Abstract
Type 2 diabetes (T2D) is a major metabolic disease and a key epigenetic risk factor for the development of additional clinical complications. Among them, periodontitis (PD), a severe inflammatory disease ascribable to a dysregulated physiology and composition of the oral microbiota, represents one of the most relevant complications. Periodontitis can impact the structure of the tooth and likely the stem and progenitor cell pool, which actively contributes to the periodontal microenvironment and homeostasis. Modifications of the oral plaque play a key role in the etiopathogenesis of PD caused by T2D. However, to what extent the biology of the progenitor pool is affected has still to be elucidated. In this short report, we aimed to explore the biological effects of oral plaque derived from T2D patients with PD in comparison to non-diabetic patients with PD. Oral plaque samples were isolated from T2D and non-diabetic subjects with PD. Dental pulp stem cells (DPSCs), derived from the premolar tooth, were conditioned for 21 days with oral plaque samples and tested for their clonogenic ability. Cultures were also induced to differentiate towards the osteogenic lineage, and ALP and osteocalcin gene expression levels were evaluated by real-time qPCR. Results have shown that the number of clones generated by DPSCs exposed to T2D oral plaque was significantly lower compared to controls (ctl). The multivariate analysis confirmed that the decreased clonogenesis was significantly correlated only with T2D diagnosis. Moreover, the effect of T2D oral plaque was specific to DPSCs. Indicators of osteogenic differentiation were not significantly affected. This study provides a new biological insight into the effects ascribable to T2D in PD.
1. Introduction
Type 2 diabetes (T2D) is a very common metabolic disease caused by resistance to insulin and consequent systemic hyperglycaemia. The pathological effects induced by T2D are chronic and exhibit multifaceted features, as they imply profound alterations in both the endocrine asset and metabolic/physiological functions of several biological systems [1–3], including the oral microenvironment [4, 5]. In this regard, periodontitis (PD), a severe inflammatory disorder of the periodontium caused by oral bacterial challenge, is a complication of T2D [6]. In fact, patients affected by T2D are more susceptible to PD. The periodontium is a highly vascularised system, and oral plaque possesses intrinsic biological properties such as the pivotal chemotactic ability for immune cells [7]. Type 2 diabetes severely impairs the architecture of periodontal tissue and interferes with basic cellular functions such as phagocytosis, migration, and more importantly the innate immune response provided by the epithelial mucosa of the periodontium. Importantly, the unbalanced inflammatory and dysmetabolic state in T2D significantly affects the composition of the bacterial film in the oral plaque, therefore impairing periodontal integrity and function [8]. This scenario is further exacerbated by modifications both in macro and microcirculation of the periodontium, ascribable to T2D due to the increase in glycation end-products (AGEs), oxidative stress and deregulation of the endothelial barrier [8]. Intriguingly, PD and diabetes mutually influence each other in a bidirectional fashion: diabetes increases proinflammatory levels of soluble mediators, therefore reinforcing periodontal inflammation; in turn, PD influences the glycaemic index in diabetic patients [9]. Moreover, epidemiological studies have demonstrated that PD is strictly associated with increased cardiovascular risk as a direct consequence of endothelial dysfunction following changes of oral plaque [10]. Notably, T2D-induced oral plaque modifications are not limited to the sole periodontium, but they could likely affect defined cell populations of dental origin and mainly progenitors of mesenchymal origin, which reside in the tooth canal opening in the periodontium. Dental pulp stem cells (DPSCs) have been defined as adult progenitor cells of ectodermal origin, exhibiting mesenchymal features [11–13], including clonogenic and self-renewal capacity, mesodermal-like differentiation, and mainly osteogenic, mineralization, and regeneration capabilities [14, 15]. Interestingly, transplanted DPSCs in streptozotocin-induced diabetic mouse models have confirmed the ability of DPSCs both to enhance glucose tolerance in a paracrine manner [16, 17] and to restore vascular function [18, 19]. Dental pulp stem cells have been also reported as effective candidates to generate insulin-producing cells [20–22], parallel to a peculiar and attractive feature of anti-inflammatory and nociceptive soluble mediator-based secretion [21]. Importantly, the progression of PD in the presence of T2D is certainly dependent on the plaque-host relationship, which may vary between individuals. To date, biological and molecular mechanisms underlying the interplay between DPSC properties and alterations of the oral microenvironment caused by T2D are still to be addressed. Here, we investigate the effects of T2D-related oral dysbiosis and its potential biological consequences that might involve DPSCs, responsible for the dental stemness homeostasis and regenerative properties. Accordingly, we hypothesize a direct biological effect of T2D oral plaque on DPSC function and differentiation beyond the sole involvement of the periodontium. We found that oral plaque obtained from T2D patients with PD significantly reduced the clonogenic ability of DPSCs compared to that obtained from nondiabetic patients with PD, without impacting osteogenic differentiation, analysed by the expression of both alkaline phosphatase (ALP) and osteocalcin, known to represent specific markers of the osteoblast lineage [23] and to be involved in the mineralization of the extracellular matrix and therefore in osteogenesis [24]. Notably, the decrease in clonogenic potential of DPSCs was independent of the main specific clinical features of diabetic patients, such as age and sex.
2. Materials and Methods
2.1. Isolation, Culture, and Characterization of Dental Pulp Stem Cells (DPSCs) by Flow Cytometry
Dental pulp stem cells (DPSCs) were obtained from explants of dental pulp from one subject undergoing tooth extraction for orthodontic treatment and according to previous reports [15, 25]. Briefly, the premolar tooth was kept in a physiological solution after extraction and then in milk. Afterwards, the pulp was collected, chopped in a petri dish containing PBS and 1% penicillin/streptomycin, and left to adhere for 2 hours at 37°C in 5% CO2 in the incubator. Afterwards, PBS was removed and replaced by complete media composed of DMEM-low glucose supplemented with 20% FBS and 1% penicillin/streptomycin (all Gibco). After 72 hours, nonadherent cells were removed and fresh complete media were added. DPSCs were expanded up to the third passage by trypsinization and subcultured at 4000 cells/cm2 in complete media. Cell growth and viability were monitored by trypan blue exclusion assay. Cultures were characterized by flow cytometry analysis. At passage 3, DPSCs were trypsinized and resuspended in FACS buffer (PBS/2% foetal bovine serum) for immunophenotype assessment. Cells were stained with indirectly or directly conjugated primary antibodies against CD44, CD105, CD117, CD90 (all Abcam, Cat. N. ab44967, ab6124, ab23894, and ab5506), CD34 (Miltenyi Biotec, Calderara di Reno, Bologna, Italy, Cat. N. 130-081-001), and CD73 (BioLegend, San Diego, CA, USA, Cat. 344005). Cells were incubated with primary antibodies for 30 minutes, followed by staining with secondary antibodies (only if indirectly conjugated) [11–13, 26]. Data was acquired by cytofluorimeter (FACSAria II, BD, San Jose, CA, USA) and analysed by Diva Software (v6.1.1, BD, San Jose, CA, USA). This study was approved by the Ethical Committee of Policlinico Umberto I, Rome, Italy (protocol number 4336, 02/02/2017). Experiments were conducted in accordance with the 1964 Helsinki Declaration regarding the study involving human participants. Informed consent was obtained from all subjects before the surgical procedure. Subjects were all deidentified by employing a code (from B-F (T2D) and from G-I (ctl)). The clinical features of all patients are reported in Table 1. Subcutaneous adipose stromal cells (ASCs, primary cell lines 55P and 56P) were isolated as previously described [11, 27], according to the approved protocol (Ethical Committee of “Sant'Andrea” Hospital in Rome, Ref. 49_2013/28.01.2016).
Table 1.
Clinical features of patients.
| Age | Sex | Smoker | |
|---|---|---|---|
| Donor DPSCs | |||
| 1 | 16 | M | No |
| Donor oral plaque | |||
| T2D-B | 52 | M | No |
| T2D-C | 91 | F | No |
| T2D-D | 79 | M | No |
| T2D-E | 94 | F | No |
| T2D-F | 94 | M | No |
| Ctl-G | 22 | M | No |
| Ctl-H | 53 | M | No |
| Ctl-I | 82 | F | No |
2.2. Oral Plaque Isolation
Oral plaque was isolated as previously reported [7]. Briefly, single plaque samples were firstly spun at 400g for 5 minutes at 4°C in order to concentrate the plaque. Afterwards, the pellet was weighed for the following data normalization and mechanically homogenised using a pestle in ice. The plaque was then diluted in 1 ml of sterile PBS and centrifuged at 4°C and 600g for 15 minutes. The supernatant was then collected and filtered by using a 0.45 μm mesh. Oral plaque suspensions were freshly used.
2.3. Clonogenic and Osteogenic Differentiation Assay
Cultures were conditioned with oral plaque from diabetic and ctl patients, by seeding DPSCs at a low density (10 cells/cm2) in complete media, as previously described [11–13, 26], then supplemented with an equal amount of oral plaque-derived suspensions (volume ratio 1 : 1) for 21 days. Fresh oral plaque suspensions were replaced every 3 days. At the end of the incubation time, cells were fixed with 4% paraformaldehyde for 1 hour at room temperature and then stained with Giemsa (Sigma) in order to identify nuclear and/or cytoplasmic morphology of colony-forming units (CFU). The number of clones was quantified by an optical microscope and then normalized to the amount (mg) of the oral plaque seeded [11–13, 26]. For osteogenic differentiation tests, DPSCs were cultured for 21 days in a StemPro® Osteogenesis Differentiation Kit (5 × 103 cells/cm2, Gibco, Cat. N. A10072-01) [12, 13] supplemented with an equal amount of oral plaque-derived suspensions (volume ratio 1 : 1). Afterwards, calcium deposition was analysed incubating the cells for 1 hour at room temperature with a 2% Alizarin red solution at pH 4.1-4.3 (Sigma, St. Louis, USA, Cat. N. A5533) [13, 26].
2.4. Real-Time PCR
Total RNA was isolated (RNeasy kit, Qiagen), and c-DNA was obtained and amplified by the SensiMix SYBR Hi-ROX Kit (Bioline, London, UK). Templates were amplified by the 7900HT Fast Real-Time PCR System (Applied Biosystems, Cheshire, UK) for 40 cycles according to the following protocol: 95°C for 15 seconds, 56-58°C for 15 seconds, and 72°C for 15 seconds. Primer sequences used were the following: forward—GATGTGGAGTATGAGAGTGAC and reverse—GGTCAAGGGTCAGGAGTTC (ALP), forward—TGAAAGCCGATGTGGTCAG and reverse—CAGCGAGGTAGTGAAGAGAC (osteocalcin), and forward—ACAGTCAGCCGCATCTTC and reverse—GCCCAATACGACCAAATCC (GAPDH). GAPDH was considered a housekeeping gene. The reaction products were analysed by SDS 2.1.1 software (Applied Biosystems, Cheshire, UK).
2.5. Statistical Analysis
Statistical analysis was performed by using GraphPad Prism 5 software (San Diego, USA). Comparison between two groups was performed by t-test. Multivariable linear regression analyses were conducted to adjust for potential confounders, and computation was performed with SPSS 20 (IBM). A p value < 0.05 has been considered statistically significant. Data are presented as mean ± standard error, unless specified.
3. Results
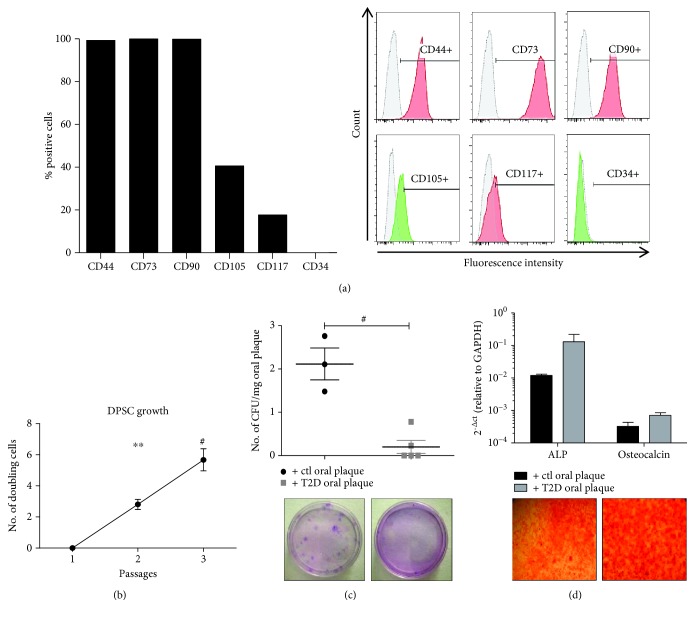
In order to test the influence of T2D patient-derived oral plaque on DPSC biology in the presence of PD, we have firstly isolated and characterized the primary cell line of DPSCs from the premolar tooth. Flow cytometry analysis showed that DPSCs exhibited a mesenchymal stem cell-like immunophenotype [11–13, 26] with high expression of stromal markers such as CD44, CD90 and CD73 and a moderate and low positivity for CD105 and CD117, respectively (Figure 1(a)). The expression of CD34 (hematopoietic marker) was undetectable. The DPSC cultures were expanded up to the third passage, when they exhibited the highest number of doubling viable cells (Figure 1(b), p < 0.0001 and p < 0.01, passage 3 vs. passages 1 and 2, respectively). In order to evaluate their clonogenic ability, cells were then cultured at a low density for 21 days by supplementing the culture media with oral plaque preparations obtained from T2D patients (N = 5) with PD. Cultures supplemented with oral plaque from non-diabetic individuals with PD were used as ctl (N = 3). The clinical features of subjects enrolled in this study are displayed in Table 1. Smoking, surgical interventions, and infections have been considered exclusion criteria. Results showed a striking and significant decrease in the number of clones generated by DPSCs when conditioned with oral plaque derived from T2D patients with PD compared to ctl (Figure 1(c), p < 0.0001). Since the average age of donors in the two groups appeared to differ, we performed a multivariable linear regression analysis to adjust for this potential confounder. The multivariable regression analysis indicated a significant association between the presence of T2D and decreased clonogenesis independently of the age of the patient (Table 2). Afterwards, we induced DPSC differentiation into the mesodermal lineage, by priming the cultures for 21 days towards the osteogenic phenotype in the presence of both types of oral plaque, and we quantified the expression of the main osteogenic genes including ALP and osteocalcin [28]. Results showed that T2D oral plaque did not significantly affect the osteogenic differentiation, although a trend of ALP to increase in cultures supplemented with oral plaque samples derived from T2D patients with respect to ctl was observed (Figure 1(d)).
Figure 1.
(a) Flow cytometry analysis of DPSCs after isolation. The graph shows that DPSC cultures exhibit a mesenchymal-like immunophenotype with positivity to the main stromal markers and negativity to the hematopoietic marker CD34. Representative cytofluorimetric histograms are displayed on the right (red and green histograms and APC and FITC fluorochromes, respectively). (b) Cell growth profile of DPSCs. The graph displays that the number of doubling cells is significantly increased at higher passages. Results were normalized to passage 1. ∗∗p < 0.01, #p < 0.0001. (c) The graph shows that the number of clones generated by DPSCs in the presence of T2D oral plaque is significantly lower compared to that in controls (ctls). The number of CFU was normalized to the amount of oral plaque (mg). Below the graph, representative optical images of CFU stained with Giemsa are shown. #p < 0.0001. (d) Osteogenic differentiation of DPSCs. The graph displays the gene expression levels of ALP and osteocalcin which are unaltered between the two treatments. Below the graph, representative optical images of osteogenic differentiation of DPSCs stained with Alizarin red. Magnification: 10x.
Table 2.
Multivariable linear regression analysis for the association of the normalized CFU yield (CFU/mg) and diabetes, adjusted for age.
| Independent variable | Regression coefficient | p |
|---|---|---|
| T2D | -1.74 | 0.01 |
| Age | -0.006 | 0.54 |
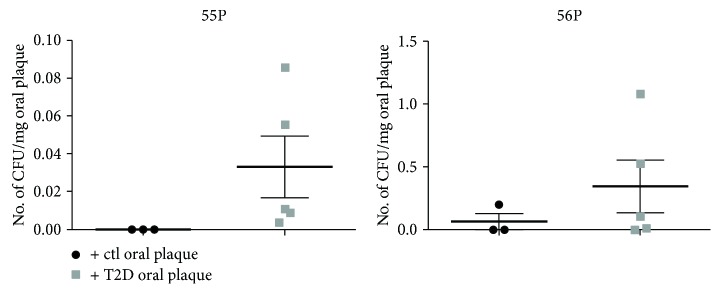
Finally, in order to verify whether the effect of T2D oral plaque was specific to DPSCs, we tested the samples on two distinct human primary subcutaneous ASC lines (55P and 56P). Results have shown that the number of CFU was not significantly affected by T2D oral plaque (Figure 2), therefore suggesting a specific effect mainly related to the dental microenvironment.
Figure 2.
The graphs display the number of clones (CFU) generated from two distinct primary lines (55P and 56P) derived from subcutaneous adipose tissue and stimulated with ctl and T2D oral plaque, highlighting that there is no significant difference between the treatments. The number of CFU was normalized to the amount of oral plaque (mg).
4. Discussion
In this study, we have shown that oral plaque derived from T2D patients with PD impacts negatively on the clonogenic ability of DPSCs, highlighting a potential biological mechanism by which diabetes may contribute to periodontal damage. To the best of our knowledge, the direct effect of T2D-derived plaque on DPSCs has never been addressed. Our set of diabetic patients has developed PD, whose etiopathogenesis is linked to a wide range of alterations triggered in the mouth. These include changes of the periodontal microbiota with respect to healthy individuals [28], associated in particular with the increase in defined pathogens [29–31], known to antagonize the immune response in patients with PD [32]. Although we did not identify the nature of the biofilm in our oral plaque samples, it is plausible that the microbiological profile has been profoundly modified in diabetic patients [8, 33, 34]. Notably, the number of CFU generated by DPSCs was negatively affected by oral plaque from this set of T2D patients, despite the absence of significant statistical correlations with clinical parameters and potential interpatient variability, known to play a role in the progression of PD even in relation to the oral microenvironment [28]. Intriguingly, when subcutaneous ASCs (stromal cells of different tissue origin from DPSCs) are similarly treated with T2D oral plaque, the effect is not reproducible in our conditions, indicating that the ability of T2D to decrease the clonogenic efficiency is specific to DPSCs and likely restricted to the dental microenvironment.
The low number of subjects enrolled in this study certainly represents a limitation. Nevertheless, we cannot rule out that the alterations of the oral plaque mediated by diabetes with regard to the intrinsic properties of DPSCs represent a priming and sufficient event able to transversely affect individuals at any age and gender, therefore highlighting non-cumulative effects over the time. Additionally, we have detected a trend towards increased osteogenic differentiation in the presence of T2D oral plaque (although not statistically significant). This result is only apparently discrepant from the majority of the studies in literature, showing that diabetes severely compromises osteogenic differentiation [28, 35, 36]. However, our result should be interpreted in the light of the clonogenesis result. In fact, normally, the biological events related to the clonogenic and differentiation properties are mutually exclusive and strictly dependent on the tissue of origin [11, 23]. In the majority of adult progenitor populations, including DPSCs, the balance between these two processes is finely tuned, and pathological insults as T2D are able to deregulate both phases. Thus, the modifications of the oral plaque caused by diabetes are likely to shift the ability of DPSCs to select the proliferative clones towards a main tendency to differentiate, therefore exhausting the original multipotent and stromal stem cell pool. From a clinical point of view, this effect would result in a deregulated ossification and in the inability of patients to exploit their own stem cell pool to trigger regeneration upon pathological conditions. Accordingly, DPSCs represent the responsible population for periodontal homeostasis, able to actively respond to different damages, by enhancing the repairing process [15]. Notably, periodontal therapy (especially the intensive kind) in diabetic patients in combination with glycaemia control is able to successfully restore a suitable periodontal microenvironment [33, 37, 38], therefore confirming the reversible epigenetic nature of T2D. Thus, it is also plausible that hyperglycaemia consequent to insulin resistance (the hallmark of T2D patients) may represent the key stimulus to change both the composition of the oral plaque and the biological features of DPSCs.
5. Conclusions
Our study strengthens the involvement of DPSCs in T2D-mediated PD. The finding that oral plaque may represent the direct biological modality by which diabetes impairs the dental pulp stem and progenitor cell pool could be of clinical significance.
Acknowledgments
We thank “Fondazione Roma” for the continuous support. This work was supported by the Department of Medical-Surgical Sciences and Biotechnologies, Sapienza University of Rome.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflicts of interest to disclose.
References
- 1.Angelini F., Pagano F., Bordin A., et al. The impact of environmental factors in influencing epigenetics related to oxidative states in the cardiovascular system. Oxidative Medicine and Cellular Longevity. 2017;2017:18. doi: 10.1155/2017/2712751.2712751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frati G., Schirone L., Chimenti I., et al. An overview of the inflammatory signalling mechanisms in the myocardium underlying the development of diabetic cardiomyopathy. Cardiovascular Research. 2017;113(4):378–388. doi: 10.1093/cvr/cvx011. [DOI] [PubMed] [Google Scholar]
- 3.Zheng Y., Ley S. H., Hu F. B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nature Reviews Endocrinology. 2018;14(2):88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 4.DeFronzo R. A., Ferrannini E., Groop L., et al. Type 2 diabetes mellitus. Nature Reviews Disease Primers. 2015;1, article 15019 doi: 10.1038/nrdp.2015.19. [DOI] [PubMed] [Google Scholar]
- 5.Nascimento G. G., Leite F. R. M., Vestergaard P., Scheutz F., Lopez R. Does diabetes increase the risk of periodontitis? A systematic review and meta-regression analysis of longitudinal prospective studies. Acta Diabetologica. 2018;55(7):653–667. doi: 10.1007/s00592-018-1120-4. [DOI] [PubMed] [Google Scholar]
- 6.Kinane D. F., Stathopoulou P. G., Papapanou P. N. Periodontal diseases. Nature Reviews Disease Primers. 2017;3, article 17038 doi: 10.1038/nrdp.2017.38. [DOI] [PubMed] [Google Scholar]
- 7.Miller R. L., Folke L. E. A., Umana C. R. Chemotactic ability of dental plaque upon autologous or heterologous human polymorphonuclear leukocytes. Journal of Periodontology. 1975;46(7):409–414. doi: 10.1902/jop.1975.46.7.409. [DOI] [PubMed] [Google Scholar]
- 8.Levine R. S. Obesity, diabetes and periodontitis – a triangular relationship? British Dental Journal. 2013;215(1):35–39. doi: 10.1038/sj.bdj.2013.627. [DOI] [PubMed] [Google Scholar]
- 9.Santos C. M. M. L., Lira-Junior R., Fischer R. G., Santos A. P. P., Oliveira B. H. Systemic antibiotics in periodontal treatment of diabetic patients: a systematic review. PLoS One. 2015;10(12, article e0145262) doi: 10.1371/journal.pone.0145262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saremi A., Nelson R. G., Tulloch-Reid M., et al. Periodontal disease and mortality in type 2 diabetes. Diabetes Care. 2005;28(1):27–32. doi: 10.2337/diacare.28.1.27. [DOI] [PubMed] [Google Scholar]
- 11.Siciliano C., Bordin A., Ibrahim M., et al. The adipose tissue of origin influences the biological potential of human adipose stromal cells isolated from mediastinal and subcutaneous fat depots. Stem Cell Research. 2016;17(2):342–351. doi: 10.1016/j.scr.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Siciliano C., Chimenti I., Bordin A., et al. The potential of GMP-compliant platelet lysate to induce a permissive state for cardiovascular transdifferentiation in human mediastinal adipose tissue-derived mesenchymal stem cells. BioMed Research International. 2015;2015:10. doi: 10.1155/2015/162439.162439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siciliano C., Ibrahim M., Scafetta G., et al. Optimization of the isolation and expansion method of human mediastinal-adipose tissue derived mesenchymal stem cells with virally inactivated GMP-grade platelet lysate. Cytotechnology. 2015;67(1):165–174. doi: 10.1007/s10616-013-9667-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nuti N., Corallo C., Chan B. M. F., Ferrari M., Gerami-Naini B. Multipotent differentiation of human dental pulp stem cells: a literature review. Stem Cell Reviews. 2016;12(5):511–523. doi: 10.1007/s12015-016-9661-9. [DOI] [PubMed] [Google Scholar]
- 15.Ledesma-Martinez E., Mendoza-Nunez V. M., Santiago-Osorio E. Mesenchymal stem cells derived from dental pulp: a review. Stem Cells International. 2016;2016:12. doi: 10.1155/2016/4709572.4709572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanafi M. M., Rajeshwari Y. B., Gupta S., et al. Transplantation of islet-like cell clusters derived from human dental pulp stem cells restores normoglycemia in diabetic mice. Cytotherapy. 2013;15(10):1228–1236. doi: 10.1016/j.jcyt.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Izumoto-Akita T., Tsunekawa S., Yamamoto A., et al. Secreted factors from dental pulp stem cells improve glucose intolerance in streptozotocin-induced diabetic mice by increasing pancreatic β-cell function. BMJ Open Diabetes Research & Care. 2015;3(1, article e000128) doi: 10.1136/bmjdrc-2015-000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Omi M., Hata M., Nakamura N., et al. Transplantation of dental pulp stem cells improves long-term diabetic polyneuropathy together with improvement of nerve morphometrical evaluation. Stem Cell Research & Therapy. 2017;8(1):p. 279. doi: 10.1186/s13287-017-0729-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Datta I., Bhadri N., Shahani P., et al. Functional recovery upon human dental pulp stem cell transplantation in a diabetic neuropathy rat model. Cytotherapy. 2017;19(10):1208–1224. doi: 10.1016/j.jcyt.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Li Y., Zhao S., Nan X., et al. Repair of human periodontal bone defects by autologous grafting stem cells derived from inflammatory dental pulp tissues. Stem Cell Research & Therapy. 2016;7(1):p. 141. doi: 10.1186/s13287-016-0404-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marei M. K., El Backly R. M. Dental mesenchymal stem cell-based translational regenerative dentistry: from artificial to biological replacement. Frontiers in Bioengineering and Biotechnology. 2018;6:p. 49. doi: 10.3389/fbioe.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Govindasamy V., Ronald V. S., Abdullah A. N., et al. Differentiation of dental pulp stem cells into islet-like aggregates. Journal of Dental Research. 2011;90(5):646–652. doi: 10.1177/0022034510396879. [DOI] [PubMed] [Google Scholar]
- 23.Mori G., Brunetti G., Oranger A., et al. Dental pulp stem cells: osteogenic differentiation and gene expression. Annals of the New York Academy of Sciences. 2011;1237(1):47–52. doi: 10.1111/j.1749-6632.2011.06234.x. [DOI] [PubMed] [Google Scholar]
- 24.Ching H. S., Luddin N., Rahman I. A., Ponnuraj K. T. Expression of odontogenic and osteogenic markers in DPSCs and SHED: a review. Current Stem Cell Research & Therapy. 2017;12(1):71–79. doi: 10.2174/1574888x11666160815095733. [DOI] [PubMed] [Google Scholar]
- 25.Spath L., Rotilio V., Alessandrini M., et al. Explant-derived human dental pulp stem cells enhance differentiation and proliferation potentials. Journal of Cellular and Molecular Medicine. 2010;14(6B):1635–1644. doi: 10.1111/j.1582-4934.2009.00848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siciliano C., Chimenti I., Ibrahim M., et al. Cardiosphere conditioned media influence the plasticity of human mediastinal adipose tissue-derived mesenchymal stem cells. Cell Transplantation. 2015;24(11):2307–2322. doi: 10.3727/096368914X685771. [DOI] [PubMed] [Google Scholar]
- 27.Businaro R., Scaccia E., Bordin A., et al. Platelet lysate-derived neuropeptide y influences migration and angiogenesis of human adipose tissue-derived stromal cells. Scientific Reports. 2018;8(1, article 14365) doi: 10.1038/s41598-018-32623-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lalla E., Papapanou P. N. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nature Reviews Endocrinology. 2011;7(12):738–748. doi: 10.1038/nrendo.2011.106. [DOI] [PubMed] [Google Scholar]
- 29.Mandell R. L., Dirienzo J., Kent R., Joshipura K., Haber J. Microbiology of healthy and diseased periodontal sites in poorly controlled insulin dependent diabetics. Journal of Periodontology. 1992;63(4):274–279. doi: 10.1902/jop.1992.63.4.274. [DOI] [PubMed] [Google Scholar]
- 30.Ebersole J. L., Holt S. C., Hansard R., Novak M. J. Microbiologic and immunologic characteristics of periodontal disease in Hispanic Americans with type 2 diabetes. Journal of Periodontology. 2008;79(4):637–646. doi: 10.1902/jop.2008.070455. [DOI] [PubMed] [Google Scholar]
- 31.Haubek D., Ennibi O. K., Poulsen K., Vaeth M., Poulsen S., Kilian M. Risk of aggressive periodontitis in adolescent carriers of the JP2 clone of Aggregatibacter (Actinobacillus) actinomycetemcomitans in Morocco: a prospective longitudinal cohort study. The Lancet. 2008;371(9608):237–242. doi: 10.1016/S0140-6736(08)60135-X. [DOI] [PubMed] [Google Scholar]
- 32.Bostanci N., Allaker R. P., Belibasakis G. N., et al. Porphyromonas gingivalis antagonises Campylobacter rectus induced cytokine production by human monocytes. Cytokine. 2007;39(2):147–156. doi: 10.1016/j.cyto.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Miranda T. S., Feres M., Retamal-Valdés B., Perez-Chaparro P. J., Maciel S. S., Duarte P. M. Influence of glycemic control on the levels of subgingival periodontal pathogens in patients with generalized chronic periodontitis and type 2 diabetes. Journal of Applied Oral Science. 2017;25(1):82–89. doi: 10.1590/1678-77572016-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Babaev E. A., Balmasova I. P., Mkrtumyan A. M., et al. Metagenomic analysis of gingival sulcus microbiota and pathogenesis of periodontitis associated with type 2 diabetes mellitus. Bulletin of Experimental Biology and Medicine. 2017;163(6):718–721. doi: 10.1007/s10517-017-3888-6. [DOI] [PubMed] [Google Scholar]
- 35.Silva J. A. F., Lorencini M., Reis J. R. R., Carvalho H. F., Cagnon V. H. A., Stach-Machado D. R. The influence of type I diabetes mellitus in periodontal disease induced changes of the gingival epithelium and connective tissue. Tissue & Cell. 2008;40(4):283–292. doi: 10.1016/j.tice.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Gurav A. N. Management of diabolical diabetes mellitus and periodontitis nexus: are we doing enough? World Journal of Diabetes. 2016;7(4):50–66. doi: 10.4239/wjd.v7.i4.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silva-Boghossian C. M., Orrico S. R. P., Gonçalves D., Correa F. O. B., Colombo A. P. V. Microbiological changes after periodontal therapy in diabetic patients with inadequate metabolic control. Brazilian Oral Research. 2014;28(1):1–9. doi: 10.1590/1807-3107BOR-2014.vol28.0007. [DOI] [PubMed] [Google Scholar]
- 38.Artese H. P. C., Longo P. L., Gomes G. H., Mayer M. P. A., Romito G. A. Supragingival biofilm control and systemic inflammation in patients with type 2 diabetes mellitus. Brazilian Oral Research. 2015;29(1):1–7. doi: 10.1590/1807-3107BOR-2015.vol29.0071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.