Abstract
Recently, miR-124 has been regarded as a critical modulator of colorectal cancer. We here summarize the studies on the role and mechanism of miR-124 in colorectal cancer. They indicate that miR-124 is methylated during carcinogenesis and functions as a tumor-suppressor in colorectal cancer. miR-124 might serve as a potential diagnostic biomarker and therapeutic target in colorectal cancer in the foreseeable future.
Keywords: miR-124, colorectal cancer, biomarker, methylation, therapeutic target
Introduction
Colorectal cancer (CRC), the third leading cause of cancer death worldwide, hits nearly 1.2 million people each year with a 5-year survival rate of 62%. Chromosomal abnormalities, epigenetic alterations, and environmental factors may contribute to the pathogenesis of CRC. Identification of key molecules involved in the disease process is helpful to find more effective therapeutic targets for CRC patients. miR-124 is the most abundantly expressed miRNA in the brain and also expressed in other tissues. Three subtypes of miR-124, namely, miR-124-1, miR-124-2, and miR-124-3, have been identified with distinct chromosome locations. Mature miR-124 shares completely identical sequence in humans, mice, and rats. miR-124 is involved in many biological processes such as affecting biological functions of the central nervous system, emerging as a critical modulator of immunity and inflammation, and acting as a negative regulator of osteogenic differentiation of stem cells, and so on.1 Recently, miR-124 has emerged as a crucial modulator of carcinogenesis.2 Especially, miR-124 may act as a potential diagnostic biomarker and therapeutic target in CRC.
miR-124 in CRC
Ueda et al3 found that three miR-124 genes are methylated during carcinogenesis in patients with ulcerative colitis. The methylation level of miR-124-3 could be recognized as a valuable marker for estimating individual risk for CRC. Zhang et al4 reported that miR-124 is significantly downregulated in CRC compared to adjacent non-tumor colorectal tissues. Downregulation of miR-124 in the mucosa samples is an independent prognostic factor in patients with CRC.5 Also, low level of serum miR-124 is reported to be correlated with poor prognosis in CRC patients.6
Growing evidence has implicated that miR-124 plays a tumor-suppressive role in the development of CRC. CRC is a major complication in patients with inflammatory bowel disease (IBD). Long chronic inflammation is able to induce miR-124 methylation and contributes to the higher epidemiologic risk of cancer. STAT3 is hyper-activated in the colonic tissues of IBD and CRC. STAT3 induces and maintains a pro-carcinogenic inflammatory microenvironment, completing the link between inflammation and cancer.7 Aberrant activities of STAT3 actively contribute to CRC, especially persistent STAT3 activation in colon cancer is associated with enhanced cell proliferation and tumor growth.8 miR-124 could target and inhibit STAT3 activation, therefore is able to suppress the growth of human CRC.4
Many other effects and targets of miR-124 in the control of CRC have been identified:
Changing genetic susceptibility to CRC by miR-124 variation. Pri-miR-124 rs531564 polymorphism is a common polymorphism (rs531564, C.G), which is significantly associated with the decreased risk of CRC and may act as a genetic modifier for developing CRC.9 Mutants of pre-miR-124-1 rs531564 increase recurrent risk in surgically resected CRC individuals receiving adjuvant chemoradiotherapy.10
Counteracting the Warburg effect by targeting pyruvate kinase muscle (PKM)-dependent glycolysis regulation. Warburg effect is the “addiction” of cancer cells to fermentative glycolysis. PKM2 is a tumor-specific isoform and promotes Warburg effect. miR-124 switches PKM gene expression from PKM2 to PKM1, resulting in the suppression of the glycolysis rate.11 Interestingly, Taniguchi et al12 reported that miR-124 contributes to the maintenance of Warburg effect in colon cancer cells by regulating positive feedback circuit of DEAD-box RNA helicase 6 (DDX6)/c-Myc/polypyrimidine tract-binding protein 1 (PTB1), indicating that miR-124 is a fine tuner of the Warburg effect.
Inhibiting DNA synthesis and proliferation of CRC cells by targeting the inhibitor of apoptosis-stimulating protein of p53,13 reducing the levels of pentose phosphate pathway enzymes phosphoribosyl pyrophosphate synthetase 1 and ribose-5-phosphate isomerase-A mRNAs,14 and modulating miR-124-p63 feedback loop.15
Inducing apoptosis and autophagy by targeting PTB1/ PKM1/PKM2 feedback cascade,16 inhibiting STAT3,4 and regulating the positive feedback loop between miR-124 and PKM1/hepatocyte nuclear factor 4α in CRC.17
Suppressing migration and invasion in several CRC cell lines via negatively regulating KAI1 C-terminal interacting tetraspanin18 and rho-associated protein kinase 1 (ROCK1).19,20
Enhancing radiosensitivity of human CRC cells by targeting paired-related homeobox 121 and increasing sensitivity to chemotherapy by directly targeting DNA methyltransferase 3B and indirectly targeting DNMT1 in CRC cells.22
Conclusion
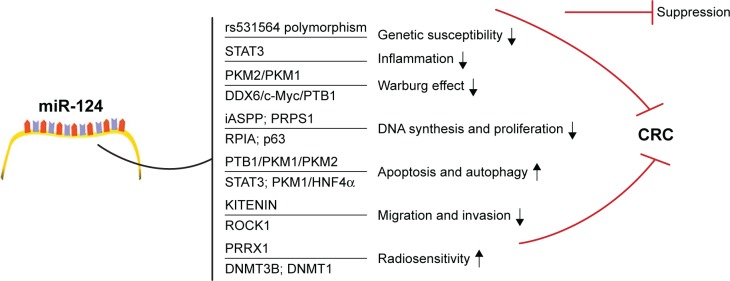
A summary schema of miR-124 signaling in CRC is indicated as Figure 1. Due to multiple effects and multiple targets in the control of CRC, miR-124 is obviously superior as a therapeutic target in the foreseeable future. The clinical application of stable miR-124 mimic or inhibitor might be practicable. For instance, nanomaterials are increasingly distinguished as highly potential drug delivery systems for cancer treatment. Nanoparticles are able to carry and release the anticancer drug in the right place and with the required dose, greatly reducing toxicity and side effects.23,24 Combining nanomaterials and miR-124 might be a potential anticancer strategy. Future research is being directed to regulate miR-124 level to offer new therapeutic avenues for the treatment of CRC.
Figure 1.
miR-124 in colorectal cancer.
Note: In CRC, miR-124 produces tumor suppressive role by targeting STAT3 to inhibit inflammation, changing genetic susceptibility, counteracting Warburg effect, inducing apoptosis and autophagy, and so on.
Abbreviations: CRC, colorectal cancer; DDX6, DEAD-box RNA helicase 6; DNMT, DNA methyltransferase; HNF4α, hepatocyte nuclear factor 4α; iASPP, inhibitor of apoptosis-stimulating protein of p53; KITENIN, KAI1 C-terminal interacting tetraspanin; PKM, pyruvate kinase muscle; PRPS1, phosphoribosyl pyrophosphate synthetase 1; PRRX1, paired-related homeobox 1; PTB1, polypyrimidine tract-binding protein 1; RPIA, ribose-5-phosphate isomerase-A; ROCK1, rho-associated protein kinase 1.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No 81473259 and 81773726 to XL).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Qadir AS, Um S, Lee H, et al. miR-124 negatively regulates osteogenic differentiation and in vivo bone formation of mesenchymal stem cells. J Cell Biochem. 2015;116(5):730–742. doi: 10.1002/jcb.25026. [DOI] [PubMed] [Google Scholar]
- 2.Qin Z, Wang PY, Su DF, Liu X. miRNA-124 in Immune System and Immune Disorders. Front Immunol. 2016;7:406. doi: 10.3389/fimmu.2016.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ueda Y, Ando T, Nanjo S, Ushijima T, Sugiyama T. DNA methylation of microRNA-124a is a potential risk marker of colitis-associated cancer in patients with ulcerative colitis. Dig Dis Sci. 2014;59(10):2444–2451. doi: 10.1007/s10620-014-3193-4. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J, Lu Y, Yue X, et al. MiR-124 suppresses growth of human colorectal cancer by inhibiting STAT3. PLoS One. 2013;8(8):e70300. doi: 10.1371/journal.pone.0070300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang MJ, Li Y, Wang R, et al. Downregulation of microRNA-124 is an independent prognostic factor in patients with colorectal cancer. Int J Colorectal Dis. 2013;28(2):183–189. doi: 10.1007/s00384-012-1550-3. [DOI] [PubMed] [Google Scholar]
- 6.Jinushi T, Shibayama Y, Kinoshita I, et al. Low expression levels of microRNA-124-5p correlated with poor prognosis in colorectal cancer via targeting of SMC4. Cancer Med. 2014;3(6):1544–1552. doi: 10.1002/cam4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bromberg J, Wang TC. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell. 2009;15(2):79–80. doi: 10.1016/j.ccr.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corvinus FM, Orth C, Moriggl R, et al. Persistent STAT3 activation in colon cancer is associated with enhanced cell proliferation and tumor growth. Neoplasia. 2005;7(6):545–555. doi: 10.1593/neo.04571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao XR, Wang HP, Zhang SL, Wang MX, Zhu ZS. Pri-miR-124 rs531564 polymorphism and colorectal cancer risk. Sci Rep. 2015;5:14818. doi: 10.1038/srep14818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ying HQ, Peng HX, He BS, et al. MiR-608, pre-miR-124-1 and pre-miR26a-1 polymorphisms modify susceptibility and recurrence-free survival in surgically resected CRC individuals. Oncotarget. 2016;7(46):75865–75873. doi: 10.18632/oncotarget.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun Y, Zhao X, Zhou Y, Hu Y. miR-124, miR-137 and miR-340 regulate colorectal cancer growth via inhibition of the Warburg effect. Oncol Rep. 2012;28(4):1346–1352. doi: 10.3892/or.2012.1958. [DOI] [PubMed] [Google Scholar]
- 12.Taniguchi K, Sugito N, Kumazaki M, et al. Positive feedback of DDX6/c-Myc/PTB1 regulated by miR-124 contributes to maintenance of the Warburg effect in colon cancer cells. Biochim Biophys Acta. 2015;1852(9):1971–1980. doi: 10.1016/j.bbadis.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 13.Liu K, Zhao H, Yao H, et al. MicroRNA-124 regulates the proliferation of colorectal cancer cells by targeting iASPP. Biomed Res Int. 2013;2013:867537. doi: 10.1155/2013/867537. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Qiu Z, Guo W, Wang Q, et al. MicroRNA-124 reduces the pentose phosphate pathway and proliferation by targeting PRPS1 and RPIA mRNAs in human colorectal cancer cells. Gastroenterology. 2015;149(6):1587.e11–1598.e11. doi: 10.1053/j.gastro.2015.07.050. [DOI] [PubMed] [Google Scholar]
- 15.Liu K, Yao H, Lei S, et al. The miR-124-p63 feedback loop modulates colorectal cancer growth. Oncotarget. 2017;8(17):29101–29115. doi: 10.18632/oncotarget.16248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taniguchi K, Sugito N, Kumazaki M, et al. MicroRNA-124 inhibits cancer cell growth through PTB1/PKM1/PKM2 feedback cascade in colorectal cancer. Cancer Lett. 2015;363(1):17–27. doi: 10.1016/j.canlet.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 17.Sun Y, Zhao X, Luo M, et al. The pro-apoptotic role of the regulatory feedback loop between miR-124 and PKM1/HNF4α in colorectal cancer cells. Int J Mol Sci. 2014;15(3):4318–4332. doi: 10.3390/ijms15034318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park SY, Kim H, Yoon S, et al. KITENIN-targeting microRNA-124 suppresses colorectal cancer cell motility and tumorigenesis. Mol Ther. 2014;22(9):1653–1664. doi: 10.1038/mt.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou L, Xu Z, Ren X, Chen K, Xin S. MicroRNA-124 (MiR-124) Inhibits Cell Proliferation, Metastasis and Invasion in Colorectal Cancer by Downregulating Rho-Associated Protein Kinase 1(ROCK1) Cell Physiol Biochem. 2016;38(5):1785–1795. doi: 10.1159/000443117. [DOI] [PubMed] [Google Scholar]
- 20.Xi ZW, Xin SY, Zhou LQ, Yuan HX, Wang Q, Chen KX. Downregulation of rho-associated protein kinase 1 by miR-124 in colorectal cancer. World J Gastroenterol. 2015;21(18):5454–5464. doi: 10.3748/wjg.v21.i18.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Zheng L, Huang J, et al. MiR-124 Radiosensitizes human colorectal cancer cells by targeting PRRX1. PLoS One. 2014;9(4):e93917. doi: 10.1371/journal.pone.0093917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Z, Liu S, Tian L, et al. miR-124 and miR-506 inhibit colorectal cancer progression by targeting DNMT3B and DNMT1. Oncotarget. 2015;6(35):38139–38150. doi: 10.18632/oncotarget.5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang K, Huang Q, Qiu F, Sui M. Non-viral Delivery Systems for the Application in p53 Cancer Gene Therapy. Curr Med Chem. 2015;22(35):4118–4136. doi: 10.2174/0929867322666151001121601. [DOI] [PubMed] [Google Scholar]
- 24.Zhang B, Wang K, Si J, Sui M, Shen Y. Charge-reversal polymers for biodelivery. In: Gu Z, editor. Bioinspired and Biomimetic Polymer Systems for Drug and Gene Delivery. Hoboken, NJ: Wiley Online Library; 2014. pp. 223–242. [Google Scholar]