Abstract
Antibiograms are important clinical tools to report and track antibiotic susceptibility and help guide empiric antimicrobial therapy. Antibiograms support compliance with antimicrobial stewardship (AMS) requirements from the Centers for Medicare and Medicaid Services and are in line with recommendations from the Centers for Disease Control and Prevention Core Elements of AMS for nursing homes/longterm care facilities (LTCFs). Unlike most acute-care settings, LTCFs are challenged in creating antibiograms because of the low number of bacterial isolates collected annually. Determining the best methodology for creating clinically useful antibiograms for LTCFs needs to be explored. Possible approaches include (1) extending the isolate data beyond 1 year, (2) combining isolate data from the same geographic region, (3) using a nearby acute-care facility’s antibiogram as a proxy, or (4) collapsing isolate data. This article discusses the benefits and limitations of each approach.
Keywords: Antibiograms, antimicrobial stewardship, long-term care facilities
Antimicrobial stewardship (AMS) initiatives consist of multidisciplinary approaches to coordinate appropriate antimicrobial use in an effort to decrease selective pressures that drive the emergence of multidrug resistant organisms (MDROs).1,2 AMS initiatives have been effectively implemented across many acute-care settings but are less well established in long-term care facilities (LTCFs).3,4 The Centers for Medicare and Medicaid Services “proposed that the facility’s infection prevention and control program must also include an antibiotic stewardship program that includes antibiotic use protocols and systems for monitoring antibiotic use and recording incidents” under Reform of Requirements for Long-Term Care facilities.5 The Centers for Disease Control and Prevention Core Elements for AMS encourages nursing homes to start implementing at least 1 AMS activity and then gradually incorporate additional strategies.6 A facility-specific antibiogram supports AMS activity for tracking and reporting antibiotic resistance and represents a tangible contribution to meet the recommendations of the Centers for Medicare and Medicaid Services and the Centers for Disease Control and Prevention.
An antibiogram summarizes a healthcare facility’s bacteria susceptibilities to antibiotics, typically over a 1-year time period.7 By displaying which bacteria have the highest rates of susceptibility to specific antibiotics in a given facility, antibiograms may help guide the establishment of antibiotic-use protocols.1,6–8 Prudent use of such protocols supports AMS efforts to reduce the prevalence of MDROs and the risks of adverse drug events in the long-term care population.9–14 Unfortunately, LTCFs may run into challenges when creating antibiograms because of the relative low number of residents in some facilities and the paucity of bacterial isolates collected for diagnostic purposes.15 To address this, we reviewed the literature to evaluate proposed methods for developing an antibiogram with low isolate counts and to address some of the common pitfalls pertaining to the long-term care environment. Articles were identified by PubMed searches with the following keywords in various combinations: acute-care antibiograms, antibiograms, development of antibiograms, long-term care facility antibiograms, nursing home antibiograms, regional antibiograms, and stratified antibiograms. Manual searches of reference lists found from initial searches were also conducted. Studies were included based on the authors’ judgment of relevance to the topic.
Guidelines for Creating an Antibiogram
The Clinical and Laboratory of Standards Institute (CLSI) publishes the M39 Analysis and Presentation of Cumulative Antimicrobial Susceptibility Test Data; Approved Guideline, which is a commonly referenced guideline on how to create antibiograms1,2,7 It provides comprehensive recommendations geared toward microbiologists, physicians, pharmacists, epidemiologists, and other healthcare personnel on how to collect, analyze, and present cumulative antimicrobial susceptibility data. When completed, the antibiogram is often displayed as a table with columns and rows dedicated to listing all individual bacterial species with all individual antibiotics to match each “drug-bug” pair’s cumulative susceptibility as a percent.
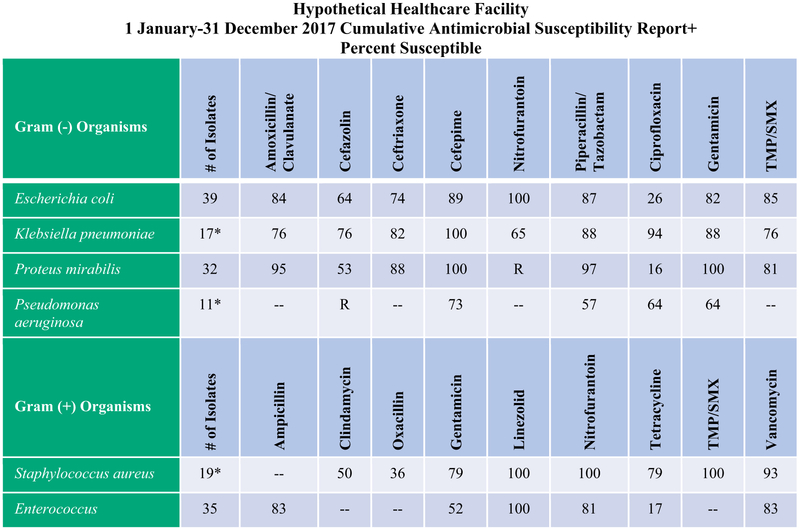
Figure 1 shows an example of an antibiogram prepared for a healthcare facility and guidance on how to use it. In that example, 29 out of 39 clinical Escherichia coli isolates collected from patients at that facility were resistant to ciprofloxacin. Accordingly, the percent susceptible listed in the antibiogram is 26% [(10/39) × 100 = 26%]. The same procedure was performed for all other antibiotics used to treat E coli infections as well as for antibiotics used to treat infections caused by other bacteria isolated.
Fig. 1.
Example of hypothetical facility antibiogram with instructions for use.7 Hypothetical healthcare facility 1 January-31 December 2017 cumulative antimicrobial susceptibility report+ percent susceptible; +The percent susceptible for each organism/antimicrobial combination was generated by including the first isolate of that organism encountered on a given patient; *Indicates <30 isolates tested and potentially low accuracy of susceptibility rates; –Indicates the antimicrobial agent is not tested, or is known to be clinically ineffective. R, intrinsic resistance; TMP/SMX Sulfamethoxazole/Trimethoprim. Instructions for Use: (1) Locate the rows that list pathogens that are most likely to cause the infection: (ie, Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis for a urinary tract infection); (2) Locate columns of antibiotics within the same pathogen rows that have the highest percent susceptible (closest to 100); (3) Identify which antibiotics have the highest percent susceptibility rates, >80%–85% preferred,15,16 and consider these as potential empiric therapeutic options; (4) To support antimicrobial stewardship, choose narrow spectrum agents when possible (eg, TMP/SMX rather than piperacillin/tazobactam).
Some of the recommendations from the CLSI M39 document on how to collect the data for these reports include the following: report at least annually, include only verified final results, include only species with data for ≥30 isolates, include only diagnostic (not surveillance) isolates, and only include the first isolate of a species obtained from a patient for each analysis period.7 Only including diagnostic isolates and the first isolate of a species obtained from a patient are recommended to prevent overestimation of bacterial resistances. The cut-off of 30 isolates is recommended to improve the accuracy of the calculated susceptibility rates. As the number of isolates decreases, the 95% confidence intervals (CIs) become wider. For example, if considering a 90% susceptibly rate for n = 30 the 95% CI is 74%–97% compared with n = 20 and n = 10, for which the 95% CIs are 69%–98% and 57%–100%, respectively. A descriptive study found that out of 32 community hospitals, only 8 followed this 30-isolate recommendation whereas the rest included footnotes of “impaired statistical validity.”15 LTCFs, often smaller than community hospitals and with a lower rate of admissions, are even less likely to satisfy this recommendation. Thus, the question becomes how do we create antibiograms for LTCFs that will best estimate bacteria susceptibility rates to empiric guide antibiotic selection and support AMS practices?
Significance of the Problem
When clinicians start antibiotics without having culture results complete with susceptibilities to inform their antibiotic choices, antibiograms can help guide selection of an antibiotic likely to be effective against the offending bacteria. Although clinical practice guidelines provide recommendations on antibiotic regimens for the initial treatment of infections, antibiograms tailor this information for the specific facility.1,2,8 Unfortunately, in LTCFs even the most frequently cultured bacteria may still be less than 30 isolates. Without an antibiogram’s information, antibiotics that have high resistance rates in that facility may be inadvertently chosen. Alternatively, broad-spectrum antibiotics might be used unnecessarily because of concerns of inadequately treating potential pathogens. In both scenarios, there is the potential for use of antibiotics with inappropriate antibacterial coverage, which can select for MDROs and increase the prevalence of these infections in LTCFs.16,17
Discussion
Potential Approaches to Creating LTCF Antibiograms
The following can be used to create antibiograms for the LTCF environment. Table 1 summarizes the advantages and limitations of each approach.
Table 1.
Advantages and Limitations of the Potential Approaches to Creating a LTCF Antibiogram
| Approach | ||
|---|---|---|
| Extending the antibiogram data beyond 1 year | Advantages | • Simple and easy to create |
| • Accurate susceptibilities over the given time period | ||
| Limitation | • Resistance rates and patterns of bacteria may change from year to year | |
| Creating a regional antibiogram | Advantage | • May be helpful if residents access healthcare facilities throughout that given region |
| Limitations | • Requires coordination between multiple microbiology laboratories and healthcare facilities | |
| • Bacteria that infect residents may not have similar antimicrobial susceptibilities to those of that region’s general population | ||
| Using antibiograms of nearby hospitals | Advantage | • Antibiograms that are already annually made by the hospitals could be used |
| Limitations | • All residents go to different hospitals | |
| • Bacteria that infect LTCF residents may not have similar antimicrobial susceptibilities to those of the general hospital population | ||
| Collapsed antibiograms | Advantage | • May help guide infection-specific empiric antibiotic choices |
| Limitation | • Intrinsic resistance of some bacteria to specific antibiotics would not be listed |
Approach 1: Extending the Antibiogram Data Beyond 1 Year
Perhaps the easiest approach to achieve the threshold of ≥30 isolates of each species is to extend collection beyond the conventional 1-year period. The CLSI M39 document promotes this approach as a solution to overcoming the problem with low number of isolates.7 The advantage is that it may report more accurate susceptibilities over that extended time period, but the disadvantage is that the resistance rates and patterns of bacteria can change over years.18–22 However, the emergence of changing bacterial resistance may be slow enough that going back a few years may not significantly change the antibiograms. This balance between permitting enough time to collect a sufficient number of isolates and changes in resistance patterns is not yet well understood or characterized. In our opinion, extending the collection period remains an option for LTCFs, especially if the resident population is stable.
Approach 2: Creating a Regional Antibiogram
Another approach promoted by the CLSI M39 document is the creation of antibiograms that combine data from several facilities in the same geographic area.7 The supporting theory is that bacteria are spread within geographic regions, and, therefore, these areas may have similar bacterial susceptibility rates.23,24 When considering LTCFs, residents often transfer to or from nearby hospitals. Transmission of bacteria, including MDROs, between acute-care hospitals and nursing homes has high bidirectional flow.25–27 Nursing home residents acquire MDRO infections in their nursing homes –60% of the time, and in hospitals ~40% of the time. Typically, there are only a handful of “parent” hospitals to which residents are admitted. When this has been studied with inpatients compared with outpatients, the results were variable and some susceptibility rates were found to be similar while others were not.28 A major barrier to this approach, however, is the relative lack of the coordination between the acute-care facilities and LTCFs, and that their laboratory methodologies may differ.29 The microbiology laboratories of LTCFs are often not on site, and some acute-care facilities may also contract to have some or all of their specimens sent to other laboratories. In addition, this approach would rely upon strong relationships and communication among key stakeholders from the facilities’ laboratories, infectious diseases teams, and/or AMS teams. Finally, this approach relies on the assumption that the bacterial susceptibilities and emerging resistances are similar within a region.23,24 Depending on multiple factors, this may not be necessarily the case.
Approach 3: Using Antibiograms of Nearby Hospitals
A third approach would be to use the nearby or “parent” hospitals’ antibiograms as representative of the LTCFs’ bacterial susceptibilities. Considering the low number of isolates that the LTCF would contribute, the parent hospital’s antibiogram may suffice. This approach has the advantage of convenience as hospitals create these annually already, and LTCFs could use them within their own facility.29–31 However, as with the regional antibiogram, this assumes that the bacterial susceptibilities and emerging resistances are similar between the facilities, which may not always be the case.28,32,33
Approach 4: Collapsed Antibiograms
A different approach to the low isolate issue is to create a collapsed antibiograms by grouping similar organisms. For example, an antibiogram in favor of collecting data by specimen site (ie, urine, skin, sputum, etc) might prove useful as decisions on empiric antibiotics are often made in this fashion. In this approach, the number of total isolates could be used instead of each bacterial species. The interpreter would be able to select antibiotics with activity against several similar species of bacteria most likely to cause specific infections. Because antibiograms are tools used for empiric decision making of antibiotic selection, this approach may be best from a functional standpoint. For example, if a collapsed urinary antibiogram was created, the user would be able to identify which antibiotics were the most likely to provide effective empiric therapy against urinary pathogens collected from a single LTCF in the previous year. LTCF studies that have shown higher prevalence of antibiotic resistant bacteria specifically isolated certain infection sites, such as the urinary tract and skin, comparatively to other infection sites.16,17 A limitation is that clinicians using the antibiograms must still be aware that bacteria have varying intrinsic resistances to certain antibiotics.7,34,35 For example, Proteus mirabilis is intrinsically resistant to nitrofurantoin, which adequately covers many other gram-negative bacteria, and an R is placed the Proteus mirabilis-nitrofurantoin cell of antibiograms. Because a collapsed antibiogram would not contain listings of individual bacterial species, dissemination with appropriate explanations for interpretations would be imperative.
Implications for Practice, Policy, and/or Research
Currently, there is not consensus on identifying the optimal approach for creating a LTCF-specific antibiogram when there are a limited number of clinical isolates. With fewer clinical specimens of bacteria isolated from infections in LTCFs compared with acute-care settings, it becomes difficult to create an antibiogram in compliance with the CLSI M39 guideline recommendations. Each of the approaches discussed has its advantages and limitations. Extending the antibiogram data beyond 1 year, creating a regional antibiogram, and using nearby hospital antibiograms all increase the likelihood of becoming more compliant with the CLSI M39 guideline recommendation of having at least 30 isolates per bacterial species reported, but it is unclear if the bacterial susceptibility rates will represent the rates of the LTCFs. In addition, coordination between LTCFs and nearby acute-care facilities may be difficult depending on the health systems. A collapsed antibiogram would allow for infection site-specific data to be presented, but intrinsic resistances of specific bacterial species would need to be noted. Further research is necessary to provide further insight, as the best approach to create antibiograms in LTCFs is currently unknown and may very well vary by facility.
Acknowledgments
This work was supported in part by the Veterans Affairs (VA) Health Services and Research Merit Award #15-120. The findings and conclusions in this document are those of the authors, who are responsible for its content, and do not necessarily represent the views of the VA or of the United States Government.
References
- 1.Fishman N. Policy statement on antimicrobial stewardship by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the Pediatric Diseases Society (PIDS). Infect Control Hosp Epidemiol 2012;33:322–327. [DOI] [PubMed] [Google Scholar]
- 2.Jump RLP, Gaur S, Katz MJ, et al. Template for an antibiotic stewardship policy for post-acute and long-term care settings. J Am Med Dir Assoc 2017;18:913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malani AN, Brennan BM, Collins CD, et al. Antimicrobial stewardship practices in Michigan long-term care facilities. Infect Control Hosp Epidemiol 2016;37:236–237. [DOI] [PubMed] [Google Scholar]
- 4.Morrill HJ, Mermel LA, Baier RR, et al. Antimicrobial stewardship in Rhode Island long-term care facilities: Current standings and future opportunities. Infect Control Hosp Epidemiol 2016;37:979–982. [DOI] [PubMed] [Google Scholar]
- 5.Department of Health and Human Services. Centers for Medicare and Medicaid Services. 42 CFR Part 483. Medicare and Medicaid Programs; Reform of Requirements for Long-Term Care Facilities [CMS-3260-F]. RIN 0938–AR61; 2016. [Google Scholar]
- 6.The core elements of antibiotic stewardship for nursing homes. Centers for Disease Control and Prevention website. 2017. Available at: https://www.cdc.gov/longtermcare/prevention/antibiotic-stewardship.html. Accessed November 20, 2017.
- 7.Clinical and Laboratory Standards Institute (CLSI). Analysis and Presentation of Cumulative Antimicrobial Susceptibility Test Data. Approved guideline M39–A4. 4th ed. Wayne, PA: CLSI; 2014. [Google Scholar]
- 8.Help Prescribing Clinicians Choose the Right Antibiotic. Content last reviewed October 2016. Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.ahrq.gov/nhguide/toolkits/help-clinicians-choose-the-right-antibiotic/index.html. Accessed March 15, 2018.
- 9.Lautenbach E, Fishman NO, Bilker WB, et al. Epidemiological investigation of fluoroquinolone resistance in infections due to extended-spectrum P. Lactamase—Producing Escherichia coli and Klebsiella pneumonia. Clin Infect Dis 2001;33:1288–1294. [DOI] [PubMed] [Google Scholar]
- 10.Raz R. The clinical impact of multiresistant gram-positive microorganisms in long-term care facilities. J Am Med Dir Assoc 2003;4:S100–S104. [DOI] [PubMed] [Google Scholar]
- 11.Yoshikawa TT. Antimicrobial resistance and aging: Beginning of the end of the antibiotic era? J Am Geriatr Soc 2002;50:S226–S229. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell SL, Shaffer ML, Loeb MB, et al. Infection management and multidrug-resistant organisms in nursing home residents with advanced dementia. JAMA Intern Med 2014;174:1660–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alshammari TM, Larrat EP, Morrill HJ, et al. Risk of hepatotoxicity associated with fluoroquinolones: A national case-control safety study. AJHP 2014;71:37–43. [DOI] [PubMed] [Google Scholar]
- 14.Hensgens MP, Goorhuis A, Dekkers OM, Kuijper EJ. Time interval of increased risk for Clostridium difficile infection after exposure to antibiotics. J Antimicrob Chemother 2012;67:742–748. [DOI] [PubMed] [Google Scholar]
- 15.Moehring RW, Hazen KC, Hawkins MR, et al. Challenges in preparation of cumulative antibiogram reports for community hospitals. J Clin Microbiol 2015;53:2977–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swami SK, Banerjee R. Comparison of hospital-wide and age and location—Stratified antibiograms of S. aureus, E. coli, and S. pneumoniae: Age- and location-stratified antibiograms. Springerplus 2013;2:63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie C, Taylor M, Howden BP, et al. Comparison of bacterial isolates and antibiotic resistance patterns of elderly nursing home and general community patients. Intern Med J 2012;42:e157–e164. [DOI] [PubMed] [Google Scholar]
- 18.Kuster SP, Ruef C, Zbinden R, et al. Stratification of cumulative antibiograms in hospitals for hospital unit, specimen type, isolate sequence and duration of hospital stay. J Antimicrob Chemother 2008;62:1451–1461. [DOI] [PubMed] [Google Scholar]
- 19.Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med 2005;352:1436–1444. [DOI] [PubMed] [Google Scholar]
- 20.File TM Jr. Clinical implications and treatment of multiresistant Streptococcus pneumoniae pneumonia. Clin Microbiol Infect 2006;12:31–41. [DOI] [PubMed] [Google Scholar]
- 21.Giske CG, Monnet DL, Cars O, Carmeli Y. Clinical and economic impact of common multidrug-resistant gram-negative bacilli. Antimicrob Agents Chemother 2008;52:813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rice LB. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J Infect Dis 2008;197:1079–1081. [DOI] [PubMed] [Google Scholar]
- 23.Farrell DJ, Jenkins SG. Distribution across the USA of macrolide resistance and macrolide resistance mechanisms among Streptococcus pneumoniae isolates collected from patients with respiratory tract infections: PROTEKT US 2001–2002. J Antimicrob Chemother 2004;54:17–22. [DOI] [PubMed] [Google Scholar]
- 24.Polik RE, Johnson CK, McClish D, et al. Predicting hospital rates of fluoroquinolone-resistant Pseudomonas aeruginosa from fluoroquinolone use in US hospitals and their surrounding communities. Clin Infect Dis 2004;39:497–503. [DOI] [PubMed] [Google Scholar]
- 25.Kahvecioglu D, Ramiah K, McMaughan D, et al. Multidrug-resistant organism infections in US nursing homes: A national study of prevalence, onset, and transmission across care settings, October 1, 2010-December 31, 2011. Infect Control Hosp Epidemiol 2014;35:S48–S55. [DOI] [PubMed] [Google Scholar]
- 26.Lee BY, Bartsch SM, Wong KF, et al. The importance of nursing homes in the spread of methicillin-resistant Staphylococcus aureus (MRSA) among hospitals. Med Care 2013;51:205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strausburgh LJ, Sukumar SR, Joseph CL. Infectious disease outbreaks in nursing homes: An unappreciated hazard for frail elderly persons. Clin Infect Dis 2003;36:870–876. [DOI] [PubMed] [Google Scholar]
- 28.McGregor JC, Bearden DT, Townes JM, et al. Comparison of antibiograms developed for inpatients and primary care outpatients. Diagn Microbiol Infect Dis 2013;76:73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Var SK, Hadi R, Khardori N. Evaluation of regional antibiograms to monitor antimicrobial resistance in Hampton Roads, Virginia. Ann Clin Microbiol Antimicrob 2015;14:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamoth F, Wenger A, Prod’hom G, et al. Comparison of hospital-wide and unit-specific cumulative antibiograms in hospital- and community-acquired infection. Infection 2010;38:249–253. [DOI] [PubMed] [Google Scholar]
- 31.Anderson DJ, Miller B, Marfatia R, Drew R. Ability of an antibiogram to predict Pseudomonas aeruginosa susceptibility to targeted antimicrobials based on hospital day of isolation. Infect Control Hosp Epidemiol 2012;33:589–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friedmann R, Hamburger R, Shulman C, et al. Antimicrobial susceptibilities of urinary pathogens in a multidisciplinary long-term care facility. Diagn Microbiol Infect Dis 2003;46:217–222. [DOI] [PubMed] [Google Scholar]
- 33.Kinschuch W, Russo D, Kariolis I, et al. Comparison of a hospital-wide antibiogram with that of an associated long-term care facility. J Am Geriatr Soc 2012;60:798–800. [DOI] [PubMed] [Google Scholar]
- 34.Gilbert DN, Chambers HF, Eliopoulos GM, Saag MS. The Sanford Guide to Antimicrobial Therapy; Antibacterial Activity Spectra. Sperryville, VA: Antimicrobial Therapy; 2016. [Google Scholar]
- 35.Clinical and Laboratory Standards Institute. 2011. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-first Informational Supplement. M100–S21. Wayne, PA: Clinical and Laboratory Standards Institute; 2011. [Google Scholar]