Abstract
Background
Giant cell tumor of bone (GCTB) in distal radius is a benign but invasive bone tumor characterized by strong aggressive behavior and frequent recurrence.
Methods
To identify recurrence related risk factors and decide suitable surgical strategy, the potential tumor- and treatment-specific factors, post-operative oncologic and functional outcomes were collected and analyzed from 58 patients with GCTB of the distal radius at our center.
Results
With the numbers available, our analysis strongly indicated soft tissue extension (with vs. without, HR: 5.645, 95% CI: 1.424 to 22.377, p = 0.014) and size of GCTB (diameter ≥ 5 cm vs. 5 cm HR: 3.893, 95% CI: 1.109 to 13.659, p = 0.034) are the two independent risk factors related to local relapse. Neither surgical procedures (curettage vs. en-bloc resection) nor other factors apparently affected the recurrence, including age, tumor nature, dominant hand involvement, pathological fracture conditions or pre-operative denosumab. However, intralesional curettage group achieved much better functional scores ((VAS: 2.5 ± 0.8 vs. 3.6 ± 1.2, p = 0.011; MSTS: 20.2 ± 3.4 vs. 16.7 ± 3.8, P = 0.034; DASH 9.1 ± 3.9 vs. 16.4 ± 5.5, p = 0.030) and much less complications (non-unions, dislocations, fractures and infections) compared to resection ones. Furthermore, denosumab provided dramatic pain reduction and strong tumor suppression, facilitating curettage with local adjuvants even in GCTB with advanced status.
Conclusions
Taken together, the radiographic presentations (soft-tissue extension and tumor size) are the strong prognostic predictors of local recurrence of GCTB in distal radius. In most tumors, an initial treatment with curettage remains feasible and first-choice, especially with the adjuvant denosumab.
Keywords: Giant cell tumor of distal radius, Recurrence, Surgical treatments, Denosumab
1. Introduction
Giant cell tumor of bone (GCTB), a locally invasive but benign bone tumor, mostly occurs in patients aged between 20–40 years. Except for the distal femur and proximal tibia, the distal radius is the most common site of GCTB [1], [2]. A stronger recurrent tendency was found at this site of GCTB [3]. To achieve adequate surgical margin, en bloc resection other than intralesional curettage was commonly adopted as a standard surgery for aggressive lesion in distal radius [4]. Lower recurrent rate might be expected, but the more destructive resection with complex surgical reconstruction generally resulted in more complications [4], [5]. In addition, with the increasing demand in wrist function of the patients, who are still young and active, a less destructive choice is preferred [6]. Therefore, to decide an individualized therapeutic strategy, a more comprehensive understanding of predictive factors regarding both oncologic and functional outcomes should be achieved [7].
Varied potential factors related to local recurrence of GCTB have been reported, including the Campanacci grade [8], pathologic fracture [9], tumor location [1], [2], [3], type of adjuvant used [10], [11], and the primary or the recurrent tumor. And most of the mentioned studies have a higher risk of confounding bias by including GCTB in all sites, weakening the predictive significance of the proposed factors.
Recently, the combined therapy of GCTB has been broadly accepted in clinical practice. Denosumab, a monoclonal antibody to the receptor activator of nuclear factor kappa-B (RANK) ligand (RANKL), showed effective bone loss prevention in osteoporotic patients by inhibiting the recruitment and activity of osteoclastic cells [12]. Surprisingly, this emergent modality turned out to be a promising neoadjuvant treatment for GCTB by its dramatic pain reduction and strong tumor suppression [13], [14], [15], [16]. The pre-operative denosumab was also estimated to have a potent effect for local control and capably degrade surgical option from resection to curettage even in advanced GCTB of distal radius [7], [17]. But the clinical outcomes were rarely reported.
In the current research, in order to perform comprehensive analysis of prognostic predictors and confirm the adjuvant efficacy of denosumab in this population, we retrospectively evaluated variables related to local recurrence in 58 patients exclusively with GCTB of distal radius, including demographic characteristics, tumor-specific or treatment-specific factors. The radiological and functional outcome after operation was assessed at a mean follow-up of 10 years. The clinical outcome of neoadjuvant of denosumab for GCTB in distal radius before and after surgical treatment was preliminarily reported as well.
2. Methods and patients
This was a retrospective, single-center study within the Department of Musculoskeletal Oncology, the First Affiliated Hospital of Sun Yat-Sen University. After obtaining Institutional Review Board approval, data were collected on patients with confirmed histological diagnosis of GCTB, followed-up at our department from January 1990 to March 2018. The inclusion criteria were: follow-up more than 20 months post-operation and with available data regarding clinical or radiological record.
Data were collected from the medical records regarding demographic characteristics (age, gender), tumor-specific factors (tumor nature, size, site, soft-tissue invasion and pathological fractures) and treatment-specific factors (surgical methods, adjuvant denosumab) (Table 1). Soft tissue extension was defined as a clear involvement of adjacent soft tissue by breaking through cortical bone according to radiographic findings.
Table 1.
Summary data of patient information.
| General information | Mean | SD | Range |
|---|---|---|---|
| Age (years) | 33.2 | 13.4 | 15–65 |
| Follow-upa (months) | 95.3 | 100.6 | 21–321 |
| Tumor size (cm) | 4.7 | 1.3 | 1.2–8 |
| Time to recurrence (months) | 23.8 | 12.3 | 9–53 |
| Potential factors | Number | Percentage | Pb-value | |
|---|---|---|---|---|
| Gender | Female | 23 | 39.7 | 0.115 |
| Male | 35 | 60.3 | ||
| Status at surgery | Primary | 42 | 72.4 | 0.001 |
| Recurrent | 16 | 27.6 | ||
| Dominant hand affected | No | 30 | 51.7 | 0.793 |
| Yes | 28 | 48.3 | ||
| Soft tissue extension | No | 34 | 58.6 | 0.189 |
| Yes | 24 | 41.4 | ||
| Pathologic fractures | No | 49 | 84.5 | 0.000 |
| Yes | 9 | 15.5 | ||
| Surgical method | Curettage | 21 | 36.2 | 0.036 |
| En-bloc | 37 | 63.8 | ||
| Pre-operative denosumab | No | 50 | 86.2 | 0.000 |
| Yes | 8 | 13.8 | ||
Time from surgery until last followup (months).
Analyzed by nonparametric Chi-Square test.
Intralesional curettage conventionally was recommended in less invasive lesion, like Campanacci grade I and II with well-maintained articular surface. Adjuvant treatments included high speed burring, iodine tincture, electrocautery. Polymethylmethacrylate (PMMA) and cancellous allograft/autograft were used to fill the bone defect after curettage. On the other hand, resection typically was recommended for patients Campanacci grade Ⅲ with extensive soft tissue mass or collapse of the articular surface. The bone defect was reconstructed with allograft or autograft of proximal fibula.
Application of denosumab in GCTB patients at our center started several years ago. Generally, denosumab is indicated in patients with advanced status (Campanacci grade Ⅲ), sever pain and a strong demand of better function. The primary purpose of pre-operative denosumab is to reduce pain, restore wrist function and provide better surgical condition. Pre-operative denosumab was given subcutaneously at dosage of 120 mg on day 1, day 8, day 15 and day 29 as the loading dosage for the first month, 120 mg per four weeks thereafter.
If a patient encountered re-operation(s) due to local recurrence, only the first surgery performed by the team was taken into the current analysis. For the deonosumab treating patients, pre- and post-treatment radiological images and pathological findings were evaluated for assessment of treatment response.
Visual analogue scale (VAS), Musculoskeletal Tumor Society (MSTS) score and the disability of the arm, shoulder and hand (DASH) questionnaire are applied to evaluate pain level, oncologic functional outcome [18], and upper extremity function of the patients, respectively before and after surgery. All the data collection were conducted by a group of independent doctors and blind to the treating surgeons. The events of recurrence, confirmed by histology, were recorded and the follow-up time was calculated as the interval between the date of surgery and that of recurrence. For the patients who were free of recurrence, then the date of final clinical visit was considered as the end-point of follow-up. Recurrence-free survival (RFS) was analyzed using follow-up time in months. The data of patients who died during follow-up but not relevant to tumor or surgery was excluded.
Categorical data were recorded by the frequency of events. Continuous variables, like the age, tumor size and follow-up time, were described as the mean and standard deviation. Log-rank tests of Kaplan–Meier survival analysis were adopted to assess the differences in the RFS between different demographic characteristics (age, gender, dominant hand involved), tumor-specific (tumor nature, size, soft-tissue extension and pathological fractures) and treatment-specific factors (surgical methods, peri-operative denosumab). If any factor reached a significant p value (p < 0.1), a sequential multivariate Cox regression analysis was conducted to rule out potential confounding factors. Surgical methods are our primary factor of interest, which wouldl be taken into multivariate Cox regression analysis even its p value > 0.1 in Log-rank tests. In regards to multivariate Cox regression analysis, p < 0.05 was considered for significant findings and the hazards ratio (HR) with 95% confidential interval (CI) was then calculated. All the statistical analysis were finished on SPSS Statistics 19 software while the Kaplan–Meier survival curve were generated from GraphPad Prism version 6.00 for Windows.
3. Results
3.1. The summary of patient and tumor information
58 patients (42 primary and 16 recurrent) with follow-up time during 95.3 ± 100.6 months (21–321 months) were included within our center from January 1990 to March 2018. The mean age of all the included patients was 33.2 ± 13.4 years, therefore 33 was chosen as the cut-off value to test whether age could affect RFS. 23 patients were female (23/58, 39.7%) while male patients consisted 60.3% (35/58). The average diameter of tumor was 4.7 ± 1.3 cm but the median diameter was 5 cm, then the later was used as the cut-off value to test whether tumor size could influence RFS. 9 of 58 patients (15.5%) encountered pathological fractures. The radiologic evidences of soft-tissue extension could be seen in 41.4% (24/58) tumors (Table 1).
3.2. The summary of treatment information
Intralesional curettage followed by high-speed burring was accomplished in 36.2% (21/58) of patients, bone defects of which were filled by autograft (n = 4), allograft (n = 8) and PMMA (n = 9). While 63.8% (37/58) patients underwent treatment with en-bloc resection and the following reconstruction choosing internal fixation with fibular autograft (n = 26) or distal allograft (n = 6) or PMMA (n = 5). For 8 patients (21.1%), which were all Campanacci grade III, pre-operative denosumab was given subcutaneously at dosage of 120 mg on day 1, day 8, day 15 and day 29 as the loading dosage for the first month, 120 mg per four weeks thereafter. En bloc resection and 7 intralesional curettages were done in the patients with denosumab treated.
3.3. Recurrence-free survival analysis using Kaplan–Meier manner
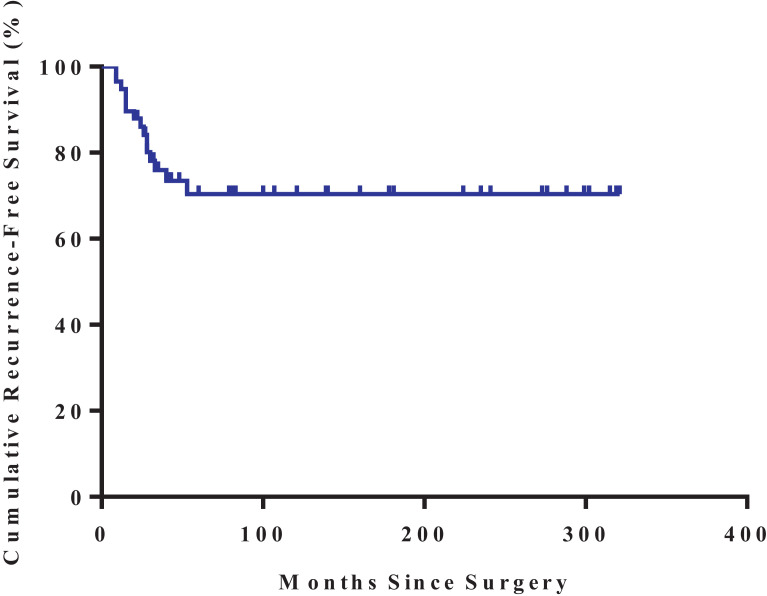
No metastasis was observed in all patients during follow-up. Totally, 15 of the 58 (25.9%) patients had a local recurrence during follow-up after surgery (Table 2). Mean time to overall recurrence was 23.8 (9–53) (median time: 24) months (Table 1). In 2 years, the cumulative RFS was 86% and was stably maintained at 70% during 5–20 years post-operation according to the Kaplan–Meier survival analysis (Fig. 1).
Table 2.
Log rank tests for potential risk factors related to recurrence.
| Variables | No. of patients | Cases of recurrence | Percentage of recurrence | HR (95% CIs) | χ2 | Pa-value | |
|---|---|---|---|---|---|---|---|
| Gender | Female | 23 | 9 | 39.1 | 2.749 (1.042–8.663) | 4.075 | 0.0435 |
| Male | 35 | 6 | 17.1 | ||||
| Surgical method | Curettage | 21 | 5 | 23.8 | 1.104 (0.3723–3.311) | 0.034 | 0.8537 |
| En-bloc | 37 | 10 | 27.0 | ||||
| Age of patients at surgery (yrs) | ≤33 | 27 | 9 | 33.3 | 1.965 (0.7192–5.547) | 1.735 | 0.1878 |
| >33 | 31 | 6 | 19.4 | ||||
| Tumor nature | Primary | 42 | 10 | 23.8 | 1.334 (0.4382–4.254) | 0.284 | 0.5943 |
| Recurrence | 16 | 5 | 31.3 | ||||
| Tumor size (cm) | ≥5 | 30 | 11 | 36.7 | 2.786 (0.9500–7.193) | 3.411 | 0.0648 |
| <5 | 28 | 4 | 14.3 | ||||
| Dominant hand affected | No | 30 | 7 | 23.3 | 1.207(0.4398–3.332) | 0.135 | 0.7134 |
| Yes | 28 | 8 | 28.6 | ||||
| Soft tissue extension | No | 34 | 5 | 14.7 | 6.120 (1.991–15.78) | 10.460 | 0.0012 |
| Yes | 24 | 10 | 41.7 | ||||
| Pathologic fracture | No | 49 | 11 | 22.4 | 2.008(0.5954–9.880) | 1.506 | 0.2197 |
| Yes | 9 | 4 | 44.4 | ||||
| Pre-operative denosumabb | No | 50 | 14 | 28.0 | 1.150(0.1708–7.801) | 0.020 | 0.888 |
| Yes | 8 | 1 | 12.5 | ||||
| Total | 58 | 15 | 25.9 | – | |||
No.: number of patients; HR: Hazard Ratio (log rank); CIs: Condenfical Intervals.
p < 0.1 indicated a potiential correlation between the risk factor and recurrence.
Pre-operative denosumab was given subcutaneously at dosage of 120 mg for 4–6 times.
Fig. 1.
The overall Kaplan–Meier estimated recurrence-free survival rate.
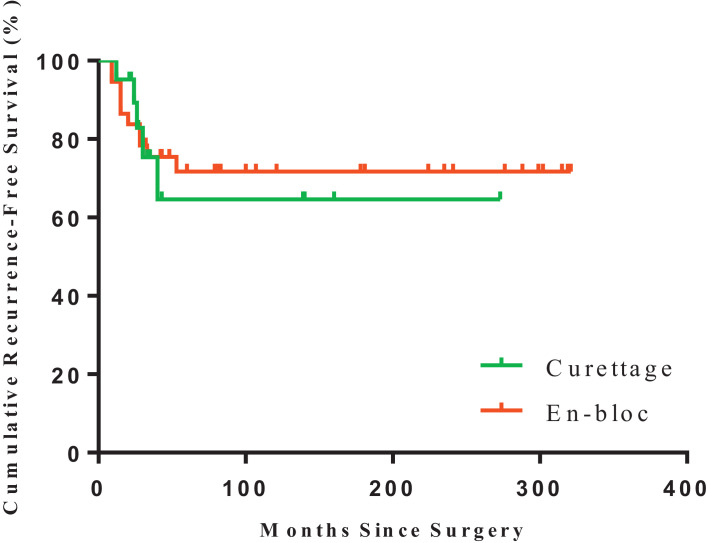
There were no obvious differences in RFS between different age, tumor nature, dominant hand involvement, pathological fracture conditions, surgical treatments (curettage vs. en-bloc resection, Fig. 2) or pre-operative denosumab (Table 2.)
Fig. 2.
Kaplan–Meier estimated recurrence-free survival of GCTB in patients underwent en-bloc resection (n = 37; red) or intralesional curettage (n = 21; green) (log-rank test, p = 0.8537). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
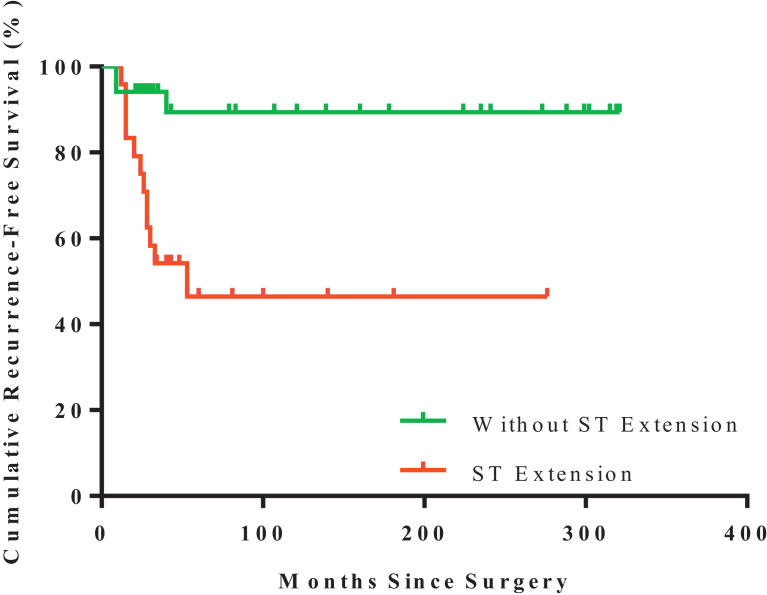
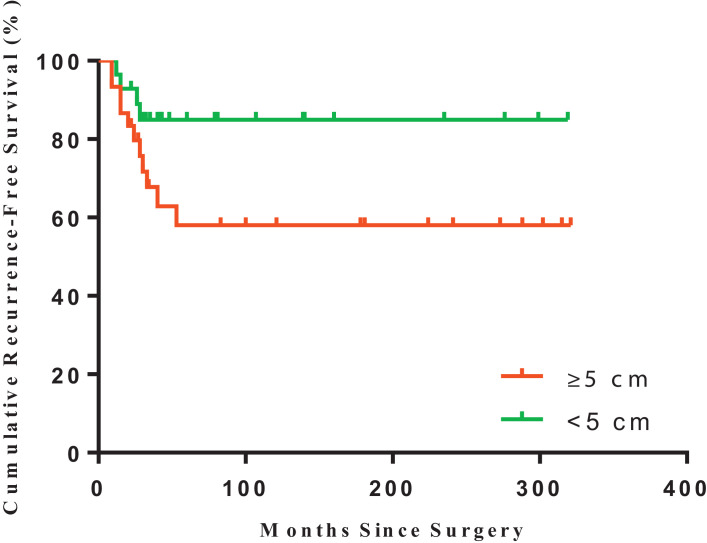
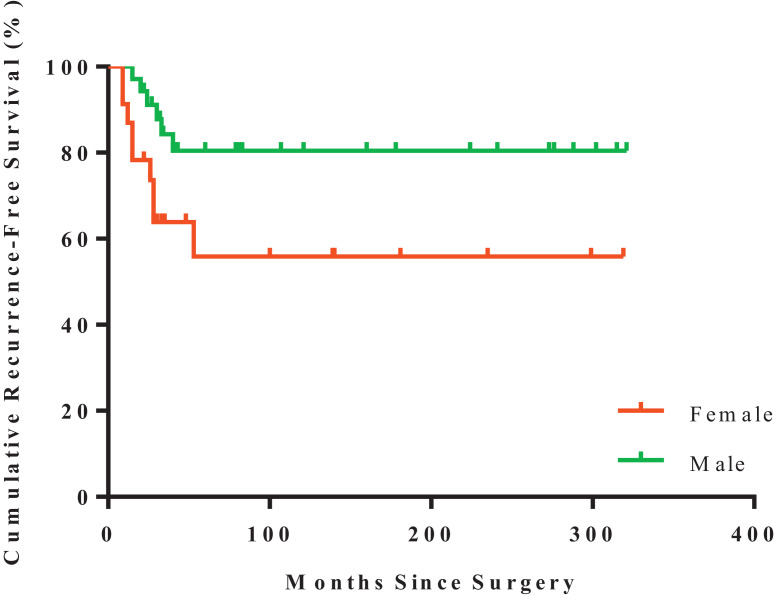
However, tumor with soft tissue extension (p = 0.012, Fig. 3) or with diameter ≥5 cm (p = 0.065, Fig. 4) were potentially related to patients’ RFS (Table 2). In addition, the risk of recurrence in female patients was higher than that of male ones (p = 0.044, Table 2 and Fig. 5).
Fig. 3.
Kaplan–Meier estimated recurrence-free survival of GCTB in patients with soft tissue extension (n = 24; red) or patients without (n = 24; green) (log-rank test, p = 0.0012). ST:soft-tissue. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 4.
Kaplan–Meier estimated recurrence-free survival of GCTB in patients with tumor diameter ≥ 5 cm (n = 30; red) or < 5 cm (n = 28; green) (log-rank test, p = 0.0648). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 5.
Kaplan–Meier estimated recurrence-free survival of GCTB in female (n = 23; red) or male patients (n = 35; green) (log-rank test, p = 0.043). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.4. Multivariate analysis by Cox regression
As mentioned before, those potential factors (p < 0.1) and surgical method were further taken into multivariate Cox regression analysis. And the results of analysis ruled out the impacts of gender or surgical method on the cumulative RFS of GCTB patients and strongly confirmed the soft tissue extension and size of GCTB were the two independent risk factors. Specially, patients with soft tissue extension had a 5.645 times higher (95% CI: 1.424 to 22.377, p = 0.014) recurrence risk as compared with patients treated without. On the other hand, a significant 3.893 times higher recurrence rate (95% CI: 1.109 to 13.659, p = 0.034) was validated in tumor diameter ≥5 cm in comparison to tumor size smaller than 5 cm (Table 3).
Table 3.
Multivariate Cox regression analysis.
| Variables | Regression coefficent | SE | P-value | HR | 95% CIs for HR |
|
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Gender | −0.85 | 0.60 | 0.155 | 0.426 | 0.131 | 1.381 |
| Surgical method | −0.95 | 0.64 | 0.139 | 0.388 | 0.111 | 1.360 |
| Size | 1.36 | 0.64 | 0.034 | 3.893 | 1.109 | 13.659 |
| Softtissue extension | 1.73 | 0.70 | 0.014 | 5.645 | 1.424 | 22.377 |
p < 0.05 indicated a strong correlation between the risk factor and recurrence; SE: Standard Errors; HR: Hazards Ratio; CIs: Condenfical Intervals.
3.5. VAS, functional scores and complication rates in the two surgical groups
According to our Kaplan–Meier survival analysis, similar recurrence rates were found between the two treatment groups. However, with the numbers available (curettage: n = 10; en-bloc resection: n = 15), intralesional curettage group achieved much better functional scores (VAS: 2.5 ± 0.8 vs. 3.6 ± 1.2, p = 0.011; MSTS: 20.2 ± 3.4 vs. 16.7 ± 3.8, P = 0.034; DASH 9.1 ± 3.9 vs. 16.4 ± 5.5, p = 0.030; Table 4).
Table 4.
Difference in functional scores between the two surgical treatments.
| Outcome measure | Mean ± SD |
||
|---|---|---|---|
| Curretage (n = 10) | En Bloc (n = 15) | p value | |
| VAS | 2.5 ± 0.8 | 3.6 ± 1.2 | 0.011 |
| MSTS | 20.2 ± 3.4 | 16.7 ± 3.8 | 0.034 |
| DASH | 9.1 ± 3.9 | 16.4 ± 5.5 | 0.030 |
VAS: Visual analogue scale; MSTS = Musculoskeletal Tumor Society; DASH = Disabilities of the Arm, Shoulder and Hand.
p < 0.05 indicated a significant difference between the two groups (t-test).
More frequent complications were observed after en-bloc resection compared to curettage group, including dislocation and subluxation of wrist joint, autograft fracture, non-union and infection. In the resection group, 32.4% (12/37) patients encountered complications related to the surgery. Displacements of the wrist joint, including carpal subluxation (1 patient) and separation of the distal radioulnar joint (2 patients) were seemed in the patients with en-bloc resection group. Furthermore, there were 5 nonunions in cases of en-bloc resection with fibular autograft reconstruction, all of which were re-operated by the autogenous bone grafting and achieved union 10 months post the 2nd operation. Fractures of bone graft were observed in two cases in the same group, which were treated by re-fixation with plate and autogenous bone grafting. Two infections occurred in the en-bloc resection with PMMA or allograft reconstruction, respectively, which need removal of implants and debridement. On the other hand, in the intra-lesional curettage group, we did not observe sever complications related to the operation needing re-operation (Table 5).
Table 5.
Complications in the en-bloc resection group.
| Complications | Event (n) |
|---|---|
| Total | 10 |
| Carpal subluxation | 1 |
| Separations of the distal radioulnar joint | 2 |
| Nonunions of the bone graft | 5 |
| Fractures of bone graft | 2 |
| Infections | 2 |
No obvious complications observed in curettage group.
3.6. The preliminary results of GCTB patients in distal radius treated with denosumab
8 patients [6 men, 2 women; mean age: 30.6 ± 12 years (range: 20–56 years)] with GCTB of distal radius using denosumab as neoadjuvant were included in the study. Average tumor size was 5.1 ± 0.4 cm, which was larger than the overall average size (4.7 ± 1.3 cm) and all the patients were primary GCTB at Campanacci grade Ⅲ. After 4–6 times administration of denosumab (Xgeva; Amgen, CA, USA) by subcutaneous injections at dosage of 120 mg on days 1, 8, 15, 29 and every 4 weeks thereafter, the pain of the wrist evaluated by VAS score was greatly reduced (pre- vs. post-treatment: 5.6 ± 1.1 vs. 1.0 ± 0.8, p < 0.0001, Table 6). 7 of 8 (87.5%) patients received intralesional curettage of the tumor saving the intactness of the involved bone, in which the anatomic structure was spared. No sever complications, including necrosis of the jaw, were observed related to denosumab.
Table 6.
The summary of patients treated with deonsumab.
| ID | Gender | Age (years) | Recurrent/primary | Diameter (cm) | Campanacci grade | Follow-upa (months) | Denosumab Administration | VAS |
||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-treament | Post-treament | Pb-value | ||||||||
| 1 | M | 40 | P | 5.3 | III | 24 | 120 mg per mo sc × 6 | 7 | 2 | <0.0001 |
| 2 | M | 20 | P | 5.2 | III | 27 | 120 mg per mo sc × 5 | 6 | 1 | |
| 3 | F | 28 | P | 4.5 | III | 26 | 120 mg per mo sc × 6 | 5 | 0 | |
| 4 | M | 20 | P | 5.2 | III | 24 | 120 mg per mo sc × 4 | 6 | 1 | |
| 5 | M | 29 | P | 4.7 | III | 22 | 120 mg per mo sc × 4 | 7 | 2 | |
| 6 | F | 56 | P | 4.8 | III | 22 | 120 mg per mo sc × 4 | 5 | 0 | |
| 7 | M | 26 | P | 5.9 | III | 22 | 120 mg per mo sc × 4 | 4 | 1 | |
| 8 | M | 26 | P | 5.0 | III | 21 | 120 mg per mo sc × 6 | 5 | 1 | |
| Mean ± SD | – | 30.6 ± 12 | – | 5.1 ± 0.4 | – | 23.5 ± 2.1 | – | 5.6 ± 1.1 | 1 ± 0.8 | – |
Time from treatment until last followup (months).
p < 0.05 indicated a significant difference between pre- and post-treament (paired t-test).sc: subcutaneously; VAS: visual analogue scale.
To further understand the underline mechanism that denosumab's clinical effect on GCTB patients, we reviewed the radiological and pathological results of those patients before and after denosumab treatment.
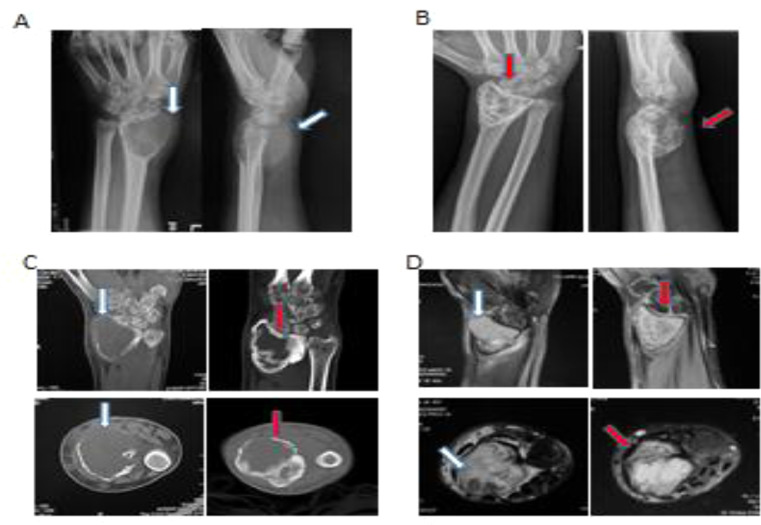
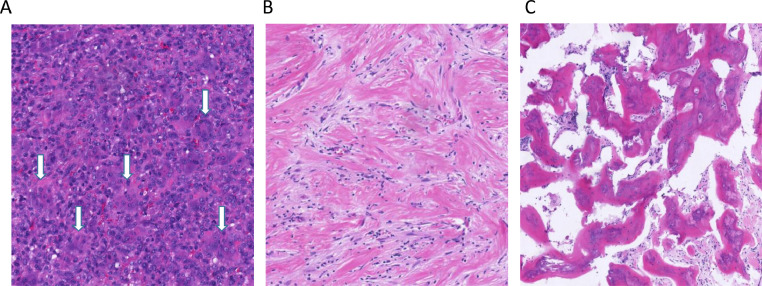
In contrast to the baseline images, radiological graphs acquired after the cessation of drug, clearly demonstrated the peri-articular lytic lesion and thinning cortex observed at diagnosis, had developed intact boundary with cortical thickening and substantial sclerosis in the subchondral/central region owing to the significant new bone formation. Even in cases which invaded into adjacent soft-tissue by breaking through cortex, there were found to form an intact layer of bone surrounding the mass after denosumab administration (Fig. 6). Accordingly, the above radiological evidences of bone formation were further confirmed by pathological assessments revealing that giant cells had been suppressed >90% and wove bone had been formed in all cases (Fig. 7).
Fig. 6.
Representative radiological images of a 40-year-old man with left wrist pain for 7 months who was diagnosed with giant cell tumor (GCT) and received denosumab treatment (four times). (A) Anteroposterior and lateral radiographs: a peri-articular lytic lesion with involvement of the articular surface, cortical bone and soft tissue (white arrows), which obtained significant cortical and subchondral thickening, reconstitution of an intact cortex circumferentially, and central sclerosis (B, red arrows) after denosumab; (C) Left: computed tomography (CT) images acquired at baseline presenting an osteolytic lesion, approximately 5 cm in size, noted in the left distal radius. Cortical thinning, destruction and soft tissue extension (white arrow) of the tumor are also observed, without a sclerotic rim. Right: in comparison with the baseline images, CT images acquired 12weeks after the first denosumab injection show definite new bone formation in the periphery of the cross-sectional lines of the tumor (red arrows); this might indicate tumor response to denosumab treatment. (D) Magnetic Resonance imaging (MRI) images indicate the soft tissue edema (white arrows) is disappeared (red arrows) after denosumab treatment. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 7.
Representative microscopic images for histological samples of pre- or post-denosumab treatment. (A) Pre-treatment biopsies were highly similar and were typical for GCTB in all cases. They were characterized by the presence of very large numbers of osteoclast-like giant cells what appear uniformly scattered amongst numerous round to ovoid mononuclear cells (tumor cells, white arrow). (B and C) In the post-treatment tumor samples, osteoclast-like giant cells were nearly absent, but fibrosis and collagenzation were present in the most area. The increased bone matrix, woven bone, or new bone were also commonly observed.
4. Discussion
GCTB in distal radius is a benign but sever bone tumor characterized by strong invasive behavior and high recurrent rate. For the primary surgical treatment, the preferred strategy is to preserve structural intactness with adequate surgical margin. Therefore comprehend understanding of prognosis factor and thorough surgical preparation are demanded to ensure the optimized balance between oncologic cure and functional satisfaction.
Generally, with the mere radiological, histological or other clinical factors, the prediction of oncologic and functional prognosis in the GCTB patients is insufficiently accurate.
Previously proposed risk factors—tumor nature or pathological fracture—could not be confirmed to affect local recurrence in the present study [19]. It had been claimed young age could be risk factor related to RFS of GCTB, which might be a result of the more active bone metabolism in younger population [20,21]. However, we have found that age less than 33 was not a major factor influencing the RFS of GCTB (Table 2), while female patients had a marginally higher RFS than male ones, which suggested the influence of gender on the recurrence rate (p = 0.0435). But when combined with other factors in a step-wise multivariate Cox regression analysis, gender was not an independent predictor impacting the recurrence (Table 3). The decisive clinic-pathologic factors for the recurrence rate change were turned out to be soft tissue extension and tumor size. Soft tissue extension existed in 24 patients in the current study, and the calculation of HR from our multivariate Cox regression is 5.321 (p = 0.011), strongly implying the increase of recurrence rate of GCTB attributed to soft tissue extension. In addition, tumor diameter ≥5 cm (30/58 patients) also turned out to be an independent predictor of local recurrence in GCTB in distal radius (HR: 3.893, p = 0.034). It can be explained by the difficulty in achieving complete surgical margin in cases of soft tissue extension or over size, which eventually resulted in the high recurrence rate [22,23].
The overall recurrence rate of GCTB in radius is 25.9% (15/58) in the present study, which is similar to previous study and relative higher than that of other long bone [1,2]. The reason might be the incomplete exposure and dissection owing to the local anatomical characteristics. In order to reduce recurrence rate, en-bloc resection was more commonly chosen than curettage in this location. In the current study with the number of patients analyzed, the recurrent rate in the curettage group (5/21, 27%) is similar to the resection group (10/37, 23.8%) according to the Kaplan–Meier survival analysis (p = 0.8537), which might indicate that with adequate local adjuvant modalities (high speed burring, iodine tincture, electrocautery and PMMA), intra-lesional curettage might have comparable local control of GCTB compared to en-bloc resection [11,20]. But this result might be biased by the choice of surgical procedure for the patients underwent en bloc resection were those with subtantial tumor mass. In those advanced GCTB patients, due to the insufficient number of patients, the impacts of the types of adjuvants to curettage on the local recurrence were not further evaluated in the current study, but it was widely reported that the risk-reducing effect of PMMA [20] and additional high speed burring was of great importance [11].
En bloc resection might achieved better local control, but the reconstruction is never easy and the function of long term was not so satisfied. In the current study, we found the complication rate and re-operation risk were much higher in the resection group, which will sacrifice the function and range of motion. Indeed, we found poorer results by VAS scores (3.6 vs. 2.5, p = 0.011), MSTS (16.7 vs. 20.2, p = 0.034) and DASH (16.4 vs. 9.1, p = 0.030) in the resection group in contrast to the intralesional curettage group, respectively. Additionally, with the increasing demand for preservation of wrist function and motion, especially in this young and active population, a less destructive option is preferred [6]. Therefore, owing to the debilitating side effects and complications, wide resection might not be considered as a standard method for GCTB treatment.
To some extent, the current study demonstrates possibility that curettage were the feasible first-choice treatment option for GCTB due to a similar RFS but a simpler performance and a better clinical outcome. Even if the recurrence were to occur after curettage, the possibility and applicability for the secondary resection were still reserved.
Consistent with previous reports [22], [23], the radiographic presentations (soft-tissue extension and tumor size) are the strong predictors related to local recurrence in the current study, providing significant decision guiding of clinical strategy for surgeons.
Recently, the emergent denosumab has been proved to be a promising neoadjuvant chemotherapeutic agent for GCTB by its dramatic pain reduction and strong tumor suppression [13], [14], [15], [16]. However, controversy exists on denosumab in patients with GCTB in the distal radius and its recurrence. The Kaplan–Meier survival analysis did not interfered denosumab as a critical factor related to RFS of survival in the current study, and a recent systematic review [24] similarly concluded that denosumab did not show any effect on reducing tumor recurrence. On the contrary, the denosumab cessation potentially resulted in subsequent recover of the tumor cells, which was recently reported in another GCT cell cultures study [25]. This study observed that the giant cells were almost totally cleared by the denosumab treatment, but the neoplastic stromal cells, which are tumor cells, remained alive and continuously proliferating [25]. These data were implying that denosumab might target on the effector cells rather than the tumor cells, therefore the withdrawal of which will lead to higher recurrence rate.
However, as a result of the pre-operative treatment with denosumab, 7 of 8 (87.5%) Campanacci Grade Ⅲ GCTB patients underwent joint preserving surgeries in the current study. The decision-making could have been expected by the significant new bone formation in the cortical and subchondral area which was evident in 8/8 (100%) patients. In addition, the preoperative denosumab treatment generated substantial pain reduction. In those patients with denosuamab treatment VAS score were sharply reduced from 5.6 to 1.0 (p < 0.0001). Our post-operative pathological evaluation of the patients with denosumab treatment supported by the results from others as well [17], [26], [27], [28], [29]: denosumab was effective in inhibiting the activity and recruitment of osteoclast-like giant cells and thereby preventing bone absorption and promoting bone repairmen. More importantly, it provides for a calcified layer covering the soft tissue mass typically seen in advanced GCTB, ensuring the feasibility for intralesional curettage with local adjuvants even in GCTB with advanced status [7].
The current study had several limitations. (1) It is a retrospective analysis with a small sample size in a single center, but a large randomized, controlled multi-center trial was impractical for such rare tumors. Nevertheless, the conclusions should be further confirmed by a larger prospective study with longer follow-up. With larger numbers, longer follow-up, some of the expected risk factors regarding RFS might have become apparent, especially the long-term adjuvant effects of denosumab on the recurrence, as in that group, there were only eight patients with almost 2-years follow-up. (2) Patients with less invasive lesion might prefer to be treated with less destructive surgery than those with higher-grade disease. Such selection bias might explain partly the better pain score and functional outcomes in curettage group than en bloc resection group. This selection bias is somewhat unavoidable as en bloc resection other than intralesional curettage was commonly adopted as a first-choice of surgery for aggressive lesion in distal radius. (3) There were substantial heterogeneities within the surgical performance even in the same group, especially the different types of bone defect filling and the varied adjuvants used during intralesional curettage. Therefore the strength of the conclusion that state intralesional curettage is a more appropriate surgical method over resection is limited. But the results of curettage in our patients provided them with better function and comparable local control.
5. Conclusion
The radiographic presentations (soft-tissue extension and tumor size) are the strong predictors related to local recurrence. Curettage could be a feasible first-choice treatment option for most GCTB due to a similar RFS but a simpler performance and a better clinical outcome compared to resections. Denosumab is a promising neoadjuvant that further ensure the appropriateness of intralesional curettage in even more severe cases. Our study might provide potential guiding significance for the eligible treatment of GCTB in the distal radius in the future.
Acknowledgments
Precis
The radiographic presentations (soft-tissue extension and tumor size) are the strong predictors related to local recurrence of giant cell tumor of distal radius. The application of denosumab is a promising neoadjuvant that ensure ideal prognosis results and surgical condition for GCTB patients with soft-tissue extension.
Funding
The project was supported by Guangdong Natural Science Foundation (2018A030313077, 2016A030313215), National Natural Science Foundation of China (81703017), Science and Technology Program of Guangzhou, China (201804010080).
Conflict of interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Author contributions
All authors contributed to the conceptions of the study and interpretation of data. The study was designed by ZCY, YJQ and SJN. ZCY, LT and ZZQ performed the literature search and collected data. ZCY, LT, ZZQ, WB, HG and LB analyze and interpret data. ZCY, LT and HG drafted the manuscript with input from all the authors. The approval of the final version of the manuscript was given by all the authors. YJQ and SJN acts as the guarantors. All the authors had full access to all of the data, including statistical reports and figures, in the study and can take responsibility for the integrity of the data and the accuracy of the analysis.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jbo.2018.100211.
Contributor Information
Junqiang Yin, Email: yinjunqiang77@163.com.
Jingnan Shen, Email: shenjingnan@126.com.
Appendix. Supplementary materials
References
- 1.Panchwagh Y. Giant cell tumor – distal end radius: do we know the answer? Indian J. Orthop. 2007;41(2):139–145. doi: 10.4103/0019-5413.32046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Errani C. Giant cell tumor of the extremity: a review of 349 cases from a single institution. Cancer Treat. Rev. 2010;36(1):1–7. doi: 10.1016/j.ctrv.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Siddiqui M.A., Seng C., Tan M.H. Risk factors for recurrence of giant cell tumours of bone. J. Orthop. Surg. (Hong Kong) 2014;22(1):108–110. doi: 10.1177/230949901402200127. [DOI] [PubMed] [Google Scholar]
- 4.Saini R. En bloc excision and autogenous fibular reconstruction for aggressive giant cell tumor of distal radius: a report of 12 cases and review of literature. J. Orthop. Surg. Res. 2011;6:14. doi: 10.1186/1749-799X-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wysocki R.W. Is intralesional treatment of giant cell tumor of the distal radius comparable to resection with respect to local control and functional outcome? Clin. Orthop. Relat. Res. 2015;473(2):706–715. doi: 10.1007/s11999-014-4054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damert H.G., Altmann S., Kraus A. Custom-made wrist prosthesis in a patient with giant cell tumor of the distal radius. Arch. Orthop. Trauma Surg. 2013;133(5):713–719. doi: 10.1007/s00402-013-1692-y. [DOI] [PubMed] [Google Scholar]
- 7.van der Heijden L. Giant cell tumour of bone in the denosumab era. Eur. J. Cancer. 2017;77:75–83. doi: 10.1016/j.ejca.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 8.Teixeira L.E. Giant cell tumors of bone: nonsurgical factors associated with local recurrence. Acta Orthop. Traumatol. Turc. 2014;48(2):136–140. doi: 10.3944/AOTT.2014.2714. [DOI] [PubMed] [Google Scholar]
- 9.O'Donnell R.J. Recurrence of giant-cell tumors of the long bones after curettage and packing with cement. J. Bone Joint Surg. Am. 1994;76(12):1827–1833. doi: 10.2106/00004623-199412000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Becker W.T. Local recurrence of giant cell tumor of bone after intralesional treatment with and without adjuvant therapy. J. Bone Joint Surg. Am. 2008;90(5):1060–1067. doi: 10.2106/JBJS.D.02771. [DOI] [PubMed] [Google Scholar]
- 11.Gouin F., Dumaine V. Local recurrence after curettage treatment of giant cell tumors in peripheral bones: retrospective study by the GSF–GETO (French Sarcoma and Bone Tumor Study Groups) Orthop. Traumatol. Surg. Res. 2013;99(6 Suppl):S313–S318. doi: 10.1016/j.otsr.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 12.McClung M.R. Denosumab in postmenopausal women with low bone mineral density. N. Engl. J. Med. 2006;354(8):821–831. doi: 10.1056/NEJMoa044459. [DOI] [PubMed] [Google Scholar]
- 13.Branstetter D.G. Denosumab induces tumor reduction and bone formation in patients with giant-cell tumor of bone. Clin. Cancer Res. 2012;18(16):4415–4424. doi: 10.1158/1078-0432.CCR-12-0578. [DOI] [PubMed] [Google Scholar]
- 14.Erdogan K.E. Morphologic evaluation of the effect of denosumab on giant cell tumors of bone and a new grading scheme. Pol. J. Pathol. 2016;67(4):392–397. doi: 10.5114/pjp.2016.65873. [DOI] [PubMed] [Google Scholar]
- 15.Martin-Broto J. Effects of denosumab on pain and analgesic use in giant cell tumor of bone: interim results from a phase II study. Acta Oncol. 2014;53(9):1173–1179. doi: 10.3109/0284186X.2014.910313. [DOI] [PubMed] [Google Scholar]
- 16.Ueda T. Objective tumor response to denosumab in patients with giant cell tumor of bone: a multicenter phase II trial. Ann. Oncol. 2015;26(10):2149–2154. doi: 10.1093/annonc/mdv307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Traub F. Efficacy of denosumab in joint preservation for patients with giant cell tumour of the bone. Eur. J. Cancer. 2016;59:1–12. doi: 10.1016/j.ejca.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Enneking W.F. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin. Orthop. Relat. Res. 1993;(286):241–246. [PubMed] [Google Scholar]
- 19.Wang C.S. Recurrence in giant cell tumour of bone: imaging features and risk factors. Radiol. Med. 2013;118(3):456–464. doi: 10.1007/s11547-012-0860-4. [DOI] [PubMed] [Google Scholar]
- 20.Klenke F.M. Recurrent giant cell tumor of long bones: analysis of surgical management. Clin. Orthop. Relat. Res. 2011;469(4):1181–1187. doi: 10.1007/s11999-010-1560-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kivioja A.H. Cement is recommended in intralesional surgery of giant cell tumors: a Scandinavian Sarcoma Group study of 294 patients followed for a median time of 5 years. Acta Orthop. 2008;79(1):86–93. doi: 10.1080/17453670710014815. [DOI] [PubMed] [Google Scholar]
- 22.Li D. Surgery methods and soft tissue extension are the potential risk factors of local recurrence in giant cell tumor of bone. World J Surg Oncol. 2016;14:114. doi: 10.1186/s12957-016-0871-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Heijden L., van de Sande M.A., Dijkstra P.D. Soft tissue extension increases the risk of local recurrence after curettage with adjuvants for giant-cell tumor of the long bones. Acta Orthop. 2012;83(4):401–405. doi: 10.3109/17453674.2012.711193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jamshidi K. Denosumab in patients with giant cell tumor and its recurrence: a systematic review. Arch. Bone Jt. Surg. 2018;6(4):260–268. [PMC free article] [PubMed] [Google Scholar]
- 25.Mak I.W. A translational study of the neoplastic cells of giant cell tumor of bone following Neoadjuvant Denosumab. J. Bone Joint Surg. Am. 2014;96(15):e127. doi: 10.2106/JBJS.M.01332. [DOI] [PubMed] [Google Scholar]
- 26.Deveci M.A. Clinical and pathological results of denosumab treatment for giant cell tumors of bone: prospective study of 14 cases. Acta Orthop. Traumatol. Turc. 2017;51(1):1–6. doi: 10.1016/j.aott.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmerini E. Denosumab in advanced/unresectable giant-cell tumour of bone (GCTB): for how long? Eur. J. Cancer. 2017;76:118–124. doi: 10.1016/j.ejca.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 28.Thomas D. Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol. 2010;11(3):275–280. doi: 10.1016/S1470-2045(10)70010-3. [DOI] [PubMed] [Google Scholar]
- 29.Yi J. Response evaluation of giant-cell tumor of bone treated by denosumab: histogram and texture analysis of CT images. J. Orthop. Sci. 2018;23(3):570–577. doi: 10.1016/j.jos.2018.01.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.