sleep apnoea syndrome (OSAS) Respirology. 2010;15(2):336–342. doi: 10.1111/j.1440-1843.2009.01697.x. [DOI] [PubMed] [Google Scholar]
- 102.Dingli K., Coleman E. L., Vennelle M., et al. Evaluation of a portable device for diagnosing the sleep apnoea/hypopnoea syndrome. European Respiratory Journal. 2003;21(2):253–259. doi: 10.1183/09031936.03.00298103. [DOI] [PubMed] [Google Scholar]
- 103.Collop N. A., Anderson W. M., Boehlecke B., et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. Journal of Clinical Sleep Medicine. 2007;3(7):737–747. [PMC free article] [PubMed] [Google Scholar]
- 104.Gupta M., Bosma H., Angeli F., et al. Impact of a multi-strategy community intervention to reduce maternal and child health inequalities in india: a qualitative study in Haryana. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0170175.e0170175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pereira S. M., Blignault I., du Toit R., Ramke J. Improving access to eye health services in rural Timor-Leste. Rural and Remote Health. 2012;12:p. 2095. [PubMed] [Google Scholar]
- 106.Reeve C., Banfield S., Thomas A., Reeve D., Davis S. Community outreach midwifery-led model improves antenatal access in a disadvantaged population. Australian Journal of Rural Health. 2016;24(3):200–206. doi: 10.1111/ajr.12249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Characteristics of adults who likely required this specialist medical care (n = 2813; 2230 households) by distance quartile.
Data Availability Statement
Original microdata files for the Saskatchewan Rural Health Study used to support the findings of this study are available from Drs. Pahwa and Dosman at the Canadian Centre for Health and Safety in Agriculture, University of Saskatchewan, upon request.