Abstract
Purpose
Evaluation of the safety and effectiveness of a TearCare® retreatment in adult subjects with clinically significant dry eye disease (DED).
Patients and methods
This was an extension of an initial 6-month, prospective, single-center, randomized, parallel-group pilot study. In the initial study, subjects with DED were randomized to either a single TearCare® treatment or 4 weeks of daily warm compress therapy. The extension study involved retreatment of those subjects assigned to the TearCare® treatment group following the initial 6-month end point. At 6 months, subjects were evaluated for the clinical signs and symptoms of DED prior to retreatment in the extension study that would measure the safety, effectiveness, and durability of a TearCare® retreatment for another 6 months through a 12-month end point. The TearCare® retreatment procedure consisted of 12 minutes of thermal eyelid treatment immediately followed by manual meibomian gland clearance. The primary effectiveness end point was the change in tear break-up time (TBUT) from baseline to 1-month follow-up. Secondary end points included meibomian gland scores, corneal and conjunctival staining scores, and assessment of dry eye symptoms. Safety was evaluated through monitoring intraocular pressure, best-corrected visual acuity, and device-related adverse events.
Results
Twelve subjects participated in the 6-month extension study. At 1-month clinic visit following retreatment, a significant improvement from baseline in mean (± SD) TBUT of 12.4 (±3.3) seconds was observed (P<0.001). Significant improvements in the mean change from baseline in meibomian gland scores, corneal and conjunctival staining scores, and symptoms of DED were also observed following retreatment. The second treatment was well tolerated.
Conclusion
The findings of the extension study through 12 months suggest that a second TearCare® treatment after 6 months provides additional improvement in the signs and symptoms of DED.
Keywords: dry eye disease, meibomian gland dysfunction, warm compresses, evaporative dry eye, blepharitis
Introduction
Dry eye disease (DED) is a chronic disease of the ocular surface frequently encountered in the eye clinic.1 In the USA, DED has been estimated to affect 5%–50% of the population, and the prevalence increases with age.2,3 Other research shows that up to 40 million people either suffer from DED or are predisposed to the condition.4 While it has been determined that there is no increased risk attributable to race, education, or US Census region, many risk factors have been found to be associated with the development of DED. These risks include female gender, increasing age, insurance status, contact lens wear, electronic device use, and hormonal dysfunction.3
The ocular surface, lacrimal apparatus, and eyelids act in a coordinated fashion to maintain a stable, effective refractive surface, to resist injury, and to resist infection. Disruption of the homeostasis of this system can cause the vicious cycle of DED.5
In more mild cases, DED may be little more than a nuisance. Subjects may experience mild fluctuating vision or foreign body sensation in the eyes. However, often visual acuity may be adversely affected, and this can interfere with common, essential visual tasks such as driving, reading, or using devices. Prolonged or more severe forms of DED can further damage the ocular surface which has direct and measurable impact on quality of life.2,6 In particularly severe cases, corneal scarring or ocular surface damage may result in profound visual loss.7,8
DED is also becoming more of a concern in perioperative patients undergoing procedures such as corneal refractive surgery and cataract surgery.9 Additionally, the increasing use of so-called “premium” intraocular lenses and femtosecond laser-assisted cataract surgery has drawn further attention to DED. This is particularly true given that patients undergoing such procedures understandably have high postoperative expectations. In the past, efforts were weighted toward managing postoperative dry eye following LASIK, for example. However, there are now efforts to “optimize the ocular surface” prior to cataract surgery or refractive surgery in order to provide patients with better calculations and outcomes.
Since DED was formally defined as an ocular disease, there has been an evolution in the understanding of this ocular surface disease. Originally, DED, as its name suggests, was felt to be a disease of aqueous insufficiency of the tear film.10 This thinking persisted for years, and the primary modalities of treatment included the use of artificial tears, ointments, and punctal plugs. These were directed at supplementing the aqueous layer or shoring up the tear film. However, this perspective changed as a result of increasing evidence-based approaches which would lead to a convergence between meibomian gland dysfunction (MGD) and dry eye. Led primarily by the International Dry Eye Workshop and also the International Workshop on Meibomian Gland Dysfunction, research has shown the involvement of meibomian glands in maintaining a healthy, evaporation resistant tear film.10,11 Conversely, in the presence of MGD, DED is frequently encountered. This association has led to the term “evaporative dry eye”.12 Note that this does not exclude existence of aqueous deficient dry eye. Rather, it appears that patients with DED may actually suffer from either aqueous deficient dry eye, evaporative dry eye, or both.
One of the primary functions of the meibomian glands is the secretion of an oily substance, meibum, which reduces evaporation. While there is great debate over the total role of the lipid component of the tear film, there is accumulating evidence that the tear film lipids impart viscoelastic properties and thereby stabilize the tear film.13 The natural blinking mechanism, presumably with a contribution from the muscle of Riolan, is believed to facilitate physiologic expression of meibum from the meibomian glands into the tear film.12 MGD has been shown to be associated with decreased tear break-up time (TBUT), increased tear evaporation, and resultant dry eye. The role of MGD with respect to tear film disturbances has been demonstrated by Nichols in 2011.11 Furthermore, Lemp showed in 2012 that while patients may demonstrate both aqueous deficient dry eye and evaporative dry eye, the majority of patients have evaporative dry eye or at least a component of evaporative dry eye.14
Obstruction of the meibomian glands by inspissated meibum is thought to be associated with the evaporative dry eye component of MGD. Studies have demonstrated that meibum in patients with MGD has higher phase transition temperatures.15 In other words, the meibum needs to reach higher temperatures in order for the fatty acids comprising it to become more disordered rendering it “softer” or more liquid. Perhaps, this is why traditional treatments with warm compresses have had variable success in treating MGD. Increasing evidence points not only to the theory of gland obstruction but also to the therapeutic role of clearing these obstructions in the successful treatment of DED.16,17
In a study by Blackie et al, it was demonstrated that stringently controlled, externally applied warm compress therapy is able to achieve and maintain the necessary temperatures to melt or soften meibum.18 While impractical in a clinical or home setting, such a controlled, properly positioned, and labor-intensive successive warm compress approach demonstrates that external heat delivery can consistently achieve therapeutic external and inner eyelid temperatures for clearing obstructed glands. Olson et al conducted a similar series of stringently controlled and successive warm compress treatments.19 In these subjects where therapeutic temperatures were achieved and maintained for a sufficient period of time using external compress therapy, they found an increase in tear film lipid layer thickness and TBUTs. Of course, it remains impractical to apply and replace precisely warmed compresses every 2 minutes at home on a daily basis to achieve such results. These studies do demonstrate, however, that external application of heat, if done in a controlled and stringent manner, can consistently achieve therapeutic temperatures all the way from the outside of the lid to the inner eyelid.
While it is widely prescribed, warm compress therapy for the treatment of MGD or evaporative dry eye suffers from many shortcomings.20–22 It has been shown that it is challenging to achieve and maintain therapeutically warm (>40°C) compresses in the clinic or at home.21 For instance, the compress may be too cool initially or may cool off too quickly. On the other hand, a compress that is too warm may cause injury to the eyelid or even the eye. Also, the surface of the warm compress may have a temperature profile that is not uniform, with >10°C variation, delivering different amounts of heat and possibly affecting patient outcomes (internal data). Warm compresses lack a conforming and ergonomic fit as well, and this may further hamper the delivery of obstruction clearing thermal energy to each meibomian gland across the eyelid’s contoured and irregular anatomy. Since warm compress therapy is performed with the eyes closed, it does not exploit the act of normal blinking’s role in expression of meibum and gland clearing. Finally, warm compress therapy is limited by the fact that it is time-consuming, labor intensive, and requires lifelong daily therapy. As a result, well-known patient compliance problems arise.
Other approaches to clearing obstructed meibomian glands include patient-administered eyelid massage, in-office gland expression performed by the physician,23 or treatments with devices such as LipiFLow® (TearScience, a Johnson and Johnson Vision company, Morrisville, NC, USA).24 In terms of patient-administered massage, drawbacks include lack of consistency and long-term patient compliance. Moreover, there is the potential of altering or disrupting the eyelid anatomy. Also, there may be associations with various forms of eye rubbing or external pressure and keratoconus, retinal detachment, and other ocular conditions.25,26 On the other hand, in-office, “cold” meibomian gland expression can be uncomfortable or painful to the patient and may be limited in its ability to evacuate hardened or inspissated meibum from the glands.23
The TearCare® system (Sight Sciences, Inc, Menlo Park, CA, USA) is an in-office treatment that delivers thermal energy to the eyelids for patients suffering from DED.27 Described here are the results of a 6-month extension to the pilot study,27 evaluating the safety and effectiveness of a second TearCare® treatment for another 6 months in subjects who had been randomized to receive TearCare® treatment as part of the initial study design.
Patients and methods
This study was a 6-month extension of an initial 6-month, prospective, single-center, randomized pilot clinical trial whose results were previously reported.27 This extension study was conducted in full accordance with the tenets of the Declaration of Helsinki and US Food and Drug Administration regulations for the protection of human subjects in medical research. Only a subset of patients, those who had received the investigational TearCare® treatment in the initial study, were enrolled in this extension study and were retreated with the TearCare® treatment and followed for another 6 months.
All of the evaluations and therapy were performed at a clinical ophthalmology practice by a single board-certified ophthalmologist and cornea subspecialist. Additionally, all participants completed self-administered questionnaires at the ophthalmologist’s practice. The study documents were reviewed and approved by the Aspire Institutional Review Board (IRB) (Aspire IRB, Santee, CA, USA) as a non-significant risk study. The study (registry number NCT03006978) was also registered at the US clinical trials website (ClinicalTrials.gov).
All trial participants were required to read and sign the IRB-approved informed consent form prior to initiation of any study-related evaluations or interventions. Participants in the study were originally derived from the patient population at the clinical trial site. It was also at this site where all evaluations and interventions were conducted. Subjects were originally screened for suitability for inclusion in the pilot study based on the following criteria: at least 18 years of age, reports of dry eye symptoms within 3 months of the screening assessed through the Standard Patient Evaluation for Eye Dryness II (SPEED II)28,29 questionnaire score of ≥6 and a Schirmer I tear test score of ≤10 mm in at least one eye, and/or a TBUT of <10 seconds in at least one eye. Since all subjects for this continuation trial had met these criteria and had also undergone an initial TearCare® treatment ~6 months previously, they were included in this trial.27 All subjects who were originally randomized to the TearCare® arm of the study volunteered to proceed with this extension of the pilot study.
Subjects were excluded from the pilot study based on several exclusion criteria. Subjects with a presence or history of ocular inflammation, infection, systemic disease, or other abnormalities that may have adversely affected their ability to complete the trial or that may have confounded the results of the assessments in the opinion of the investigator. Also excluded were subjects with a history of recent ocular surgery; recent history (within past month) of topical medication use, including antibiotics, steroids, NSAIDs; or who required the chronic use of topical ophthalmic medications. Subjects using prescription medications for the treatment of their DED or MGD had to undergo a washout period between the screening and baseline evaluations to be eligible for participation in the study. For topical anti-inflammatory medications such as cyclosporine or lifitegrast, the washout period was 2 months. For oral tetracycline class agents, such as minocycline or doxycycline, the washout period was 2 weeks. However, subjects using artificial tears or topical lubricants were allowed to continue using their lubricants without need for cessation.27
In the extension study, subjects were required to complete a total of four visits: extension baseline/retreatment and at 1, 3, and 6 months post-retreatment. At the extension baseline/retreatment visit, the subject’s ocular history was reviewed. At the extension baseline and all subsequent visits, the subject’s best-corrected visual acuity (BCVA) was evaluated, a slit-lamp examination was conducted, and corneal and conjunctival staining were assessed.27 TBUT, intraocular pressure (IOP), and meibomian gland secretions were measured at each visit. Subjects completed the SPEED, Symptom Assessment in Dry Eye (SANDE),30 and Ocular Surface Disease Index (OSDI)31 questionnaires at each visit. In order to avoid iatrogenic ocular surface staining confounding any results, the evaluations were conducted in the following sequence: BCVA assessment, TBUT, corneal fluorescein staining, conjunctival lissamine green staining, meibomian gland assessment, and IOP measurement. Both eyes were evaluated for each subject.
Adverse events (AEs) were recorded at each study visit.
Study design
Subjects who had previously qualified for the original clinical trial described, had successfully completed the trial, and had initially received the investigational TearCare® treatment were invited to participate as potential subjects in this extension trial. Since all patients received a repeat TearCare® treatment, there was no randomization or masking.
TearCare® System
The TearCare® system consists of the SmartHub, charging nest, charging adaptor, and the single-use SmartLid™ devices (Sight Sciences, Inc) that are attached to the upper and lower eyelids of both eyes. In order to ensure good adhesion of the SmartLid™ device, the subject’s eyelids were cleaned with an off-the-shelf makeup wipe prior to treatment to remove any makeup, debris, or skin oils. A SmartLid™ device was affixed to the external eyelid surfaces of each eyelid parallel to the eyelid margin to overlay all the meibomian glands in all four eyelids. Subsequently, the SmartHub was activated to initiate a treatment. Treatment consists of 12 minutes of controlled and targeted delivery of thermal energy, ranging from 41°C to 45°C, to the eyelids. Subjects were queried throughout the treatment to ensure comfort throughout the procedure. Additionally, the subjects were encouraged to blink normally throughout the procedure in order to take advantage of the natural meibum expression forces at a time when meibum is in a more melted or disordered phase. Next, the SmartLid™ devices were removed, and a drop of topical 0.5% tetracaine was applied into the conjunctival fornix of each eye. Meibomian gland clearing was performed under slit lamp visualization using meibomian gland forceps (Rhein Medical Inc, St Petersburg, FL, USA).
Study end points
The primary efficacy end point in the extension study was the change from baseline in TBUT at 4 weeks after retreatment. The secondary efficacy end points included the change from baseline in: meibomian gland assessment scores, corneal and conjunctival staining scores, and patient-reported symptom scores from the SPEED, OSDI, and SANDE questionnaires. Safety was assessed through the recording of AEs, changes in IOP, and BCVA.
Statistical analyses
No calculations were conducted to determine the size of the study population due to the exploratory nature of this pilot study. For each variable, the differences from baseline (Day 0) were calculated as the difference between the measurements at each follow-up visit and the Day 0 measurement. Then mean and SD were calculated for change from baseline at each follow-up time.
For differences from baseline (Day 0), the P-values were the result of separate tests of whether the mean difference =0 for each follow-up time listed. There were no corrections for multiplicity. For eye-level measurements (TBUT, corneal staining, conjunctival staining, meibomian gland score), the P-values were from simple mixed-effects linear models (with no covariates) that allow for within-subject correlation. For person-level measurements (SPEED, SANDE, and OSDI scores), the P-values were from a paired t-test. A P-value ≤0.05 was the threshold for determining statistical significance. All statistical analyses were carried out using R (version 3.3.3).
Results
Study populations
All subjects who were originally randomized to the TearCare® system (12 subjects; 24 eyes) at initiation of the initial pilot study were enrolled in this extension study. The mean ± SD age of this group at the initial baseline evaluation was 69.3±11.5 years, and all participants were female. All of the subjects were also white. The baseline characteristics of the study population with respect to the efficacy end points at study initiation (Day 0) and prior to retreatment at Month 7 is presented in Table 1.
Table 1.
Baseline characteristics of the study population
| Characteristics
|
Study initiation (n=12) | Prior to second treatmenta (n=12) |
|---|---|---|
| End point – mean (SD) | ||
|
| ||
| Tear break-up time (seconds) | 3.1 (0.8) | 5.0 (1.1) |
| Meibomian gland score | 6.3 (3.6) | 22.0 (8.2) |
| Corneal staining score | 3.5 (1.8) | 0.2 (0.4) |
| Conjunctival staining score | 3.7 (2.5) | 1.0 (1.4) |
| SPEED score | 15.7 (5.2) | 10.0 (5.4) |
| SANDE score | 64.9 (25.9) | 47.1 (25.8) |
| OSDI score | 41.0 (18.4) | 31.4 (18.6) |
Note:
Baseline characteristics of the study population at the Month 7 clinic visit prior to the second treatment by the TearCare® system.
Abbreviations: OSDI, Ocular Surface Disease Index; SANDE, Symptom Assessment in Dry Eye; SPEED, Standard Patient Evaluation for Eye Dryness.
Tear break-up time (primary end point)
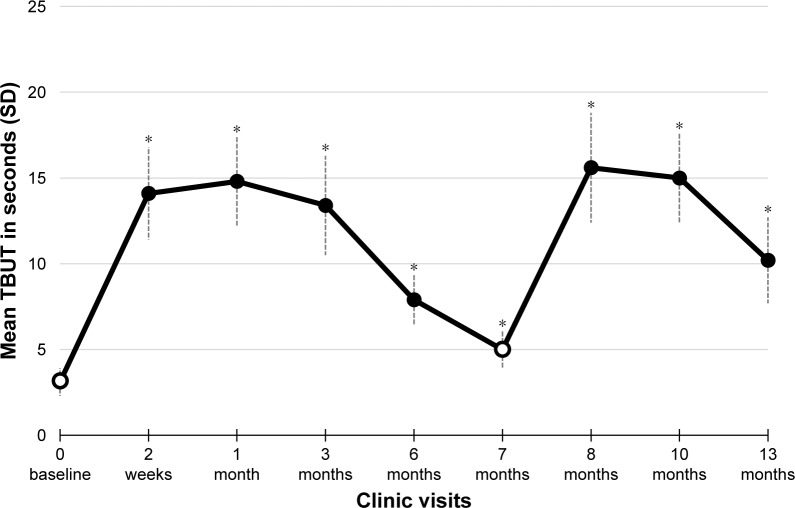
There was a significant improvement in TBUT after both the initial TearCare® treatment and upon retreatment. At initial baseline, TBUT averaged 3.1±0.8 seconds for this group. Over the initial 6 months, it reached an average value as high as 14.8 seconds and gradually decreased to 5.0 seconds before the retreatment at 7 months. Upon retreatment, TBUT improved again peaking at Month 8 with a mean ± SD of 15.6±3.2 seconds, indicating a mean change from baseline (Day 0) of 12.4±3.3 seconds (P<0.001). Six months later, the value of TBUT had decreased to 10.2±2.5 seconds which was well above the two original baseline values, initiation of trial and at retreatment. The mean TBUT for the study population is presented in Figure 1.
Figure 1.
Primary efficacy end point – tear break-up time (TBUT) results.
Notes: The mean (± SD) TBUT values measured in seconds are presented by study visit. Baseline assessments (indicated by open circles) were conducted prior to TearCare® treatment at study initiation (Day 0) and at Month 7. Significant improvements in change from baseline (increases in the score from baseline Day 0) in TBUT were observed at each post-baseline visit. *P<0.001.
Corneal and conjunctival staining
Subjects receiving a second TearCare® treatment demonstrated significant improvements in both corneal and conjunctival staining. At initial baseline, mean corneal and conjunctival staining scores were 3.5±1.8 and 3.7±2.5, respectively. Before the second treatment was administered at Month 7, corneal and conjunctival staining averaged 0.2±0.4 and 1.0±1.4, respectively. Upon retreatment, corneal staining scores dropped to an average of 0 and eventually reached 0.5±0.7 (P<0.001) at the 6-month conclusion of this extension study, or ~13 months after initial baseline. Conjunctival staining also dropped to as low as an average of 0 and at the 6-month conclusion of the extension study was at 0.2±0.6, which was also well below the initial baseline value (P<0.001). These mean corneal and conjunctival staining results are summarized by study visit in Table 2.
Table 2.
Outcomes for ocular surface staining assessments
| TearCare® treatment | Time from first treatment | Corneal staining, mean (SD) (n=12) | Conjunctival staining, mean (SD) (n=12) |
|---|---|---|---|
|
| |||
| First treatment | Baseline (Day 0) | 3.5 (1.8) | 3.7 (2.5) |
| 2 weeks | 0.2 (0.4)a | 0.2 (0.4)a | |
| 1 month | 0.2 (0.4)a | 0.1 (0.3)a | |
| 3 month | 0.2 (0.4)a | 0.2 (0.6)a | |
| 6 months | 0.5 (0.7)a | 0.3 (0.7)a | |
|
| |||
| Second treatment | 7 months | 0.2 (0.4)a | 1.0 (1.4)a |
| 8 months | 0.0 (0.0)a | 0.5 (1.2)a | |
| 10 months | 0.0 (0.0)a | 0.0 (0.0)a | |
| 13 months | 0.5 (0.7)a | 0.2 (0.6)a | |
Notes: TearCare® treatment performed at the end of the baseline (Day 0) and Month 7 clinic visits.
P<0.001 in comparison to Day 0.
Meibomian gland scores
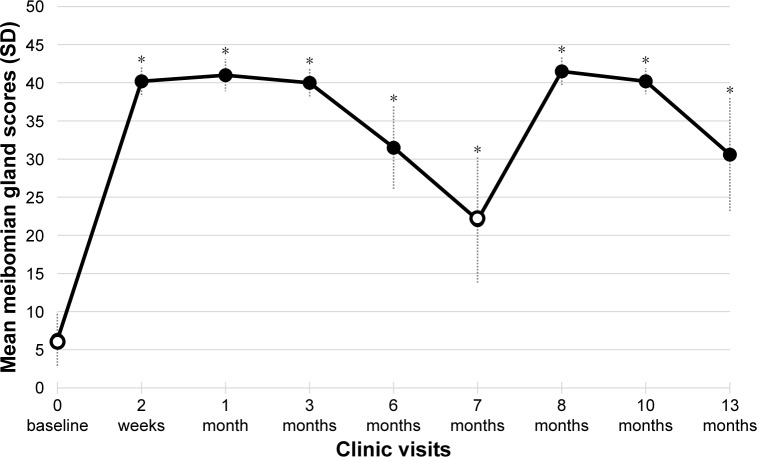
At baseline of the initial study, the mean ± SD meibomian gland score was poor at 6.3±3.6. Following the initial treatment, mean meibomian gland scores peaked to 41.0±2.1 at the 1-month visit. At the retreatment baseline of the extension study, the mean meibomian gland scores remained elevated from initial baseline score of 22.0±8.2. After the second treatment, the gland scores again climbed (improved). The score peaked to 41.5±1.8 1-month post-retreatment (Month 8). At the very conclusion of the study, the final meibomian score average was 30.6±7.5 which was well above initial baseline of 6.3 (P<0.001). The mean meibomian gland scores are summarized by clinic visit in Figure 2.
Figure 2.
Secondary efficacy end point – meibomian gland score results.
Notes: The mean (± SD) values for the meibomian gland assessment scores is presented by study visit. Baseline assessments (indicated by open circles) were conducted prior to TearCare® treatment at study initiation (Day 0) and at Month 7. Significant improvements in the change from baseline (increasing score from baseline Day 0) in meibomian gland scores were observed at each post-baseline visit. *P<0.001.
Dry eye symptoms
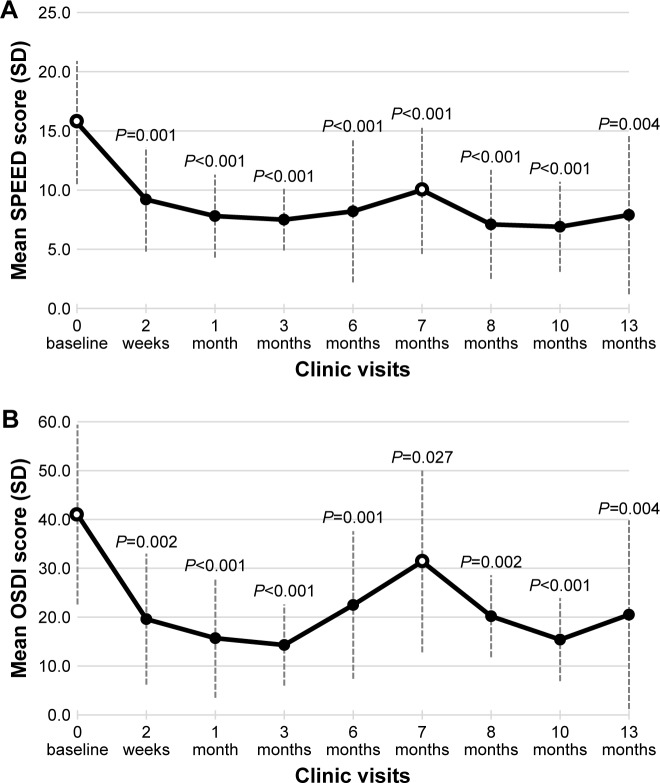
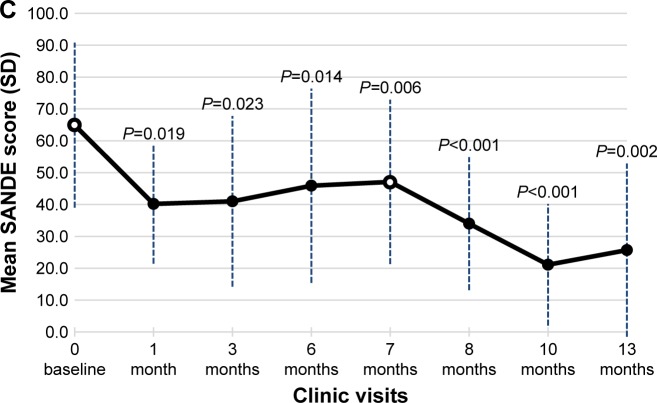
All 12 subjects completed three well-validated patient questionnaires to assess the degree and impact of their DED symptoms at the baseline and at follow-up visits. These included the SPEED, OSDI, and SANDE patient-based outcome measures (Figure 3A–C).
Figure 3.
Secondary efficacy end points – questionnaire results.
Notes: The mean (± SD) values for the subject responses are presented for the Standard Patient Evaluation of Eye Dryness (SPEED) questionnaire (A), the Ocular Surface Disease Index (OSDI) questionnaire (B), and the Symptom Assessment in Dry eye (SANDE) questionnaire (C). Baseline assessments (indicated by open circles) were conducted prior to TearCare® treatment at study initiation (Day 0) and at Month 7. Significant improvements in the change from baseline in mean scores (decreases in the score from baseline Day 0) are noted with the P-values for each panel.
Standard Patient Evaluation for Eye Dryness
At baseline of the initial study, subjects had a mean ± SD SPEED score of 15.7±5.2. After initial treatment, the SPEED score dropped, indicating improvement in DED symptoms, to a value as low as a mean of 7.5±2.6. At the retreatment baseline before the second TearCare® treatment at 7 months, the SPEED score remained lower at 10.0±5.4. After the second TearCare® treatment it dropped to as low as 6.9±3.8 and a final score, 6 months later, of 7.9±6.7 (P=0.004 compared to Day 0). The treatment effect seemed to be most pronounced at 3 months after the second treatment, but it persisted overall until the conclusion of the trial. These SPEED questionnaire score results are summarized in Figure 3A.
Ocular Surface Disease Index
Subjects averaged an OSDI score of 41.0±18.4 at the initial study baseline, and this dropped to as low as 14.3±8.3 at Month 3 after the initial treatment. At the retreatment baseline (Month 7), it remained reduced at 31.4±18.6. After the second treatment, it again reached its best (lowest score) at Month 3 (15.4±8.5). At the conclusion of the trial, it remained reduced from original and retreatment baseline at 20.5±19.3 (P=0.004 compared to Day 0). These results are summarized in Figure 3B.
Symptom Assessment in Dry Eye
The mean ± SD SANDE score at the initial baseline for the pool of 12 subjects was 64.9±25.9. The mean SANDE score dropped to between 40 and 41 at Month 1 and Month 3 follow-up visits, respectively. At retreatment baseline, SANDE scores remained reduced at 47.1±25.8. After the second TearCare® treatment, mean scores dropped to as low as 21.1±19.1 3 months later (Month 10). At the conclusion of the trial, SANDE scores remained reduced at 25.7±27.2 (P=0.002 compared to Day 0). Mean scores for the SANDE questionnaire are summarized by study visit (Figure 3C).
Safety
The TearCare® retreatment at Month 7 and evaluations were well tolerated, and there were no heat- or procedure-related AEs at the time of retreatment. One subject developed a chalazion at 3 weeks post-retreatment which resolved 1 week later. One other subject experienced severe allergic conjunctivitis 5 weeks post-retreatment which resolved 5 weeks later. Both of these events were judged to be unrelated to the TearCare® procedure. Additionally, there was no significant impact on IOP or BCVA (ie, >2-line decline) throughout this extension study.
Discussion
The primary purpose of this study was to test the safety, degree of therapeutic effectiveness, and durability through 12 months of a second TearCare® treatment to subjects ~6 months after the first TearCare® treatment. During the initial clinical trial upon which this current extension study is based, it was demonstrated that the TearCare® procedure was significantly more effective objectively and subjectively against daily, standardized warm compress treatments through 6 months. Objective end points in the original and extension study (ie, this study) included TBUT, corneal staining, conjunctival staining, and meibomian gland scores. Subjective end points included the well validated OSDI, SPEED, and SANDE questionnaires.
Examination of the initial 6-month data indicated that, as a general trend, both objective and subjective end points appear to improve with the treatment and gradually return in the direction of baseline over 6 months.27 MGD is a chronic disease, and it is believed that meibomian gland obstructions will recur over time. Thus, there was an interest in determining if a similar therapeutic effect and similar treatment durability could be achieved with a repeat TearCare® treatment after 6 months. Based on the results of this extension study, it appears that TearCare® treatments can be successfully repeated for patients and that they will yield significant, durable improvement in the signs and symptoms of dry eye.
When examining the objective measurements following retreatment, including TBUT, meibomian gland scores, corneal staining, and conjunctival staining, the results were similar to the initial study. Subjects demonstrated a rapid improvement in all of the objective measurements. While it falls under the objective end point category, the fact that meibomian gland scores improved again suggests that the procedure does not adversely affect meibomian gland function or output. This provides some evidence for the safety of the treatment and that the therapeutic benefit of the procedure is repeatable.
Similar to the repeatability of the objective results, the subjective end points such as OSDI, SPEED, and SANDE scores improved with the second TearCare® treatment. In particular, the SANDE patient-based outcomes continued to improve significantly from initial baseline, to secondary baseline, and finally to study termination.
In the first TearCare® clinical study, an initial TearCare® treatment was demonstrated to be effective throughout the entire 6-month study period. Objective and subjective efficacy highs were seen at Month 1 and Month 3 end points, and these efficacy levels appeared to recede from their highs at Month 6.27 This retreatment study was designed to determine if a retreatment would restore the efficacy highs seen in the first study and if those results would last for another 6 months. It is encouraging that the retreatment procedure restored the maximum objective and subjective efficacy levels seen in the first study through 12 months.
The TearCare® system used in this study delivered regulated, targeted thermal energy (41°C–45°C) to the outer surface of the eyelid for 12 minutes. This temperature range is intended to allow for effective softening or melting of meibum in vivo and in a safe manner. These temperature ranges have been shown to be safe not only in our experience but also in the previously published literature.18 In addition, the results of this study again confirm the safety of this approach in that no significant, device-related adverse effects were observed.
There are some limitations to this extension study. For example, this was a single-treatment, single-investigator study. As a result, it was not possible to mask subjects or the investigator. Also, the sample size was 12 subjects who had previously undergone a TearCare treatment. A larger, multi-center, randomized and masked, prospective trial is currently being planned. A larger study will enhance the evidence base for the use of TearCare® in the treatment of DED.
While previous trials, such as the LipiFlow trial,24 followed treated patients up to 1 month, this trial followed patients up to 13 months. Patients received a treatment at entry and then at 7 months. Objective and subjective end points improved in a repeatable fashion. In other words, the signs and symptoms of DED improved in patients up to 13 months. Further studies comparing TearCare® to other treatments are currently being prepared.
Conclusion
Based on the statistically significant improvement in the signs and symptoms of DED following a second treatment with the TearCare® system observed in this extension study, repeated application appears to provide additional benefit to patients with DED. Larger, multicenter, randomized, prospective trials are planned to further evaluate this treatment for DED.
Acknowledgments
The author would like to thank Gerry Gray, PhD, for performing the statistical analysis of the data and Bridge Over Brook, Inc. for assistance in preparing and editing the manuscript.
Footnotes
Disclosure
In addition to practicing as a board-certified ophthalmologist, David Badawi is an employee of Sight Sciences, Inc. (Menlo Park, CA, USA). This study was sponsored by Sight Sciences, Inc. The sponsor participated in the design of the study and data collection. The sponsor also reviewed the manuscript for accuracy and assessment of the presentation of any proprietary information.
References
- 1.Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–283. doi: 10.1016/j.jtos.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15(3):334–365. doi: 10.1016/j.jtos.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Farrand KF, Fridman M, Stillman IÖ, Schaumberg DA. Prevalence of diagnosed dry eye disease in the united states among adults aged 18 years and older. Am J Ophthalmol. 2017;182:90–98. doi: 10.1016/j.ajo.2017.06.033. [DOI] [PubMed] [Google Scholar]
- 4.Ding J, Sullivan DA. Aging and dry eye disease. Exp Gerontol. 2012;47(7):483–490. doi: 10.1016/j.exger.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rolando M, Zierhut M. The ocular surface and tear film and their dysfunction in dry eye disease. Surv Ophthalmol. 2001;45(Suppl 2):S203–S210. doi: 10.1016/s0039-6257(00)00203-4. [DOI] [PubMed] [Google Scholar]
- 6.Fiscella RG. Understanding dry eye disease: a managed care perspective. Am J Manag Care. 2011;17(Suppl 16):S432–S439. [PubMed] [Google Scholar]
- 7.Ciurtin C, Ostas A, Cojocaru VM, Walsh SB, Isenberg DA. Advances in the treatment of ocular dryness associated with Sjögren’s syndrome. Semin Arthritis Rheum. 2015;45(3):321–327. doi: 10.1016/j.semarthrit.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Petroustsos G, Paschides CA, Kitsos G, Drosos AA, Psilas K. Sterile corneal ulcers in dry eye II. Treatment, complications, and course. J Fr Ophthalmol. 1992;15(2):106–111. French. [PubMed] [Google Scholar]
- 9.Dell SJ. Intense pulsed light for evaporative dry eye disease. Clin Ophthalmol. 2017;11:1167–1173. doi: 10.2147/OPTH.S139894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The definition and classification of dry eye disease: report of the definition and classification subcommittee of the international dry eye workshop (2007) Ocul Surf. 2007;5(2):75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 11.Nichols KK, Foulks GN, Bron AJ, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011;52(4):1922–1929. doi: 10.1167/iovs.10-6997a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knop E, Knop N, Schirra F. Meibomian glands. Part II: physiology, characteristics, distribution and function of meibomian oil. Ophthalmologe. 2009;106(10):884–892. doi: 10.1007/s00347-009-2019-9. [DOI] [PubMed] [Google Scholar]
- 13.Willcox MDP, Argüeso P, Georgiev GA, et al. TFOS DEWS II Tear Film Report. Ocul Surf. 2017;15(3):366–403. doi: 10.1016/j.jtos.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31(5):472–478. doi: 10.1097/ICO.0b013e318225415a. [DOI] [PubMed] [Google Scholar]
- 15.Borchman D, Foulks GN, Yappert MC, et al. Human meibum lipid conformation and thermodynamic changes with meibomian-gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52(6):3805–3817. doi: 10.1167/iovs.10-6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korb DR, Blackie CA. Restoration of meibomian gland functionality with novel thermodynamic treatment device-a case report. Cornea. 2010;29(8):930–933. doi: 10.1097/ICO.0b013e3181ca36d6. [DOI] [PubMed] [Google Scholar]
- 17.Friedland BR, Fleming CP, Blackie CA, Korb DR. A novel thermodynamic treatment for meibomian gland dysfunction. Curr Eye Res. 2011;36(2):79–87. doi: 10.3109/02713683.2010.509529. [DOI] [PubMed] [Google Scholar]
- 18.Blackie CA, Solomon JD, Greiner JV, Holmes M, Korb DR. Inner eyelid surface temperature as a function of warm compress methodology. Optom Vis Sci. 2008;85(8):675–683. doi: 10.1097/OPX.0b013e318181adef. [DOI] [PubMed] [Google Scholar]
- 19.Olson MC, Korb DR, Greiner JV. Increase in tear film lipid layer thickness following treatment with warm compresses in patients with meibomian gland dysfunction. Eye Contact Lens. 2003;29(2):96–99. doi: 10.1097/01.ICL.0000060998.20142.8D. [DOI] [PubMed] [Google Scholar]
- 20.Lam AK, Lam CH. Effect of warm compress therapy from hard-boiled eggs on corneal shape. Cornea. 2007;26(2):163–167. doi: 10.1097/01.ico.0000248380.86401.4d. [DOI] [PubMed] [Google Scholar]
- 21.Freedman HL, Preston KL. Heat retention in varieties of warm compresses: a comparison between warm soaks, hard-boiled eggs and the re-heater. Ophthalmic Surg. 1989;20(12):846–848. [PubMed] [Google Scholar]
- 22.Blackie CA, Solomon JD, Greiner JV, Holmes M, Korb DR. Inner eyelid surface temperature as a function of warm compress methodology. Optom Vis Sci. 2008;85(8):675–683. doi: 10.1097/OPX.0b013e318181adef. [DOI] [PubMed] [Google Scholar]
- 23.Blackie CA, Korb DR. Meibomian gland expression: forces of expression, types of secretion and the limitation of resulting pain. Invest Ophthalmol Vis Sci. 2010;51(13):3385. [Google Scholar]
- 24.Lane SS, Dubiner HB, Epstein RJ, et al. A new system, the LipiFlow, for the treatment of meibomian gland dysfunction. Cornea. 2012;31(4):396–404. doi: 10.1097/ICO.0b013e318239aaea. [DOI] [PubMed] [Google Scholar]
- 25.Davidson AE, Hayes S, Hardcastle AJ, Tuft SJ. The pathogenesis of keratoconus. Eye. 2014;28(2):189–195. doi: 10.1038/eye.2013.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panikkar K, Manayath G, Rajaraman R, Saravanan V. Progressive keratoconus, retinal detachment, and intracorneal silicone oil with obsessive-compulsive eye rubbing. Oman J Ophthalmol. 2016;9(3):170–173. doi: 10.4103/0974-620X.192285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Badawi D. A novel system, TearCare®, for the treatment of the signs and symptoms of dry eye disease. Clin Ophthalmol. 2018;12:683–694. doi: 10.2147/OPTH.S160403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ngo W, Situ P, Keir N, Korb D, Blackie C, Simpson T. Psychometric properties and validation of the standard patient evaluation of eye dryness questionnaire. Cornea. 2013;32(9):1204–1210. doi: 10.1097/ICO.0b013e318294b0c0. [DOI] [PubMed] [Google Scholar]
- 29.Asiedu K, Kyei S, Mensah SN, Ocansey S, Abu LS, Kyere EA. Ocular Surface Disease Index (OSDI) versus the Standard Patient Evaluation of Eye Dryness (SPEED) Cornea. 2016;35(2):175–180. doi: 10.1097/ICO.0000000000000712. [DOI] [PubMed] [Google Scholar]
- 30.Schaumberg DA, Gulati A, Mathers WD, et al. Development and validation of a short global dry eye symptom index. Ocul Surf. 2007;5(1):50–57. doi: 10.1016/s1542-0124(12)70053-8. [DOI] [PubMed] [Google Scholar]
- 31.Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. 2000;118(5):615–621. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]