Abstract
The present study evaluates the crosslinking of electrospun gelatin nanofibers by physical and chemical methods to further elucidate the importance of the application of gelatin scaffold platforms for cell-based assays. The dehydrothermally cross-linked electrospun gelatin scaffolds were unable to retained their structure morphology and integrity upon exposure to 1X PBS or cell-culture media. The DHT and EDC/Sulfo-NHS cross-linked gelatin scaffolds exhibited fiber diameter on average in the nanometer range. Subsequently, we utilized 1X PBS and cell culture media to evaluate the stability of the nanofibers in solution. The immersion evaluation indicated that the chemically cross-linked gelatin nanofibers maintained their random nanofiber distribution and morphology. However, a high degree of swelling was observed in the presence of cell culture media. Overall, the gelatin scaffold demonstrated good performance in PBS and cell culture media. Hence, EDC/Sulfo-NHS cross-linked electrospun gelatin nanofibrous scaffolds have good biocompatibility and are promising bio-scaffolds for cell-based assays.
I. INTRODUCTION
Electrospinning technique offers the ability to highly control fiber architecture, fiber size, alignment, porosity, mechanical properties, depth, and degradation rate [1]. These properties are highly important for the design and development of alternative biocompatible scaffold construct for cell and tissue engineering. Electrospun scaffold may be able to recapitulate the microenvironment of cells compared to tissue culture plastic. These porous scaffolds enable a platform for the transport of nutrient, removal of wastes, and proliferation and migration of cells. The development of such a platform is critical in the pharmaceutical industry as more drugs need further in vitro assessments prior to preclinical studies. Additionally, these tissue-specific scaffolds may enable better understanding of tissue and/ or cell response to drug therapy and provide an insight into how molecules permeate the biological barrier for drug design and delivery, thereby obtaining good clinical outcomes of the desired drug therapy.
Until now, many different synthetic biodegradable polymers such as PLA, PGA, PLGA, and PCL have been used in the electrospinning for tissue scaffold constructs [2]. On the other hand, natural polymers have gain significant attention in their application as scaffolds for tissue engineering. Gelatin is a biopolymer derived from partial hydrolysis of natural collagen, whereas collagen is the most abundant protein in the extracellular matrix (ECM) and the human body [3–5]. Gelatin electrospun nanofibers can provide safe and well-functioning construct for tissue engineering because of its biological origin, nonimmunogenicity, biocompatibility, ability to biodegrade and availability at relatively low cost. Gelatin has been widely used in the preparation of three-dimensional scaffolding in the pharmaceutical and medical field because of its enhanced biocompatibility in comparison to synthetic polymers [6].
The gelatin electrospun nanofiber scaffolds are water soluble, thereby for long-term biomedical application, it must be cross-linked. Depending on the desired properties, various crosslinking agents and crosslinking techniques are used. There are three methods of crosslinking: chemical, physical, and enzymatic crosslinking. Gelatin electrospun fibers can be cross-linked using physical method such as dehydrothermal treatment. Also, among the numerous chemical crosslinking agents, glutaraldehyde is predominantly used. Glutaraldehyde vapors is a simple, effective, and low-cost method to stabilize gelatin-based materials [7, 8]. However, the release of its unreacted residues into the host upon the degradation of the fiber have been shown to be toxic to cells and tissue [9].
Among the many crosslinking agents, genipin has been reported to provide scaffolds with higher biocompatibility and less cytotoxicity [5, 8, 10–12]. However, up to now genipin crosslinking procedures results in the discoloration of the electrospun scaffolds. By considering diverse types of fluorescence microscopy utilized in cell-based assays, the color changed in the electrospun nanofiber from white to dark blue makes this crosslinking technique not promising as a viable crosslinker for cell-based assay applications. In the present study, we investigated the use of dehydrothermal (DHT) crosslinking and 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysulfosuccinimide (Sulfo-NHS) to crosslink electrospun gelatin nanofiber constructs. Moreover, we also evaluated the morphology and characteristic of cross-linked electrospun gelatin nanofiber constructs via scanning electron microscopy before and after incubation in various media to determine the integrity of the as-electrospun gelatin nanofiber constructs. Our research indicated that EDC/Sulfo-NHS cross-linked electrospun gelatin scaffold construct retain excellent structural and morphological integrity and could be a versatile scaffold for tissue engineering.
II. MATERIALS AND METHODS
A. Gelatin scaffold preparation
Gelatin nanofibrous scaffolds were fabricated via electrospinning. Gelatin from Bovine Skin Type B Powder (Sigma-Aldrich) was prepared by dissolving gelatin in 70/30 vol.% acetic acid/double distilled water at a concentration of 30% (w/v). The solution was stirred at 50 °C for 1h. The electrospinning apparatus comprise a high voltage power supply (Gamma High Voltage Research, Ormond Beach, FL), a syringe pump (KD Scientific, KDS 101 Legacy Syringe Pump), a plastic syringe, a 22-gauge stainless steel blunt-ended needles (BSTEAN, inner diameter 0.21 mm) connected to the power supply electrode and a grounded collector. The collector consisted of a vertically positioned board covered with aluminum foil. The prepared electrospun solution was dispensed through the syringe which was placed vertical to the collecting plate. The prepared gelatin solution was electrospun into nanofiber mats using the following conditions: applied voltage 15 kV, needle to collector distance 15 cm, solution flow rate 5 μl/min, at room temperature and a relative humidity of less than 40%. Electrospun gelatin scaffolds were kept at room temperature overnight in order to allow residual solvents to evaporate. Afterward the electrospun mat was then fixed to plastic rings (CellCrown™, Scaffdex) before proceeding with the cross-linking treatments.
B. Crosslinking methods
In the physically DHT crosslinking method, the as-electrospun gelatin nanofiber was incubated in a conventional oven at 160 °C for 24 hours. The chemical crosslinking process utilizing EDC (Sigma-Aldrich) and NHS (Sigma-Aldrich was used at a ratio of 2:1) [13]. The solution was prepared in 90% ethanol/water to prevent the hydrolysis of gelatin proteins in the presence of water. The gelatin nanofiber scaffold construct was soaked in the EDC/NHS solution and kept on an orbital shaker for 7 to 24 hours at room temperature.
C. Disinfection of as-electrospun gelatin scaffold constructs
Various concentrations of ethanol (100, 90, 80, and 70%) were used to disinfect the scaffolds, wherein the samples were immersed in 12 well plates containing 2 mL of the desired ethanol concentration for 10 min. The scaffolds were sterilized at room temperature via UV irradiation for 30 min.
D. Physical characterization
EDC/NHS cross-linked electrospun gelatin scaffolds were washed with 1X PBS at room temperature for 30 mins under mild agitation. The scaffolds were then stored in 1X PBS or ell culture media (DMEM–Dulbecco’s Modified Eagle Medium, Thermo Fisher Scientific) for a period of time ranging from 5h to 5 days to monitor the structural integrity of the scaffolds. For physical characterization, the scaffolds were dried in a conventional at 37 °C overnight. Scanning electron microscopy (SEM; FEI Nova NanoSEM 450) was performed to examine the surface morphology of the electrospun gelatin scaffolds. The scaffolds were sputtered with a thin layer of gold to reduce the surface charging of the gelatin scaffolds. The SEM was operated at 20 kV to analyze the surface structure and fiber diameter of the electrospun gelatin before and after crosslinking. The results are given as the average diameter ± standard deviation.
E. Cell Culture
PC-12 cells (ATCC CRL-1721) were cultured in DMEM (Dulbecco’s Modified Eagle Medium, Thermo Fisher Scientific) supplemented with 10% calf serum in an incubator at 37 °C with 5% CO2. Nanofibrous scaffolds were fixed in plastic crowns and then disinfected with 70% ethanol followed by UV irradiation for 30 min. Subsequently, the sterilized nanofibrous scaffolds were rinsed three times with 1X PBS before seeding with PC-12 cells. A cell density of 2 × 106 cells ml−1 was seeded onto the surface of gelatin nanofibrous scaffold and was incubated at 37 °C for 20 min to allow the cells to adhere. Adherent PC-12 cells cultured for 2 days were visualized and analyzed by calcein-AM (Invitrogen) and ethidium homodimer-1 (EthD-1) (Invitrogen) to determine the cytocompatibility of the crosslinked gelatin nanofibrous scaffold in comparison to tissue culture polystyrene. Stained PC-12 cells were imaged by confocal microscopy (Leica SP5, Leica Microsystems, Germany). PC-12 cells adhesion on the crosslinked gelatin nanofibrous construct was fixed on with 4% formaldehyde and then permeabilized by immersion in 0.2% Triton for 5 min. Afterwards, the nuclei was stained with 4′,6-diamino-2′-phenylindole (DAPI) (Thermo Fisher Scientific).
III. RESULTS
A. Dehydrothermally cross-linked gelatin scaffolds
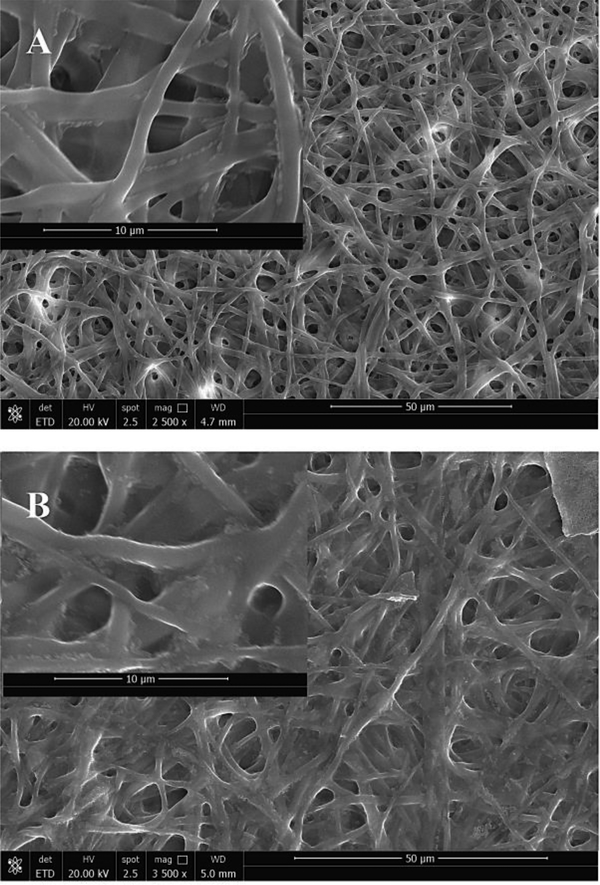
In this physical crosslinking method, non-covalent bonding is utilized to attract different gelatin molecules together in order to avoid the application of the traditional chemical cross-linkers. The optimized temperature has been observed to be in the range of 120 to 180 °C [14]. When the temperature is increased beyond 180 °C, there is an increased risk of disrupting the non-covalent bonds and/or disulfide bonds responsible for maintaining the three-dimensional shape and activity of the uncross-linked biopolymer nanofibers. As expected, the surface morphology and fiber diameter were observed to remain relatively unchanged when compared to uncross-linked electrospun gelatin scaffolds. The SEM image of the uncross-linked and DHT cross-linked gelatin scaffolds are depicted in Figure 1. The gelatin nanofibers presented randomly oriented morphology and smooth surfaces. The distributions of the fiber diameter for uncross-linked gelatin scaffolds are observed to be on average 675±30 nm, and the DHT cross-linked gelatin scaffolds are observed to be on average 524±40 nm. The slight reduction in fiber diameter size may be attributed to the loss of moisture and acetic acid residues when incubated at 160 °C and may indicate that the DHT treated gelatin scaffolds exhibited larger surface area. Upon immersing the DHT treated gelatin scaffolds in 1X PBS or media, they completely and immediately dissolved, thereby confirming the weak non-covalent bounding between gelatin molecules and low degree of crosslinking.
Figure 1.
(A) Uncross-linked, (B) DHT Crosslinked gelatin scaffold.
B. EDC/NHS cross-linked gelatin scaffolds
It has been observed that EDC/NHS crosslink lysine and asparagine amino acids of gelatin and acts as a zero length crosslinker. Figure 2A depicts the surface morphology and distributions of EDC/NHS cross-linked gelatin nanofibers. The electrospun gelatin scaffolds were immersed in 1X PBS for 5h at 22 °C and then dried at 37 °C overnight. The cross-linked gelatin scaffolds did not dissolve upon exposure to 1X PBS and maintained their surface morphology in PBS. The porous structure of the cross-linked gelatin scaffolds is maintained upon exposure to PBS. The gelatin nanofibers experienced swelling when immersed in 1X PBS. The fiber diameter and pour sizes were observed to be 827±100 nm and 4.651 μm, respectively.
Figure 2.
Cross-linked gelatin scaffold stored in (A) 1X PBS and (B) cell culture media for 5h.
In order to assess the application of the cross-linked gelatin scaffold construct for cell-based assay, scaffolds were immersed in cell culture media for 5h at 22 °C and then dried at 37 °C overnight. The cross-linked gelatin scaffolds also retained their surface morphology and porosity as shown in Figure 2B. The fiber diameter and pore sizes were observed to be on average 1.630±0.120 μm and 5.589 μm. Significant amount of swelling is observed in the presence of cell culture media. This increased swelling rate is attributed to the penetration of water molecule into the gelatin nanofibers. However, in the presence of 1X PBS, the rate of swelling is observed to be much lower. This lower rate of swelling in PBS is attributed to the presence of ions in PBS, which shielded the nanofibers and prevented the penetration of water molecules into the nanofibers. Physicochemical parameters, such as the crosslinking density, nanofiber composition, network structure, and ambient situation are factors that can affect swelling rate. Additionally, the surface morphology of the cross-linked gelatin scaffolds in 1X PBS and cell culture media resulted in a slightly roughened surface, wherein it can be observed that salt particulates, along will other particulates found in cell culture media deposited on the surface of the nanofibers.
C. Ethanol Disinfection
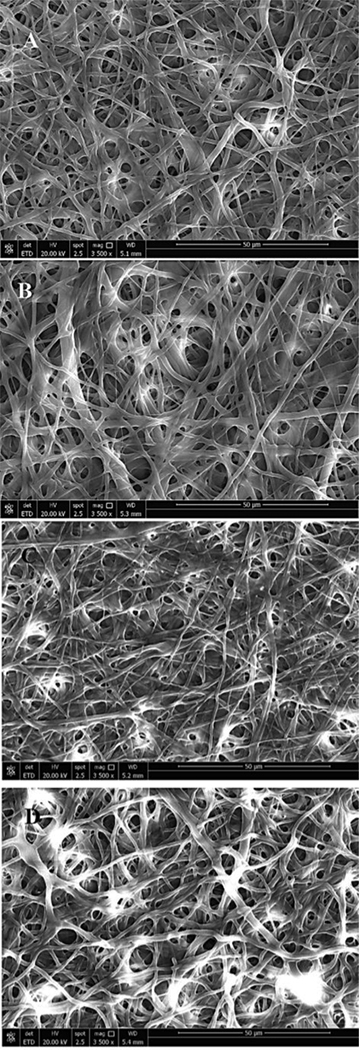
EDC/NHS cross-linked gelatin scaffolds must be sterilized before performing any kind of cell-based assays, which is the intended application of the fabrication of these 3D scaffolds. For this purpose, different concentrations of ethanol were explored in the disinfection of the cross-linked gelatin scaffolds followed by UV sterilization. The scaffolds were soaked in ethanol for 10 min and then dried at 37 °C overnight. The SEM images of these cross-linked gelatin scaffolds upon treatment in various concentrations of ethanol (100, 90, 80, and 70%) are depicted in Figure 3. It is clear that the cross-linked gelatin scaffold can be disinfected in 100% ethanol without influencing the surface morphology, fiber diameter distributions and pore sizes.
Figure 3.
Gelatin biopaper disinfection using (A) 100, (B) 90, (C) 80, and (D) 70% of Ethanol for 10 min.
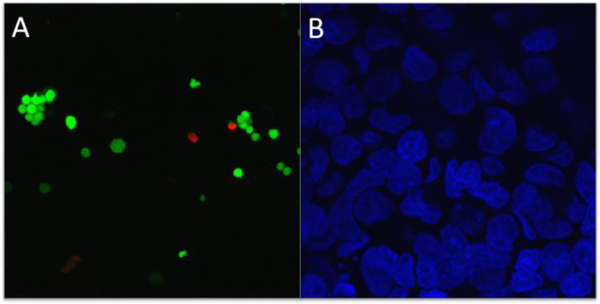
The biocompatibility of the crosslinked gelatin scaffold was evaluated using PC-12 cells double-stained for fluorescence using calcien-AM (green) and EthD-1 (red), respectively. Figure 4A shows the live/dead assays of cell viability after 7 days.
Figure 4.
PC-12 cell viability: representative confocal images of (A) live/dead assay and (B) DAPI staining of the nuclei.
The prepared nanofibers exhibited very good cell viability of 88% compared to the control sample viability of 95%. Figure 4B shows the PC-12 cell attachment and growth on crosslinked electrospun gelatin scaffold after cell fixation with DAPI (blue) to highlight the nuclei. It can be seen that PC-12 cells adhered and spread on the surface of the crosslinked gelatin nanofiber. The crosslinked gelatin nanofibrous scaffold has potential application in the development of 3D scaffolds for tissue engineering.
*
This work was supported by Maryland Industrial Partnership (Award# 6002).
REFERENCES
- [1].Bhardwaj N and Kundu SC, 2010. Electrospinning: a fascinating fiber fabrication technique. Biotechnology Advances, 28(3), pp.325–347. [DOI] [PubMed] [Google Scholar]
- [2].Huang ZM, Zhang YZ, Ramakrishna S and Lim CT, 2004. Electrospinning and mechanical characterization of gelatin nanofibers. Polymer, 45(15), pp.5361–5368. [Google Scholar]
- [3].Sung HW, Liang IL, Chen CN, Huang RN and Liang HF, 2001. Stability of a biological tissue fixed with a naturally occurring crosslinking agent (genipin). Journal of Biomedical Materials Research Part A, 55(4), pp.538–546. [DOI] [PubMed] [Google Scholar]
- [4].Kim MS, Jun I, Shin YM, Jang W, Kim SI and Shin H, 2010. The Development of Genipin-Crosslinked Poly (caprolactone)(PCL)/Gelatin Nanofibers for Tissue Engineering Applications. Macromolecular Bioscience, 10(1), pp.91–100. [DOI] [PubMed] [Google Scholar]
- [5].Ko JH, Yin H, An J, Chung DJ, Kim JH, Lee SB and Pyun DG, 2010. Characterization of cross-linked gelatin nanofibers through electrospinning. Macromolecular Research, 18(2), pp.137–143. [Google Scholar]
- [6].Ofner CM III and Bubnis WA, 1996. Chemical and swelling evaluations of amino group crosslinking in gelatin and modified gelatin matrices. Pharmaceutical Research, 13(12), pp.1821–1827. [DOI] [PubMed] [Google Scholar]
- [7].Zhang YZ, Venugopal J, Huang ZM, Lim CT and Ramakrishna S, 2006. Crosslinking of the electrospun gelatin nanofibers. Polymer, 47(8), pp.2911–2917. [Google Scholar]
- [8].Sisson K, Zhang C, Farach-Carson MC, Chase DB and Rabolt JF, 2009. Evaluation of cross-linking methods for electrospun gelatin on cell growth and viability. Biomacromolecules, 10(7), pp.1675–1680. [DOI] [PubMed] [Google Scholar]
- [9].Damink LO, Dijkstra PJ, Van Luyn MJA, Van Wachem PB, Nieuwenhuis P and Feijen J, 1995. Glutaraldehyde as a crosslinking agent for collagen-based biomaterials. Journal of Materials Science: Materials in Medicine, 6(8), pp.460–472. [Google Scholar]
- [10].Sung HW, Huang DM, Chang WH, Huang LL, Tsai CC and Liang IL, 1999. Gelatin-derived bioadhesives for closing skin wounds: an in vivo study. Journal of Biomaterials Science, Polymer Edition, 10(7), pp.751–771. [DOI] [PubMed] [Google Scholar]
- [11].Sung HW, Huang DM, Chang WH, Huang RN and Hsu JC, 1999. Evaluation of gelatin hydrogel crosslinked with various crosslinking agents as bioadhesives: in vitro study. Journal of Biomedical Materials Research Part A, 46(4), pp.520–530. [DOI] [PubMed] [Google Scholar]
- [12].Kim MS, Jun I, Shin YM, Jang W, Kim SI and Shin H, 2010. The Development of Genipin-Crosslinked Poly (caprolactone)(PCL)/Gelatin Nanofibers for Tissue Engineering Applications. Macromolecular Bioscience, 10(1), pp.91–100. [DOI] [PubMed] [Google Scholar]
- [13].Lai JY, 2013. Corneal stromal cell growth on gelatin/chondroitin sulfate scaffolds modified at different NHS/EDC molar ratios. International Journal of Molecular Sciences, 14(1), pp.2036–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Madaghiele M, Calò E, Salvatore L, Bonfrate V, Pedone D, Frigione M, and Sannino A, 2014. “Effect of dehydrothermal treatment on collagen crosslinking and denaturation.” Biomaterials for Medicine: Atti del Convegno Nazionale della Società Italiana Biomateriali. pp. 37–40. [Google Scholar]