Abstract
Rexin-G is a replication-incompetent retroviral vector displaying a cryptic SIG-binding peptide for targeting abnormal Signature (SIG) proteins in tumors and encoding a dominant-negative human cyclin G1 construct. Herein we report on the safety and antitumor activity of escalating doses of Rexin-G in gemcitabine-refractory pancreatic adenocarcinoma, with one 10-year survivor. For the safety analysis (n = 20), treatment-related grade 1 adverse events included fatigue (n = 6), chills (n = 2), and headache (n = 1), with no organ damage and no DLT. No patient tested positive for vector-neutralizing antibodies, antibodies to gp70, replication-competent retrovirus (RCR), or vector integration into genomic DNA of peripheral blood lymphocytes (PBLs). For the efficacy analysis (n = 15), one patient achieved a complete response (CR), two patients had a partial response (PR), and 12 had stable disease (SD). Median progression-free survival (PFS) was 2.7, 4.0, and 5.6 months at doses 0–I, II, and III, respectively. Median overall survival (OS) and 1-year OS rate at dose 0–I were 4.3 months and 0%, and at dose II–III they were 9.2 months and 33.3%. To date, one patient is still alive with no evidence of cancer 10 years after the start of Rexin-G treatment. Taken together, these data suggest that Rexin-G, the first targeted gene delivery system, is uniquely safe and exhibits significant antitumor activity, for which the FDA granted fast-track designation.
Keywords: gene therapy, cell cycle control, 10-year cancer-free survivor, pancreas adenocarcinoma, cyclin G1 inhibitor, retrovector, tumor targeting, targeted gene delivery, CCNG1
Introduction
Pancreatic adenocarcinoma (PDAC) is projected to become the second leading cause of cancer death in the United States and is rising worldwide.1, 2 For patients with advanced disease, first-3, 4 and second-line5 treatment options improve survival modestly, but they are not curative. Unfortunately, there have been few successful targeted therapy options,6 in part because the most common mutations (KRAS and TP53) have not been targetable, and others are uncommon (BRCA1, BRCA2, and MSI). As with most cancers, genetic dysfunction of the normal cell division cycle and its checkpoint control elements is critical to progression of PDAC;7 therefore, targeting the executive elements of cell cycle checkpoint control represents a promising strategy.8
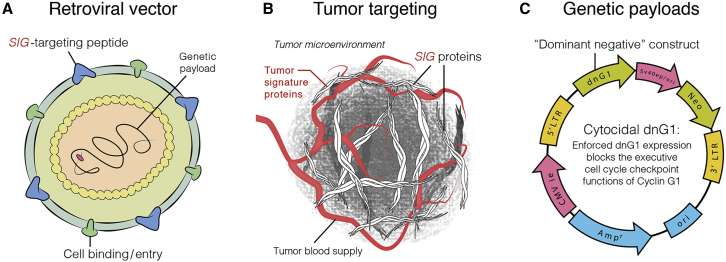
Rexin-G (former names: Mx-dnG1) is the first targeted injectable vector to be approved for clinical trials in the treatment of metastatic cancers.9 Rexin-G (Figure 1) is a non-replicative MLV-based amphotropic retrovector displaying a cryptic collagen-binding motif on its gp70 surface membrane for targeting abnormal Signature (SIG) proteins in the tumor microenvironment (TME)10 and encoding a dominant-negative mutant construct (dnG1) of human cyclin G1 (CCNG1).11 The vector also contains a neomycin resistance (neor) gene, which is driven by the SV40 early promoter. When injected intravenously, Rexin-G seeks out and accumulates in cancerous lesions by binding to exposed abnormal collagenous SIG proteins deposited as a result of tumor invasion, tumor-associated angiogenesis, and stroma formation, elevating the vector concentration in the TME in the vicinity of cancer cells. Upon gaining entry into the rapidly proliferating cells, within the TME,12 Rexin-G produces a cytocidal dnG1 protein that effectively blocks a pivotal checkpoint of the cell division cycle, resulting in apoptosis and, thus, the elimination of cancer cells, proliferative tumor vasculature, and associated malignant fibroblasts.13, 14
Figure 1.
Graphic Illustration of Rexin-G Vector
The Rexin-G vector displaying a SIG-targeting peptide (A), for binding to Signature (SIG) proteins in the tumor microenvironment (TME) (B), and encoding a dominant-negative human cyclin G1 inhibitor gene (C). Injected intravenously, Rexin-G nanoparticles seek out and bind to abnormal SIG proteins in the TME, which augments effective vector concentration in tumors.
Based on encouraging clinical data from the Philippines in patients with metastatic PDAC,15, 16 clinical trials began in the United States using Rexin-G for standard chemotherapy-resistant PDAC, sarcoma, osteosarcoma, and breast cancer.17, 18, 19 In this report, we provide updated results, along with new mechanistic and pharmacological insights, from an advanced phase I-II study evaluating overall safety and potential antitumor activity of intravenous infusions of Rexin-G in metastatic gemcitabine-resistant PDAC.
Results
Patients and Treatment
This phase I-II trial enrolled 20 patients with metastatic gemcitabine-refractory PDAC. Table 1 shows the patient demographics. The patients had failed a median of two regimens, one of which contained gemcitabine. All patients exhibited metastatic disease. Two patients had one target lesion, and 17 patients had 2–7 target lesions in the pancreas, lymph node, omentum, mesentery, adrenal, bone, lung, and the liver in 16 patients (Table 2). Aside from the target lesions in Table 2, all patients had many non-target lesions, malignant ascites, pleural effusion, and peritoneal carcinomatosis. Therefore, target lesions alone do not reflect the patients’ total tumor burden.
Table 1.
Patient Demographics (N = 20)
| Patient Characteristics | Number, range, or n (% of N) |
|---|---|
| Age | |
| Median | 62 |
| Range | 50–83 |
| Gender | |
| Female | 12 (60%) |
| Male | 8 (40%) |
| Race | |
| White | 16 (80%) |
| Asian | 4 (20%) |
| Disease stage | |
| Metastatic (1–7 target lesions + non-target lesions, ascites, pleural effusion, peritoneal carcinomatosis) | 20 (100%) |
| Performance score (ECOG) | |
| 1 | 20 (100%) |
| Number of previous chemotherapy regimens | |
| Median | 2 |
| Range | 1–7 |
Table 2.
Locations and Sizes of Lesions in 20 Patients with Metastatic PDAC
| Subject ID | Location of Target Lesions (Size, Longest Diameter, mm) |
|---|---|
| 1 | left periumbilical (85) |
| 2 | preaortic node (40) |
| right lobe liver, post aspect (27) | |
| pancreas (20) | |
| celiac node (11) | |
| 3 | right lobe liver, upper anterior aspect (23) |
| body of pancreas (22) | |
| anterior right lower lobe, liver (17) | |
| anterior right lobe liver, porta hepatis (18) | |
| posterior to porta hepatis (22) | |
| inferior tip of right liver lobe (11) | |
| 4 | left liver lobe, lateral tip (32) |
| left liver lobe (36) | |
| mid-right liver lobe (17) | |
| body of pancreas (10) | |
| 5 | left lobe liver at splenic hilum (76) |
| right lobe liver, lateral dome (22) | |
| liver, segment 4A (58) | |
| anterior omentum (42) | |
| left lung, hilar (27) | |
| right pre-tracheal node (10) | |
| 6 | perihilar right ML, lung (10) |
| left lobe liver, lateral segment (35) | |
| posterior right lobe, liver (41) | |
| left lobe liver, lateral segment (29) | |
| lateral left lobe liver (26) | |
| pancreatic tail (22) | |
| left adrenal apex (11) | |
| 7 | right hepatic lobe, posterior (88) |
| right hepatic lobe, upper portion (45) | |
| 8 | right liver lobe posterior aspect (24) |
| left liver lobe (55) | |
| anterior to left kidney (11) | |
| slightly lower to S1 (19) | |
| level of S1 first lesion (12) | |
| level of S1 second lesion (14) | |
| 9 | lateral tip left liver lobe (14) |
| inferior anterior margin, left liver lobe (16) | |
| right anterior subcapsular (23) | |
| right liver lobe dome (19) | |
| left liver lobe anterior superficial subcapsular (10) | |
| 10 | RLL lung posterior subpleural upper (12) |
| RLL lung posterior subpleural lower (15) | |
| 11 | anterior segment 5, liver (64) |
| segment 4 liver (48) | |
| pancreatic body, preaortic (52) | |
| 12 | right liver lobe, posterior (21) |
| head of pancreas medial to biliary stent (32) | |
| 13 | tail of pancreas (66) |
| left adrenal (10) | |
| mid segment 4, liver (40) | |
| 14 | dome right hepatic lobe (20) |
| lateral tip left hepatic lobe (18) | |
| head of pancreas (30) | |
| nodule anterior abdominal wall to right of midline at T12 (12) | |
| 15 | head and body of pancreas (67) |
| mesenteric mass slightly left of midline (19) | |
| circular lesion posterior to porta hepatis (34) | |
| right hepatic lobe anterior and superior to gall bladder (36) | |
| 16 | head of pancreas (90) |
| aortocaval mass at right hepatic lobe (20) | |
| 17 | pancreatic head (28) |
| 18 | subhepatic area below anastomosis (28) |
| subperitoneal lymph node (22) | |
| 19 | right posterior sulcus lung (15) |
| B01 | lateral left lower lobe lung (14) |
| medial left lower lobe lung (21) | |
| right of hepatic art. (13) | |
| left periaortic lymph node (15) | |
| left adrenal nodule (11) |
ML, middle lobe; RLL, right lower lobe; art., artery.
Dose Escalations
Six patients were treated at dose levels 0–I; 7 were treated at dose level II; and 7 were treated at dose level III. One patient was included in the dose II cohort because he received an (FDA-approved) intrapatient dose escalation from dose 0 to dose II. The number of Rexin-G infusions, the number of completed cycles of Rexin-G, and the total exposure (colony-forming units [CFU]) to Rexin-G are summarized by dosage group in Table 3.
Table 3.
Total Exposure to Rexin-G in 20 Patients with Metastatic PDAC
| Dose Group for Analysis |
||||
|---|---|---|---|---|
| Parameter | 0–I(N = 6) | II(N = 7) | III(N = 7) | All(N = 20) |
| Number of all infusions | ||||
| Total for all patients | 72 | 356 | 404 | 832 |
| Median (minimum, maximum) per patient | 9 (5, 23) | 30 (11, 157) | 52 (10, 151) | 24 (5, 157) |
| Number of all completed cycles | ||||
| Total for all patients | 5 | 27 | 31 | 63 |
| Median (minimum, maximum) per patient | 2 (1, 2) | 3 (1, 13) | 4 (1, 12) | 3 (1, 13) |
| CFU of Rexin-G | ||||
| Total (×10e11 CFU) | 73 | 676 | 1212 | 1961 |
| Median (minimum, maximum) (×10e11 CFU) per patient | 9 (5, 24) | 60 (22, 314) | 156 (30, 453) | 49 (5, 453) |
The median number of infusions varied from 9 in dose group 0–I to 52 in dose group III. A total of 832 infusions were administered for all patients. The total number of completed infusion cycles varied from 5 in dose group 0–I to 31 in dose group III. The median cumulative dose of Rexin-G increased from 9 × 10e11 CFU in dose group 0–I to 60 × 10e11 CFU in dose group II to 156 × 10e11 CFU in dose group III. Total exposure to Rexin-G for all patients was 1,927 × 10e11 CFU, with a range from 30 to 453 × 10e11 CFU.
Safety Analysis
There were no dose-limiting toxicities observed at any dose level. Unrelated adverse events were reported for all 20 patients. Related but clinically non-significant adverse events occurred in 7 patients, and all were grade 1 (Table 4). These consisted of chills (1 patient), fatigue (2 patients), and headache (1 patient) at dose level II and fatigue (4 patients) at dose level III. There was no treatment-related loss of hair; nausea; vomiting; anemia; thrombocytopenia; neutropenia; or liver, lung, or kidney dysfunction reported. There were no serious drug-related adverse events (AEs).
Table 4.
Clinically Non-significant Drug-Related Adverse Events by Dose Level and Toxicity Grade (n = 20)
| MedRA System Organ Class | Preferred Term | Dose Level | Toxicity Grade |
|---|---|---|---|
| General disorders and administration site conditions | fatigue | 2 | 1 |
| General disorders and administration site conditions | fatigue | 2 | 1 |
| Musculoskeletal and connective tissue disorders | chills | 2 | 1 |
| Nervous system disorders | headache | 2 | 1 |
| General disorders and administration site conditions | fatigue | 3 | 1 |
| General disorders and administration site conditions | fatigue | 3 | 1 |
| General disorders and administration site conditions | fatigue | 3 | 1 |
| General disorders and administration site conditions | fatigue | 3 | 1 |
The most frequent clinically non-significant unrelated grade 3 AEs were hypoalbuminemia (4 patients) and increased alanine aminotransferase (3 patients). Anemia, hyperglycemia, increased aspartate aminotransferase, and hypocalcemia were reported in 2 patients each. Other clinically non-significant unrelated grade 3 AEs were reported in 1 patient each. Several types of unrelated AEs appeared to be more frequent at higher doses: anemia, hyperbilirubinemia, increased aspartate aminotransferase, and decreased appetite (Table 5).
Table 5.
Clinically Non-significant, Unrelated, Grade 3 Adverse Events Reported in ≥2 Patients by Rexin-G Dose Level
| MedRA System Organ Class/Preferred Term | Dose Level |
Total |
|||
|---|---|---|---|---|---|
| N = 6 |
N = 7 |
N = 7 |
|||
| 0 |
I |
II |
III |
||
| N = 2 | N = 4 | N = 7 | N = 7 | N = 20 | |
| Blood and lymphatic system disorders | |||||
| Anemia | 1 | 1 | 2 | ||
| Endocrine disorders | |||||
| Hyperglycemia | 1 | 1 | 2 | ||
| Hepatobiliary disorders | |||||
| Hypoalbuminemia | 1 | 3 | 4 | ||
| Investigations | |||||
| Alanine aminotransferase increased | 1 | 1 | 1 | 3 | |
| Aspartate aminotransferase increased | 1 | 1 | 2 | ||
| Blood alkaline phosphatase increased | 2 | 2 | |||
| Metabolism and nutrition disorders | |||||
| Hypocalcemia | 1 | 1 | 2 | ||
13 patients experienced 25 serious AEs, all of which were deemed not related to the study drug. Details regarding these AEs are provided in Table 6.
Table 6.
Serious Unrelated Adverse Event Listings by Dose Level and Toxicity Grade
| Patient | System Organ Class | MedDRA Preferred Term | Dose Level | Toxicity Grade |
|---|---|---|---|---|
| 1 | gastrointestinal disorders | obstruction gastric | 0 | 3 |
| gastrointestinal disorders | intestinal perforation | 0 | 3 | |
| 2 | neoplasms: benign, malignant, and unspecified | malignant pleural effusion | 0 | 3 |
| 3 | infection and infestations | sepsis | 1 | 3 |
| 4 | injury, poisoning, and procedural complications | overdose | 1 | 3 |
| 5 | gastrointestinal disorders | abdominal pain | 1 | 3 |
| 6 | vascular disorders | pulmonary embolism | 2 | 3 |
| 7 | metabolism and nutrition disorders | dehydration | 2 | 3 |
| blood and lymphatic system disorders | thrombocytopenia | 2 | 4 | |
| metabolism and nutrition disorders | hyponatremia | 2 | 4 | |
| 8 | gastrointestinal disorders | obstruction gastric | 2 | 3 |
| 9 | vascular disorders | pulmonary embolism | 3 | 3 |
| infection and infestations | sepsis | 3 | 3 | |
| 10 | gastrointestinal disorders | fecaloma | 3 | 3 |
| gastrointestinal disorders | constipation | 3 | 3 | |
| metabolism and nutrition disorders | hyponatremia | 3 | 4 | |
| endocrine disorders | hyperglycemia | 3 | 4 | |
| 11 | hepatobiliary disorders | cholangitis | 3 | 3 |
| 12 | psychiatric disorders | mental status changes | 3 | 3 |
| gastrointestinal disorders | ascites | 3 | 3 | |
| nervous system disorders | altered state of consciousness | 3 | 3 | |
| 13 | respiratory, thoracic, and mediastinal disorders | pneumothorax | 3 | 3 |
| psychiatric disorders | delirium | 3 | 3 | |
| metabolism and nutrition disorders | dehydration | 3 | 3 | |
| investigations | aspartate aminotransferase increased | 3 | 4 |
No patient tested positive for any of the following: vector-neutralizing antibodies, antibodies to gp70, replication-competent retrovirus in peripheral blood lymphocytes (PBLs), or vector integration into genomic DNA of PBLs.
To date, 19 of the 20 patients enrolled in the study have died. None of the deaths were considered related to Rexin-G. The cause of death was progressive disease in all but one patient where the cause of death was sepsis. Remarkably, the long-term survivor exhibited lymphatic metastasis prior to intravenous Rexin-G infusions as salvage therapy.
Efficacy Analysis
Of the 20 enrolled patients, 15 received at least one complete cycle (4 weeks) of treatment and had a follow-up positron emission tomography-computed tomography (PET-CT) scan, and, therefore, they were considered evaluable for efficacy (modified intent-to-treat [mITT] population) in terms of response, progression-free survival, and overall survival. In the first cohort (dose level I), three patients were withdrawn from the study prior to completion of one treatment cycle either due to disease-related complications (n = 1; worsening malignant pleural effusion) or due to a personal decision to discontinue treatment (n = 2; one patient had worsening ascites and the other decided to take an alternative medicine). In the second cohort (dose level II), one patient had worsening ascites and clinical deterioration, and in the third cohort (dose level III), one patient had worsening malignant pleural effusion.
Table 7 shows the evaluation of tumor response using Response Evaluation Criteria in Solid Tumors (RECIST) version (v.)1.0, Choi,20 and modified international PET criteria in the mITT population. In the overall cohort, the median tumor burden was 32.6 × 10e9 cells; the range in tumor burden was wide across patients, with a minimum of 5.0 × 10e9 cells and a maximum of 115.5 × 10e9 cells. Notably, patients at dose level III had significantly larger tumor loads (52.1 × 10e9 cancer cells) than those in dose group 0–I or II (32.6 × 10e9 and 31.5 × 10e9 cancer cells, respectively). Patients were assigned to dose levels on a first-come first-served basis. No significant relationship was noted between estimated tumor burden and response, progression-free survival (PFS), or overall survival (OS).
Table 7.
Summary of Responses, Tumor Burden, Progression-Free Survival, and Overall Survival
| Category |
Dose Levela |
|||
|---|---|---|---|---|
| 0–I | II | III | All | |
| mITT Patientsb | N = 3 | N = 6 | N = 6 | N = 15 |
| Median tumor burden (number × 10e9 cancer cells) | 13.5 | 37.1 | 38.8 | ND |
| Best response | ||||
| RECIST v.1.0 (n = 15) | 3 SD | 1 PR, 5 SD | 1 CR, 1 PR, 4 SD | 1 CR, 2 PR, 12 SD |
| PET (n = 15) | 1 PR, 2 SD | 1 PR, 5 SD | 1 CR, 2 PR, 3 SD | 1 CR, 4 PR, 10 SD |
| Choi (n = 14) | 1 PR, 2 SD | 2 PR, 4 SD | 1 CR, 3 PR, 1 SD | 1 CR, 5 PR, 8 SD |
| Median PFS (month) | ||||
| RECIST | 2.7 | 4.0 | 5.6 | ND |
| PET | 2.7 | 4.1 | 4.2 | ND |
| Choi | 2.7 | 4.1 | 6.9 | ND |
| Median OS (month) | 4.3 | 9.2 | 9.2 | ND |
| OS (%) | ||||
| 1 year | 0 | 33.3 | ||
| 1.5 years | 25.0 | |||
| ITT populationc | N = 6 | N = 7 | N = 7 | N = 20 |
| Median OS (month) | 2.6 | 9.0 | 7.8 | ND |
| OS (%) | ||||
| 1 year | 0 | 28.6 | ND | |
| 1.5 years | 0 | 21.4 | ND | |
| Number Alived | 0 | 0 | 1 10-year survivor in remission | 1 10-year survivor in remission |
ITT, intent-to-treat; mITT, modified ITT; RECIST v.1.0, Response Evaluation Criteria in Solid Tumors; PET, positron emission tomography; Choi, modified RECIST as described by Choi et al.;20 PFS, progression-free survival; OS, overall survival; ND, not determined; BIW, two times a week; TIW, three times a week.
Dose level 0 = 1 × 1011 CFU BIW, dose level I = 1 × 1011 CFU TIW, dose level II = 2 × 1011 CFU TIW, dose level III = 3 × 1011 CFU TIW.
mITT population defined as all patients who received at least one cycle and had a follow-up PET CT scan.
ITT population defined as all patients who received at least one infusion of Rexin-G.
As of December 1, 2018.
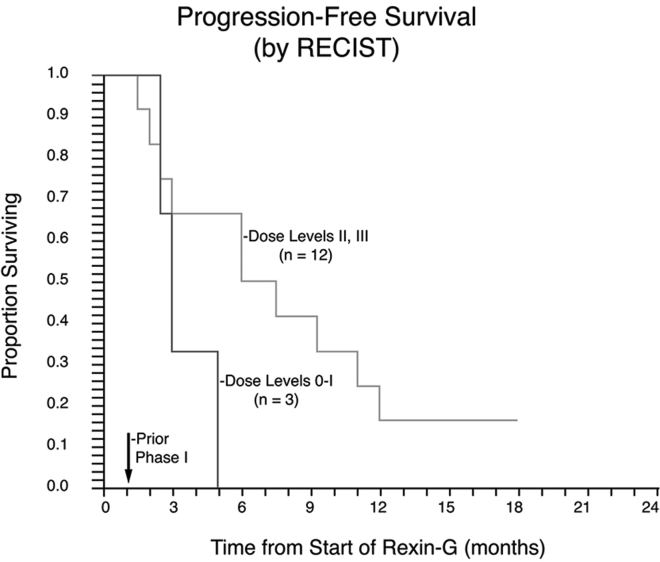
By RECIST, one patient achieved a complete response (CR), two patients had a partial response (PR), and 12 had stable disease (SD). The tumor control rate (CR + PR + SD) by RECIST v.1.0 was 100% (15/15 patients). Responses were more frequent when assessed using modified international PET criteria or Choi criteria. By PET, one patient achieved a CR, 4 patients had a PR, and 10 patients had SD. By Choi, one patient had a CR, 5 had a PR, and 8 had SD. One patient did not have a Choi analysis because the lesions were too small. By RECIST, PRs and CRs occurred only at dose levels II and III, suggesting a dose-dependent relationship between Rexin-G dose and response. PFS by RECIST was 2.7, 4.0, and 5.6 months at dose levels 0–I, II, and III, respectively, suggesting a dose-dependent relationship between Rexin-G dose and PFS. Kaplan-Meier analysis of PFS (Figure 2) in the mITT group suggested a trend toward a dose-response relationship between PFS and Rexin-G dosage. It is important to note that a higher tumor burden was observed for patients in doses II and III compared with doses 0–I, providing evidence in support of Rexin-G’s antitumor activity.
Figure 2.
Kaplan-Meier Plot of Progression-free Survival in Rexin-G-Treated Gemcitabine-Resistant PDAC
Progression-free survival data for the modified intent-to-treat population are displayed. The proportion of patients surviving progression-free are plotted on the vertical axis as a function of time from the beginning of treatment, plotted on the horizontal axis.
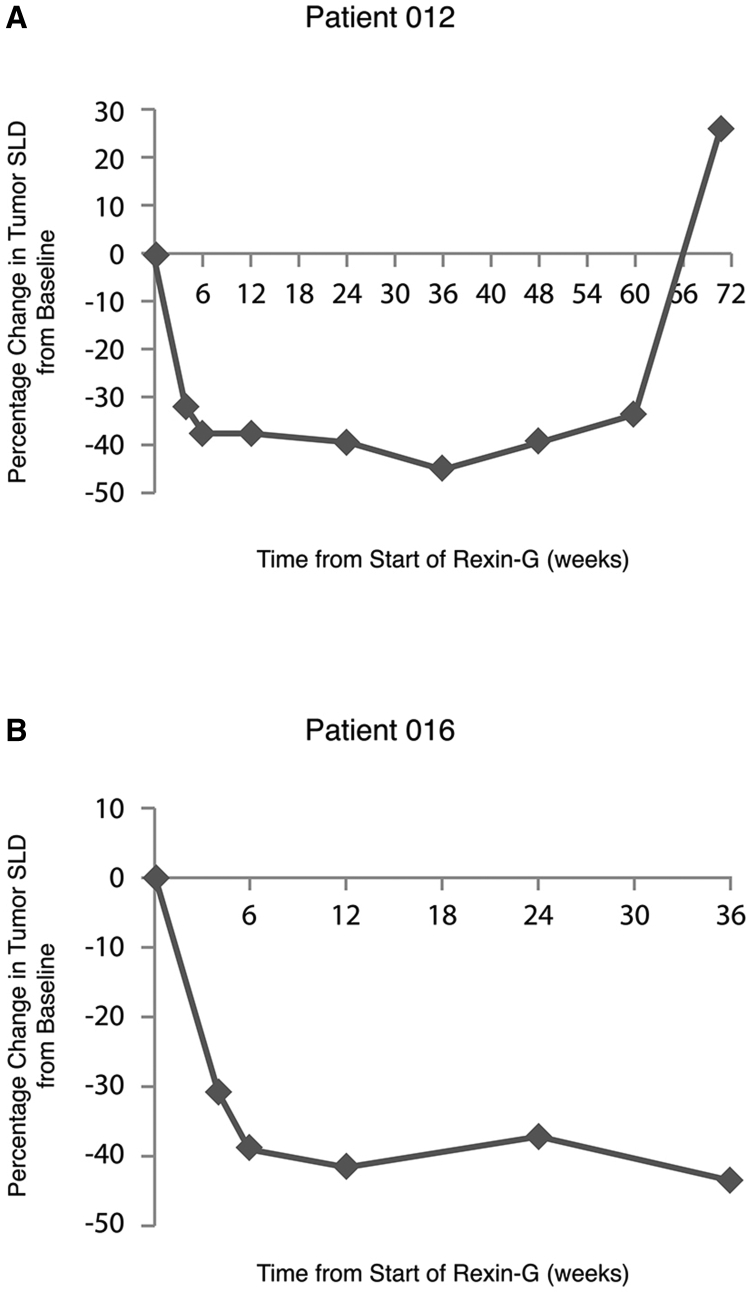
Table 8 shows three patients with durable tumor response patterns when assessed by RECIST, Choi, and PET (patients 12, 16, and 18). Patient 12-CJP is a 56-year-old white female with status post (s/p) biliary stent placement and radiation therapy for poorly differentiated PDAC, who failed gemcitabine and had target lesions at the pancreatic head medial to the biliary stent and right liver lobe. She achieved a best response of PR by RECIST at week 4 and continued in PR through week 36 (Figure 3A). She had definitive disease progression at week 60. Patient 016-JLM is a 59-year-old white female with poorly differentiated PDAC, who had failed four chemotherapy regimens, including gemcitabine, 5FU, oxaliplatin, and capecitabine, with target lesions at the head of pancreas and an aortocaval mass by the right hepatic lobe. She achieved a best response of PR by RECIST at weeks 4, 6, 12, 24, and 36 (Figure 3B). She discontinued treatment with Rexin-G on week 42 due to an AE (bile duct obstruction). She withdrew from the study due to symptomatic progression without a confirmatory CT scan.
Table 8.
Notable Tumor Response Patterns at Each Assessment Point for Patients with Partial and Complete Responses by RECIST v.1.0 Compared to Choi and PET Criteria
| Patient | Assessment Time Point (Week) | Overall Response by RECIST | Overall Response by Choi | Overall Response by PET |
|---|---|---|---|---|
| 12 | 4 | PR | SD | SD |
| 6 | PR | SD | PR | |
| 12 | PR | PR | PR | |
| 24 | PR | SD | SD | |
| 36 | PR | SD | PD | |
| 48 | SD | PD | PD | |
| 60 | PD | PD | PD | |
| 72 | PD | PD | PD | |
| 16 | 4 | PR | SD | SD |
| 6 | PR | SD | SD | |
| 12 | PR | PR | SD | |
| 24 | PR | PR | PD | |
| 36 | PR | PR | PD | |
| 18 | 4 | SD | SD | PD |
| 6 | SD | PR | PD | |
| 12 | SD | SD | SD | |
| 24 | PR | PR | PR | |
| 36 | CR | CR | CR | |
| 48 | CR | CR | CR | |
| 60 | CR | CR | CR | |
| 72 | CR | CR | CR |
Figure 3.
Tumor Regression during Treatment with Rexin-G
Patients 12 (A) and 16 (B). Percentage change in tumor size (sum of longest diameter [SLD]) is plotted on the vertical axis, as a function of time from the beginning of Rexin-G treatment, plotted on the horizontal axis.
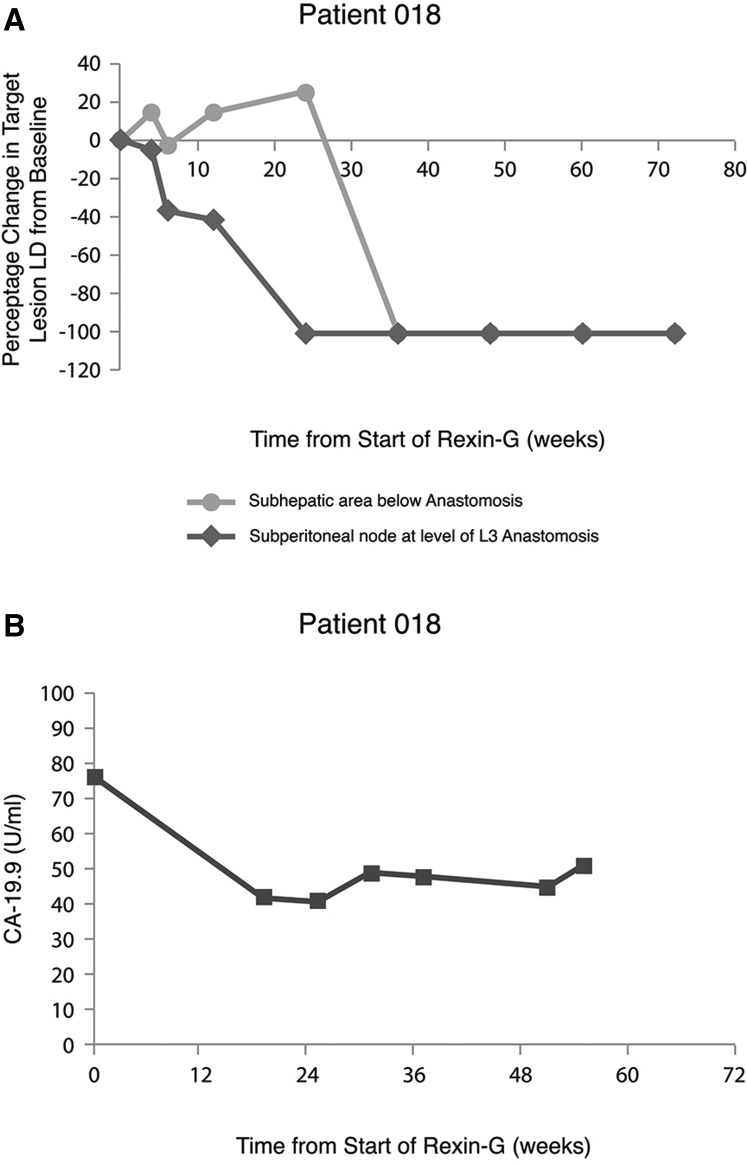
Patient 18 is a white female (72 years of age at study entry) who had been initially diagnosed with non-metastatic, poorly differentiated adenocarcinoma of the pancreas; she underwent a Whipple’s resection with postoperative radiation therapy, and she received chemotherapy with 5FU and gemcitabine for 1 year. A year later, she presented with hepatic and lymph node metastases (target lesions) and mesenteric stranding indicative of peritoneal carcinomatosis (non-target lesions) with a rising serum CA19.9 level. She was advised to receive further chemotherapy, but she decided in favor of participating in the phase I-II study using Rexin-G. The patient completed a total of 17.9 months of therapy with Rexin-G, and she did not achieve CR until week 36 of treatment; this patient has remained in CR at the time of study completion. Notably, when examined separately, the two target lesions were found to have different disappearance profiles. As shown in Figure 4A, the lymph node metastasis decreased more rapidly, starting at week 6, than the hepatic metastasis, which increased in size before ultimately completely resolving in week 36. Serum level of the tumor marker CA-19.9 decreased by 45%, from 76 to 42 U/mL (normal level is <37 U/mL) by week 19, and then it remained relatively constant thereafter (Figure 4B). She received no additional chemotherapy or alternative treatment after the discontinuation of Rexin-G therapy, and she remains in sustained remission with no evidence of disease or late-onset AEs as of November 2018.
Figure 4.
Tumor Regression and CA-19.9 Levels during Treatment with Rexin-G in Patient 18
(A) Percentage changes in tumor size (longest diameter [LD]) of metastatic hepatic and lymph node sub-peritoneal lesions are individually plotted on the vertical axis, as a function of time from the beginning of Rexin-G treatment, plotted on the horizontal axis. (B) Serum levels of tumor marker CA-19.9 (U/mL) are plotted on the veritical axis, as a function of time from the beginning of Rexin-G treatment, plotted on the horizontal axis.
Of note, continuation of treatment contributed to efficacy or clinical benefit in at least five patients. These patients survived from 10.6 months to 10 years after starting Rexin-G. Antitumor effects differed for individual target lesions in some patients. These data suggest that patients may benefit from extended treatment with Rexin-G despite signs of apparent progression (pseudoprogression), which may result from the known mechanism of action of Rexin-G: induction of apoptosis via cell cycle blockade of cancer cells, tumor vasculature, and malignant tumor-associated fibroblasts without bone marrow suppression, which may initially cause lesions to appear larger due to inflammatory or immunologic responses seen in published reports.20, 21, 22
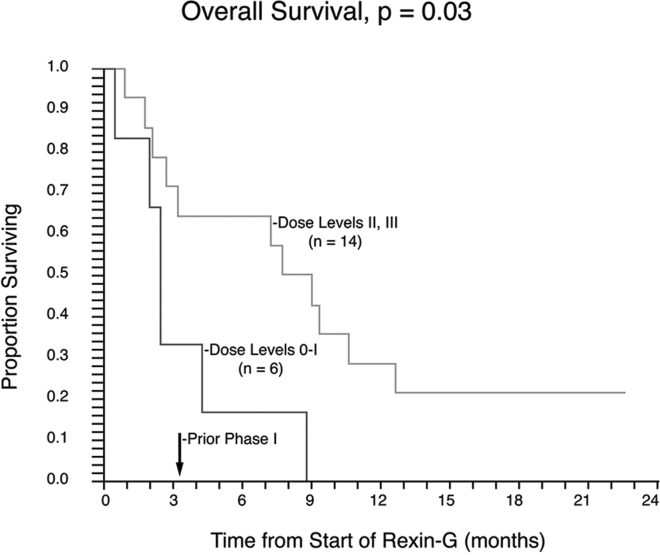
Median OS in the mITT group was calculated to be 4.3 months at dose 0–I, 9.2 months at dose II, and 9.2 months at dose III. The OS estimates in the efficacy evaluable mITT population among the combined groups of dose levels 0–I was 0% at 1 year. In contrast, OS estimates in the combined groups dose levels II–III were 33.3% at 1 year and 25% at 1.5 years. The median OS in the ITT group was 2.6 months in the dose 0–I cohort versus 9.0 and 7.8 months in the dose II and III cohorts, respectively. The OS rates in the dose 0–I group was 0% at 1 year. In contrast, OS rates among the combined groups of dose II–III were 28.6% at 1 year and 21.4% at 1.5 years (p = 0.03) compared with dose 0–I. Kaplan-Meier analysis of OS in the ITT population suggests a dose-response relationship between OS and Rexin-G dosage (p = 0.03; Figure 5).
Figure 5.
Kaplan-Meier Plot of Overall Survival of PDAC Patients following Rexin-G Treatment at Escalating Dose Levels
Analysis of overall survival in the ITT population suggests a dose-response relationship between overall survival and Rexin-G dosage (p = 0.03). The proportion of patients surviving are plotted on the vertical axis as a function of time from beginning of treatment, plotted on the horizontal axis.
Using the one-sided Fisher’s test, we compared tumor control responses (TCRs by RECIST) in this advanced phase I-II study (n = 15; TCR 1 CR, 2 PR, and 12 SD) with those in the prior phase I study where patients received up to a total dose of 6 × 1011 CFU per cycle (TCR 1 SD and 11 progressive disease [PD]).14 With “tumor control response” designated as CR, PR, or SD at any given time during the Rexin-G treatment period, the proportions are 15/15 for the current study and 1/12 in the prior study, with p < 0.0001 by the one-sided Fisher’s test. These data indicate a dose-response relationship between TCR and Rexin-G dosage across studies.
Discussion
This report updates and extends a phase I-II study of safety and efficacy using Rexin-G in gemcitabine-refractory PDAC with additional analysis and new mechanistic insights. The initial clinical data were previously reported on 13 patients by Chawla et al.19 Safety was established with no dose limiting toxicity (DLT) following multiple Rexin-G infusions at all four dose levels, and the maximum tolerated dose (MTD) was not reached. It is important to note that there was no treatment-related loss of hair, bone marrow suppression, or organ dysfunction at all dose levels. The serious AEs experienced by these patients were due to disease-related complications and not related to Rexin-G treatment, as assessed by the principal investigators. Further, there were no vector-related safety issues raised, as evidenced by no detected anti-vector-neutralizing antibodies, antibodies to gp70, replication-competent retrovirus in PBLs, or vector integration into genomic DNA of PBLs. These data indicate the exceptional safety of Rexin-G when compared to FDA-approved therapies such as nab-paclitaxel, gemcitabine, FOLFORINOX, and erlotinib.3, 4, 6, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32
Regarding efficacy, we report durable response rates (3/15; 20%) lasting 36–72 weeks during Rexin-G treatment. Two patients were progression-free for more than 1 year: patient 12 had a PFS of 13.8 months; patient 18 had a PFS >17.9 months. The best overall response rates (20%) noted in this study were significantly better than those reported by Galanis et al.,17 which used much lower Rexin-G doses. In support of this observation, a significant dose-response relationship was shown between OS and Rexin-G dose in the intent-to-treat population. Remarkably, one patient is still alive 10 years later with no evidence of PDAC; the documented eradication of cancer within the lymphatic system has compelling implications that warrant additional studies of the anaplastic Signature (SIG) proteins involved.33
Other viral gene therapy approaches for PDAC include an ongoing phase 1 trial combining oncolytic adenovirus-mediated cytotoxic and interleukin (IL)-12 gene therapy with chemotherapy in metastatic PDAC (https://www.clinicaltrials.gov/) and a recently completed phase III randomized, controlled clinical trial of PANVAC-VF for the treatment of patients with advanced pancreatic cancer. PANVAC-VF is a vaccine regimen composed of a priming dose of recombinant vaccinia virus and booster doses of recombinant fowl pox virus expressing carcinoembryonic antigen, mucin-1, and a triad of costimulatory molecules (TRICOM), given subcutaneously, followed by injection of recombinant granulocyte-macrophage colony-stimulating factor at the vaccination site.34, 35 However, the phase III randomized trial did not meet its primary endpoint of improving OS when compared with the physician’s choice of palliative therapy.36 Both viral gene therapies for PDAC involve either intratumor or subcutaneous viral vector injections. In contrast, Rexin-G involves a systemically (intravenously) administered tumor-targeted gene delivery approach (Figure 1).10, 13, 14, 18, 19, 22, 37, 38
Rexin-G is a potent inhibitor of the human cyclin G1 pathway (CCNG1 proto-oncogene). CCNG1 gene expression plays a powerful executive role in cell cycle regulation, exerting significant influence on critical oncogenic drivers, including the potent Mdm2 and cMyc oncoproteins and the p53 tumor suppressor protein, gatekeeper of DNA fidelity.38 CCNG1 is overexpressed in over 50% of various malignancies, including pancreas, breast, prostate, ovarian, and colon cancer.39 Albeit a small study in patient number, the single-agent antitumor activity of Rexin-G in metastatic PDAC is evident. In addition to the single-agent efficacy observed in the oncology clinic, molecular mechanisms were histologically revealed, as repeated intravenous infusion of Rexin-G induced the apoptosis of cancer cells, stromal fibroblasts, and associated tumor vasculature in biopsied tumors of Rexin-G-treated patients.12, 19, 40 Conceivably, patients whose tumors overexpress CCNG1, revealing a pathological distortion in growth control pathways, will respond favorably to cyclin G1 inhibitor therapy, delivered precisely. Studies currently in progress aim to confirm that the Rexin-G-induced tumor eradication observed histologically by enforced apoptosis of cancer cells, as well as supportive neo-vasculature and associated malignant fibroblasts of the TME, is the executive mechanism of Rexin-G anticancer activity. Moreover, the demonstrated eradication of refractory, chemoresistant pancreatic cancer (that is, progressive eradication upon continued intravenous infusions) is certainly noteworthy and potentially important—prompting us to closely examine the anaplastic Signature (Sig) proteins with an aim toward further optimizing these pioneering aspects of tumor-targeted gene delivery in future investigations.33
In conclusion, the clinical data gleaned from this phase I-II study of precision, tumor-targeted genetic medicine suggest that (1) Rexin-G is exceptionally safe with a wide margin of safety, and (2) Rexin-G exhibits dose-dependent antitumor activity in patients with gemcitabine-refractory metastatic PDAC. Based on the analysis of clinical data, Rexin-G gained fast-track designation from the FDA for the conduct of a planned phase II-III study using the optimal dose level III treatment schedule of Rexin-G versus physician’s choice in a larger number of patients. This planned phase II-III study will include correlations of CCNG1 gene expression in tumors, along with pertinent companion diagnostics, histology, and treatment outcome parameters.
Materials and Methods
Study Drug and FDA-Approved Vector Production
Rexin-G (Figure 1) is a non-replicative murine leukemia virus (MLV)-based amphotropic retrovector displaying a cryptic collagen-binding motif on its gp70 surface membrane, for targeting abnormal Signature (SIG) proteins in the TME,10, 33 and encoding a dominant-negative mutant construct (dnG1) of CCNG1.11 The vector also contains a neor gene, which is driven by the SV40 early promoter. The Rexin-G vector is produced by transient co-transfection of HEK293T cells. Clinical vector production and characterization have been described elsewhere.15, 16, 17 The final product exhibits a vector titer of 5 × 109 CFU/mL, a biologic potency of 50%–70% growth inhibitory activity in target cancer cells, less than 550 bp residual DNA, no detectable E1A or SV40 large T antigen, and no detectable replication-competent retrovirus (RCR), in compliance with FDA recommendations for retroviral vector-based gene therapy products.41 The vector formulation is stored in aliquots of 23 mL in a 30-mL glass vial or 40 ml in 150 ml cryobag and kept frozen at −70° to −90°C until used. Preparation of the Rexin-G vector for patient administration consisted of rapid thawing of the vector in the vial or cryobag in a 34°C water bath. The vector was thawed 15–30 min prior to infusion into the patient and given intravenously at 4 mL/min.18, 19 All personnel who handled and disposed of the vector observed Biosafety level 2 compliance in accordance with the NIH Guidelines for Research Involving Recombinant DNA molecules.
Vector-Related Testing and Biodistribution Studies
Detection of anti-vector antibodies in serum, testing for the presence of RCR, and vector DNA integration studies in patient PBLs were performed as described previously.17
Study Design
This was an open-label, single-arm, dose-seeking study that incorporated a modification of the standard Cohort of 3 design, which allowed patients to continue the study drug into phase II.18, 19 Treatment with Rexin-G comprised 6-week cycles that encompassed 4 weeks of treatment, followed by 2 weeks of rest. Four dose levels were given, beginning at 1.0 × 1011 CFU given by intravenous (i.v.) infusion two times per week. Three patients were to be treated at each dose level with expansion to 6 patients per cohort if DLT was observed in any 1 of the first 3 patients at each dose level. The MTD was defined as the highest dose in which 0 of 3 or ≤1 of 6 patients experienced a DLT, with the next higher dose level having at least 2 patients who experienced a DLT. A DLT was defined as any National Cancer Institute Common Toxicity Criteria for Adverse Events (CTCAE) grade 3, 4, or 5 AE, considered possibly, probably, or definitely related to the study drug, excluding the following: grade 3 absolute neutrophil count lasting <72 h; grade 3 alopecia; or any grade 3 or higher incident of nausea, vomiting, or diarrhea in a patient who did not receive maximal supportive care.21
Adaptive Design
For the phase II part of the study, patients who had no toxicity or in whom toxicity had resolved to grade 1 or less could receive additional cycles of therapy. Protocol Amendments I and II permitted an intrapatient dose escalation up to dose level II for patients who had no toxicity or in whom toxicity had resolved to grade 1 or less, once safety had been established at the higher dose level in a simultaneously conducted phase I-II study for sarcoma.18 Additionally, each cohort also could be expanded to 6 or 7 patients if significant biologic activity (SD or better) was noted at each dose level. The principal investigator was allowed to recommend surgical resection or debulking after at least one treatment cycle has been completed. Response was evaluated first using the RECIST v1.0.42 Additional evaluations used the International PET criteria43 and a modified RECIST, as described by Choi et al.20 Safety and efficacy analyses were conducted by the site principal investigators (S.P.C., H.B., and M.A.M.).
Patient Population and Treatment
Inclusion Criteria
Candidates included in the study had to have a histologically or cytologically confirmed pathologic diagnosis of advanced or metastatic PDAC that was resistant to gemcitabine or a gemcitabine-containing regimen; be ≥18 years of age; have an Eastern Cooperative Oncology Group (ECOG) performance score of 0–1; and acceptable hematologic, hepatic, and kidney functions.
Exclusion Criteria
Exclusion criteria included HIV, hepatitis B virus, or hepatitis C virus positivity; clinically significant ascites; medical or psychiatric conditions that could compromise proper adherence to the protocol; and unwillingness to employ effective contraception during treatment with Rexin-G and for 6 weeks following treatment completion.
The clinical protocol was reviewed and approved by the Western Institutional Review Board, Olympia, WA. The patients were recruited on a first-come first-served basis, and a written informed consent was obtained from each patient at the time of enrollment. All personnel who handled and disposed of the vector observed Biosafety level 2 compliance in accordance with the NIH Guidelines for Research Involving Recombinant DNA molecules.
Evaluation of Tumor Burden
Estimated tumor burden was determined for each patient using the following formula:
*Note: 20 × 10e9 cancer cells for each occurrence of ascites, pleural effusion, and/or too-many-to-count non-target lesions.
Safety Analysis
Pretreatment evaluation included history; physical exam; complete blood count with differential and platelet count; a serum chemistry panel, including aspartate transaminase, alanine transaminase, alkaline phosphatase, creatinine, and total bilirubin; assessment of coagulation status, including prothrombin time, international normalized ratio, and activated partial thromboplastin time; and testing for HIV, hepatitis B virus, and hepatitis C virus. All patients had a complete blood count and serum chemistry panel performed weekly during treatment. Toxicity was evaluated before each vector infusion, as well as before beginning an additional treatment cycle. Toxicity was graded using NCI CTCAE version 321and MedDRA. Patients’ serum was collected for the detection of vector-neutralizing antibodies and antibodies to gp70. The peripheral blood mononuclear cells were also collected to test for the presence of vector DNA integration and RCR at the end of 4 weeks, at 6 weeks, or before the start of a treatment cycle. Vector-related studies were performed as previously described.18 Rexin-G was stored in volumes of 23 mL in 30-mL vials or 40 mL in 150-mL cryobags at −70 to −90°C. Preparation of the vector for patient administration consisted of rapid thawing in the vial in a 34°C water bath 15–30 min prior to infusion, and it was given intravenously over 5–10 min. All personnel who handled and disposed of the vector observed Biosafety level 2 compliance in accordance with the NIH Guidelines for Research Involving Recombinant DNA molecules.
Efficacy Analysis
Prior to beginning treatment, imaging evaluations such as whole-body fluorodeoxyglucose (FDG)-PET-CT scan, electrocardiography, and chest X-ray were performed. FDG-PET-CT scan was done for efficacy assessment at the end of 4 weeks, at the end of 6 weeks, or before starting an additional treatment cycle up to 12 weeks and every 12 weeks thereafter. RECIST v.1.0 criteria was used to assess the tumor responses (CR, PR, or SD).42 Tumor control rate was defined as the percentage of patients who had CR, PR, or SD at any time during the Rexin-G treatment period. Tumor responses were also evaluated using modifications of the international PET criteria43 and the Choi criteria.20 The modified international PET criteria define a CR as disappearance of FDG avid uptake in target and non-target lesions with no new lesions; PR as a decrease in maximum standard uptake value of >25% from baseline with no new lesions along with no obvious progression of non-target lesions; PD as an increase in maximum standard uptake value of >25% from baseline, any new lesions, and obvious progression of non-target lesions; and SD as not meeting the criteria for CR, PR, or PD, and no symptomatic deterioration attributed to tumor progression. The modified Choi criteria define CR as the disappearance of all disease and no new lesions; PR as a decrease in size of ≥10% or a decrease in CT density (Hounsfeld units) ≥15% with no new lesions and no obvious progression of non-measurable disease; PD as an increase in tumor size of >10% and did not meet criteria for PR by CT density, any new lesions, including new tumor nodules in a previously cystic tumor; and SD as not meeting the criteria for CR, PR, or PD, and no symptomatic deterioration attributed to tumor progression.
Author Contributions
S.P.C., H.B., and M.A.M. are the principal investigators of the clinical trial; they conducted the study; evaluated patients’ tumor responses, survival, and safety; and wrote parts of the manuscript and reviewed and edited the final manuscript. E.M.G. and F.L.H. wrote the clinical protocol and informed consent, submitted the investigational new drug (IND) application to the FDA, wrote parts of the manuscript, reviewed the published literature, oversaw the clinical trial, and reviewed and edited the final manuscript. N.A. reviewed the clinical data and published literature, wrote the manuscript, and edited and reviewed the final manuscript. All authors approved the submission of the manuscript for publication.
Conflicts of Interest
E.M.G. and F.L.H. are co-inventors of Rexin-G, including its targeted gene delivery system, which was originally developed at the University of Southern California Keck School of Medicine, and they are co-founders of Delta Next-Gene, LLC. The other authors have no competing interests.
Acknowledgments
The authors gratefully acknowledge Heather C. Gordon for graphic illustrations and previous funding from the National Science Foundation, NIH, NATO Scientific Exchange Program, American Heart Association, and the Whittier Family Foundation in support of this pioneering biomedical research. Supported in part by an RO1 grant from the FDA Office of Orphan Products Division awarded to F.L.H. and E.M.G. The authors understand that the materials included in the manuscript, including all relevant raw data, will be made freely available to any researchers who wish to use this for non-commercial purposes, while preserving any necessary confidentiality and anonymity. The clinical protocol was approved by the FDA, the Western IRB, and the Institutional Biosafety Committee. A written informed consent was obtained from each patient prior to treatment with Rexin-G.
References
- 1.Rahib L., Smith B.D., Aizenberg R., Rosenzweig A.B., Fleshman J.M., Matrisian L.M. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society (2018). Cancer facts & figures 2018. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2018/cancer-facts-and-figures-2018.pdf.
- 3.Conroy T., Desseigne F., Ychou M., Bouché O., Guimbaud R., Bécouarn Y., Adenis A., Raoul J.L., Gourgou-Bourgade S., de la Fouchardière C., Groupe Tumeurs Digestives of Unicancer. PRODIGE Intergroup FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 4.Von Hoff D.D., Ervin T., Arena F.P., Chiorean E.G., Infante J., Moore M., Seay T., Tjulandin S.A., Ma W.W., Saleh M.N. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang-Gillam A., Li C.P., Bodoky G., Dean A., Shan Y.S., Jameson G., Macarulla T., Lee K.H., Cunningham D., Blanc J.F., NAPOLI-1 Study Group Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016;387:545–557. doi: 10.1016/S0140-6736(15)00986-1. [DOI] [PubMed] [Google Scholar]
- 6.Moore M.J., Goldstein D., Hamm J., Figer A., Hecht J.R., Gallinger S., Au H.J., Murawa P., Walde D., Wolff R.A., National Cancer Institute of Canada Clinical Trials Group Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y., Elsawa S.F., Almada L.L. Primers on molecular pathways - cycling toward pancreatic cancer. Pancreatology. 2010;10:6–13. doi: 10.1159/000283646. [DOI] [PubMed] [Google Scholar]
- 8.Matera R., Saif M.W. New therapeutic directions for advanced pancreatic cancer: cell cycle inhibitors, stromal modifiers and conjugated therapies. Expert Opin. Emerg. Drugs. 2017;22:223–233. doi: 10.1080/14728214.2017.1362388. [DOI] [PubMed] [Google Scholar]
- 9.Waehler R., Russell S.J., Curiel D.T. Engineering targeted viral vectors for gene therapy. Nat. Rev. Genet. 2007;8:573–587. doi: 10.1038/nrg2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall F.L., Liu L., Zhu N.L., Stapfer M., Anderson W.F., Beart R.W., Gordon E.M. Molecular engineering of matrix-targeted retroviral vectors incorporating a surveillance function inherent in von Willebrand factor. Hum. Gene Ther. 2000;11:983–993. doi: 10.1089/10430340050015293. [DOI] [PubMed] [Google Scholar]
- 11.Xu F., Prescott M.F., Liu P.X., Chen Z.H., Liau G., Gordon E.M., Hall F.L. Long term inhibition of neointima formation in balloon-injured rat arteries by intraluminal instillation of a matrix-targeted retroviral vector bearing a cytocidal mutant cyclin G1 construct. Int. J. Mol. Med. 2001;8:19–30. doi: 10.3892/ijmm.8.1.19. [DOI] [PubMed] [Google Scholar]
- 12.Gordon E.M., Hall F.L. Rexin-G, a targeted genetic medicine for cancer. Expert Opin. Biol. Ther. 2010;10:819–832. doi: 10.1517/14712598.2010.481666. [DOI] [PubMed] [Google Scholar]
- 13.Gordon E.M., Liu P.X., Chen Z.H., Liu L., Whitley M.D., Gee C., Groshen S., Hinton D.R., Beart R.W., Hall F.L. Inhibition of metastatic tumor growth in nude mice by portal vein infusions of matrix-targeted retroviral vectors bearing a cytocidal cyclin G1 construct. Cancer Res. 2000;60:3343–3347. [PubMed] [Google Scholar]
- 14.Gordon E.M., Chen Z.H., Liu L., Whitley M., Liu L., Wei D., Groshen S., Hinton D.R., Anderson W.F., Beart R.W., Jr., Hall F.L. Systemic administration of a matrix-targeted retroviral vector is efficacious for cancer gene therapy in mice. Hum. Gene Ther. 2001;12:193–204. doi: 10.1089/104303401750061258. [DOI] [PubMed] [Google Scholar]
- 15.Gordon E.M., Cornelio G.H., Lorenzo C.C., 3rd, Levy J.P., Reed R.A., Liu L., Hall F.L. First clinical experience using a ‘pathotropic’ injectable retroviral vector (Rexin-G) as intervention for stage IV pancreatic cancer. Int. J. Oncol. 2004;24:177–185. [PubMed] [Google Scholar]
- 16.Gordon E.M., Lopez F.F., Cornelio G.H., Lorenzo C.C., 3rd, Levy J.P., Reed R.A., Liu L., Bruckner H.W., Hall F.L. Pathotropic nanoparticles for cancer gene therapy Rexin-G IV: three-year clinical experience. Int. J. Oncol. 2006;29:1053–1064. [PubMed] [Google Scholar]
- 17.Galanis E., Carlson S.K., Foster N.R., Lowe V., Quevedo F., McWilliams R.R., Grothey A., Jatoi A., Alberts S.R., Rubin J. Phase I trial of a pathotropic retroviral vector expressing a cytocidal cyclin G1 construct (Rexin-G) in patients with advanced pancreatic cancer. Mol. Ther. 2008;16:979–984. doi: 10.1038/mt.2008.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chawla S.P., Chua V.S., Fernandez L., Quon D., Saralou A., Blackwelder W.C., Hall F.L., Gordon E.M. Phase I/II and phase II studies of targeted gene delivery in vivo: intravenous Rexin-G for chemotherapy-resistant sarcoma and osteosarcoma. Mol. Ther. 2009;17:1651–1657. doi: 10.1038/mt.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chawla S.P., Chua V.S., Fernandez L., Quon D., Blackwelder W.C., Gordon E.M., Hall F.L. Advanced phase I/II studies of targeted gene delivery in vivo: intravenous Rexin-G for gemcitabine-resistant metastatic pancreatic cancer. Mol. Ther. 2010;18:435–441. doi: 10.1038/mt.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi H., Charnsangavej C., Faria S.C., Macapinlac H.A., Burgess M.A., Patel S.R., Chen L.L., Podoloff D.A., Benjamin R.S. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J. Clin. Oncol. 2007;25:1753–1759. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 21.NCI Common terminology criteria for adverse events v3.0. 2006. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf
- 22.Dy P.S.G., Chawla S.P., Hall F.L., Gordon E.M. Immune cell trafficking in the tumor microenvironment of human cyclin G1 (CCNG1) inhibitor-treated tumors. Br. J. Cancer Res. 2018;1:202–207. [Google Scholar]
- 23.Burris H.A., 3rd, Moore M.J., Andersen J., Green M.R., Rothenberg M.L., Modiano M.R., Cripps M.C., Portenoy R.K., Storniolo A.M., Tarassoff P. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J. Clin. Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 24.Berlin J.D., Catalano P., Thomas J.P., Kugler J.W., Haller D.G., Benson A.B., 3rd Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J. Clin. Oncol. 2002;20:3270–3275. doi: 10.1200/JCO.2002.11.149. [DOI] [PubMed] [Google Scholar]
- 25.Rocha Lima C.M., Green M.R., Rotche R., Miller W.H., Jr., Jeffrey G.M., Cisar L.A., Morganti A., Orlando N., Gruia G., Miller L.L. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J. Clin. Oncol. 2004;22:3776–3783. doi: 10.1200/JCO.2004.12.082. [DOI] [PubMed] [Google Scholar]
- 26.Heinemann V., Quietzsch D., Gieseler F., Gonnermann M., Schönekäs H., Rost A., Neuhaus H., Haag C., Clemens M., Heinrich B. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J. Clin. Oncol. 2006;24:3946–3952. doi: 10.1200/JCO.2005.05.1490. [DOI] [PubMed] [Google Scholar]
- 27.Colucci G., Giuliani F., Gebbia V., Biglietto M., Rabitti P., Uomo G., Cigolari S., Testa A., Maiello E., Lopez M. Gemcitabine alone or with cisplatin for the treatment of patients with locally advanced and/or metastatic pancreatic carcinoma: a prospective, randomized phase III study of the Gruppo Oncologia dell’Italia Meridionale. Cancer. 2002;94:902–910. [PubMed] [Google Scholar]
- 28.Louvet C., Labianca R., Hammel P., Lledo G., Zampino M.G., André T., Zaniboni A., Ducreux M., Aitini E., Taïeb J., GERCOR. GISCAD Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J. Clin. Oncol. 2005;23:3509–3516. doi: 10.1200/JCO.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 29.Poplin E., Feng Y., Berlin J., Rothenberg M.L., Hochster H., Mitchell E., Alberts S., O’Dwyer P., Haller D., Catalano P. Phase III, randomized study of gemcitabine and oxaliplatin versus gemcitabine (fixed-dose rate infusion) compared with gemcitabine (30-minute infusion) in patients with pancreatic carcinoma E6201: a trial of the Eastern Cooperative Oncology Group. J. Clin. Oncol. 2009;27:3778–3785. doi: 10.1200/JCO.2008.20.9007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herrmann R., Bodoky G., Ruhstaller T., Glimelius B., Bajetta E., Schüller J., Saletti P., Bauer J., Figer A., Pestalozzi B., Swiss Group for Clinical Cancer Research. Central European Cooperative Oncology Group Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J. Clin. Oncol. 2007;25:2212–2217. doi: 10.1200/JCO.2006.09.0886. [DOI] [PubMed] [Google Scholar]
- 31.Burris H., 3rd, Rocha-Lima C. New therapeutic directions for advanced pancreatic cancer: targeting the epidermal growth factor and vascular endothelial growth factor pathways. Oncologist. 2008;13:289–298. doi: 10.1634/theoncologist.2007-0134. [DOI] [PubMed] [Google Scholar]
- 32.Senderowicz A.M., Johnson J.R., Sridhara R., Zimmerman P., Justice R., Pazdur R. Erlotinib/gemcitabine for first-line treatment of locally advanced or metastatic adenocarcinoma of the pancreas. Oncology (Williston Park) 2007;21:1696–1706. [PubMed] [Google Scholar]
- 33.Gordon, E.M., and Hall, F.L. Cyclin G1 inhibitors and related methods of treating cancer. US patent WO/2018/144863, filed February 2, 2018 and granted September 8, 2018.
- 34.Schuetz T., Kaufman H.L., Marshall J.L., Safran H. Extended survival in second-line pancreatic cancer after therapeutic vaccination. J. Clin. Oncol. 2005;23:2576. [Google Scholar]
- 35.Petrulio C.A., Kaufman H.L. Development of the PANVAC-VF vaccine for pancreatic cancer. Expert Rev. Vaccines. 2006;5:9–19. doi: 10.1586/14760584.5.1.9. [DOI] [PubMed] [Google Scholar]
- 36.FDA News (2018). Therion reports results of PANVAC-VF trial. https://www.fdanews.com/articles/87938-therion-reports-results-of-panvac-vf-trial.
- 37.Kim S., Federman N., Gordon E.M., Hall F.L., Chawla S.P. Rexin-G®, a tumor-targeted retrovector for malignant peripheral nerve sheath tumor: A case report. Mol. Clin. Oncol. 2017;6:861–865. doi: 10.3892/mco.2017.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gordon E.M., Ravicz J.R., Liu S., Chawla S.P., Hall F.L. Cell cycle checkpoint control: The cyclin G1/Mdm2/p53 axis emerges as a strategic target for broad-spectrum cancer gene therapy - A review of molecular mechanisms for oncologists. Mol. Clin. Oncol. 2018;9:115–134. doi: 10.3892/mco.2018.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ravicz J., Liu S., Andrali S.S., Reddy S.K., Sellappan S., Leong B., Chawla S.P., Hall F.L., Gordon E.M. Differential expression of human cyclin G1 (CCNG1) in cancer: A novel biomarker in development for CCNG1 inhibitor therapy. J. Clin. Oncol. 2018;36:e24315. [Google Scholar]
- 40.Hall F.L., Levy J.P., Reed R.A., Petchpud W.N., Chua V.S., Chawla S.P., Gordon E.M. Pathotropic targeting advances clinical oncology: tumor-targeted localization of therapeutic gene delivery. Oncol. Rep. 2010;24:829–833. doi: 10.3892/or.2010.829. [DOI] [PubMed] [Google Scholar]
- 41.FDA (2006). Guidance for industry: supplemental guidance on testing for replication competent retrovirus in retroviral vector based gene therapy products and during follow-up of patients in clinical trials using retroviral vectors. https://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/ucm078723.pdf. [DOI] [PubMed]
- 42.Therasse P., Arbuck S.G., Eisenhauer E.A., Wanders J., Kaplan R.S., Rubinstein L., Verweij J., Van Glabbeke M., van Oosterom A.T., Christian M.C., Gwyther S.G. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 43.Young H., Baum R., Cremerius U., Herholz K., Hoekstra O., Lammertsma A.A., Pruim J., Price P., European Organization for Research and Treatment of Cancer (EORTC) PET Study Group Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. Eur. J. Cancer. 1999;35:1773–1782. doi: 10.1016/s0959-8049(99)00229-4. [DOI] [PubMed] [Google Scholar]